Abstract
We investigated the morphology and evolutionary relationships of Torodinium spp. and Katodinium glaucum, unarmoured dinoflagellates characterized by a small hyposome. An emended generic description of Torodinium was proposed based on light and scanning electron microscopy. Torodinium exhibited a unique combination of morphological features including a minute hyposome, a long episome with longitudinal ribs and a canal of unknown function on the dextro-lateral side. Unlike any known dinoflagellate both cingulum and sulcus extended in the episome. The apex surface showed ribs that converged in a bill-like projection. The shape of the apical groove was a circular spiral that extended around the apex running in 2.5 turns in an anticlockwise direction. The type species T. teredo was usually longer than T. robustum. The longitudinal outline of T. teredo was linear, with almost parallel margins, a circular transversal section, a relatively large hyposome and a conspicuous bill-like projection. The longitudinal outline of T. robustum was oblong, widened in the middle, with an ellipsoidal transversal section, a small hyposome and a less prominent bill-like projection. Several morphological features of Katodinium glaucum (=Gyrodinium glaucum) resembled Gyrodinium, such as the cingular displacement, longitudinal ribs, trichocysts, rod-shaped and refractile bodies and a capsule that surrounded the spherical nucleus. Distinctive features of K. glaucum were the horseshoe-shaped apical groove under a tongue-shaped notch pointed towards the dorsal side, and a bifurcated proximal end of the cingulum. Phylogenetic analysis revealed that Torodinium spp. and K. glaucum formed two independent lineages with no close relationships with other known dinoflagellates. The morphology of K. glaucum was distant from the type species of Katodinium. We propose the new genus and combination Lebouridinium glaucum gen. nov., comb. nov. for the species Katodinium glaucum.
INTRODUCTION
Two major groups of dinoflagellates can be distinguished based on morphological criteria: thecate or armoured species with discernible thecal plates and athecate, unarmoured or ‘naked’ species without plates or with plates that are barely visible under the light microscope. Unarmoured dinoflagellates, especially gymnodinioid forms, tend to be delicate, easily damaged by net sampling and often too distorted by fixation to be identified. Live specimens can be easily deformed when they are examined under the microscope (Kofoid & Swezy, Citation1921).
Gymnodinium teredo C.H.G. Pouchet was described as a fusiform unarmoured dinoflagellate with a very large episome that occupied most of the cell body, a posterior cingulum and a much reduced hyposome (Pouchet, Citation1885). Schütt (Citation1895) illustrated G. teredo in more detail, including the cell shape variability, particularly the length of the episome, as later remarked by Lebour (Citation1917). Kofoid & Swezy (Citation1921) used the marked torsion of the sulcus, amounting to 0.5 turns, to support the erection of Torodinium Kof. & Swezy as a genus independent from Gymnodinium F. Stein. Kofoid & Swezy proposed the type, Torodinium teredo (C.H.G. Pouchet) Kof. & Swezy, and the new species Torodinium robustum Kof. & Swezy based on their own observations and some of the illustrations of G. teredo from Schütt (Citation1895). Apparently Kofoid & Swezy (Citation1921) did not observe any specimens of T. teredo. However, they considered that the apex of T. teredo lacked the apical groove (which they called reversed terminal anterior loop of the sulcus). This feature, together with the relative ratio between the length and the transdiameter, with a stouter episome in T. robustum, was used for species distinction.
The two Torodinium species have been commonly reported in the literature and further authors agreed with the diagnosis given by Kofoid & Swezy, with no new taxonomic information (Elbrächter, Citation1979; Dodge, Citation1982; Sournia, Citation1986; Hansen & Larsen, Citation1992; Steidinger & Tangen, Citation1997; Gárate-Lizárraga & Muciño-Márquez, Citation2013). Gómez (Citation2009) reported that some specimens of Torodinium showed a body extension that protrudes from the hyposome and accumulation bodies (tentative food vacuoles). These features supported the mixotrophic character of Torodinium.
Kofoid & Swezy (Citation1921, p. 390) reported a link between Torodinium and Spirodinium glaucum M. Lebour. The latter species was further reported as Gyrodinium glaucum (M. Lebour) Kof. & Swezy, Massartia glauca (M. Lebour) J. Schiller and Katodinium glaucum (M. Lebour) A.R. Loebl. Katodinium glaucum is devoid of plastids, with a postmedian cingulum, marked cingular displacement, apex with a tongue-shaped notch, and the cell surface is covered with longitudinal ribs (Takayama, Citation1985, Citation1998). Daugbjerg et al. (Citation2000) placed K. glaucum again into the genus Gyrodinium Kof. & Swezy. Kim & Kim (Citation2007) retained the name Katodinium glaucum because they did not find a close relationship with Gyrodinium or other dinoflagellates in their LSU rDNA phylogenetic analysis. More than 40 species have been described or transferred into the genus Katodinium Fott. The type species, K. nieuportense (W. Conrad) Loebl. & A.R. Loebl., only known from the original description, is an unarmoured dinoflagellate with two to four plate-like, yellowish chloroplasts and numerous, minute oil droplets. Its apex is rounded and the cell surface smooth (Conrad, Citation1926). Further studies have revealed that Katodinium was a polyphyletic group that even included thecate species (Hansen, Citation1995; Murray et al., Citation2007; Calado, Citation2011; Kang et al., Citation2015). Reñé et al. (Citation2015) provided new sequences of Torodinium and K. glaucum. In their SSU rDNA phylogeny, T. robustum and K. glaucum branched together with very low support (Reñé et al., Citation2015). In this study, we provide a detailed study of the morphology of the two species of Torodinium and Katodinium glaucum. We re-examined the molecular phylogeny of these taxa with new sequences of Torodinium spp.
Materials and methods
Sampling and isolation of material
In the Mediterranean Sea, the specimens of Torodinium spp. were collected from October 2007 to September 2008 by slowly filtering surface seawater taken from the pier of the Station Marine d’Endoume at Marseille (43°16′48.05″N, 5°20′56.22″E, bottom depth 3 m). A strainer of 20 µm mesh size was used to collect planktonic organisms from water volumes ranging between 10 and 100 l, depending on particle concentration. The plankton concentrate was scanned in settling chambers at 100× magnification with an inverted microscope (Nikon Eclipse TE200, Nikon Inc., Tokyo, Japan). Cells were photographed alive at 200× or 400× magnification with a Nikon Coolpix E995 digital camera. During the sampling on 17–21 December 2007, the distinction between species was not defined and we pooled a total of 20 specimens of Torodinium spp. into a single sample for PCR amplification and cloning (isolated cells FG21–2, FG21–3, FG21–4, GenBank accession numbers KR139781, KR139782, KR139783). Sporadic samplings were carried out in the Bay of Marseille at the SOMLIT (Service d’Observation en Milieu LITtoral) site (43º14′52.8″N, 5º17′52.8″E). Samples were collected with Niskin bottles at the surface and 55 m depth and analysed following the procedure described above. In this case, a single specimen (#FG187) was analysed by single-cell PCR (GenBank accession number KR139784).
Further specimens were collected using the same method from October 2008 to August 2009 in the surface waters (depth of 2 m) of the port of Banyuls-sur-Mer, France (42º28′50″N, 3º08′09″E). The concentrated sample was examined in Utermöhl chambers with an inverted microscope (Olympus IX51, Olympus Inc., Tokyo, Japan) and photographed with an Olympus DP71 digital camera. Sampling continued from September 2009 to February 2010 in the Bay of Villefranche-sur-Mer, Ligurian Sea. For this location, sampling was performed at the long-term monitoring site Point B (43º41′10″N, 7º19′00′′E, water column depth ~80 m). Water column samples (0–80 m) were obtained using a phytoplankton net (53 µm mesh size, 54 cm diameter, 280 cm length). Samples were prepared according to the same procedure as described above and specimens were observed with an inverted microscope (Olympus IX51) and photographed with an Olympus DP71 digital camera. Sampling continued from May 2012 to February 2013 in the port of Valencia, Spain (39°27′38.13″N, 0°19′21.29″W, water column depth of 4 m). Specimens were obtained using a phytoplankton net (20 µm mesh size). Samples were prepared according to the same procedure as described above and specimens were observed with an inverted microscope (Nikon Eclipse T2000) and photographed with an Olympus DP71 digital camera.
In addition, samples were collected during the BOUM (Biogeochemistry from the Oligotrophic to the Ultra-oligotrophic Mediterranean) cruise on board R/V L’Atalante from the south of France to the south of Cyprus (20 June–18 July 2008). Seawater samples were collected with Niskin bottles from 30 stations. At each station 6 depths were sampled between 5 and 125 m, with an additional sample at 250 m depth. These samples were preserved with acid Lugol’s solution and stored at 5ºC. Samples of 500 ml were concentrated via sedimentation in glass cylinders. The top 450 ml of sample was slowly siphoned off with small-bore tubing over 6 days. The remaining 50 ml of concentrate, representing 500 ml whole water, was then settled in composite settling chambers. The sample was examined in Utermöhl chambers at 100× magnification with a Nikon inverted microscope (Nikon Eclipse TE200) and the specimens were photographed with a digital camera (Nikon Coolpix E995).
In the North Pacific Ocean, samples were collected with a plankton net (30 µm mesh size) from the coastal Inland Sea of Japan at Kure (34°10′30″N, 132°33′21.6″E). The living concentrated samples were observed at 400× and 1000× magnification with an upright microscope (Olympus BH2), and photographed with a digital camera (Canon EOS Kiss F., Canon Inc., Tokyo, Japan).
Scanning electron microscopy
Seawater samples were collected with a bucket from the coastal areas of the Inland Sea of Japan along Hiroshima Prefecture in 1980–1985 as described in Takayama (Citation1998). For scanning electron microscopy, dinoflagellate cells were pipetted individually, rinsed three times in filtered seawater and placed on poly-lysine coated coverslips. They were fixed in 2% osmium tetroxide in seawater for 20 min. After washing in distilled water for 30 min, cells were dehydrated in an ethanol series, 10 min in each change of 30%, 50%, 70%, 90% and 95%, followed by two 30 min changes in absolute ethanol, and finally transferred to amyl acetate. The cells were critical-point dried using liquid carbon dioxide and ion sputter-coated with gold. They were observed using a scanning electron microscope (Hitachi S–430, Hitachi Ltd, Tokyo, Japan) operated at 15 kV. The method is explained in detail in Takayama (Citation1998). Pictures were scanned and presented on a black background using Adobe Photoshop CS3 (Adobe Systems Inc., San José, California, USA).
PCR amplification of small subunit rRNA genes (SSU rDNAs) and sequencing
For molecular analysis, each specimen was photographed and then micropipetted individually with a fine capillary into a clean chamber and washed several times in a series of drops of 0.2 µm-filtered and sterilized seawater. Finally, the specimen was placed in a 0.2 ml tube (ABgene; Thermo Fisher Scientific Inc., Courtaboeuf, France) filled with several drops of absolute ethanol. The sample was kept at room temperature and in darkness until the molecular analysis could be performed. The specimens fixed in ethanol were centrifuged for 5 min at 504 × g. The ethanol was then evaporated in a vacuum desiccator and single cells were resuspended directly in 25 µl of Ex TaKaRa buffer (TaKaRa, distributed by Lonza, Levallois-Perret, France). PCRs were done in a volume of 30–50 µl reaction mix containing 10–20 pmol of the eukaryotic-specific SSU rDNA primers EK-42F (5′–CTCAARGAYTAAGCCATGCA–3′) and EK-1520R (5′–CYGCAGGTTCACCTAC–3′) (López-García et al., Citation2001). PCRs were performed under the following conditions: 2 min denaturation at 94ºC; 10 cycles of ‘touch-down’ PCR (denaturation at 94ºC for 15 s; a 30 s annealing step at decreasing temperature from 65 down to 55ºC, employing a 1ºC decrease with each cycle, extension at 72ºC for 2 min); 20 additional cycles at 55ºC annealing temperature; and a final elongation step of 7 min at 72ºC. A nested PCR was then carried out using 2–5 µl of the first PCR products in a GoTaq (Promega, Lyon, France) polymerase reaction mix containing the eukaryotic-specific primers EK-82F (5′–GAAACTGCGAATGGCTC–3′) and EK-1498R (5′–CACCTACGGAAACCTTGTTA–3′) (López-García et al., Citation2001) and similar PCR conditions as described above. Negative controls without template DNA were used at all amplification steps. Amplicons of the expected size (~1700 base pairs) were then sequenced bi-directionally using primers EK-82F and EK-1498R using an automated 96-capillary ABI PRISM 3730xl sequencer (BC Genomics, Takeley, UK). In other samples, the amplified product was subsequently cloned using the Topo TA Cloning system (Invitrogen, Life Technologies, Saint Aubin, France) following the instructions provided by the manufacturers. Three clones were picked and the corresponding insert amplified using vector primers. Amplicons of the expected size were fully sequenced (Cogenics, Meylan, France) with vector primers using the same automated sequencer.
Phylogenetic analyses
The new SSU rDNA sequences were aligned to a large multiple sequence alignment containing ~1500 publicly available complete or nearly complete (>1300 base pairs) dinoflagellate sequences using the profile alignment option of MUSCLE 3.7 (Edgar, Citation2004). The resulting alignment was manually inspected using the program ED of the MUST package (Philippe, Citation1993). Ambiguously aligned regions and gaps were excluded in phylogenetic analyses. Preliminary phylogenetic trees with all sequences were constructed using the Neighbour joining method (Saitou & Nei, Citation1987) implemented in the MUST package (Philippe, Citation1993). These trees allowed identification of the closest relatives of our sequences together with a sample of other dinoflagellate species, which were selected to carry out more computationally intensive Bayesian inference analyses. These analyses were done with the program MrBayes 3.2.3 (Ronquist et al., Citation2012) applying a GTR + Γ4 model of nucleotide substitution, taking into account a Γ-shaped distribution of substitution rates with four rate categories. Four chains (three heated and one cold) were run for 2 million generations with trees sampled every 100 generations. The first 5000 trees were discarded as burn-in and a majority-rule consensus tree was constructed with the remaining trees. Our sequences were deposited in DDBJ/EMBL/GenBank under accession numbers KR139781–KR139784.
Results
Light microscopy of Torodinium spp
The observations of Torodinium, with more or less slender co-existing specimens, were sporadic in the sampling areas. The separation of the two species of Torodinium in the literature has traditionally been based on morphometric parameters: T. teredo for larger slender specimens [length more than 3.5–4 depths (=transdiameter sensu Kofoid & Swezy)] and T. robustum for shorter and stouter specimens. We propose the separation of both species based on cell length, with Torodinium teredo (55–100 µm long) usually longer than T. robustum (40–75 µm long), and outline of the cells. The outline of T. teredo was linear, with almost parallel margins, while the outline of T. robustum was oblong, widened in the middle (–). The hyposome and the bill-like projection, the latter further described in detail, were more conspicuous in T. teredo than in T. robustum. From the pier of the Marine Station of Endoume, some records corresponded to larger and more slender specimens in agreement with the definition of T. teredo (–). Other observations corresponded to shorter specimens with an oblong shape in agreement with our definition of T. robustum (–). One of the specimens of T. teredo showed an interesting morphological feature with a kind of edging of crenate margin (with rounded teeth) that extended longitudinally in the episome ().
Figs 1–24. Light microscopy (LM) images of Torodinium teredo and T. robustum. Bright field optics, except by epifluorescence microscopy. –. Specimens from Endoume, Marseille, France. –6. T. teredo. –11. T. robustum. . Lugol-fixed specimen of T. robustum from the open Algerian Basin, western Mediterranean Sea (BOUM cruise, station #21). The arrow points to the body extension. . T. robustum from the Gulf of Lions, isolated cell #FG187 (GenBank accession number KR139784). –. T. teredo from Gulf of Lions. Note the chloroplasts in epifluorescence microscopy. . T. robustum from Banyuls-sur-Mer. The arrow points to the longitudinal flagellum. . Torodinium sp. from the Bay of Villefranche-sur-Mer, France. –. T. robustum from the port of Valencia, Spain. The arrows point to the longitudinal ribs. . T. teredo from the port of Valencia. The arrow indicates the apical groove. ag = apical groove. bp = bill-like projection. lf = longitudinal flagellum. lr = longitudinal rib. Scale bars: 10 µm.
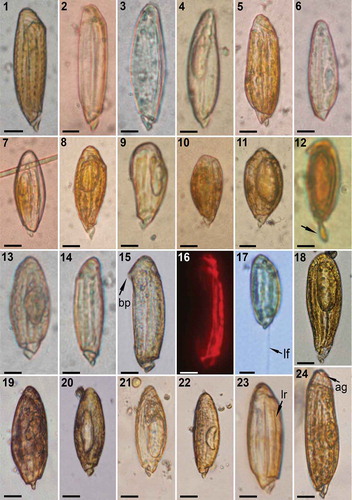
Numerous specimens were observed in Lugol-fixed samples collected from the open Mediterranean Sea, from Cyprus to the Gulf of Lions in summer 2008. Torodinium reached an abundance of up to 64 cells l−1. Several of these fixed specimens showed posterior body extension in samples collected in the open waters of the Sicily Strait, Algerian Basin and Gulf of Lions ().
One specimen was isolated from a sample collected off the Bay of Marseille at 55 m depth in July 2008 (). This specimen, ascribed to T. robustum, showed a prominent vacuole and a stouter cell body (). It was isolated for single-cell PCR analysis (isolated cell FG187, GenBank accession number KR139784).
Other specimens were observed in live samples from Banyuls-sur-Mer (–). The presence of chlorophyll a was confirmed with epifluorescence microscopy (). The cell showed longitudinal chloroplasts along the antero-posterior axis in the dorsal side. The chloroplasts were oblique or transversal in the apex and the cingulum (). All the observed specimens of Torodinium showed slow swimming and the length of the longitudinal flagellum was similar to the cell length (; see video S1 as supplementary information, https://youtu.be/Y7RPT-UdqeE). Few specimens were observed from samples collected at Villefranche-sur-Mer (). This was very likely due to the inappropriate sampling using a coarse plankton net (53 µm mesh size) (). Torodinium teredo and T. robustum co-existed in the samples from the port of Valencia, with more frequent observations of T. robustum. Under light microscopy the episomes of T. robustum (–) and T. teredo () were covered by longitudinal ribs between the cingulum and the basis of the apex. Both species showed an apical groove (named reversed terminal anterior loop of the sulcus sensu Kofoid & Swezy) that was hardly visible in LM (–).
Emended generic description of Torodinium based on scanning electron microscopy
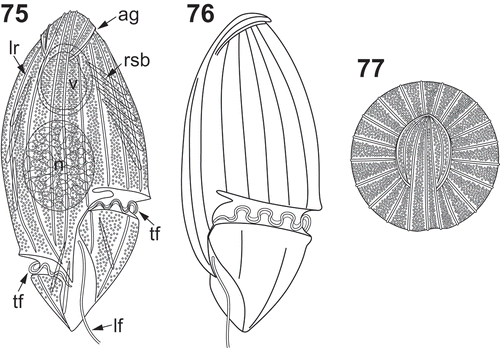
The detailed morphology was examined from specimens of Torodinium spp. collected from the south of Japan (–). We first established the orientation of the cells, whose ventral side was defined by the position of the sulcus and the pore of the longitudinal flagellum. The hyposome was small and conical. The ventral side of the hyposome was concave and occupied by the posterior end of the sulcus below the pore of the longitudinal flagellum (–).
Figs 25–34. Scanning electron microscopy (SEM) images focused on the hyposome of five specimens of Torodinium. –. Three specimens of T. teredo. –. Two specimens of T. robustum. The micrographs 29–34 correspond to the same specimen (also –). . Ventral view. . Ventro-antapical view. . Ventral view. . Antapical view. . Ventro-antapical view. –. Ventral view. . Dorsal view. . Dextro-lateral view. . Ventral-dextro-lateral view. ac = anterior cingulum. as = anterior sulcus. bp = bill-like projection. ci = cingulum. lc = lateral canal. lf = longitudinal flagellum. lfp = longitudinal flagellar pore. sl = sulcal lip. su = sulcus. tf = transversal flagellum. tfp = transversal flagellum pore. Scale bar: 5 µm.
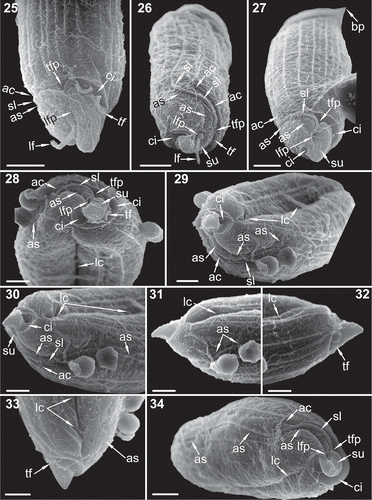
The sulcus extended for almost the entire ventral side of the hyposome, occupying about 1/3 of the contour of the hyposome (–). The pore of the longitudinal flagellum was located in the sulcus between the transversal flagellar pore and the posterior end of the cingulum (–). The posterior end of the sulcus, directed posteriorly from the longitudinal flagellar pore, was placed in a wide concave area surrounded by the cingulum and the hyposome in the left and right margins, respectively (–). The texture of the sulcus surface was rugose, similar to the surface of the rest of the cell. Towards the episome and after the longitudinal flagellar pore, the sulcus became thinner and extended anteriorly describing a loop of about 1/6 of the cell contour towards the dextral side (–). Anteriorly, from the longitudinal flagellar pore, the anterior margin of the sulcus showed an overhanging tube-like structure that separated it from the anterior extension of the cingulum (–, ). We named this structure the sulcal lip. The sulcus extended along the episome to end below the beginning of the apical groove ().
The transversal and longitudinal flagellar pores were separated by the sulcal lip (). The pore of the transversal flagellum delimited the two sections of the cingulum. The transversal flagellum encircled the posterior section of the cingulum above the hyposome and the posterior end of the sulcus (). In contrast to the sulcus, the surface of the posterior cingulum was smooth and lacked any ornamentation (, ). The anterior section of the cingulum was thinner than the posterior one. The anterior cingulum continued parallel to the anterior (left) margin of the sulcus, describing the same looping (, ). The anterior extensions of the sulcus and cingulum were separated by the sulcal lip from the flagellar pore to the convex basis of the episome. The anterior cingulum diverged from the sulcal lip and shortly ended ().
Based on these observations, we established that the cell of T. teredo showed a circular transversal section, whereas T. robustum was laterally compressed instead of dorsoventrally compressed as previously reported (). The transdiameter sensu Kofoid & Swezy corresponded to the cell depth (ventral to dorsal distance). The episome occupied about 9/10 of the cell length and ended in a hemispheric bonnet-shaped apex (, –). The cell surface of the episome was covered by well-marked longitudinal ribs. There were 12–14 longitudinal ribs (~0.35 µm wide) that were equidistant and separated by 3–4 µm (–). In addition to the ribs, at least on the dextro-lateral side, the episome surface was covered in fine longitudinal striae (). In addition to the anterior extensions of the sulcus and cingulum, the episome showed a third groove. This deep groove appeared in the middle of a concave area on the dextro-lateral side (, , , ). This concave area was not observed in live cells and it could not be ruled out that the depression of the deep groove was due to a sample preparation artefact. The anterior and posterior ends of this groove were different (, ). The anterior one ended in a straight line between two longitudinal ribs that converged at their anterior ends (). The posterior end of the groove was located above the distal end of the anterior cingulum and showed a short loop towards the left side (–, ). We were unable to find an analogous structure in any other dinoflagellate. This straight deep groove was named a ‘slender canal, anterior pusule’ by Kofoid & Swezy (Citation1921, p. 391). Following this terminology, we named this groove the lateral canal.
Figs 35–50. SEM focused on the hyposome of five specimens of Torodinium. –. T. robustum. Micrographs 35–43 correspond to the same specimen (also –). –. T. teredo. . Dextro-lateral view. . The arrows point to the longitudinal striae. . Apex. . Ventral view. . Apex. –. Dextro-lateral dorsal view. –. Apex. . Another specimen of T. robustum in apical view. . T. teredo in apical-dorsal view. –. Another specimen in dorsal view. . Another specimen in sinistro-lateral view. . Dorsal view. ag = apical groove. ar = apical rib. as = anterior sulcus. bp = bill-like projection. lc = lateral canal. lr = longitudinal rib. sl = sulcal lip. su = sulcus. Scale bar: 5 µm.
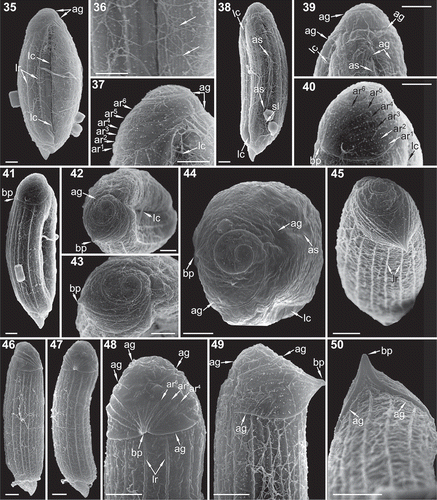
The hemispherical apex showed unique morphological structures such as a spiral-shaped apical groove and ribs that converged in a pointed projection (–). The posterior end of the apical groove began above the anterior end of the sulcus (, , ). The apical groove continued below the basis of the pointed projection and took 2.5 turns describing an anticlockwise spiral that ended in the dextro-lateral side, pointing to the lateral canal in T. teredo (–, ).
The hemispherical apex showed a pointed projection oriented towards the sinistro-lateral or dorsal sides in T. teredo and T. robustum, respectively (, –, , ). This structure was here named ‘bill-like projection’. Six or seven ribs coming from the basis of the apical groove converged from each side into the bill-like projection (, –). These transversal or oblique apical ribs were thinner and they were not connected with the prominent longitudinal ribs on the episome. The most posterior apical rib was placed above the apical groove (–, , ). The first apical rib emerged in the dextro-lateral side from the base of the apical groove. Each rib emerged at each side of the basis of the apical groove and converged between the sinistro-lateral and dorsal sides. The last pair of apical ribs joined in a triangular structure (, , ).
Figs 51–58. Line drawings of different views of Torodinium teredo (–) and T. robustum (–). , . Ventral view. , . Dorsal view. , . Apical view. , . Antapical view. ac = anterior cingulum. ag = apical groove; as = anterior sulcus. bp = bill-like projection. ci = cingulum. lc = lateral canal. sl = sulcal lip. su = sulcus.
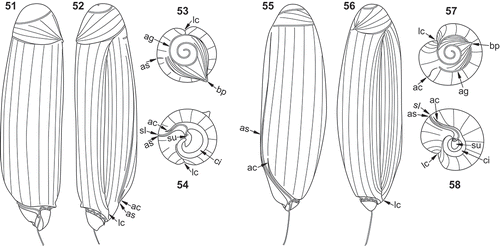
Differences between T. teredo and T. robustum
As reported above, under light microscopy T. teredo was usually longer than T. robustum. The longitudinal outline of T. teredo was linear, with almost parallel margins, a circular transversal section, a relatively large hyposome and a conspicuous bill-like projection. The longitudinal outline of T. robustum was oblong, widened in the middle, with an ellipsoidal transversal section, a small hyposome and a less prominent bill-like projection. Based on SEM, the transversal section was circular (–) and ellipsoidal (–) in T. teredo and T. robustum, respectively. Torodinium teredo (–, –, –, ) showed a larger hyposome than T. robustum (–, , , , –, ). In T. teredo, the proximal part of the anterior extension of the sulcus and cingulum extended transversally towards the dextral side and then described a marked loop (, ). In contrast, in T. robustum the proximal part of the anterior extension of the sulcus and cingulum extended obliquely towards the episome (, ). The anterior extension of the sulcus and cingulum was more displaced towards the dextral side in T. teredo than in T. robustum (, , , ). Consequently, the proximal part of the anterior extension of the cingulum and sulcus were visible in ventral view in T. robustum () and in dorsal view in T. teredo (). In the apex of T. teredo, the bill-like projection was more conspicuous, overlying the episome, and oriented between the ventral and sinistro-lateral sides (–, ). The bill-like projection of T. robustum was more reduced, and oriented between the sinistro-lateral and dorsal sides (–, ).
Morphology of Katodinium glaucum
Cells were spindle-shaped, tapering at both the apex and the antapex, and about 35–40 µm long and about 14–22 µm wide (–). The cells under division reached – µm long (–). The hyposome was about 1/4 of the cell length. The descending cingulum was displaced by three to four cingular widths (–). Cells lacked chloroplasts. Vacuoles and refractile bodies were observed in the upper episome (–). Groups of trichocysts and some rod-shaped bodies were situated along the cell margin in the episome and the hyposome (–). The nucleus was spherical and located in the posterior part of the episome at the middle of the cell (–). A capsule surrounded the nucleus (, ). The proximal end of the cingulum was bifurcated, with a short anterior extension almost parallel to the cingulum (). The cell surface showed longitudinal ribs, hard to see on the hyposome (, ). Under SEM, the proximal end of the cingulum was bifurcated (–). The transverse flagellum emerged from the posterior end of the bifurcation (). The anterior end of the bifurcation extended parallel to the cingulum. This structure could be interpreted as an anterior extension of the cingulum. However, it could also be interpreted as a notch that invaded and dissected the proximal end of the cingulum (). The apex showed a tongue-shaped notch (, –). It was bordered by two longitudinal ribs, and contained five other longitudinal ribs that converged towards a pointed end in the dorsal side (–, ). From the five longitudinal ribs, the three central ones extended posteriorly along the episome (, –). This tongue-shaped notch extended over a horseshoe-shaped apical groove with the ends oriented towards the ventral side (, ). The cell surface of the episome contained 24 equidistant longitudinal ribs (, ). Those longitudinal ribs ended at the basis of the apical groove, with the exception of three ribs that extended towards the pointed end of the tongue-shaped notch (–). The texture of the surface between the ribs was rugose, with two or three transversal granules between each two ribs (–).
Figs 59–74. LM (–) and SEM (–) images of Katodinium glaucum from South Japan. . Specimen with a large vacuole. –. Another specimen. Note the capsule of the nucleus, the rod-shaped bodies, refractile bodies and trichocysts. –. Another specimen. Note the longitudinal ribs and the bifurcation of the proximal end of the cingulum, named anterior cingular extension. –. Specimen undergoing binary division. . Ventral view. . Dextro-lateral view. . Dorsal-apical view. . Ventral view. –. Sinistro-lateral and ventral view. The inset shows the proximal end of the cingulum. –. Detail of the apex. ac = anterior extension of the cingulum. ag = apical groove. ci = cingulum. lf = longitudinal flagellum. lr = longitudinal rib. n = nucleus. rb = refractile body. rsb = rod-shaped body. su = sulcus. tf = transversal flagellum. tr = trichocyst. v = food vacuole. Scale bars: 10 µm.

Molecular phylogeny
We obtained sequences of Torodinium from two samples. One sample (#FG21) contained a mix of 20 specimens of T. teredo and T. robustum, collected from a pier at Marseille over five days in December 2007 (–). PCR amplification and cloning provided three almost complete SSU rDNA sequences (GenBank accession numbers KR139781, KR139782, KR139783). These sequences differed by 4–9 base pairs. The second sample (#FG187) corresponded to a single specimen of T. robustum collected from offshore Marseille at 55 m depth (). Sample #FG187 was analysed by single-cell PCR and provided an almost complete SSU rDNA sequence (GenBank accession number KR139784). The sequences of T. robustum and the clones of Torodinium spp. were 99% identical and differed by 11 base pairs. The three clones of sample #FG21 have been assigned to T. teredo.
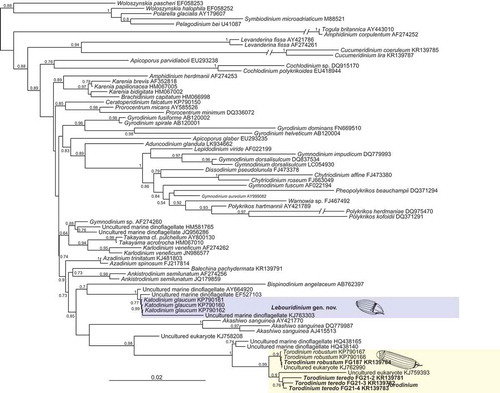
We examined the phylogenetic position of Torodinium spp. and Katodinium glaucum using a data set including a variety of dinoflagellate SSU rDNA sequences. The Bayesian tree showed that all Torodinium spp. sequences branched in a well-supported clade [posterior probability (PP) of 0.99] together with several environmental sequences (). The new sequence of T. robustum was very similar to two sequences of T. robustum from the NW Mediterranean Sea available in GenBank (KP790166–7) and an environmental clone (KJ762990) retrieved from California off San Diego. The three clones assigned to T. teredo were closer to an environmental clone (KJ759393) retrieved from the Gulf Stream and formed the sister group of the sequences of T. robustum. Two environmental sequences retrieved from the under-ice waters of the North Pole (HQ438140, HQ438165) were basal to the whole clade of Torodinium spp. All these sequences formed a strongly supported clade (PP = 1) that was sister group of an environmental clone (KJ758208) retrieved from the Ross Sea, Antarctica (PP = 0.98). The three sequences of Katodinium glaucum available in GenBank (KP790160–2) formed a lineage not closely related to Torodinium spp. or to any other dinoflagellate group. The sequences of Torodinium and Katodinium glaucum branched within the large lineage comprising Gymnodiniales, Peridiniales, Dinophysales and Prorocentrales but with poor support, making it difficult to infer the phylogenetic affinities of these orders ().
Fig. 78. Bayesian phylogenetic tree of dinoflagellate SSU rDNA sequences, based on 1584 aligned positions. Names in bold represent sequences obtained in this study. Numbers at nodes are posterior probabilities (values <0.50 are omitted). The scale bar represents the number of substitutions for a unit branch length.
Taxonomic description
Detailed study of the morphology of Katodinium glaucum confirmed that this species is not related to the type species of Katodinium, K. nieuportense. Molecular and morphological data did not support a close relationship of K. glaucum with Torodinium, Gyrodinium or any other known dinoflagellate genus. Therefore, a new genus is proposed here for K. glaucum.
Lebouridinium F. Gómez, H. Takayama, D. Moreira & P. López- García, gen. nov. (–)
DIAGNOSIS: Unarmoured spindle-shaped cells with the hyposome about 1/4 of the cell length. The descending cingulum was displaced by three to four cingular widths. The cells were devoid of plastids and the cell surface was covered with longitudinal ribs. The apex contained a tongue-shaped notch pointed towards the dorsal side. The apical groove was horseshoe-shaped and extended below the border of the tongue-shaped notch. The proximal end of the cingulum showed a bifurcation, alternatively interpreted as a short leftwards notch that transversally divided the cingulum.
Etymology: In honour of M.V. Lebour who first described the type species. The suffix ‘–dinium’, meaning ‘vortex’ is commonly applied to dinoflagellates. The gender is neuter.
Type species: Lebouridinium glaucum (M. Lebour) F. Gómez, H. Takayama, D. Moreira & P. López-García, gen. & comb. nov. See description above.
Basionym: Spirodinium glaucum M. Lebour Citation1917 (J. Mar. Biol. Ass. U.K. ser. 2, 11: 196, fig. 13).
Synonyms: Gyrodinium glaucum (M. Lebour) Kof. & Swezy Citation1921, p. 308, fig. DD16, plate 9, fig. 94; Massartia glauca (M. Lebour) J. Schiller 1933, p. 436, fig. 462; Katodinium glaucum (M. Lebour) A.R. Loebl. 1965, p. 16 (non Gymnodinium glaucum W. Conrad Citation1926, nec Gymnodinium glaucum J. Schiller 1955).
Epitype: .
Discussion
Comparison of Torodinium with previous descriptions
Our knowledge on the morphology of Torodinium has remained almost unchanged since the original generic description. Kofoid & Swezy (Citation1921) contributed greatly to the understanding of the unarmoured dinoflagellates from specimens collected in the summer of 1917 off San Diego, California. However, in numerous cases Kofoid & Swezy proposed new species based on the observation of single or few specimens, consequently ignoring the potential intraspecific morphological variability. In addition, sometimes they erroneously described life stages as new species based exclusively on the illustrations from other authors (i.e. Gymnodinium fulgens Kof. & Swezy, Gyrodinium falcatum Kof. & Swezy). In the case of Torodinium, Kofoid & Swezy (Citation1921, p. 390) reported ‘we have found only the stouter of these two species, Torodinium robustum, in which we include the first two of Schütt’s figures (Citation1895, pl. 23, figs 74, 1–3)’. Kofoid & Swezy (Citation1921) described the genus Torodinium with T. teredo as type species, based on the illustrations of Gymnodinium teredo in Schütt (Citation1895). However, it is questionable to propose a new genus and species with no personal observations of the type species. In that case, Kofoid & Swezy were right and the SSU rDNA molecular phylogeny confirms that T. robustum and T. teredo are independent species ().
Unarmoured dinoflagellates tend to show high morphological variability, especially in the extension of the cell body (Gómez et al., Citation2004, Citation2005). Thus, the relative elongation is a poor diagnostic criterion for species separation. Kofoid & Swezy (Citation1921) established T. teredo for specimens with a length greater than 4 transdiameters, and less than 3.5 transdiameters for T. robustum. The first problem of this diagnostic criterion is the discrimination of specimens with ratios between 3.5 and 4 length-transdiameter. In most recent literature, this has been solved by assigning to T. teredo specimens with cell length > 3× width (Steidinger & Tangen, Citation1997). The difficulty is to establish where the dorsoventral or lateral sides are. Kofoid & Swezy (Citation1921) erroneously used the term transdiameter (= width) for the cell depth [i.e. the length along the lateral sides (ventral to dorsal distance)].
The distinction between the two species proposed by Kofoid & Swezy was not restricted to only one morphometric character (length-depth ratio). They added that T. robustum possessed an apex with the apical groove–reversed terminal apical loop of the sulcus, which was absent in T. teredo. It should be noted that apparently Kofoid & Swezy (Citation1921) did not examine specimens of T. teredo and this was based on Schütt’s illustrations. Kofoid & Swezy and later authors represented the apical groove of T. robustum as a looping of the sulcus in the apex, while the type species lacked the apical groove (–). Elbrächter (Citation1979) illustrated T. robustum with the apical groove as an anterior extension of the sulcus (), which was absent in T. teredo (). Torodinium teredo and T. robustum are closely related in the SSU rDNA molecular phylogeny, so that the absence of the apical groove in one of the species would be very unusual. In contrast to previous studies exclusively based on LM observations, we have to consider that both Torodinium species may possess an apical groove which is independent of the sulcus (, ).
Figs 79–90. Line drawings of Torodinium teredo and T. robustum in the literature. . Gymnodinium teredo redrawn from Paulsen (Citation1908). –. T. robustum redrawn from Kofoid & Swezy (Citation1921). . Ventral view. . Dextro-lateral view. . Sinistro-lateral view. . T. teredo redrawn from Kofoid & Swezy (Citation1921). . T. robustum redrawn from Lebour (Citation1925). . T. teredo redrawn from Elbrächter (Citation1979). . T. robustum redrawn from Elbrächter (Citation1979). . T. robustum redrawn from Dodge (Citation1982). . T. robustum redrawn from Sournia (Citation1986). . T. robustum redrawn from Hansen & Larsen (Citation1992). . T. teredo redrawn from Steidinger & Tangen (Citation1997).
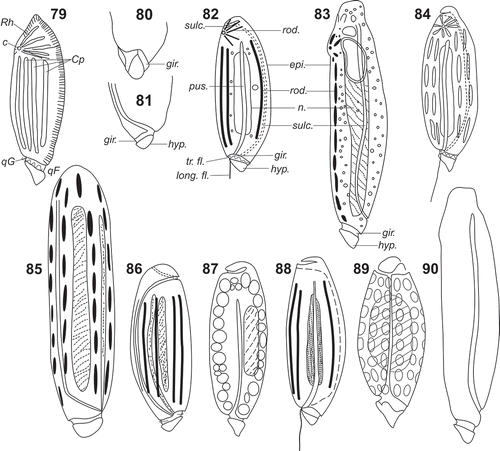
Kofoid & Swezy (Citation1921) reported also that both species of Torodinium lacked striae on the cell surface. However, the surface of the episome is covered with prominent longitudinal ribs as is even revealed by light microscopy (). Some micrographs in the literature also showed the ribs in the episome (Sournia, Citation1986; Gárate-Lizárraga & Muciño-Márquez, Citation2013). The pigmentation of Torodinium is another controversial matter. Elbrächter (Citation1979) reported that the chloroplasts were greenish-yellow to pale brown for T. teredo, and brown for T. robustum. In contrast, Steidinger & Tangen (Citation1997) reported that the pigmentation was brown and green for T. teredo and T. robustum, respectively. In our observations, some specimens showed scarce pigmentation (, , ), while it was greenish in others (). The first micrograph of Torodinium under epifluorescence microscopy showed a specimen with few long longitudinal plastids restricted to the ventral side of the cell ().
The occurrence of three grooves in the episome of Torodinium was not reported in previous studies. The anterior extension of the cingulum is short and it very probably went unnoticed in studies based on light microscopy. An anterior extension of the cingulum has been reported in some species of Gymnodinium, Cochlodinium F. Schütt and Warnowia Lindemann (Takayama, Citation1985, Citation1998). To the best of our knowledge, Torodinium is the only known genus with extensions of both sulcus and cingulum in the episome (, ). Another distinctive character of Torodinium is the sulcal lip (–). A tentatively analogous feature has been reported as a tube-like structure in the genus Takayama de Salas, Bolch, L. Botes & Hallegr. (de Salas et al., Citation2003).
Kofoid & Swezy (Citation1921, p. 391) reported that ‘From the anterior flagellar pore there runs anteriorly at the left of the nucleus a slender canal, the anterior pusule’. The lateral canal was erroneously reported in further literature as reaching the cingulum, reaching the anterior flagellar pore or being confused with the sulcus (–). All previous studies have illustrated the lateral canal in contact with the cingulum (Lebour, Citation1925; Elbrächter, Citation1979; Dodge, Citation1982; Sournia, Citation1986). Kofoid & Swezy denoted the lateral canal as a pusule (). Several functions have been attributed to the dinoflagellate pusule, including the incorporation of particles (Klut et al., Citation1987). The distribution of Torodinium in oligotrophic surface oceanic waters, the scarce chloroplasts, the presence of food vacuoles and the body extension suggest that Torodinium is indeed able to ingest particulate matter (Gómez, Citation2009). We have not yet observed the mechanism of prey capture and ingestion. The body extension was noticed only in specimens that were fixed immediately after collection (Gómez, Citation2009; ). During observations of live specimens the body extension could be retracted due to manipulation stress. The projection of a body extension from the hyposome is a feature known in other gymnodinioid dinoflagellates (Persson et al., Citation2013). Gymnodinioid dinoflagellates typically ingest their prey by direct engulfment through the sulcal area in the hyposome [e.g. Gyrodinium spirale (Bergh) Kof. & Swezy; Hansen, Citation1992]. However, Torodinium has a minute hyposome and posterior sulcus, probably insufficient for the ingestion of large prey. The lateral canal is a structure unknown in any other dinoflagellate and its function remains uncertain. It can be hypothesized that the body extension that emerged from the hyposome may facilitate prey capture and the subsequent ingestion through the lateral canal (, ).
The apex of Torodinium is also highly distinctive. Schütt (Citation1895) illustrated a group of plastids around a central plastid or oil globule forming a star of eight or nine rays, further re-drawn by other authors (, –). This unusual star-shaped distribution of the plastids coincides with the apical ribs that form the bill-like projection (). In one of the earliest dinoflagellate studies, Schütt (Citation1895) was probably confusing the apical ribs with plastids. The function of the bill-like projection is unknown.
Previous observations of Lebouridinium
Our observations of Lebouridinium glaucum unequivocally correspond to the taxon described as Spirodinium glaucum by Lebour (Citation1917, Citation1925) (–). However, L. glaucum have been reported earlier in the literature because it is a common species (Lebour, Citation1917). Schütt (Citation1895) described Gymnodinium vestificii F. Schütt with a larger episome, lacking the surface striae and with an intrusion of the sulcus into the episome (). Later, Kofoid & Swezy (Citation1921), in the absence of personal observations, added surface striations to the illustration of G. vestificii (). Lebour (Citation1925, p. 50) reported on G. vestificii ‘This species is not sufficiently defined, but bears so strong a resemblance to Gyrodinium glaucum if turned upside down that one does not feel justified in regarding it as a Gymnodinium until the flagella have been described’. Even assuming that the orientation of G. vestificii was turned upside down and it is covered with surface striations, the prominent anterior extension of the sulcus and the low cingular displacement do not indicate L. glaucum. Amphidinium extensum A. Wulff was described from four illustrations in dorsal view, lacking information on the sulcus or flagella (Lebour, Citation1925) (). Due to the poor descriptions, it is difficult to determine whether G. vestificii or A. extensum corresponded to the earlier observations of L. glaucum and, consequently, if any of these taxa have priority versus Spirodinium glaucum.
Figs 91–100. Line drawings of Katodinium nieuportense, Lebouridinium glaucum, Gymnodinium vestificii and Amphidinium extensum. . Katodinium nieuportense redrawn from Conrad (Citation1926). . Spirodinium glaucum redrawn from Lebour (Citation1917). . Gyrodinium glaucum redrawn from Lebour (Citation1925). . G. glaucum redrawn from Kofoid & Swezy (Citation1921). . K. glaucum redrawn from Elbrächter (Citation1979). . Gyrodinium glaucum redrawn from Dodge (Citation1982). . K. glaucum redrawn from Steidinger & Tangen (Citation1997). . Gymnodinium vestificii redrawn from Paulsen (Citation1908). . G. vestificii redrawn from Kofoid & Swezy (Citation1921). . Amphidinium extensum redrawn from Lebour (Citation1925).
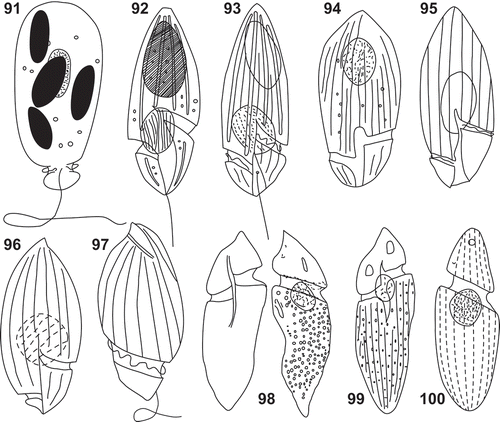
Kofoid & Swezy (Citation1921) and Elbrächter (Citation1979) illustrated Lebouridinium glaucum with an intrusion of the sulcus in the episome (–). However, we did not observe that feature (–). We observed by light and scanning electron microscopy (, –) that the proximal end of the cingulum showed a short bifurcation or, alternatively, a leftwards notch that transversally divided the cingulum (–). This feature was not reported in the literature. The tongue-shaped notch (–) was first reported by Takayama (Citation1985, Citation1998; ).
Evolutionary affinities of Lebouridinium
The morphology of Lebouridinium glaucum is very different from the type of Katodinium, K. nieuportense (), an insufficiently described species that is only known from the original description (Conrad, Citation1926). Some morphological features such as the cingular displacement, longitudinal ribs, trichocysts, rod-shaped and refractile bodies and a capsule that surrounded the spherical nucleus resemble the type of Gyrodinium (Hansen & Daugbjerg, Citation2004; Takano & Horiguchi, Citation2004). However, other features such as the apical groove, tongue-shaped notch or the cingular structure, as well as the molecular data, do not support a relationship between Lebouridinium and Gyrodinium (; Kim & Kim, Citation2007). Since the earlier studies, the small hyposome of L. glaucum invited consideration of a relationship with Torodinium (Lebour, Citation1917; Kofoid & Swezy, Citation1921). Reñé et al. (Citation2015) reported that Torodinium robustum and L. glaucum branched together in the SSU rDNA phylogenetic analysis, although with weak statistical support (bootstrap value < 80%). In our SSU rDNA phylogeny, including more sequences of Torodinium and environmental clones, we did not find a relationship between Torodinium spp. and L. glaucum (). The detailed study of the morphology of Torodinium and Lebouridinium does not reveal similarities in the distinctive diagnostic characters between the two genera. Morphological features such as the reduced hyposome or the cell surface covered with longitudinal ribs are common characters in the unarmoured dinoflagellates (Takano & Horiguchi, Citation2004; Gómez et al., Citation2015).
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Fernando Gómez
F. Gómez: collection, isolation, light microscopy and drafting; H. Takayama: collection, isolation, light and electron microscopy; P. López-García: molecular analysis; D. Moreira: phylogenetic analysis.
Haruyoshi Takayama
F. Gómez: collection, isolation, light microscopy and drafting; H. Takayama: collection, isolation, light and electron microscopy; P. López-García: molecular analysis; D. Moreira: phylogenetic analysis.
David Moreira
F. Gómez: collection, isolation, light microscopy and drafting; H. Takayama: collection, isolation, light and electron microscopy; P. López-García: molecular analysis; D. Moreira: phylogenetic analysis.
Purificación López-García
F. Gómez: collection, isolation, light microscopy and drafting; H. Takayama: collection, isolation, light and electron microscopy; P. López-García: molecular analysis; D. Moreira: phylogenetic analysis.
References
- Calado, A.J. (2011). On the identity of the freshwater dinoflagellate Glenodinium edax, with a discussion on the genera Tyrannodinium and Katodinium, and the description of Opisthoaulax gen. nov. Phycologia, 50: 641–649.
- Conrad, W. (1926). Recherches sur les flagellates de nos eaux saumâtres. 1e partie: dinoflagellates. Archiv für Protistenkunde, 55: 63–100.
- Daugbjerg, N., Hansen, G., Larsen, J. & Moestrup, Ø. (2000). Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia, 39: 302–317.
- De Salas, M.F., Bolch, C.J.S., Botes, L., Nash, G., Wright, S.W. & Hallegraeff, G.M. (2003). Takayama gen. nov. (Gymnodiniales, Dinophyceae), a new genus of unarmoured dinoflagellates with sigmoid apical grooves, including the description of two new species. Journal of Phycology, 39: 1233–1246.
- Dodge, J.D. (1982). Marine dinoflagellates of the British Isles. Her Majesty’s Stationery Office, London.
- Edgar, R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797.
- Elbrächter, M. (1979). On the taxonomy of unarmoured dinophytes (Dinophyta) from the Northwest African upwelling region. “Meteor” Forschungs-Ergebnisse, Reihe D, 31: 1–22.
- Gárate-Lizárraga, I. & Muciño-Márquez, R.E. (2013). New data on the distribution of Torodinium robustum and T. teredo (Dinophyceae: Gymnodiniales) in the Gulf of California. Check List, 9: 809–812.
- Gómez, F. (2009). Torodinium and Pavillardia (Gymnodiniales, Dinophyceae): two unarmoured dinoflagellates with a body extension, collected from the open Pacific Ocean. Protistology, 6: 131–135.
- Gómez, F., Nagahama, Y., Fukuyo, Y. & Furuya, K. (2004). Observations on Ceratoperidinium (Dinophyceae). Phycologia, 43: 416–421.
- Gómez, F., Nagahama, Y., Takayama, H. & Furuya, K. (2005). Is Karenia a synonym of Asterodinium–Brachidinium? (Gymnodiniales, Dinophyceae). Acta Botanica Croatica, 64: 263–274.
- Gómez, F., López-García, P., Takayama, H. & Moreira, D. (2015). Balechina and the new genus Cucumeridinium gen. nov. (Dinophyceae), unarmoured dinoflagellates with thick cell coverings. Journal of Phycology 51: 1088–1105.
- Hansen, G. (1995). Analysis of the thecal plate pattern in the dinoflagellate Heterocapsa rotundata (Lohmann) comb. nov. (=Katodinium rotundatum (Lohmann) Loeblich). Phycologia, 34: 166–170.
- Hansen, G. & Larsen, J. (1992). Dinoflagellater i danske farvande. In Plankton i de indre danske farvande (Thomsen, H.A., editor), 45–155. Havforskning fra Miljøstyrelsen, n. 11, Copenhagen.
- Hansen, G. & Daugbjerg, N. (2004). Ultrastructure of Gyrodinium spirale, the type species of Gyrodinium (Dinophyceae), including a phylogeny of G. dominans, G. rubrum and G. spirale deduced from partial LSU rDNA sequences. Protist, 155: 271–294.
- Hansen, P.J. (1992). Prey size selection, feeding rates and growth dynamics of heterotrophic dinoflagellates with special emphasis on Gyrodinium spirale. Marine Biology, 114: 327–334.
- Kang, N.S., Jeong, H.J., Moestrup, Ø., Jang, T.Y., Lee, S.Y. & Lee, M.J. (2015). Aduncodinium gen. nov. and A. glandula comb. nov. (Dinophyceae, Pfiesteriaceae), from coastal waters off Korea: morphology and molecular characterization. Harmful Algae, 41: 25–37.
- Kim, K.-Y. & Kim, C.-H. (2007). Phylogenetic relationships among diverse dinoflagellate species occurring in coastal waters off Korea inferred from large subunit ribosomal DNA sequence data. Algae, 22: 57–67.
- Klut, M.E., Bisalputra, T. & Antia, N.J. (1987). Some observations on the structure and function of the dinoflagellate pusule. Canadian Journal of Botany, 65: 736–744.
- Kofoid, C.A. & Swezy, O. (1921). The free-living unarmoured Dinoflagellata. Memoirs of the University of California, 5: 1–562.
- Lebour, M.V. (1917). The Peridiniales of Plymouth Sound from the region beyond the breakwater. Journal of the Marine Biological Association, Plymouth, 11: 183–200.
- Lebour, M.V. (1925). The Dinoflagellates of Northern Seas. Marine Biological Association of the United Kingdom, Plymouth.
- López-García, P., Rodríguez-Valera, F., Pedrós-Alió, C. & Moreira, D. (2001). Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature, 409: 603–607.
- Murray, S., de Salas, M., Luong-Van, J. & Hallegraeff, G. (2007). Phylogenetic study of Gymnodinium dorsalisulcum comb. nov. from tropical Australian coastal waters (Dinophyceae). Phycological Research, 55: 176–184.
- Paulsen, O. (1908). Peridiniales. In Nordisches Plankton (Brandt, K. & Apstein, C., editors), 1–124. Lepsius & Tischer, Leipzig.
- Persson, A., Smith, B.C., Morton, S., Shuler A. & Wikfors, G.H. (2013). Sexual life stages and temperature dependent morphological changes allow cryptic occurrence of the Florida red tide dinoflagellate Karenia brevis. Harmful Algae, 30: 1–9.
- Philippe, H. (1993). MUST, a computer package of management utilities for sequences and trees. Nucleic Acids Research, 21: 5264–5272.
- Pouchet, G. (1885). Nouvelle contribution á l´histoire des Péridiniens marins. Journal de l´Anatomie et de la Physiologie Normale et Pathologique de l´Homme et des Animaux, Paris, 21: 28–88.
- Reñé, A., Camp, J. & Garcés, E. (2015). Diversity and phylogeny of Gymnodiniales (Dinophyceae) from the NW Mediterranean Sea revealed by a morphological and molecular approach. Protist, 166: 234–263.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematics Biology, 61: 539–542.
- Saitou, N. & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4: 406–425.
- Schütt, F. (1895). Die Peridinien der Plankton-Expedition. Ergebnisse der Plankton-Expedition der Humboldt-Stiftung, 4: 1–170.
- Sournia, A. (1986). Atlas du phytoplancton marin. Volume I: Cyanophycées, Dictyophycées, Dinophycées, Raphidophycées. Éditions du CNRS, Paris.
- Steidinger, K.A. & Tangen, K. (1997). Dinoflagellates. In Identifying Marine Phytoplankton (Tomas, C.R., editor), 387–598. Academic Press, San Diego, CA.
- Takano, Y. & Horiguchi, T. (2004). Surface ultrastructure and molecular phylogenetics of four unarmored heterotrophic dinoflagellates, including the type species of the genus Gyrodinium (Dinophyceae). Phycological Research, 52: 107–116.
- Takayama, H. (1985). Apical grooves of unarmored dinoflagellates. Bulletin of the Plankton Society of Japan, 32: 129–137.
- Takayama, H. (1998). Morphological and taxonomical studies on the free-living unarmored dinoflagellates occurring in the Seto Inland Sea and adjacent waters. Ph.D. dissertation, The University of Tokyo, Tokyo.