Abstract
Diatoms inhabiting intertidal flats are subject to strongly changing salinities due to exposure to rain and desiccation at low tide. In order to determine the physiological responses of marine benthic diatoms to salinity changes, cultures of Navicula phyllepta, Achnanthes delicatula subsp. hauckiana and Nitzschia constricta, isolated from the Solthörn tidal flat (lower Saxony, southern North Sea), were used to study the effect on growth rates of different salinities (0.5, 10, 20, 30, 40, 50). During short (1, 3, 10, 60 min) and long exposures (30 days), the composition of free amino acids and the accumulation of polyols, saccharides, quaternary ammonium compounds and β-dimethylsulphoniopropionate (DMSP) were determined. The growth rates of N. phyllepta were not affected by salinity in the tested range, synthesizing and accumulating proline as the only response to salinity increases in the growth medium. The growth responses of A. delicatula subsp. hauckiana were significantly weaker at the lowest and highest salinities in comparison to the control treatment (at a salinity of 30), producing proline, glycine betaine, glycerol and the quaternary ammonium compound homarine. In contrast, N. constricta showed the highest growth rate in the low-salinity treatment and the lowest proline concentrations in the high-salinity assay in comparison to the other species. In addition, N. constricta was the only species that synthesized the tertiary sulphonium compound DMSP. Furthermore, results from the salinity treatments showed particular similarities in compound accumulation. While quaternary ammonium compounds as well as DMSP were synthesized during high-salinity treatments, polyols and carbohydrates were found predominantly in the low-salinity experiments. Enzyme activities specific to proline metabolism in A. delicatula and N. constricta indicated that the high salinity-induced free proline accumulation may be due to a stimulation of synthesis via the ornithine pathway, whereas in N. phyllepta the glutamate pathway may contribute significantly to the free proline accumulation. In conclusion, significant differences in osmolyte compositions were found in the three diatom species during exposure to different salinities, suggesting specific intracellular acclimation processes that provide possible explanations of the species’ insensitivity towards environmental short-time salinity variations.
Introduction
The Wadden Sea is among the most productive marine biotopes on earth (Underwood & Kromkamp, Citation1999). The unconsolidated sediment of intertidal mudflats represents a highly unstable environment, due to continuously changing water levels and currents as well as temporary exposure to the air (Reineck, Citation1983). Here, salinity reduction may be caused by freshwater input, e.g. from rain, while a salinity increase may be caused by evaporation due to insolation. For instance, as a result of wind-induced evaporation, salinities on tidal flats may reach 100 (Visscher & Van Gemerden, Citation1991). On sandy and muddy substrates, edaphic microalgal assemblages living on a benthic surface are often dominated by diatoms (Colijn & Koeman, Citation1975; Colijn & Nienhuis, Citation1977; Admiraal et al., Citation1984; Colijin & De Jonge, Citation1984; Vos et al., Citation1988; De Jonge & Colijn, Citation1994; Agatz et al., Citation1999). These biofilms are particularly important in the ecology of mudflats as they exhibit high rates of primary production (Pinckney & Zingmark, Citation1991), influence erosion and deposition of sediment (Underwood & Paterson, Citation1993; Paterson, Citation1994), and affect sediment–water nutrient fluxes (Sundbäck & Granéli, Citation1988; Rysgaard et al., Citation1995).
Salinity influences diatom physiology directly by exerting an osmotic stress. Upon this stress, algae exhibit a wide range of adaptations at the molecular, cellular and organism level (Hare & Cress, Citation1997; Bohnert et al., Citation2001). To counteract the negative effects of osmotic stress on metabolism, organisms accumulate organic osmolytes (‘compatible solutes’, Brown & Simpson, Citation1972). Compatible solutes are highly soluble, low molecular weight organic molecules without net charge at physiological pH (Kirst & Wiencke, Citation1995; Chen & Murata, 2002; DasSarma & Arora, Citation2002). In algae, the osmoprotectants are restricted to four major classes of solutes: sugars and polyols, free amino acids and derivates, quaternary ammonium compounds (QUARCS), and tertiary sulphonium compounds (TSCS) (Bisson & Kirst, Citation1995; Erdmann & Hagemann, Citation2001). Among the compatible solutes, proline appears to be the most widely distributed osmolyte accumulated under osmotic stress (Kirst, Citation1990; van Bergeijk et al., Citation2003; Plettner, 2002; Krell, Citation2006). In eukaryotes, proline is synthesized from glutamate via Δ 1 -pyrroline-5-carboxylate (P5C) in two successive reductions catalysed by Δ 1 -pyrroline-5-carboxylate synthase (P5CS) and Δ 1 -pyrroline-5-carboxylate reductase (P5CR) (Krell et al., Citation2007). The synthesis of proline via ornithine as a precursor is mediated by ornithine δ-aminotransferase (δ-OAT).
However, the existence of seasonal and spatial patterns in species abundances within salt marsh, sand and mudflat habitats (Oppenheim, Citation1991; Laird & Edgar, Citation1992; Underwood, Citation1994; Saburova et al., Citation1995; Peletier, Citation1996) indicates that benthic diatom taxa may have niches to which they specifically adapt (Underwood, Citation2005). The adaptive potential of intertidal microphytobenthic diatom populations, however, is not well understood. Most diatom species isolated from intertidal environments have shown only slight differences in growth responses to salinity fluctuations in laboratory trials (e.g. Admiraal, Citation1977; Scholz & Liebezeit, Citation2012a ). Based on these observations, we hypothesized that benthic marine diatom species could have developed similar adaptation strategies in response to fluctuating salinities in the special environment of intertidal flats. In this context it is important to differentiate between acclimation and adaptation. While the former is defined as a temporary physiological adjustment to environmental change by an organism, the latter occurs over time scales covering multiple generations of a population. In the present investigation we studied acclimation. Thus, the main objectives of the present investigation were (1) to observe effects of different salinities (0.5, 10, 20, 30, 40, 50) on free amino acid composition; (2) to ascertain enzyme activities that might give additional information on pathways in the proline metabolism; and finally (3) to detect the presence of further osmolytes, such as polyols, carbohydrates, DMSP or quaternary ammonium compounds.
Materials and methods
Chemicals
If not otherwise mentioned, all of the chemicals used in this study were of the highest purity from Sigma/Aldrich.
Organisms
The benthic diatoms Navicula phyllepta, Achnanthes delicatula subsp. hauckiana and Nitzschia constricta were isolated by dilution series of samples from microbial biofilms on sediments in the intertidal zone of the Solthörn tidal flat, which is located in the eastern part of the Inner Jade near the village Tossens in Lower Saxony, Germany (53° 34′ 2.03′′ N, 8° 13′ 54.66′′ E). Samples were taken at low tide from sediments characterized by mixed sands at bi-weekly intervals from 9 June to 22 August 2008; the three taxa reached their highest abundances during this time, which was determined by analysis of permanent slides (cf. Scholz & Liebezeit, Citation2012b ). In the first step in the isolation of clones, subsamples of approximately 2–3 g were placed in glass Petri dishes, diluted with 10 ml sterile filtered sea water from the sampling area, and stored until isolation at 18 ± 2°C and a photosynthetic active radiation (PAR) of 400 µmol photons m–2 s−1 (Phillips, Germany, Master TL-D 18 W/840). PAR (400–700 nm) was measured with an underwater quantum sensor LI-192 (Q 17079) connected to a Licor Data Logger LI-1000. Both epipsammic and epipelic species were collected from the stored rough cultures with shortened glass pipettes under the microscope and spread over agar plates (spreading plate method), using f/2 medium (Guillard, Citation1975). The diatoms were purified from bacterial contaminants by spreading cells on 1.5% f/2 agar plates containing 5 µg ml−1 tetracycline and 5 µg ml−1 kanamycin. The absence of bacterial contaminants was verified by epifluorescence microscopy using the dyes 4′,6-diamidino-2-phenylindol (DAPI). Artificial seawater salt (Tropic Marin®, Aquarientechnik, Wartenberg, Germany) dissolved in de-ionized water was used with a salinity of 30 and pH of 8.0 (defined as the standard conditions in the following account). All salinities are according to the Practical Salinity Scale (psu). Diatom identification was as described in Scholz & Liebezeit (Citation2012b ).
Culture conditions and experimental design
Each isolate was cultivated in 2–l batch cultures under sterile conditions at 18 ± 2°C and a light intensity of 800 µmol photons m−2 s−1 (Phillips, Germany, Master TL-D 18 W/840), corresponding to summer temperatures and irradiances in the environment (Scholz & Liebezeit, Citation2012c ). Axenic isolates were grown under a 12:12 h light:dark regime. Cultures were adapted to the standard conditions described above at a saturating nitrogen concentration (800 µM), reducing the N level to 90% relative to full-strength f/2. The N:P ratio of the medium in all treatments was 17:1, which is comparable to low-tide ratios in the Solthörn tidal flat (Scholz & Liebezeit, Citation2012b ). The initial nitrogen concentration was five times higher than found in the environment of the isolates during winter 2008/2009, which was the season with the highest nutrient concentrations in the Solthörn tidal flat. The phosphate source was NaH2PO4.H2O, while both nitrate (NaNO3) and ammonium (NH4Cl) were used as nitrogen sources in a mixture of 2:1. Salinity, pH and conductivity were measured using handheld probes (YK-31SA, YK-2001PH SI Model 33, Engineered Systems and Designs-Model 600, Philips W9424). For biofilm development, a layer of glass beads (Sartorius, Ø 479–700 µm) approximately 3 mm deep was inserted on the bottom of culture vessels as a substratum. Two weeks after biofilm development the culture broths were removed circumspectly with a sterile tube until only a thin liquid film remained over the biofilms, with the exception of the initial (t = 0 min) samples, which remained unaltered. Subsequently, the experiments were started by addition of fresh culture medium with different salinities (0.5, 10, 20, 30, 40 and 50), obtained by dissolving defined quantities of the artificial seawater salt (Tropic Marin®, as above) in de-ionized water. The treatments with a salinity of 30 were used as controls. While the short-term experiment took only 60 min and included five sampling times (0, 1, 3, 10, 60 min), the long-term one continued for 30 days. The experiments started with 18 replicate flasks per treatment and species. At each sampling time three flasks per treatment were removed. The positions of replicate flasks were changed randomly every other day to eliminate any location effect due to minor changes in external conditions, especially lighting.
Cell counts
The monitoring of cell volumes as well as counting of samples during short- and long-term experiments were performed microscopically, using a Neubauer Improved counting chamber with 0.1 mm depth (LO Laboroptik, Germany). During the long-term exposure, cell counts were made every second day. Only photosynthetic active cells were counted, recognized by their red fluorescence under UV light. At least 1000 cells were counted in each sample at 200 × magnification. The specific growth rate (μ) was calculated according to the following formula: μ = ln c 1 − ln c 0/t 1 − t 0, where c 1 denotes the cell amount at time t 1, and c 0 is the cell number at time t 0.
Harvesting and sample treatment
Generally, biofilms were allowed to grow without physical disturbance on the surface of the glass beads, from which diatoms could be easily removed for sampling by shaking on a rotating shaking device (25 × g), thus dislodging the biofilm from the substrate and homogenizing the culture. Microscopical observation of the beads after shaking confirmed the almost complete release of the cells. Biomass from the short-term (0, 1, 3, 10, 60 min) and the long-term experiments (30 days) was harvested by centrifugation at 60 × g for 10 min. The three replicates of diatom biomass were each subdivided into five aliquots and used for different extraction procedures as described below.
Biochemical analysis
Free amino acids
Proline concentration was determined and quantified spectrophotometrically with ninhydrin, according to the method described by Bates et al. (Citation1973) and modified by Nothnagel (Citation1995). For free amino acid analysis by HPLC, cold perchloric acid extraction, was applied according to the method of Bligny et al. (Citation1989), as modified by Plettner (Citation2002). The supernatant from the extraction was removed, fixed in liquid nitrogen and lyophilized (Alpha 1–4 LSC lyophilization system, Christ, Germany). The samples were stored at −80°C until analysis. HPLC analysis involved pre-column derivatization with o-phthaldialdehyde (OPA) and 2-mercaptoethanol according to the method of Liebezeit & Behrends (Citation1999), using a 250 mm × 4.0 mm ID column Nucleosil 100 C18 with 5 µm particle diameters as stationary phase (AB, Bischoff Chromatography, Leonberg, Germany). In detail, 1 ml of sample or standard and 100 µl sodium borate buffer (0.1 M, pH 10.5) were mixed for 50 s on a vortex mixer. Then 50 µl of the OPA reagent (prepared daily by dissolving 20 mg OPA in 5 ml methanol and 200 µl 2-mercaptoethanol) were added and mixed. After 2 min, 100 µl of this mixture was manually injected with a glass syringe (Hamilton 710 United States, Rheodyne 8125, Wertheim–Mondfeld, Germany). The column was eluted using a ternary gradient consisting of solvent A (0.05 M sodium acetate, pH 8.4), solvent B (0.05 M sodium acetate, pH 5.4) and solvent C (100% MeOH) as follows: 0–70% B and 20–30% C over 13 min, then 70–56% B and 30–36% C in 9 min, 100% C in 28 min, back to 0% B and 20% C in 5 min, and continuing at 0% B and 20% C for 5 min. The flow rate was 1.0 ml min−1. The amino acids were detected fluorometrically at an excitation wavelength of 330 nm and an emission wavelength of 450 nm. For calibration and quantification of amino acids, a standard mixture of 18 amino acids (AA-S-18, Sigma) as well as L-ornithine was used. Quantification was carried out with the program EZChrom Elite 3.1.7 (Scientific Software, Agilent Technologies, United States).
Polyols, carbohydrates, DMSP and quaternary ammonium compounds
The extraction of these compounds followed the method of Karsten et al. (Citation1991), using 70% EtOH. Saccharides and polyols accumulated during the experiments were identified by thin layer chromatography (TLC Silica Gel 60 F254; Merck) following different methods. While mono- and disaccharides were identified and quantified by the method described by Zhang et al. (Citation2009), polyols were quantified according to the method of Klaus & Ripphahn (Citation1983). As standards, xylose, sorbose, sucrose, mannose, galactose, glucose, fructose, glycerol and erythritol were used. Since all tested saccharides and polyols were present in the samples, we decided to consider only compounds above a limiting concentration in the following analyses (20 fmol cell−1), on the basis that a higher concentration level would equate to importance in the adaptation process. Homarine (N-methyl picolinic acid) and glycine-betaine were detected according to the methods of Bregoff et al. (Citation1953) and Hayashi & Konosu (Citation1977). Quantification was carried out by standard curve equations, using homarine and glycine-betaine (Merck) as standard. 3-Dimethylsulphoniopropionate (DMSP) was separated on 0.25 mm silica gel G plates (Machery–Nagel, Düren, Germany) developed with methanol:acetone:HCl (90:10:4, v/v/v) (Summers et al., 1998), and visualized by Dragendorff's reagent according to the method of Awwad & Adelstein (Citation1966). DMSP was estimated by area measurement of Dragendorff-positive TLC zones (Aronoff, Citation1967). The plots of zone area versus the logarithm of the quantity of standard (Fisher Scientific) were linear (r 2 ≥ 0.96, P > 0.01). After preparative TLC, products were eluted with aqueous methanol or ethanol and lyophilized.
Protein content
Protein was measured using the Lowry method as described by Herbert et al. (Citation1971) using bovine serum albumin as a standard.
Enzyme assays
Extractions for the 1, 10 and 60 min assays were done at 4°C, following the method described by Plettner (Citation2002). Cells were homogenized in a pre-chilled mortar and pestle with 50 ml of 100 mM 2-(N-morpholino)ethanesulphonic buffer (MES, pH 6.5) containing 10 mM MgCl2.6H2O, 0.5% saccharose, 1 mM 1,1,1 -trichloro-2,2-bis (4-chlorophenyl) ethane (DDT), 10% glycerin, 20 mg Polyclar AT, 15 mg Amberlite XAD-4, 1 tablet protease-inhibitor, 1 mM Pepstatin and 2 mM Na-EDTA. One gram of silica sand was added and the mortar was shock-frozen in liquid nitrogen. The homogenates were ground by pestle, incubated for 15 min and centrifuged (15 500 × g for 30 min). The enzymes were precipitated from the culture supernatant by adding ammonium sulphate to 80% saturation. The assay was left overnight and the precipitate collected by centrifugation at 3000 × g for 30 min, dissolved in resuspension buffer (100 mM MES buffer, pH 6.5., without Polyclar, Amberlite and Pepstatin) and frozen in 50-µl portions at −80°C. Activities are expressed as units (U), defined as the amount of the enzyme that catalyses the conversion of 1 µmol of substrate per minute in relation to the protein concentration (mg). As blanks, 100 µl of de-ionized water were used in the following enzyme assays.
The Δ1-pyrroline-5-carboxylate synthase (P5CS) assay, which is the ATP- and NADPH-dependent reduction of glutamate to γ-glutamic semialdehyde, was carried out in K3PO4 buffer (500 mM pH 7.0), 100 mM glutamic acid, 100 mM MgCl2.6H2O, 5 mM ATP, 10 mM NADPH and 100 µl enzyme extract at 20°C. The reaction velocity was measured as the rate of consumption of NADPH, monitored as decrease in absorption at 340 nm as a function of time as described by Stines et al. (Citation1999).
Δ1-pyrroline-5-carboxylate reductase (P5CR) activity was determined spectrophotometrically at 20°C according to the method of Treichel (Citation1986), using an extinction coefficient of 6.317 l−1 mM−1 cm−1 of NADPH at 340 nm, in a reaction mixture containing 75 mM DDT, 5 mM Δ1-pyrroline-5-carboxylate (P5C), 15 mM NADPH, 50 µl of enzyme solution, and 500 mM K3PO4 buffer (pH 7.0), in a total volume of 900 µl.
Ornithine-δ-aminotransferase (δ-OAT) activity was assayed according to Vogel & Kopac (Citation1960), as modified by Yang & Kao (Citation1999). The assay mixture contained 100 µl enzyme extract and 160 µl K3PO4 buffer (500 mM, pH 7.5) containing 60 mM L-ornithine, 20 mM α-ketoglutarate and 10 mM pyridoxal-5′-phosphate. The reaction medium was incubated at 30°C for 30 min. The reaction was stopped by adding 200 µl trichloroacetic acid (10%) and the colour was developed by incubating the reaction mixture with 200 µl o-aminobenzaldehyde (0.5%) in ethanol (95%) for 30 min in the dark. After centrifugation at 14 200 × g for 30 min, the absorbance at 444 nm of the clear supernatant fraction was measured.
Statistical analysis
For all measured parameters, effects of the experimental salinity treatments were analysed with XLSTAT 2011, version 2011.2.08 Addinsoft, Andernach, Germany. Effects of high salinity (50) versus low salinity (0.5) in comparison to the control (30) were tested with 1-factorial ANOVAs; P-values of <0.05 were accepted as representing significant differences.
Results
Growth responses and protein contents
Growth rates (μ) varied considerably between individual species, ranging from 0.459 d−1 (Nitzschia constricta) and 0.536 d−1 (Achnanthes delicatula), to 0.829 d−1 (Navicula phyllepta) in the control treatments (salinity of 30; ). The μ values obtained from the short-time experiment did not differ significantly between the salinity treatments (t = 1 to 60 min, ANOVA F 5,36 = 4.8, P = 0.46).
Fig. 1. A–L. Protein concentrations (left y-axes) and specific growth rates (μ, right y-axes) of Achnanthes delicatula subsp. hauckiana, Nitzschia constricta and Navicula phyllepta, obtained from short (1, 3, 10, 60 min) and long (30-day) experiments, including means ± SD (n = 3). The following salinities were tested: A–C: 0.5, D–F: 10, G–I: 20, J–L: 30. M–O: 40, P–R: 50. The x-axes are broken after 60 min.
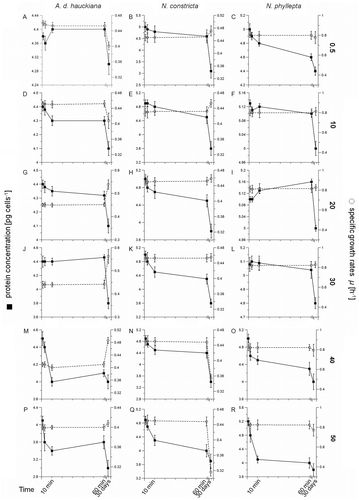
In contrast, with long exposures (t = 30 days) to the different salinities, the three species exhibited distinct differences in their growth responses. The greatest changes in growth rate, compared with the control treatment, were observed in A. delicatula (average difference between 0.5, 10 and 50, 0.155 d−1, ANOVA F1,9 = 21.8, P < 0.0001; , J, P), in which growth rates declined at salinities of 0.5 and 10, but increased from 20–50, relative to the growth rates during the short-term treatments at each salinity. In N. constricta, the highest specific growth rates occurred in the low-salinity treatments (compare the 0.5 and 30 salinity treatments in B and K, difference 0.016 d−1), while a significant decrease was recorded in the high-salinity treatments (difference 0.139 d−1, ANOVA F 1,6 = 19.2, P < 0.0001, ). The growth rates of N. phyllepta did not vary significantly between the different salinity treatments (ANOVA F 1,6 = 4.8, P = 0.32, C, R).
The initial protein content per cell did not vary significantly among the three species (4.2 to 5.1 pg cell−1) but decreases were recorded from the first and third minute onwards after salinity exposure, being significant in N. phyllepta (up to 37%, ANOVA F 1,6 = 36.5, P < 0.0001, ).
Composition of free amino acids
Due to only slight variations and low final concentrations of the amino acids histidine, cystine, ornithine, threonine, arginine, tyrosine, methionine, valine, phenylalanine, isoleucine and leucine, we suggest that these amino acids were not involved in salinity adaptation. Therefore, these amino acids have not been considered in detail in the following analyses; they have been added together and designated as ‘other’ in and .
Fig. 2. Main amino acid compositions (means ± SD, n = 3) of Achnanthes delicatula subsp. hauckiana (A), Nitzschia constricta (B) and Navicula phyllepta (C) at the beginning of the experiments (t = 0), obtained by a colorimetric method (*) and HPLC. The amino acids collectively designated as ‘other’ are those suggested to be insignificant for salinity adaptation.
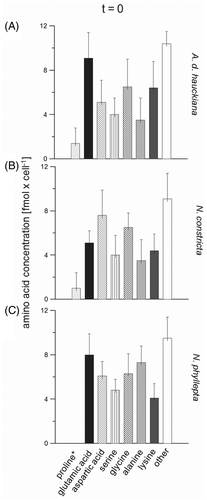
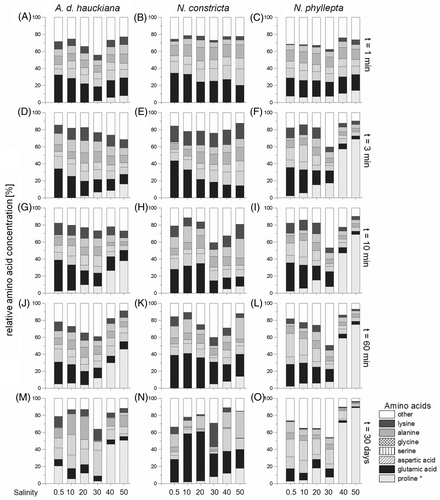
Fig. 3. Relative amino acid concentrations of Achnanthes delicatula subsp. hauckiana, Nitzschia constricta and Navicula phyllepta after 1 min (A–C), 3 min (D–F), 10 min (G–I), 60 min (J–L), and finally, 30 days’ salinity exposure (M–O), obtained by a colorimetric method (*) and HPLC. Values are expressed as per cent of total free amino acids detected.
With the exception of alanine, aspartic acid and glutamic acid, the initial free amino acid compositions of N. phyllepta, A. delicatula and N. constricta were similar, varying over only a narrow range (). Navicula phyllepta contained the highest proportion of glutamic acid (19.6% of total amino acids, ), whereas A. delicatula showed higher concentrations of aspartic acid (18.8%, ). Nitzschia constricta displayed nearly double the amount of alanine as the other two species (16%, ).
The relative amino acid composition varied significantly during the experiments, the pattern and timing of changes depending on the species (). The high and, in comparison to the other species, rapid increase of proline in N. phyllepta was particularly notable (C, F, I). In the high-salinity treatment of this species, a proline concentration of 103 fmol cell−1 was reached after 60 min (79%, ) and the final proline concentration after 30 days was 166 fmol cell−1 (91%, ). In contrast, N. constricta and A. delicatula showed significantly smaller amounts of proline in the high-salinity treatments, with proline concentrations ranging between 38 and 106 fmol cell−1 after 30 days (, M). Among the other free amino acids only alanine and glutamic acid were significantly involved in the short-time adaptation process to high salinities in N. constricta (10–15%, ANOVA F 1,6 = 30.1, P < 0.0001, ). In contrast, N. phyllepta and A. delicatula did not show any significant differences.
In all low-salinity treatments, the regulation of glutamic acid concentration seemed to be the most relevant feature during the short-time exposure (t = 1–60 min), independent of the species (); proline was generally not involved in the short-term response. The highest amounts of aspartic acid per cell occurred in Achnanthes delicatula after 60 minutes of the short-time acclimation (), while the highest amounts of serine were recorded in N. phyllepta (36 fmol cell−1, ). High relative concentrations of lysine were detected in most assays of A. delicatula and N. constricta 3 minutes after the start of the experiment (8–16%, D, E). However, due to the fact that the control assay (salinity 30) was also affected, the significant decrease of lysine (ANOVA F 1,6 = 30.1, P < 0.0001) was not related to the short-term acclimatization process. Besides the high proportions of glutamic acid (54 fmol cell−1) in N. constricta during the long-time exposure, the high relative concentrations of alanine in the high-salinity experiment and of lysine in the control one are noteworthy (36–41 fmol cell−1, ). In contrast, A. delicatula showed the highest amounts of glycine compared with the other two taxa in the long-term response to salinity stress, varying between 46 and 83 fmol cell−1 in the assays at the high salinities of 40 and 50 ().
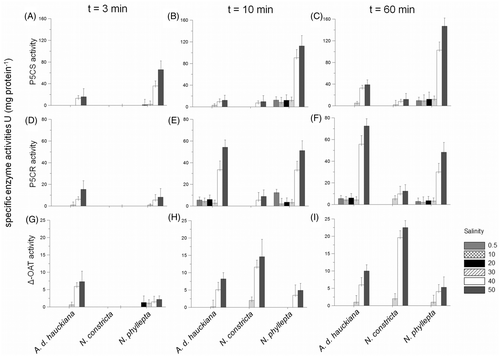
Enzyme activities
The specific activities of P5CS, P5CR and δ-OAT in the high-salinity assays were completely different from those of the low-salinity ones (). Generally, the highest enzyme activities were recorded in the high-salinity treatments, confirming the observation (see above, ‘Composition of free amino acids’) that proline was not significantly involved in the low-salinity adaptation process in N. phyllepta, A. delicatula and N. constricta. Moreover, distinct differences between the three tested species were detected in the accumulation of enzymes. For example, N. phyllepta displayed the highest P5CS activity from the third minute onwards (), indicating that the glutamate pathway may contribute significantly here to the high-salinity induced accumulation of free proline. In contrast, P5CR was more important in assays with A. delicatula (), whereas N. constricta showed the highest δ-OAT activities (). The similarity in the time-course of changes in δ-OAT and P5CR activities and in free proline levels during the later periods of the high-salinity treatments suggests that the proline accumulation induced by high salinity may be due to a stimulation of synthesis via the ornithine pathway in A. delicatula and N. constricta.
Presence of DMSP, polyols, carbohydrates and quaternary ammonium compounds
While polyols and carbohydrates were found in higher amounts only in the low-salinity treatments in extracts of all three species, the presence of quaternary ammonium compounds (QUARCS) was restricted to the high-salinity treatments of N. constricta and A. delicatula (, ). Of the two tested polyols, only glycerol was present. The highest glycerol amounts were detected in the low-salinity treatments of all three species, ranging between 16 to 22% of the entire compatible solute composition after 60 min exposure (Figs ). Although the times of occurrence of different levels of these varied significantly between the individual assays, glucose, galactose and mannose constituted the majority of the sugars detected in the three diatom taxa. Among carbohydrates, only mannose was observed in extracts of N. constricta in higher amounts in both high-salinity treatments (up to 36.8 fmol cell−1), whereas in N. phyllepta and A. delicatula it occurred only in the low-salinity ones. In addition, glucose was found in all three species from the third minute onward (average 21.5 fmol cell−1). Furthermore, N. constricta extracts indicated a relatively rapid occurrence of glycine-betaine and homarine, fromthe first minute onward, whereas DMSP accumulated only after 60 min exposure (). In N. constricta DMSP comprised 20–39% of the entire compatible solute composition in the response to both high salinity-treatments (H, K). In contrast, QUARCS showed basically lower values, varying between 23 and 36% in N. constricta, which was very similar to the relative amounts found in A. delicatula (27–29%, G, J).
Fig. 5. Relative composition of compatible solutes in Achnanthes delicatula subsp. hauckiana, Nitzschia constricta and Navicula phyllepta after 60 min exposure to salinities of 0.5 (A–C), 10 (D–E), 40 (G–I) and 50 (J–L). Values are expressed as per cent of total compatible solutes detected.
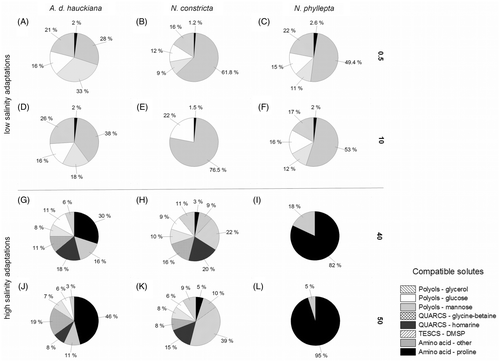
Table 1. Presence of quaternary ammonium compounds (I–II), DMSP (III), carbohydrates (IV–IX), and polyols (X–XI) in Achnanthes delicatula subsp. hauckiana, Nitzschia constricta and Navicula phyllepta, in response to treatment with different salinities (0.5, 10, 20, 30, 40 and 50) for short (0–60 min) and long (30-day) periods.
Discussion
Growth responses
The growth rates of Navicula phyllepta, Achnanthes delicatula and Nitzschia constricta, measured over the 30-day experiment, ranged between 0.4 and 0.8 d−1. These values are within the range of published μ values (Underwood & Smith, Citation1998; Smith, Citation1999) and doubling times (Admiraal et al., Citation1984; Peletier, Citation1996) for benthic marine diatoms. However, the species-specific growth rates did not vary significantly over the tested salinity range during short-term exposures. Only during the long-time experiment were distinct variations found, occurring in the lowest as well the highest salinity treatments. This is in part consistent with the conclusions of Admiraal (Citation1984), who stated that inhibition of growth of marine diatom taxa occurred generally only below a salinity of 4. In contrast, we observed N. constricta to exhibit high growth rates at lower salinities. Similar results to ours were obtained by Lam et al. (Citation2005) and Pasztaleniec & Połeć (Citation2006), whereas Kashima et al. (Citation1997) described N. constricta as having an optimum salinity between 25 and 56. However, different growth responses among clones of the same diatom species have been observed by different authors (e.g. Guillard & Ryther, Citation1962). In summary, based on the observations in the present study, only N. phyllepta can be regarded as ‘holoeuryhaline’, while A. delicatula and N. constricta may be labelled as ‘α-mesohalobous’ (salinities of 10–30) and ‘β-mesohalobous’ (salinities of 0.2–10) taxa, respectively (Pankow, Citation1990).
Proline as major compatible solute
In general, two phases can be distinguished in the response of algae to a change in salinity (Kirst, 1989). The first phase is a very rapid change (seconds) in turgor pressure caused by water fluxes in or out of the cells following the osmotic gradient (van Bergeijk et al., Citation2003), which was not considered in the present investigation. The second phase is the relatively slow change (minutes to hours) in the cellular concentrations of osmotically active solutes (osmolytes) until a new steady state is achieved. Changes in ionic contents generally precede those in organic osmolytes (Kirst, 1989). In addition, proline has been found to be the most effective of the compatible solutes tested (Kirst, 1989). An increasing importance of proline as a compatible solute with increasing salinity, observed for N. phyllepta in the present study, has also been found in Antarctic ice-diatoms (e.g. Nothnagel, Citation1995; Plettner, Citation2002; Krell et al., Citation2007), Phaeodactylum tricornutum (Besnier et al., Citation1969; Schobert, Citation1980) and Cyclotella meneghiniana (Schobert, Citation1974). However, there is some controversy about the protective function of proline. For instance, it has been shown that in some cases the accumulation of high proline levels made organisms even more susceptible to salt stress (Liu & Zhu, Citation1997; Rout & Shaw, Citation1998), and Nanjo et al. (Citation2003) even attributed toxic effects to elevated proline concentrations. In the present study, N. phyllepta did not show decreased growth rates over time, nor did it synthesize other osmoprotectants. This suggests that the increasing cellular levels of proline are (1) not toxic and (2) sufficient as an adaptation osmolyte to high salinities. Furthermore, Hellebust (Citation1976) argued that the accumulation of proline from glutamate or arginine under high salinity conditions appears to be at least in part due to a decrease in end-product control of proline biosynthesis, and probably also partly due to a general inhibition of protein synthesis, allowing accumulation of free amino acids. This observation fits well with the decreasing protein levels in N. phyllepta and A. delicatula during the first and third minutes, respectively, by which time proline biosynthesis had started in both these species. Similarly, the decelerated decrease of soluble protein in N. constricta at high salinity indicates that proteolysis might also be involved in proline accumulation in this peculiar species, which showed the lowest proline concentrations during the experiments.
Also, the biochemical pathway could be more important for the energy balance of the cells than the accumulation of osmolyte itself. For example, during salt stress, NADPH is accumulated and can cause photoinhibition as result of change in the chloroplast redox state. The activation of proline synthesis could regenerate NADP+and protect the photosynthesis system from the highly reducing environment (Hare & Cress, Citation1997). Such different physiological functions could explain the accumulation of different osmoprotectants (Garza-Sánchez et al., Citation2009), as found in the present study in A. delicatula and N. constricta. In addition, energetic aspects of proline synthesis might play a role in the preference of either the glutamate or ornithine route to proline (Krell, Citation2006). Generally, proline can be accumulated using either glutamate or ornithine as a substrate. Although both routes involve the consumption of one molecule of ATP and two molecules of NADPH for the formation of one molecule of proline, ornithine might also be derived via the urea cycle from arginine originating from protein degradation. In this context, the recent finding of a full functional urea cycle in diatoms is of great importance (Armbrust et al., Citation2004). In the latter case, proline synthesis from ornithine would require only one molecule of NADPH consumed by P5CR (Hare & Cress, Citation1997). In the Antarctic diatom Fragilariopsis cylindrus, Krell (Citation2006) found that under optimal growth conditions, expression of P5CS was high and δ-OAT expression was low, indicating that proline was synthesized via P5CS from glutamate, while ornithine could be used for the synthesis of arginine. In the present study, high external salt concentrations led to an accumulation of proline in N. phyllepta, primarily synthesized via the glutamate route, whereas A. delicatula and N. constricta synthesized proline via the ornithine route. Interestingly, only low concentrations of ornithine were found as a precursor amino acid, independent of species or salinity treatment. According to Krell (Citation2006), proline accumulation caused a feedback inhibition of P5CS in F. cylindrus, possibly to overcome a shortage in reduction equivalents caused by a severe inhibition of linear electron transport. Thus, the ornithine pathway might be preferred under circumstances of energy deficiency, leading to an elevated activity of δ-OAT, thus contradicting the obvious preference of the glutamate route in N. phyllepta. Further investigations at the transcriptional level are needed, in order to understand the physiological characteristics of this particular diatom species.
Other amino acids as compatible solutes
Besides proline, several other free amino acids have been detected in marine diatoms that may function as organic osmolytes; these include glutamic acid (Fujii et al., Citation1995; Nothnagel Citation1995), alanine (Nothnagel, Citation1995) and taurine (Jackson et al., Citation1992). Most previous studies were conducted in conjunction with high-salinity acclimatization, whereas only few dealt with low-salinity ones. While taurine was not considered in the present investigation, serine, alanine, aspartic and glutamic acid were found in higher amounts, particularly in the low-salinity treatments. The high amounts of glutamic acid at low salinity in all three species tested were especially notable. Several authors have described increasing levels of glutamic acid with increases in salinity (e.g. Nothnagel, Citation1995), even as an alternative to proline as the main osmolyte (e.g. Fujii et al., Citation1995), as found for N. constricta in the present investigation. Besides glutamic acid, aspartic acid was also reported as one of the dominant free amino acids in Chaetoceros muelleri (Fujii et al., Citation1995). Generally, the synthesis of asparagine is stimulated by starving cells of N in the absence of light, while the synthesis of macromolecules from glutamate is strongly depressed under these conditions (Liu & Hellebust, Citation1976). In the present study, aspartic acid was only detected in higher amounts in the second highest salinity treatment of N. constricta, as well as the control (salinity of 30) treatment of A. delicatula. If these high concentrations were caused only by cell starvation, then all assays should have been affected equally, at least in the 30 days exposure. Hence, other factors seemed to regulate the aspartic acid levels in these both species.
Rijstenbil et al. (Citation1989a ) found that, as a result of freshwater pulses, the cellular concentrations of glucose, glutamic acid and glutamine decreased concurrently with the respiration rate in Skeletonema costatum, and furthermore, that the share of glutamic acid in the amino acid pool increased with the specific growth rate μ. Haberstroh & Ahmed (Citation1986) reported that this relative increase of glutamic acid is also obtained after ammonium spiking, and may indicate activity of glutamate-dehydrogenase (GDH) supplementary to glutamine synthetase/glutamate synthase (GS/GOGAT). Additionally, this shift occurred in presence of large ammonium pools (Eppley & Rogers, Citation1970; Falkowski & Rivkin, Citation1976). Finally, our observation that free amino acid pools increased at lower as well as at higher salinities is consistent with the results of Rijstenbil et al. (Citation1989b ) and Fujii et al. (Citation1995).
Importance of polyols, quaternary ammonium and tertiary sulfonium compounds
Besides the free amino acids, several other organic osmolytes have been detected in marine and estuarine diatoms, such as glycine betaine (Dickson & Kirst, Citation1987b ), homarine (Dickson & Kirst, Citation1987b ; Nothnagel, Citation1995), cyclohexanetetrol (Fujii et al., Citation1995; Garza-Sánchez et al., Citation2009), glycerol (Dickson & Kirst, Citation1987b ), and finally, mannose (Paul, Citation1979). While glycerol and mannose are direct products of photosynthesis, like sugars (e.g. sucrose, trehalose), polyols (e.g. glycerol, mannitol, sorbitol) and heterosides (e.g. floridoside, isofloridoside), glycine betaine, homarine and cyclohexanetetrol are not. In microalgae, sugars and polyols are commonly the most important osmoprotectants in terms of cellular osmotic responses to a salinity change (Hu, Citation2004). Studies in vitro suggest that the polyols could serve as compatible solutes, acting as low-molecular-weight chaperones and as oxygen radical scavengers (Parida & Das, Citation2005). In diatoms, the only reported carbohydrates and polyols accumulating as a response to salinity increases are mannose in Cylindrotheca fusiformis, 1,2,4,5 cyclohexanetetrol in Chaetoceros muelleri, and glycerol in Phaeodactylum tricornutum (Paul, Citation1979; Dickson & Kirst, Citation1987b ; Fujii et al., Citation1995). In the present investigation, mannose was found to exhibit the fastest response to low-salinity treatments in all three species. Also, in the high-salinity assays of N. constricta, mannose was found in higher amounts.
Generally, not all organic solutes have equal protective properties. Based on a comparison of the general properties of compatible solutes and in vitro enzyme assays, Kirst (Citation1996) concluded that DMSP may be less effective as compatible solute than e.g. betaine, proline or glycerol. For instance, DMSP does not seem to be synthesized rapidly following changes in water potential. In the present study, most observations indicated that under such conditions DMSP is produced only slowly, if at all (Reed, Citation1983; Dickson & Kirst, Citation1986; Edwards et al., Citation1987; Stefels et al., Citation1996). On the other hand, it is quite possible that lower concentrations of this compound were not detected due to the method used. However, when cultured for a prolonged period of time, DMSP has been found to increase with salinity in several micro- and macroalgal species (Vairavamurthy et al., Citation1985; Dickson & Kirst, Citation1986, Citation1987a , Citation1987b ; Karsten et al., Citation1992). Due to its usually high intracellular concentrations in the present investigation, DMSP may thus be considered as a constitutive compatible solute, but not as an osmoticum in the strict sense of being responsible for osmotic balance (Reed, Citation1984). Kirst (Citation1996) suggested that DMSP may act as a buffer during the initial period after hyperosmotic shocks, when immediate changes in cell volume result in concomitant changes of intracellular solute concentrations, an effect which takes place without active production or degradation of the solute concentration.
Importance of nutrient availability
Generally, in nitrogen-sufficient cells, maximum amino acid pools of 160 mM contribute 20% of osmolality (Mague et al., Citation1980; Dickson & Kirst, Citation1987a ). These pools decrease considerably under nitrogen limitation (Admiraal et al., Citation1986; Haberstroh & Ahmed, Citation1986). Thus, an important aspect in this context may be the nutrient status of the tested species, especially the availability of nitrogen, since most of the organic osmolytes produced in algae are nitrogenous compounds. According to Krell (Citation2006), enhancement of protein degradation might increase the supply of nitrogen via ornithine. This might at least in part determine whether the glutamate or ornithine synthesis routes are preferred under salt stress conditions. Although isomolar concentrations of these solutes have comparable osmotic potentials, the energy costs and the amounts of carbon and nitrogen required for synthesis of the various solutes may differ considerably. Consequently, the physiological condition of the cells affects the production of solutes, which results in changes in their relative concentrations. A preference for carbohydrates versus amino acids, as found for N. constricta in the present study in response to low salinities, might reflect nitrogen limitation. On the other hand, our results showed that carbohydrates were also found during early responses to low salinities, so N deprivation should not have occurred. Thus the preference for carbohydrates might not be totally a reflection of nitrogen limitation.
Conclusions
The present study suggests that benthic marine Wadden Sea diatoms show a high potential for synthesis of three or more distinct classes of major solutes in response to salinity increases or decreases. Generally, all the taxa in the current study accumulated osmolytes in response to changes in water potential, but the responses were not equal, the osmolytes varying in both concentration and composition. While N. phyllepta synthesized first and foremost proline in response to high salinities, N. constricta was found to produce an impressive array of compounds instead e.g. DMSP, glycine-betaine, homarine and glycerol. In contrast, glutamic acid and to a lesser extent saccharides were the most important osmolytes in the adaptation to low salinities. The intracellular concentrations of different osmolyte compounds depend on environmental conditions and on the physiological potential of each diatom species. In summary, some diatoms are able to produce more of these osmoprotectants and are therefore able to grow under wider salinity ranges. These different acclimation potentials reflect niche separation in intertidal flats.
Acknowledgements
We are grateful to Dr Daniel Ziehe and Alex Rúa-Cardona for their helpful comments on the manuscript.
References
- Admiraal , W . 1977 . Salinity tolerance of benthic estuarine diatoms as tested with a rapid polarographic measurement of photosynthesis . Marine Biology , 39 : 11 – 18 .
- Admiraal , W . 1984 . “ The ecology of estuarine sediment inhabiting diatoms ” . In Progress in Phycological Research , Edited by: Round , FE and Chapman , DJ . Vol. 3 , 269 – 322 . Bristol : Biopress .
- Admiraal , W , Peletier , H and Brouwer , T . 1984 . The seasonal succession patterns of diatom species on an intertidal mudflat: an experimental analysis . Oikos , 42 : 30 – 40 .
- Admiraal , W , Peletier , H and Laane , RWPM . 1986 . Nitrogen metabolism of marine planktonic diatoms; excretion, assimilation and cellular pools of free amino acids in seven species with different cell size . Journal of Experimental Marine Biology and Ecology , 98 : 241 – 263 .
- Agatz , M , Asmus , RM and Deventer , B . 1999 . Structural changes in the benthic diatom community along a eutrophication gradient on a tidal flat . Helgoland Marine Research , 2 : 92 – 101 .
- Armbrust , EV , Berges , JA , Bowler , C , Green , BR , Martinez , D , Putnam , NH and Zhou , S . 2004 . The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism . Science , 306 : 79 – 86 .
- Aronoff , S . 1967 . Techniques of Radiobiochemistry Hafner Publishing, New York
- Awwad , HK and Adelstein , SJ . 1966 . A quantitative method for the determination of the specific radioactivity of sulfur-containing amino acids separated by paper chromatography . Analytical Biochemistry , 16 : 433 – 437 .
- Bates , LS , Waldren , RP and Teare , ID . 1973 . Rapid determination of free proline for water-stress studies . Plant and Soil , 39 : 205 – 207 .
- Besnier , V , Bazin , M , Marchelidon , J and Genevet , M . 1969 . A study of the pool of intracellular amino acids (free) in a marine diatom as a function of salinity . Bulletin de la Société de chimie biologique , 51 : 1255 – 1262 .
- Bisson , MA and Kirst , GO . 1995 . Osmotic acclimation and turgor pressure regulation in algae . Naturwissenschaften , 82 : 461 – 471 .
- Bligny , R , Roby , C and Douce , R . 1989 . “ Phosphorus-31 nuclear magnetic resonance studies in higher plant cells ” . In Nuclear Magnetic Resonance in Agriculture , Edited by: Pfeffer , PE and Gerasimowicz , WV . 72 – 87 . Boca Raton : CRC Press .
- Bohnert , HJ , Ayoubi , P , Borchert , C , Bressan , RA , Burnap , RL , Cushman , JC , Cushman , MA , Deyholos , M , Fischer , R and Galbraith , DW . 2001 . A genomics approach towards salt stress tolerance . Plant Physiology and Biochemistry , 39 : 295 – 311 .
- Bregoff , HM , Roberts , E and Delwiche , CCD . 1953 . Paper chromatography of quaternary ammonium bases and related compounds . Journal of Biological Chemistry , 205 : 565 – 574 .
- Brown , AD and Simpson , JR . 1972 . Water relations of sugar-tolerant yeast: the role of intracellular polyols . Journal of General Microbiology , 72 : 589 – 591 .
- Chen , T and Murata , N . 2002 . Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes . Current Opinion in Plant Biology , 5 : 250 – 257 .
- Colijn , F and De Jonge , VN . 1984 . Primary production of microphytobenthos in the Ems-Dollard Estuary . Marine Ecology Progress Series , 14 : 185 – 196 .
- Colijn , F and Koeman , R . 1975 . Das Mikrophytobenthos der Watten. Strande und Riffe um den Hohen Knechtsand in der Wesermündung . Forschungsstelle Norderney, Jahresbericht , 26 : 53 – 83 .
- Colijn , F and Nienhuis , H . 1977 . The intertidal microphytobenthos of the ‘Hohe Weg’ shallows in the German Wadden Sea . Forschungsstelle Norderney, Jahresbericht , 29 : 149 – 174 .
- DASSARMA, S. & ARORA, P. (2002). Halophiles. In Encyclopedia of Life Sciences, vol. 8, 458–466. Nature Publishing Group, London. (Available at http://halo.umbi.umd.edu/~dassarma/halophiles.pdf)
- De Jonge , VN and Colijn , F . 1994 . Dynamics of microphytobenthos biomass in the Ems estuary measured as chlorophyll a and carbon . Marine Ecology Progress Series , 104 : 185 – 196 .
- Dickson , DMJ and Kirst , GO . 1986 . The role of β-dimethylsulphoniopropionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis . Planta , 167 : 536 – 543 .
- Dickson , DMJ and Kirst , GO . 1987a . Osmotic adjustment in marine eukaryotic algae: the role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes: II. Prasinophytes and haptophytes . New Phytologist , 106 : 657 – 666 .
- Dickson , DMJ and Kirst , GO . 1987b . Osmotic adjustment in marine eukaryotic algae: the role of inorganic ions, quaternary ammonium, tertiary sulfonium and carbohydrate solutes: I. Diatoms and a rhodophyte . New Phytologist , 106 : 645 – 655 .
- Edwards , DM , Reed , RH , Chudek , JA , Foster , R and Stewart , WDP . 1987 . Organic solute accumulation in osmotically-stressed Enteromorpha intestinalis . Marine Biology , 95 : 583 – 592 .
- Eppley , RW and Rogers , JN . 1970 . Inorganic nitrogen assimilation of Ditylum brightwellii, a marine plankton diatom . Journal of Phycology , 6 : 344 – 351 .
- Erdmann , N and Hagemann , M . 2001 . “ Salt acclimation of algae and cyanobacteria: a comparison ” . In Algal Adaptation to Environmental Stresses , Edited by: Rai , LC and Gaur , JP . 323 – 397 . Heidelberg , , Germany : Springer-Verlag .
- Falkowski , PG and Rivkin , RB . 1976 . The role of glutamine synthetase in the incorporation of ammonium in Skeletonema costatum (Bacillariophyceae) . Journal of Phycology , 12 : 448 – 450 .
- Fujii , S , Nishimoto , N , Notoya , N and Hellebust , JA . 1995 . Growth and osmoregulation of Chaetoceros muelleri in relation to salinity . Plant Cell Physiology , 36 : 759 – 764 .
- Garza-Sánchez , F , Chapman , DJ and Cooper , JB . 2009 . Nitzschia ovalis (Bacillariophyceae) mono lake strain accumulates 1,4/2,5 cyclohexanetetrol in response to increased salinity . Journal of Phycology , 45 : 395 – 403 .
- Guillard , RRL . 1975 . “ Culture of phytoplankton for feeding marine invertebrates ” . In Culture of marine invertebrate animals , Edited by: Smith , WL and Chanley , MH . 26 – 60 . New York, USA : Plenum Press .
- Guillard , RRL and Ryther , JH . 1962 . Studies of marine diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Gran . Canadian Journal of Microbiology , 8 : 229 – 239 .
- Haberstroh , PR and Ahmed , SI . 1986 . Resolution by high pressure liquid chromatography of intracellular and extracellular free amino acids of a nitrogen deficient marine diatom, Skeletonema costatum (Grev.) Cleve, pulsed with nitrate or ammonium . Journal of Experimental Marine Biology and Ecology , 101 : 101 – 117 .
- Hare , PD and Cress , WA . 1997 . Metabolic implications of stress-induced accumulation in plants . Plant Growth Regulation , 21 : 79 – 102 .
- Hayashi , T and Konosu , S . 1977 . Quaternary ammonium bases in the adductor muscle of fan-mussel . Bulletin of the Japanese Society for the Science of Fish , 43 : 343 – 348 .
- Hellebust , JA . 1976 . Osmoregulation . Annual Review of Plant Physiology , 27 : 485 – 505 .
- Herbert , D , Phipps , PJ and Strange , RE . 1971 . “ Chemical analysis of microbial cells ” . In Methods in Microbiology, vol. 5B , Edited by: Norris , JR and Ribbons , DW . 210 – 244 . New York : Academic Press .
- Hu , Q . 2004 . “ Environmental effects on the cell composition ” . In Handbook of Microalgal Culture. Biotechnology and Applied Phycology , Edited by: Richmond , A . 83 – 93 . Oxford : Blackwell Publishing .
- Jackson , AE , Ayer , SW and Laycock , MV . 1992 . The effect of salinity on growth and amino acid composition in the marine diatom Nitzschia pungens . Canadian Journal of Botany , 70 : 2198 – 2201 .
- Karsten , U , Wiencke , C and Kirst , GO . 1991 . Growth pattern and β-dimethylsulphonio-propionate (DMSP) content of green macroalgae at different irradiance . Marine Biology , 108 : 151 – 155 .
- Karsten , U , Wiencke , C and Kirst , GO . 1992 . Dimethylsulphoniopropionate (DMSP) accumulation in green macroalgae from polar to temperate regions: interactive effects of light versus salinity and light versus temperature . Polar Biology , 12 : 603 – 607 .
- Kashima , K , Matsubara , H , Kuzucuoğlu , C and Karabiýkoğlu , M . 1997 . Diatom assemblages from inland saline lakes in the central part of Turkey – their application for quantitative reconstructions of paleosalinity changes during the late Quaternary . Japan Review , 8 : 235 – 249 .
- Kirst , GO . 1989 . Salinity tolerance of eukaryotic marine algae . Annual Review of Plant Physiology and Plant Molecular Biology , 40 : 21 – 53 .
- Kirst , GO . 1990 . Salinity tolerance of eukaryotic marine algae . Annual Review of Plant Physiology and Plant Molecular Biology , 40 : 21 – 53 .
- Kirst , GO . 1996 . “ Osmotic adjustment in phytoplankton and macroalgae: the use of dimethylsulfoniopropionate (DMSP) ” . In Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds , Edited by: Kiene , RP , Visscher , PT , Keller , MD and Kirst , GO . 121 – 129 . New York : Plenum Press .
- Kirst , GO and Wiencke , C . 1995 . Ecophysiology of polar algae . Journal of Phycology , 31 : 181 – 199 .
- Klaus , R and Ripphahn , J . 1983 . Quantitative dünnschichtchromatographische Analyse von Zuckern, Zuckersäuren und Polyalkoholen . Journal of Chromatography , 244 : 99 – 124 .
- KRELL, A. (2006). Salt stress tolerance in the psychrophilic diatom Fragilariopsis cylindrus. Ph.D. Thesis, University of Bremen, Germany
- Krell , A , Funck , D , Plettner , I , John , U and Dieckmann , G . 2007 . Regulation of proline metabolism under salt stress in the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae) . Journal of Phycology , 43 : 753 – 762 .
- Laird , K and Edgar , R . 1992 . Spatial distribution of diatoms in the surficial sediments of a New England salt marsh . Diatom Research , 72 : 267 – 279 .
- Lam , CC , Harder , T and Qian , P-Y . 2005 . Induction of larval settlement in the polychaete Hydroides elegans by extracellular polymers of benthic diatoms . Marine Ecology Progress Series , 286 : 145 – 154 .
- Liebezeit , G and Behrends , B . 1999 . “ Determination of amino acids ” . In Methods of Seawater Analysis , Edited by: Grasshoff , K , Kremling , K and Ehrhardt , M . Vol. 3 , 541 – 546 . Weinheim : Wiley–VCH .
- Liu , MS and Hellebust , JA . 1976 . Effects of salinity changes on growth and metabolism of the marine centric diatom Cyclotella cryptica . Canadian Journal of Botany , 54 : 930 – 937 .
- Liu , J and Zhu , J-K . 1997 . Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis . Plant Physiology , 114 : 591 – 596 .
- Mague , TH , Friberg , E , Hughes , DJ and Morris , I . 1980 . Extracellular release of carbon by marine phytoplankton; a physiological approach . Limnology and Oceanography , 25 : 262 – 279 .
- Nanjo , T , Fujita , M , Seki , M , Kato , T , Tabata , S and Shinozaki , K . 2003 . Toxicity of free proline revealed in an Arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase . Plant and Cell Physiology , 44 : 541 – 548 .
- NOTHNAGEL, J. (1995). The effects of salinity and light intensity on the osmolyte concentrations, cell volumes and growth rates of the Antarctic sea-ice diatoms Chaetoceros sp. and Navicula sp. with emphasis on the amino acid proline. PhD thesis, University of Bremen
- Oppenheim , DR . 1991 . Seasonal changes in epipelic diatoms along an intertidal shore, Berrow Flats, Somerset . Journal of the Marine Biological Association of the United Kingdom , 71 : 579 – 596 .
- Pankow , H . 1990 . Ostsee-Algenflora Gustav Fischer, Jena
- Parida , AK and Das , AB . 2005 . Salt tolerance and salinity effects on plants: a review . Ecotoxicology and Environmental Safety , 60 : 324 – 349 .
- Pasztaleniec , A and Połeć , A . 2006 . Distribution of benthic diatoms in the Świnka River (Polesie Region) in relation to salinity . International Journal of Oceanography and Hydrobiology , 35 : 227 – 236 .
- Paterson , DM . 1994 . “ Microbiological mediation of sediment structure and behaviour ” . In Microbial Mats , Edited by: Stal , LJ and Caumette , P . Vol. G35 , 97 – 109 . Berlin : Springer . NATO ASI Series
- Paul , JS . 1979 . Osmoregulation in the marine diatom Cylindrotheca fusiformis . Journal of Phycology , 15 : 280 – 284 .
- Peletier , H . 1996 . Long-term changes in intertidal estuarine diatom assemblages related to reduced input of organic waste . Marine Ecology Progress Series , 137 : 265 – 271 .
- Pinckney , J and Zingmark , R . 1991 . Effects of tidal stage and sun angles on intertidal benthic microalgal productivity . Marine Ecology Progress Series , 76 : 81 – 89 .
- PLETTNER, I. (2002). Stressphysiologie bei antarktischen Diatomeen – Ökophysiologische Untersuchungen zur Bedeutung von Prolin bei der Anpassung an hohe Salinitäten und tiefe Temperaturen. PhD thesis, University of Bremen, Germany
- Reed , RH . 1983 . The osmotic response of Polysiphonia lanosa from marine and estuarine sites: evidence for incomplete recovery of turgor . Journal of Experimental Marine Biology and Ecology , 68 : 169 – 193 .
- Reed , RH . 1984 . The effects of extreme hyposaline stress upon Polysiphonia lanosa from marine and estuarine sites . Journal of Experimental Marine Biology and Ecology , 76 : 131 – 144 .
- Reineck , H-E . 1983 . “ Sediment and dynamical processes ” . In Ecology of the Wadden Sea , Edited by: Wolff , WJ . 6 – 49 . Rotterdam : A.A. Balkema .
- Rijstenbil , JW , Mur , LR , Wijnholds , JA and Sinke , JJ . 1989a . Impact of temporal salinity decrease on growth and nitrogen metabolism of the marine diatom Skeletonema costatum in continuous cultures . Marine Biology , 101 : 121 – 129 .
- Rijstenbil , JW , Wijnholds , JA and Sinke , JJ . 1989b . Implications of salinity fluctuations for growth and nitrogen metabolism of the marine diatom Ditylum brightwellii in comparison with Skeletonema costatum . Marine Biology , 101 : 131 – 141 .
- Rout , NP and Shaw , BP . 1998 . Salinity tolerance in aquatic macrophytes: probable role of proline, the enzymes involved in its synthesis and C4 type of metabolism . Plant Science , 136 : 121 – 130 .
- Rysgaard , S , Christensen , PB and Nielsen , LP . 1995 . Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna . Marine Ecology Progress Series , 126 : 111 – 121 .
- Saburova , MA , Polikarpov , IG and Burkovsky , IV . 1995 . Spatial structure of an intertidal sandflat microphytobenthos community as related to different spatial scales . Marine Ecology Progress Series , 129 : 229 – 239 .
- Schobert , B . 1974 . The influence of water stress on the metabolism of diatoms. I. Osmotic resistance and proline accumulation in Cyclotella meneghiniana . Zeitschrift für Pflanzenphysiologie , 74 : 106 – 120 .
- Schobert , B . 1980 . Proline catabolism, relaxation of osmotic strain and membrane permeability in the diatom Phaeodactylum tricornutum . Plant Physiology , 50 : 37 – 42 .
- Scholz , B and Liebezeit , G . 2012a . Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions . Diatom Research , 27 : 65 – 73 .
- Scholz , B and Liebezeit , G . 2012b . Microphytobenthic communities in the Solthörn tidal flat (Southern North Sea) – Part II: Seasonal and spatial variability of non-diatom components in relation to abiotic parameters . European Journal of Phycology , 47 : 120 – 137 .
- Scholz , B and Liebezeit , G . 2012c . Microphytobenthic communities in the Solthörn tidal flat (Southern North Sea) – Part I: Seasonal and spatial variations of diatoms in relation to macronutrient supply . European Journal of Phycology , 47 : 105 – 119 .
- SMITH, D.J. (1999). Exopolymer production by epipelic diatoms. Ph.D. Thesis, University of Essex, UK
- Stefels , J , Gieskes , WWC and Dijkhuizen , L . 1996 . “ Intriguing functionality of the production and conversion of DMSP in Phaeocystis sp ” . In Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds , Edited by: Kiene , RP , Visscher , PT , Keller , MD and Kirst , GO . 305 – 315 . New York : Plenum Press .
- Stines , AP , Naylor , DJ , Hoj , PB and van Heeswijck , R . 1999 . Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of Δ 1-pyrroline-S-carboxylate synthetase mRNA or protein . Plant Physiology , 120 : 923 – 931 .
- Summers , PS , Nolte , KD , Cooper , AJL , Borgeas , H , Leustek , T , Rhodes , D and Hanson , AD . 1998 . Identification and stereospecificity of the first three enzymes of 3-dimethyl-sulfoniopropionate biosynthesis in a chlorophyte alga . Plant Physiology , 116 : 369 – 378 .
- Sundbäck , K and Granéli , W . 1988 . Influence of microphytobenthos on the nutrient flux between sediment and water: a laboratory study . Marine Ecology Progress Series , 43 : 63 – 69 .
- Treichel , S . 1986 . The influence of NaCl on Δ1-pyrroline-5-carboxylate reductase in proline-accumulating cell suspension cultures of Mesembryanthemum nodiflorum and other halophytes . Plant Physiology , 67 : 173 – 181 .
- Underwood , GJC . 1994 . Seasonal and spatial variation in epipelic diatom assemblages in the Severn estuary . Diatom Research , 9 : 451 – 472 .
- Underwood , GJC . 2005 . Microalgal (microphytobenthos) biofilms in shallow coastal waters: how important are species? . Proceedings of the California Academy of Science , 56 : 162 – 169 .
- Underwood , GJC and Kromkamp , J . 1999 . Primary production by phytoplankton and microphytobenthos in estuaries . Advances in Ecological Research , 29 : 93 – 153 .
- Underwood , GJC and Paterson , DM . 1993 . Seasonal changes in diatom biomass, sediment stability and biogenic stabilization in the Severn estuary . Journal of the Marine Biological Association of the United Kingdom , 73 : 871 – 887 .
- Underwood , GJC and Smith , DJ . 1998 . Predicting epipelic diatom exopolymer concentrations in intertidal sediments from sediment chl a . Microbial Ecology , 35 : 116 – 125 .
- Vairavamurthy , A , Andreae , MO and Iverson , RL . 1985 . Biosynthesis of dimethylsulfide and dimethylpropiothetin by Hymenomonas carterae in relation to sulfur source and salinity variations . Limnology and Oceanography , 30 : 59 – 70 .
- Van Bergeijk , SA , Van der Zee , C and Stal , LJ . 2003 . Uptake and excretion of dimethylsulphoniopropionate is driven by salinity changes in the marine benthic diatom Cylindrotheca closterium . European Journal of Phycology , 38 : 341 – 349 .
- Visscher , PT and Van Gemerden , H . 1991 . Production and consumption of dimethylsulfonio-propionate in marine microbial mats . Applied and Environmental Microbiology , 57 : 3237 – 3242 .
- Vogel , RH and Kopac , MJ . 1960 . Some properties of ornithine δ-transaminase from Neurospora . Biochimica et Biophysica Acta , 37 : 539 – 540 .
- Vos , PC , de Boer , PL and Misdorp , R . 1988 . “ Sediment stabilization by benthic diatoms in intertidal sandy shoals: qualitative and quantitative observations ” . In Tide-influenced Sedimentary Environments and Facies , Edited by: de Boer , PL , Van Gelder , A and Nio , SD . 511 – 526 . Dordrecht : D. Reidel .
- Yang , CW and Kao , CH . 1999 . Importance of ornithine-δ-aminotransferase to proline accumulation caused by water stress in detached rice leaves . Plant Growth Regulation , 27 : 189 – 192 .
- Zhang , Z , Xiao , Z and Linhardt , RJ . 2009 . Thin layer chromatography for the separation and analysis of acidic carbohydrates . Journal of Liquid Chromatography and Related Technologies , 32 : 1711 – 1732 .