Abstract
Purpose
Follow-up care (FU-care) and self-management are recognized as important to ensure prolonged effects of rehabilitation. Objectives of this study were to explore current FU-care and self-management after specialized rehabilitation for patients with rheumatic and musculoskeletal diseases.
Materials and methods
This multicentre cohort study included 523 patients who self-reported need and plans for FU-care and plans for self-management activities (SMAs) at rehabilitation discharge. The FU-care received and adherence to SMA were self-reported after 4-, 8-, and 12-months. Predictors for received FU-care and adherence to SMA were explored in multiple logistic regression models.
Results
Plans for FU-care were significantly associated with received FU-care. Younger age, better coping skills, and performing regular social activities and hobbies were significant predictors for received FU-care. Throughout the follow-up year, 221 (51%) participants had adherence to their SMA plans. Older age, regular physical activity, more severe pain, and performing regular social activities and hobbies were significant predictors for adherence to SMA. Participants with SMA adherence more often reported planned FU-care, and more frequently received the FU-care they needed.
Conclusions
Planning FU-care should be integrated in specialized rehabilitation. Patients with poor coping skills and sedentary lifestyle may need more support over longer time to implement behavioral changes for healthy self-management.
Planning follow-up should be integrated in specialized rehabilitation as it supports self-management and receiving follow-up at home.
Patients with sedentary lifestyle, poor coping skills, and depression may need more support over longer time to implement healthy self-management.
Structure and routines in daily life enhance self-management.
Implications for rehabilitation
Introduction
Rheumatic and musculoskeletal diseases (RMDs) are highly prevalent worldwide and have profound personal, economic, and social impacts on individuals and societies [Citation1–3]. The prevalence of RMDs is expected to rise in the future, due to increases in life expectancy and overweight status [Citation4]. Successful management of these conditions largely relies on the implementation of behavioral adjustments and healthy self-management [Citation5–8], which are commonly introduced to patients in multidisciplinary rehabilitation settings [Citation9,Citation10].
Self-management is the “individual’s ability to manage the symptoms, treatment, physical and psychological consequences, and life-style changes inherent in living with a chronic condition” [Citation7,Citation11], and described as the key to success in health-behavioral adjustments [Citation5]. Adherence to self-management improves active participation in the rehabilitation process [Citation6,Citation8] and it strengthens the planning and pacing of participation in everyday life activities [Citation7,Citation8,Citation12]. Adherence has commonly been described as the degree to which behavior and actions comply with established health care recommendations [Citation13–15]. However, adherence is considered a complex phenomenon [Citation14,Citation16]. Recent revisions to the definition of adherence add the implication of self-management, which includes the patient as an active participant in the rehabilitation process, rather than as a passive recipient [Citation5,Citation14–16]. A low rate of adherence in chronic conditions is associated with an additional burden for the health care systems [Citation17]. Although research has identified patient beliefs and resources as significant factors in adherence to health recommendations [Citation17,Citation18], we lack knowledge about factors that might predict adherence to healthy self-management in rehabilitation [Citation7].
Rehabilitation in specialized care leads to beneficial health effects, but the effects and maintenance of health-behavioral adjustments appear to be brief, and they decline over time [Citation9,Citation19–21]. The World Health Organization (WHO) defines rehabilitation as "a set of measures that assist individuals who experience, or are likely to experience, disability to achieve and maintain optimal functioning in interactions with their environment" [Citation22]. Moreover, for individuals with chronic conditions, rehabilitation is increasingly acknowledged as a long-term continuum of complex care [Citation23–25]. Recent evidence indicates a suboptimal transition between the rehabilitation process introduced in specialized care and the expected continuation in primary health care [Citation26], and that coordination across health care levels is the weakest element in the rehabilitation trajectory [Citation25,Citation27,Citation28]. Moreover, research evidence has emphasized that large variations exist in the content and quality of rehabilitation in rheumatology care [Citation21,Citation25,Citation29], noteworthy as variations in structure and process variables [Citation9,Citation26], as lack of patient and health care professional involvement in planning of follow-up care (FU-care) [Citation25,Citation26] and as variation in the design of follow-up interventions [Citation30]. Thus, it was suggested that rehabilitation trajectories should also include planned follow-up interventions and support in primary health care [Citation25,Citation27,Citation28,Citation31]. These changes were proposed to prolong the beneficial health effects and quality of specialized rehabilitation [Citation21,Citation23,Citation25], and maintain healthy behavioral adjustments [Citation5,Citation30,Citation32]. Nonetheless, patients have reported that they felt poorly prepared for self-managing activities in their everyday lives, and that they lacked knowledge on how to access proper support and health care services in their community after a rehabilitation discharge [Citation23,Citation32,Citation33].
Rehabilitation trajectories should be based on evidence from current practice to ensure adequate coordination and communication between health care levels, FU-care tailored to patient needs, and support of healthy self-management [Citation21]. Still, details in such evidence concerning the existing practices in RMD rehabilitation are limited. Therefore, the present study aimed to explore current FU-care, adherence to plans for self-management after specialized rehabilitation, and predictors that influence FU-care and adherence to self-management activities (SMAs) for patients with RMDs, to provide additional evidence on how to optimize rehabilitation trajectories in rheumatology care.
Materials and methods
Study design and participants
The present pragmatic, multicentre cohort study included patients with RMDs that had participated in in-patient or out-patient rehabilitation in specialized care in Norway between November 2015 and January 2017. Participants were enrolled by local project coordinators from four rheumatology hospital departments and five specialized rehabilitation institutions across Norway.
Participants were eligible when they were 18 years or older, had sufficient understanding of both spoken and written Norwegian language, had access to the internet, and had acquired a Bank-ID that allowed secure login to a digital data reporting system containing patient-reported questionnaires.
Inclusion criteria were one of the following diagnoses: inflammatory rheumatic diseases (spondyloarthritis (SpA), psoriatic arthritis (PsA), and rheumatoid arthritis (RA)), osteoarthritis, chronic low back pain, chronic neck/shoulder pain, chronic widespread pain (fibromyalgia), osteoporosis, connective tissue diseases (systemic lupus erythematosus (SLE), myositis, etc.), or fractures or orthopaedic surgery that required rehabilitation. Exclusion criteria were: reduced cognitive function or severe mental illness, determined at rehabilitation admission in the doctors’ anamnesis.
Data collection
Data were collected in self-reported questionnaires that addressed health status and function, FU-care, and SMAs. At both baseline and discharge, participants had personal guidance from a health care professional from the multidisciplinary rehabilitation team when completing the questionnaires, when reporting on needs and plans for FU-care, and on plans for SMAs in the digital data reporting system. At 4-, 8-, and 12-months after rehabilitation, participants received a text message and an e-mail with a link to the digital data reporting system to complete the questionnaires. One reminder was sent after one week to those that did not respond. The data collection was completed by January 2018.
Health status and function
Baseline data were collected on patient demographic characteristics (age, gender, body mass index (BMI), education level, and employment status), referral diagnoses, comorbidities, smoking status, and the frequency of physical and social activities. Furthermore, nine aspects of health and function were self-reported by completing the musculoskeletal disease (MSD) core set, a consensus-base set of outcome measures for rehabilitation in MSDs [Citation34]. The core set included Numeric Rating Scales (NRSs) for pain, fatigue, and motivation for goal attainment (scale: 0–10, where 0 = no pain, fatigue, or motivation) [Citation35]; physical fitness (the 30-s Sit-to-Stand test) [Citation36]; mental health (Hopkins Symptom Checklist (SCL-5); score range: 0–4, where 0 = no symptoms) [Citation37]; daily activities (Hannover Functional Questionnaire; range: 0–24, where 0 = best function) [Citation38]; goal attainment (Patient Specific Functional Scale (PSFS); range: 0–10, where 10 = best function) [Citation39]; health-related quality of life (EuroQol: 5 Dimensions of health status (EQ-5D) rated on a Visual Analogue Scale of 0–100, where 100 = best status) [Citation40]; social participation (the social participation item from the COOP/WONCA; range: 1–5, where 1 = highest participation) [Citation41]; and coping (Effective Musculoskeletal Consumer Scale-17 (EC-17); scale: 0–100, where 100 = best coping skills) [Citation42].
Follow-up care
In our study, FU-care comprises the care received in primary health care after rehabilitation, as reported by the participants. This includes the participants perceived need for FU-care, their perception on whether FU-care was planned before discharge from rehabilitation, and their experience on whether needed FU-care was received within the year after rehabilitation.
At discharge, participants self-reported their general needs and plans for FU-care, by responding yes/no to the following questions: (1) “Do you consider yourself in need of follow-up care after the rehabilitation stay?” and (2) “Is there any form of follow-up care planned?”. The FU-care received was self-reported at 4, 8, and 12 months after rehabilitation, by answering the following yes/no question: (3) “Have you received the follow-up care that you felt you needed from the primary health care system?”.
Additionally, participants self-reported the specific needs, plans, and receipt of primary health care from the following list of health care services: “general practitioner” (GP), “physiotherapy” (PT), “Norwegian Labour and Welfare Service” (Norwegian abbreviation is NAV), “occupational therapy” (OT), “nursing”, “psychiatric nurse”, “psychologist”, “new stay for multidisciplinary rehabilitation”, “community-based healthy life centre”, or “other”.
Self-management activities
The participants recorded plans for up to five SMAs at rehabilitation discharge. SMAs comprise the activities patients planned to do to maintain or improve healthy behavioral adjustments and/or reach their rehabilitation goals.
Adherence to each SMA was self-reported at 4, 8, and 12 months after rehabilitation, as follows: “I have mainly followed the plan” (score = 2), “I have occasionally followed the plan” (score = 1), or “I have not followed the plan” (score = 0).
To evaluate SMA adherence, we calculated an individual SMA adherence score for each participants’ self-reported SMAs, and dichotomized this score into adherence or non-adherence. We first calculated a potential maximum SMA adherence score for each participant as “the participants’ number of listed SMAs at discharge (ranging from 1 to 5)” multiplied by “the highest possible score for self-reporting adherence to each SMA (=2, ‘I have mainly followed the plan’)” multiplied by “the participants self-reported number of measurement time points after rehabilitation (ranging from 1 to 3 corresponding to reporting at 4, 8, and/or 12 months after rehabilitation)”. The potential maximum adherence score for each participant ranged from 6 (if only one SMA was listed and scored three times) to 30 (if five SMAs were listed and scored three times). Based on the actual scorings of each participant, an individual actual adherence score was calculated following the same methods as described above. Finally, an individual SMA adherence score was calculated as “the actual individual adherence score” divided by “the participants potential maximum adherence score”. The scores were normalized to 100 to allow us to report the results in percentages (0–100%, 100%=best score). If the individual SMA adherence score was more than 66% (i.e., 2/3 fulfilment of the potential maximum adherence score), the participant was categorized as adhering to their SMAs.
Statistical analyses
All statistical analyses were performed with IBM SPSS version 21 (Armonk, NY). Descriptive statistics were conducted with appropriate parametric or non-parametric tests at all measurement time points. Significance level was set to p < 0.05.
Multivariate logistic regression models were constructed to explore predictors of the FU-care received and of SMA adherence. We first explored potential predictive values with univariate associations between the dependent variable and the demographic baseline characteristics and the baseline scores for the health variables in the MSD core set. All independent variables with p < 0.25 from the univariate models were included in the adjusted multivariate models. Age and gender were forced into both models. The adjusted models were checked for multicollinearity and interacting variables.
Drop-out analysis on between group differences of the demographic baseline characteristics and the baseline scores for the health variables in the MSD core set were performed for participants and non-participants at 12 months after discharge from rehabilitation.
Ethics
Prior to signing informed consent forms, all participants received oral and written information about the study. All patients at the participating centres received the rehabilitation program and FU-care they would have received, irrespective of participation in the study. Inclusion protocols and data were anonymized and stored in password protected files. Ethical principles of the Helsinki Declaration and privacy requirements were followed, and the study was approved by the Norwegian Social Science Data Services, Oslo University Hospital (2015/16099).
Results
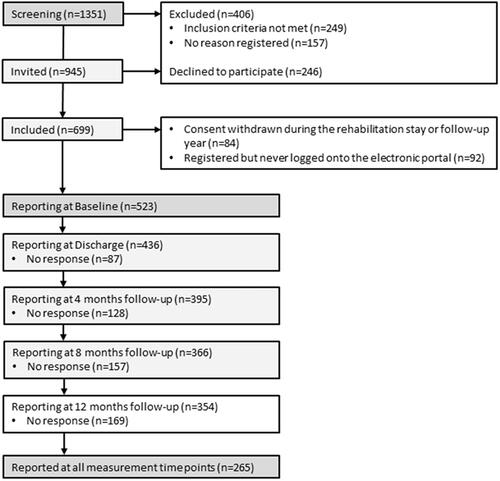
Of the 945 patients invited to participate after screening, 523 were enrolled in the study and completed the baseline assessments; at rehabilitation discharge, 436 (83%) patients completed the assessments. However, study attrition following rehabilitation discharge declined at 4, 8, and 12 months, and a total of 265 participants reported at all measurement time points (). Baseline characteristics are presented in . The majority of participants were female and middle aged, and nearly half were working. In total, 50% were diagnosed with an inflammatory rheumatic disease, and 29% were diagnosed with chronic widespread pain (fibromyalgia). More than 2/3 of patients reported that they carried out social activities and hobbies on a regular basis, and 2/3 self-reported that they were regularly physical active.
Table 1. Descriptive baseline data.
Non-participants at 12 months after rehabilitation were significantly younger (p= <0.001), more frequently were smoking (p = 0.003), had higher BMI (p = 0.03), shorter disease duration (p = 0.04), and were less physically active (p = 0.02) and social participating (p = 0.01). They more often reported depression (p = 0.01) and they were significantly more often diagnosed with chronic widespread pain (fibromyalgia) (p = 0.002) than the participants reporting at 12 months after rehabilitation.
Follow-up care
Self-reported needs and plans for follow-up care at discharge
Of the 436 participants that completed the self-reports at discharge, 429 (98%) reported a need for FU-care in primary health care, of which 72 (14%) participants reported a need for FU-care from one health care professional, 139 (27%) participants needed FU-care from two health care professionals, 118 (23%) from three health care professionals, and 68 (13%), 25 (5%), six (1%), and one (0.2%) from 4, 5, 6, and 7 health care professionals, respectively. A total of 400 (92%) participants reported FU-care needs from health care professionals other than a GP.
Of the 429 participants reporting a need for FU-care, 239 (56%) reported that FU-care was planned at discharge. Patients reported a need for FU-care from a GP (n = 362, 84%) most frequently, followed by FU-care from a PT (n = 357, 83%) and the NAV (n = 150, 35%). These health care professionals and services were also the most frequent types of planned FU-care reported at discharge ((n = 140, 39%), (n = 153, 43%), and (n = 53, 35%), respectively) (). Patients reported need for FU-care least frequently from a psychiatric nurse (n = 16, 4%) and a nurse (n = 4, 1%). The health care professionals and services least frequently planned at discharge were FU-care from a psychologist (n = 13, 33%) and a community-based healthy-life centre (n = 16, 30%), followed by FU-care from a new multidisciplinary rehabilitation stay (n = 14, 30%) and an OT (n = 17, 26%).
Figure 2. The numbers of participants that self-reported a need and a plan for follow-up care (FU-care) at discharge are shown together with the actual primary health care received during the follow-up year, from specific health care services (n = 429 participants).
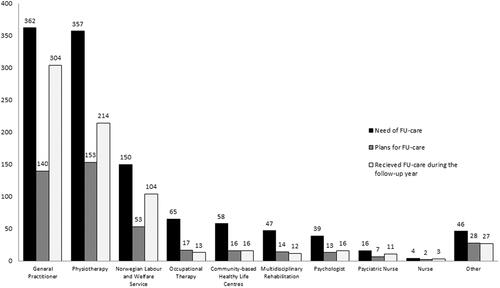
In the open category of “others” (n = 46, 11%), participants reported most frequently an FU-care need for “access to different exercise groups and other exercise facilities” (n = 9, 20%) and from a rheumatologist (n = 7, 15%). FU-care need from a psychomotor physiotherapist, a pain clinic, orthopaedist, social worker, chiropractor, dietitian, and a podiatrist were mentioned (n= <3, for each, respectively).
The chronic widespread pain (fibromyalgia) patients reported significantly more often a need for FU-care from the GP (p = 0.03), NAV (p = 0.01), psychologist (p < 0.001), and a community-based healthy-life centre (p = 0.04) compared to participants with inflammatory rheumatic diseases.
Receipt of follow-up care within the year after rehabilitation
shows the FU-care received within the year after rehabilitation. Of the 436 participants that completed self-reports at discharge, 211 (49%) received FU-care within 4 months, 271 (63%) received FU-care within 8 months, and 302 (70%) received FU-care within the year after rehabilitation. Further analysis revealed that having a plan for FU-care at discharge was significantly associated with receiving FU-care within the year after rehabilitation. Among participants that had received the FU-care they needed, 181 (60%) had a specific FU-care plan at discharge from rehabilitation. Among participants that had not received the FU-care they needed, 32 (42%) had a specific follow-up plan at discharge (p = 0.014).
Patients received FU-care most frequently from a GP (n = 304, 84%), a PT (n = 214, 60%), or the NAV (n = 104 69%), and less frequently from a psychologist (n = 16, 41%), a community-based healthy-life centre (n = 16, 28%), or an OT (n = 13, 20%).
Patients with chronic widespread pain (fibromyalgia) reported significantly less often received FU-care within the year after rehabilitation (p = 0.04) compared to participants with inflammatory rheumatic diseases.
The adjusted model for predicting the types of patients that were most likely to receive FU-care is shown in . No statistical interactions were found between the independent variables. We found three significant predictors that a patient would receive FU-care in the year after rehabilitation: younger age (adjusted odds ratio (aOR)=0.97, 95% confidence interval (95% CI): 0.94, 0.99; p = 0.02), better coping skills (EC-17) (aOR = 1.04, 95% CI: 1.02, 1.07; p= <0.001), and performing social activities and hobbies on a regular basis (aOR = 2.00, 95% CI: 1.14, 3.51; p = 0.02) at baseline.
Table 2. Prediction model of received follow-up care (FU-care).
Self-management activities
Of the 436 participants that completed the self-reports at discharge, 434 (99%) had recorded at least one plan for SMA after rehabilitation. At least three SMA plans were reported by 405 (93%) participants, and 4–5 SMA plans were reported by half the participants. In summary, the SMAs focused on physical health and general well-being by managing everyday routines and pacing, prioritizing rest and recovery, together with applying coping strategies and utilization of acquired new knowledge about health management.
Adherence to plans for self-management activities within the year after rehabilitation
In total, 221 (51%) participants were categorized with adherence to their SMAs throughout the year after rehabilitation. The adjusted model for predicting adherence is summarized in . No statistically significant interactions were found between the independent variables. The significant predictors of adherence to SMAs were an older age (aOR = 1.03, 95% CI: 1.01, 1.05; p = 0.01), performing physical activity on a regular basis (aOR = 3.84, 95% CI: 2.41, 6.12; p < 0.001), more severe pain (NRS Pain) (aOR = 1.14, 95% CI: 1.00, 1.30; p = 0.05), and performing social activities and hobbies on a regular basis (aOR = 1.66, 95% CI: 1.02, 2.71; p = 0.04) at baseline. One other factor that nearly reached significance was a low degree of depression and anxiety (SCL-5) (aOR = 0.79, 95% CI: 0.61, 1.02; p = 0.06).
Table 3. Prediction model of adherence to self-management activities (SMAs).
Associations between follow-up care and adherence to self-management activities
At discharge from rehabilitation, planned FU-care (59%) was reported more often by participants with adherence to SMAs compared to those without adherence to SMAs (51%) (p = 0.07). There was, however, a significant positive association between adherence to SMAs and received FU-care (p < 0.001).
Discussion
This study is one of the few studies to explore current FU-care and adherence to self-management in rehabilitation among individuals with RMDs. Our results revealed that the majority of patients experienced a need for FU-care after specialized rehabilitation. Patients most frequently reported that they needed care from a GP, but they also frequently needed care from a PT and the NAV. Nonetheless, only 56% of participants had plans for FU-care at discharge. Importantly, having FU-care plans was significantly associated with receiving FU-care in the year following rehabilitation. Additionally, we identified three patient characteristics that significantly predicted the receipt of FU-care: a younger age, better coping, and performing social activities and hobbies on a regular basis.
Our findings also showed that half of the participants had adherence to their planned SMAs. We identified four patient characteristics that significantly predicted SMA adherence: performing physical and social activities and hobbies on a regular basis, a higher age, and higher levels of pain. Adherence to SMAs was also associated with having a plan for FU-care and receiving FU-care more frequently. These associations highlight the importance of both providing support and encouraging active participation in the rehabilitation and self-management processes.
GPs are assigned the role of coordinating patient care, and they are typically charged with referring patients to rehabilitation with specialized health care. Consequently, the discharge report is routinely returned to the GP [Citation9]. However, the majority of individual consultations in Norwegian rehabilitation settings are provided by physical therapists [Citation9]. Indeed, the most common long-term goal put forward by participants in a previous rehabilitation study was to improve physical fitness [Citation43]. Thus, it was not surprising that the most frequently reported types of FU-care planned and received during the first year following rehabilitation were provided by GPs and PTs. Still, recent studies have indicated that closer collaboration between GPs and PTs might lead to an even higher quality of care and more integrated cooperation [Citation44].
Recently, political focus on supporting people with chronic conditions in continuing or returning to work has increased. Also, all people registered on sick leave or on Work Assessment Allowance (AAP) in Norway have a sanctuary requirement on regularly follow-up from the NAV. FU-care from the NAV is then initiated as social support rather than a direct consequence of the rehabilitation stay. This focus might explain why patients frequently reported the need and receipt of support from the NAV.
Participants less frequently reported the need for FU-care from an OT, a community-based healthy-life centre, or a psychologist, the probability of receiving those types of care was rather low. One reason for these findings could be the current lack of OTs and psychologists in primary care. Thus, the high numbers of patients receiving FU-care by GPs, PT, and the NAV compared to the small number for the other health care services might be related to how the Norwegian health care system organize the involvement and accessibility of the health services in primary care and not to whether the FU-care was planned in the first place. However, starting in 2020, OTs and psychologists are statutory services in Norwegian municipalities. Future research should evaluate whether this change will lead to improved access to those professionals.
Only half of the participants in our study had plans for FU-care at discharge. In two recent studies, using a newly developed quality of care indicator set for the rehabilitation of individuals with RMDs, planning FU-care and involving external health care professionals in this planning were the two quality indicators with lowest pass-rates [Citation25,Citation45]. Our study results support the notion that these are essential quality indicators, since having a plan for FU-care was significantly associated with receiving FU-care. We also found that a younger age, better coping skills, and being socially active on a regular basis were significant predictors of receiving FU-care. Moreover, participants in diagnosed with chronic widespread pain (fibromyalgia) reported less frequently received needed FU-care, which supports research indicating scarce primary health care services offered particularly for this patient group [Citation46,Citation47], even though international recommendations state that optimal management requires advanced FU-care [Citation48]. These findings suggested that FU-care planning might be particularly important for older and socially isolated patients, and for patients diagnosed with chronic widespread pain (fibromyalgia). Thus, although there is currently no consensus regarding the design of follow-up interventions [Citation30], our results imply that there should be more focus on everyday life skills after specialized rehabilitation. Indeed, our findings suggest that discussing and planning the follow-up should be an integral part of rehabilitation in specialized health care. Furthermore, structured patient pathways after rehabilitation for the RMD patient group should be developed and implemented in the Norwegian health care system; as such structured follow-up programmes have been proved beneficial in similar patient groups in other countries [Citation49]. Additionally, participants in a qualitative study that evaluated follow-up after rehabilitation reported that follow-up phone calls from the rehabilitation centre were perceived as positive and motivating, and helped them redefine eventual relapses and return them to adhering to behavioral adjustments and self-management [Citation32]. However, methods to improve patient coping skills should also be targeted, both in clinical practice and in research.
Consistent with results from a WHO report on adherence to long-term therapies [Citation15], approximately half of our participants were categorized as adhering to their SMA plan(s). Our results identified four factors that predicted adherence to SMA: older age, higher pain scores, and performing physical and social activities and hobbies on a regular basis. Depression has previously been identified as an important risk-factor for non-adherence to self-management in two systematic reviews [Citation8,Citation50], and was also close to significant in our study, suggesting that screening for depression should be part of the initial assessment in rehabilitation. However, in contrast to our results, one of the reviews identified high levels of pain and fatigue symptoms as obstacles to psychological adjustment in chronic disease [Citation8]. Therefore, future studies should investigate whether more pain might increase the motivation to engage in self-management for pain relief. Research has also shown that autonomous motivation and being active prior to an exercise intervention were important for sustaining behavioral changes over the long term [Citation50–52]. Our findings showed that having structure and routines in daily life enhanced self-management and promoted successful adjustments to the challenges posed by chronic conditions. Consequently, patients with a sedentary lifestyle, poor coping skills, and depression might need support over a longer time period to implement behavioral changes. More research is needed to identify factors that might be associated with positive long-term outcomes of rehabilitation and to develop interventions that fit patient needs and resources.
Strengths of our study are the pragmatic design, the inclusion of participants with a wide variety of RMDs, and the inclusion of specialized rehabilitation programs at nine different rehabilitation centres from all over Norway. These features may increase the generalizability of our findings in a Norwegian context. However, our results may not be applicable for settings and rehabilitation structure that differ significantly from the Norwegian health care system.
This study also has some other limitations. One potential weakness is that our results were based on self-reported data; thus, the results might have been influenced by recall bias and the fact that people tend to overestimate normative behaviors, such as adherence to health recommendations [Citation53,Citation54]. Compared to the general Norwegian population, the proportion of people with high education was large in our sample. Any generalization to other populations should therefore be done bearing this in mind. Moreover, reported specific FU-care received in our study did allow participants to register specific health care professionals at 4, 8, and 12 after rehabilitation, which might have led to an overestimation of specific FU-care received. Currently, there is no reference tool for measuring adherence [Citation55]; consequently, the method for collecting this information was developed for this study and has not been tested elsewhere. Also, the calculation of adherence did not consider how, e.g., one SMA scored with high adherence was valued compared to several SMAs with low adherence.
In summary, our results indicated that discussing and planning FU-care should be an integral part of rehabilitation in specialized health care. We showed that participants with plans for FU-care were more likely to receive the FU-care they needed. Furthermore, rehabilitation should be tailored according to individual patient needs; in particular, patients with a sedentary lifestyle, poor coping skills, depression, or being diagnosed with chronic widespread pain (fibromyalgia) might need more support over a longer time period to implement behavioral changes for healthy self-management.
Author contributions
All authors contributed to analyses and interpretation of data. All authors contributed substantially to drafting the article or revising it critically.
Disclosure statement
All author(s) declare no conflict of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2197–2223.
- van der Heijde D, Daikh DI, Betteridge N, et al. Common language description of the term rheumatic and musculoskeletal diseases (RMDs) for use in communication with the lay public, healthcare providers and other stakeholders endorsed by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR). Ann Rheum Dis. 2018;77(6):829–832.
- Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7):e002014.
- Al Maini M, Adelowo F, Al Saleh J, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the world forum on rheumatic and musculoskeletal diseases. Clin Rheumatol. 2015;34(5):819–829.
- Knittle K, De Gucht V, Maes S. Lifestyle- and behaviour-change interventions in musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2012;26(3):293–304.
- Dures E, Hewlett S. Cognitive-behavioural approaches to self-management in rheumatic disease. Nat Rev Rheumatol. 2012;8(9):553–559.
- Iversen MD, Hammond A, Betteridge N. Self-management of rheumatic diseases: state of the art and future perspectives. Ann Rheum Dis. 2010;69(6):955–963.
- de Ridder D, Geenen R, Kuijer R, et al. Psychological adjustment to chronic disease. Lancet. 2008;372(9634):246–255.
- Klokkerud M, Hagen KB, Lochting I, et al. Does the content really matter? A study comparing structure, process, and outcome of team rehabilitation for patients with inflammatory arthritis in two different clinical settings. Scand J Rheumatol. 2012;41(1):20–28.
- McCuish WJ, Bearne LM. Do inpatient multidisciplinary rehabilitation programmes improve health status in people with long-term musculoskeletal conditions? A service evaluation. Musculoskelet Care. 2014;12(4):244–250.
- Barlow J, Wright C, Sheasby J, et al. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187.
- Kralik D, Koch T, Price K, et al. Chronic illness self-management: taking action to create order. J Clin Nurs. 2004;13(2):259–267.
- Sackett DL, Haynes RB. Compliance with therapeutic regimens. Baltimore (MD): John Hopkins University Press; 1976. p. 293.
- Bailey DL, Holden MA, Foster NE, et al. Defining adherence to therapeutic exercise for musculoskeletal pain: a systematic review. Br J Sports Med. 2018.
- World Health Organization. Adherence to long term therapies. Evidence for action. Geneva: World Health Organization; 2003.
- McKay CD, Verhagen E. 'Compliance' versus 'adherence' in sport injury prevention: why definition matters. Br J Sports Med. 2016;50(7):382–383.
- Horne R, Chapman SC, Parham R, et al. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8(12):e80633.
- Horne R, Albert A, Boone C. Relationship between beliefs about medicines, adherence to treatment, and disease activity in patients with rheumatoid arthritis under subcutaneous anti-TNFα therapy. Patient Prefer Adherence. 2018;12:1099–1111.
- Kjeken I, Bo I, Ronningen A, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med. 2013;45(3):260–267.
- Bearne LM, Byrne AM, Segrave H, et al. Multidisciplinary team care for people with rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int. 2016;36(3):311–324.
- Berdal G, Bø I, Dager TN, et al. Structured goal planning and supportive telephone follow-up in rheumatology care: results from a pragmatic, stepped-wedge, cluster-randomized trial. Arthritis Care Res. 2018;70(11):1576–1586.
- World Health Organization. World report on disability. Geneva: World Health Organization; 2011.
- Cott CA. Client-centred rehabilitation: client perspectives. Disabil Rehabil. 2004;26(24):1411–1422.
- World Health Organization. Rehabilitation in health systems. Geneva: World Health Organization; 2017.
- Johansen I, Klokkerud M, Anke A, et al. A quality indicator set for use in rehabilitation team care of people with rheumatic and musculoskeletal diseases; development and pilot testing. BMC Health Serv Res. 2019;19(1):265.
- Sand-Svartrud AL, Berdal G, Valaas HL, et al. Associations between patient-reported quality of healthcare and clinical outcomes. A rehabilitation cohort study; submitted for publication to BMC Musculoskeletal Disorders on 12 November 2021 UTC.
- World Health Organization. Continuity and coordination of care: a practice brief to support implementation of the WHO framework on integrated people-centred health services. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2018.
- World Health Assembly, 69. Framework on integrated, people-centred health services: report by the Secretariat. Document number A69/39. World Health Organization; 2016.
- Clemente D, Leon L, Foster H, et al. Systematic review and critical appraisal of transitional care programmes in rheumatology. Semin Arthritis Rheum. 2016;46(3):372–379.
- Berdal G, Smedslund G, Dagfinrud H, et al. Design and effects of supportive followup interventions in clinical care of patients with rheumatic diseases: a systematic review with meta-analysis. Arthritis Care Res. 2015;67(2):240–254.
- Yun DW, Choi JS. Person-centered rehabilitation care and outcomes: a systematic literature review. Int J Nurs Stud. 2019;93:74–83.
- Dager TN, Kjeken I, Berdal G, et al. Rehabilitation for patients with rheumatic diseases: patient experiences of a structured goal planning and tailored follow-up programme. SAGE Open Med. 2017;5:2050312117739786.
- Hamnes B, Berdal G, Bø I, et al. Patients' experiences with goal pursuit after discharge from rheumatology rehabilitation: a qualitative study. Musculoskelet Care. 2021;19(3):249–258.
- Klokkerud M, Dagfinrud H, Uhlig T, et al. Developing and testing a consensus-based core set of outcome measures for rehabilitation in musculoskeletal diseases. Scand J Rheumatol. 2018;47(3):225–234.
- Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093.
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119.
- Strand BH, Dalgard OS, Tambs K, et al. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003;57(2):113–118.
- Magnussen LH, Lygren H, Anderson B, et al. Validation of the Norwegian version of Hannover Functional Ability Questionnaire. Spine. 2010;35(14):E646–E653.
- Westaway MD, Stratford PW, Binkley JM. The Patient-Specific Functional Scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther. 1998;27(5):331–338.
- Johnsen LG, Hellum C, Nygaard OP, et al. Comparison of the SF6D, the EQ5D, and the oswestry disability index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet Disord. 2013;14:148.
- Bentsen BG, Natvig B, Winnem M. Questions you didn't ask? COOP/WONCA charts in clinical work and research. World organization of colleges, academies and academic associations of general practitioners/family physicists. Fam Pract. 1999;16(2):190–195.
- Hamnes B, Garratt A, Kjeken I, et al. Translation, data quality, reliability, validity and responsiveness of the Norwegian version of the Effective Musculoskeletal Consumer Scale (EC-17). BMC Musculoskelet Disord. 2010;11:21.
- Berdal G, Sand-Svartrud A-L, Bø I, et al. Aiming for a healthier life: a qualitative content analysis of rehabilitation goals in patients with rheumatic diseases. Disabil Rehabil. 2018;40(7):765–778.
- Osteras N, Moseng T, Bodegom-Vos LV, et al. Implementing a structured model for osteoarthritis care in primary healthcare: a stepped-wedge cluster-randomised trial. PLoS Med. 2019;16(10):e1002949.
- Sand-Svartrud AL, Berdal G, Valaas HL, et al. A quality indicator set for rehabilitation services for people with rheumatic and musculoskeletal diseases demonstrates adequate responsiveness in a pre-post evaluation. BMC Health Serv Res. 2021;21(1):164.
- Kinge JM, Knudsen AK, Skirbekk V, et al. Musculoskeletal disorders in Norway: prevalence of chronicity and use of primary and specialist health care services. BMC Musculoskelet Disord. 2015;16:75.
- Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328.
- The Norwegian Directorate of Health. Muskel- og skjelettsmerter – uten leddhevelser, uten inflammasjonparametre: Helsedirektoratet; 2015. Available from: https://www.helsedirektoratet.no/veiledere/prioriteringsveiledere/revmatologi/tilstander-for-revmatologi/muskel-og-skjelettsmerter/muskel-og-skjelettsmerter-uten-leddhevelser-uten-inflammasjonparametre-ikke-rett#null-begrunnelse
- Fechtner S, Bethge M. Effects of rehabilitation aftercare on work participation in patients with musculoskeletal disorders: a propensity score-matched analysis. Int J Rehabil Res. 2018;41(1):74–80.
- Picorelli AM, Pereira LS, Pereira DS, et al. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. 2014;60(3):151–156.
- Knittle K, De Gucht V, Hurkmans E, et al. Explaining physical activity maintenance after a theory-based intervention among patients with rheumatoid arthritis: process evaluation of a randomized controlled trial. Arthritis Care Res. 2016;68(2):203–210.
- Schoo AMM, Morris ME, Bui QM. Predictors of home exercise adherence in older people with osteoarthritis. Physiother Can. 2005;57(3):179–187.
- Garfield S, Clifford S, Eliasson L, et al. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149.
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71 Suppl. 2:1–14.
- Hall AM, Kamper SJ, Hernon M, et al. Measurement tools for adherence to non-pharmacologic self-management treatment for chronic musculoskeletal conditions: a systematic review. Arch Phys Med Rehabil. 2015;96(3):552–562.