ABSTRACT
This study investigated the effect of dietary α-tocopherol (α-Toc), pomegranate peel extract (PPE), and pomegranate peel (PP) on phenolic content and breast meat quality of broilers during 11 days of storage with the addition of α-Toc, PPE, and PP. Broilers were fed eight dietary treatments, including: control diet, α-Toc diet (200 mg/kg), PPE diets (100, 200, and 300 mg/kg), and PP diets (1, 2, and 3 g/kg) during 0–42 days. The extents of lipid oxidation in samples were assessed by measuring thiobarbituric acid-reactive substances and the 1, 1-Diphenyl-2-picrylhydrazyl radical-scavenging activities were determined. The feed efficiency was improved significantly in chickens fed diet containing 0.2 g/kg PPE, and the growth performance of chickens was impaired in chickens fed with PP diet. Long-chain polyunsaturated fatty acids n-3 were increased in breast muscles of broilers fed α-Toc and high levels of PPE diets (P < .05). Total phenolic contents and antioxidant activity in the breast meat were improved significantly when chickens fed diets containing α-Toc and PPE (P < .05). In conclusion, dietary supplementation with 200 and 300 mg/kg PPE may improve the antioxidant potential and quality indices of broilers breast meat. The antioxidant potential of PPE was equal to that of α-Toc in refrigerated meat.
1. Introduction
Poultry meat is an accepted valuable source of nutrients for consumers. In general, consumer preference, nutrient profile, availability, and low production cost have made poultry meat as a major source of animal food protein worldwide. Poultry meat enriched with long-chain polyunsaturated fatty acids n-3 (PUFA LC n-3) can make a nutritionally meaningful contribution to Western diets in which consumption of PUFA LC n-3 is low. Enrichment of poultry meat with fatty acid (FA) is usually achieved by inclusion of fish oil in broiler diet (Rymer & Givens Citation2010). However, meat enriched in this way is susceptible to quality deterioration by lipid oxidation during storage or cooking, leading to reduction of nutritive value and accumulation of lipid oxidation products (Aziza et al. Citation2010).
The oxidative stability of poultry meat and products depends upon the balance of anti- and pro oxidants and the oxidative substrates, including polyunsaturated fatty acids (PUFA), birds’ diet, and stressors. We have previously shown that exposing broilers to stressors such as heat stress and feed withdrawal leads to higher meat oxidation in broilers (Akbarian et al. Citation2014).
Oxidative stability has been improved by antioxidant supplementation for foods of animal origin (Flachowsky et al. Citation2002). α-Tocopherols (α-Toc) or other synthetic antioxidants have been used to control lipid oxidation in meat. However, due to consumer concern about the safety and toxicity of synthetic antioxidants, recent research has focused on naturally occurring antioxidants.
Negative outcome of lipid oxidation in chicken meat can be attenuated by the use of diets containing phenolic compounds (Sahin & Kucuk Citation2003; Goñi et al. Citation2007). In this respect, our recent results have shown that supplementation of broilers diets with phenolic rich materials could exert beneficial effects on antioxidant status and meat quality of broilers (Akbarian et al. Citation2014; Akbarian et al. Citation2015). However, some researchers reported that the pork meat derived from the pig fed oleoresins of rosemary did not show any antioxidant effect (Lopez-Bote et al. Citation1998). In the lights of these inconsistent results, many researchers have been investigating a variety of other natural antioxidants that possesses a superior protective activity.
Iran is one of the major important pomegranate (Punica granatum L.) producers and exporters in the world, and its total production was 1010,000 tons in 2012 (Anonymous Citation2013). Pomegranate peel (PP) is the residue left after juice extraction by pressing pomegranates in the juicer industry. Recent investigations have stressed the importance of by-products from plant materials particularly rich in phenolic compounds (Akbarian et al. Citation2013a, Citation2013b). Studies have shown that poly phenols have the ability to act as powerful antioxidants by scavenging free radicals and terminating oxidative reactions (Gonzalez-Parama’s et al. Citation2004; Yilmaz & Toledo Citation2004). The peel and rind are good sources of tannins, anthocyanins, and flavonoids (Naveena et al. Citation2008). Hydrolysable tannins (HT) are the most abundant polyphenols and antioxidant compounds in pomegranates and include gallotannins, ellagitannins, and gallagyl esters such as punicalagin and punicalin than condense tannins (Madrigal-Carballo et al. Citation2009). HT are usually more soluble in water and methanol solvents than condensed tannins (CT) (Terrill et al. Citation1992; Reed Citation1995). It has been documented that products rich in phenolic compounds do have a considerable antioxidant potential (Li et al. Citation2006; Akbarian et al. Citation2014).
The objective of this study was to evaluate the effects of dietary α-Toc, pomegranate peel extract (PPE), and PP on phenolic contents, fatty acid (FA) composition, oxidation susceptibility, and quality of the thigh meat of broilers during refrigeration.
2. Materials and methods
2.1. Preparation of extracts
Peels of pomegranate were harvested from pomegranate trees (Ardestani variety) in Khorasan Razavi province (North-East, Iran). The peels were manually removed and air dried under ambient conditions and then powdered in a grinder to pass 40-mesh. The peels were finally packed and stored at −20°C pending extraction. The proximate analysis of PP is shown in . Dried powders of peels (2.5 g) were extracted with 40 mL of methanol solvent at room temperature for 6 h. The extract was filtered through Whatman No. 42 filter paper (Sigma–Aldrich, Munich, Germany) to remove fine particles. After extraction, the solvent was evaporated using a rotary evaporator (Bio-Equip RE-52-3-5; Shanghai Qingpu Huxi Instruments Factory, Shanghai, China) at 30°C and the concentrated extracts were stored in a freezer (Ben Nasr et al. Citation1996). Total polyphenol contents (TPP) were determined by the Folin–Ciocalteu method reported by Elfalleh et al. (Citation2009). The TPP of each fraction were converted into milligram gallic acid equivalents per gram of dry weight (mg GAE/g DW). HT content was determined by the method of Çam and Hişil (Citation2010). The results were expressed as milligram tannic acid equivalent per gram of dry weight (mg TAE/g DW). Methanol extraction treated with 5 mL/L of HCl-butanol during 3 h at 100°C (Makkar & Becker Citation1993) for CT determination. CT were determined from the absorbance at 550 nm of the anthocyanidin solutions.
Table 1. Analyses of PP and PPE.
2.2. Animals, diets, and experimental design
The experimental protocol was approved by the Animal Care Committee of the Ferdowsi University of Mashhad (Mashhad, Khorasan Razavi, Iran). Three hundred and eighty four 1-day-old male broiler chicks (Ross 308) were obtained from a commercial hatchery (Seamorgh Co., Quchan, Mashhad, Iran). Broilers were randomly allotted to 8 groups with 4 replicates of 12 birds each. Water and mash diets were provided ad libitum. Chickens were vaccinated for Infectious Bronchitis on day 1, for Newcastle Disease and Avian Influenza on day 7, and for Infectious Bursal Disease on day 14 of age. Ambient temperature on day 1 was set at 32°C and was gradually reduced to 22°C by day 21. The lighting programme was 23 h light: 1 h dark during the entire period.
A completely randomized design was used with eight dietary treatments including control diet without feed additives, control diet mixed with 200 mg/kg α-Toc (Razak, Tehran, Iran), control diet mixed with PPE (100, 200, and 300 mg/kg), and control diet mixed with PP (1, 2, and 3 g/kg). The source of dietary fibre for control and PPE diets were Sawdust. Peel Pomegranate was replaced by cellulose in PP diets. Ingredients and nutrient composition of experimental diets are shown in . All diets contained 2% fish oil to enhance the enrichment of unsaturated FA n-3 in birds. All diets were isocaloric and isonitrogenous according to Ross 308 recommendation (Aviagen Citation2009) with slight modifications. Broilers were weighed, and feed efficiency was calculated at the end of the experimental period
Table 2. Ingredients and nutrient composition of experimental diets in (g/kg as fed).
2.3. Sample collection and storage
At 42 days of age, four birds from each pen were randomly selected. Breast muscles were trimmed and sorted at −20°C pending analysis of FA composition. Breast meat samples were minced twice (4 mm plate) using a grinder and stored at 4°C pending antioxidant potential and meat quality measurements. Antioxidant potential and meat quality indices were determined on 0, 7, and 11 days of refrigerated storage.
2.4. Meat quality
2.4.1. Total phenolic contents
Each meat sample (5 g) was placed in distilled water (15 mL) and homogenized (Ultra-Turrax T 10, Ika Works, Staufen, Germany) at 1130×g for 2 min. Chloroform (9 mL) was added to the homogenates and the mixture was shaken two to three times vigorously to separate the lipids. Total phenols contents in the aqueous supernatant were estimated by the Folin–Ciocalteu method (Subramanian et al. Citation1965). One-mL of diluted sample (1:4, vol/vol) was added to the Folin–Ciocalteu reagent (500 μL), followed by addition of 1 mL of sodium carbonate solution (10%). The reaction mixture was vortexes and the absorbance was measured with a spectrophotometer (UV 1600 PC, Shimadzu, Japan) at 700 nm after incubation for 1 h at room temperature. The quantification of phenolic compounds was done based on the standard curve generated with gallic acid, and expressed as gallic acid equivalent.
2.4.2. DPPH radical-scavenging activity
The 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity was estimated with the aqueous supernatant obtained from breast meat according to the method described by Blois (Citation1958), with slight modifications. Briefly, a 200-μL of diluted aqueous supernatant was added to 800 μL of water and 1 mL of methanolic DPPH solution (0.2 mM). The mixture was vortexes and left to stand at room temperature for 30 min. A tube containing 1 mL of distilled water and 1 mL of methanolic DPPH solution (0.2 mM) served as the control. The absorbance of the solution was measured at 517 nm (UV 1600 PC, Shimadzu, Japan) using a spectrophotometer. The percentage of DPPH radical scavenging was obtained from the following equation:
2.4.3. Thiobarbituric acid-reactive substances
Each meat sample (5 g) from different storage periods was placed in 15 mL distilled water and homogenized at 1130×g for 1 min (Ultra-Turrax T 10, Ika Works, Staufen, Germany). Sample homogenate (5 mL) was transferred to a test tube and lipid oxidation was determined as the 2-thiobarbituric acid-reactive substance (TBARS) value by using the method described by Ahn et al. (Citation1999). Briefly, 50 μL of butylated hydroxyanisol (7.2%) and 5 mL of TBA-trichloroacetic acid solution (20 mM TBA in 15% trichloroacetic acid) were added to the test tube. Tubes were heated in a boiling water bath for 15 min, cooled, and then centrifuged (966 g, 15 min at 4°C). Absorbance of the supernatants was measured at 532 nm with a spectrophotometer (UV 1600 PC, Shimadzu, Japan). Lipid peroxidation was reported as milligram of MDA per kilogram of meat.
2.4.4. FA composition
Total lipid contents in samples were extracted using chloroform–methanol (2:1, v/v) according to the procedure of Folch et al. (Citation1957). The FA methyl esters were prepared from the extracted lipids with BF3-methanol (Sigma–Aldrich, Chemie GmbH, Germany). The FA methyl esters were then separated on a gas chromatograph in a UNICAM (Ion Path, Road three, Winford, Cheshire, CW7 3GA, UK) equipped with a Flame Ionization Detector. A split inlet (split ratio, 50:1) was used to inject samples into an Sun Grid Engine capillary column (30 m × 0.22 mm × 0.25 μm), and ramped oven temperature was used (160°C for 3 min, increased to 180°C a 2.5°C/min and maintained for 5 min, then increased to 220°C at 2.5°C/min and maintained for 25 min). The injector temperature was programmed at 240°C and the detector temperature was 280°C. Helium was the carrier gas at constant flow of 0.7 mL/min. FAs (gram of weight per 100 g of total FAs) were identified by comparison of retention times to known standards.
2.5. Statistical analysis
Data were subjected to ANOVA using the generalized linear model procedures of SAS (SAS Institute Citation2003), and single degree of freedom linear contrast was used to separate treatments. Linear and quadratic effects were also analysed. Differences among treatments were separated by Duncan multiple comparison tests. In all cases, P < .05 was considered as significant, unless otherwise stated.
3. Results
3.1. Broiler performance
The effect of dietary PP, PPE, and α-Toc acetate on feed intake, body weight gain, and feed conversion ratio is shown in . The addition of PP diets impaired growth performance of chickens (BW, Feed consumption, and Feed efficiency) when compared to those fed the unsupplemented or supplemented with PPE and α-Toc diets (P < .05). Feeding 0.2 g/kg PPE diet significantly improved the daily weight gain and decreased the feed to gain ratio, whereas these diets had no effect on daily feed intake.
Table 3. Effect of dietary supplementation of the α-Toc, PPE, and PP on feed intake, daily weight gain, and feed conversion ratio of broilers (0–42 days).
3.2. FA composition
The effects of dietary PP, PPE, and α-Toc on FA composition of breast meat of broilers are shown in . The FA composition of the diets, particularly PUFA LC n-3, was increased by the inclusion of fish oil into diet. The concentration of myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0) in the breast meat were not influenced by dietary PP, PPE, and α-Toc when compared to that of control birds (P < .01). The results indicated that broilers’ tissues have limited capacity for alteration of their SFAs. Palmitoleic acid (C16:1), oleic acid (C18:1), and total MUFA content in breast were decreased in birds fed diet containing PP, PPE, and α-Toc compared to control birds. Linoleic acid (LA; C18: 2), Arachidonic acid (AA; C20:4), and n-6 FA composition in the breast meat were significantly increased by dietary treatments (P < .01). The level of n-6 FAs in breast of broilers fed diets containing dietary additives (PPE, PP, and α-Toc) were the highest and almost the same in all experimental diets. Concentrations of Linolenic acid (LNA), Eicosapentaenoic acid (EPA, C22:5), Docosapentaenoic acid (DPA, C: n-3), and Docosahexaenoic acid (DHA, C22:6, n-3) in the breast meat of broilers fed diet containing PP, PPE, and α-Toc were higher than that of control birds(P < .01). The n-6: n-3 in the breast meat was decreased by dietary PP, PPE, and α-Toc.
Table 4. FA composition of breast meat in broilers fed α-Toc, PPE, and PP.
3.3. Total phenolic content and antioxidant activity
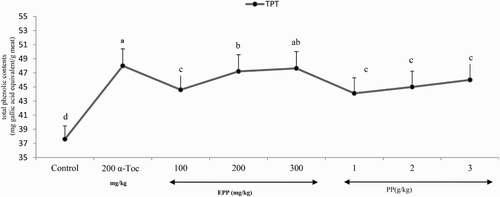
Total phenolic contents of the breast meat in broilers fed PP, PPE, and α-Toc diets are presented in . Breast meat of the broilers fed α-Toc and PPE diets had significantly higher levels of total phenolic contents when compared to control or PP diets (P < .01).
Figure 1. Effect of dietary PPE, PP, and α-TOC acetate on total phenolic content (mg gallic acid equivalent/g meat) of raw chicken breast meat. All data points represent mean total phenolic contents concentrations from four analyses and their standard deviations.
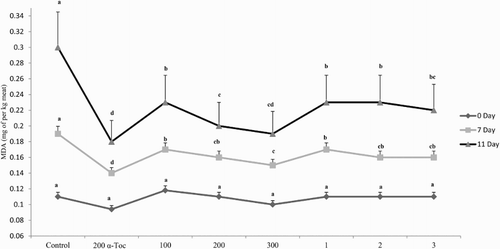
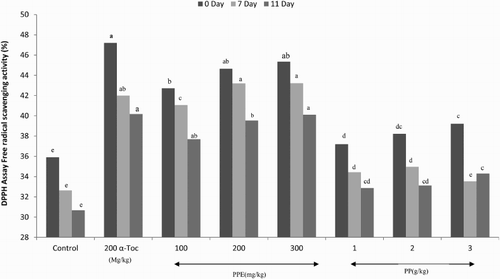
Two indicators (DPPH and TBARS) were used to evaluate the antioxidant potential of dietary PP, PPE, and α-Toc ( and ). The DPPH radical-scavenging activity of the breast meat in broilers fed α-Toc and 200 and 300 mg/kg PPE were significantly higher than that of the control birds during the entire storage period, whereas lower significant differences were found in broilers fed PP diets when compared to control birds (P < .01). The development of lipid oxidation in the breast meat was delayed by diets containing antioxidants (PP, PPE, and α-Toc) after 7 and 11 days of refrigeration (P < .05). A linear effect was observed for TBARS values with increasing the levels of dietary PP and PPE (P < .05). The inclusion of α-Toc and PPE was more effective than PP in order to reduce the oxidation process. In this respect and according to the results of the current experiment, PPE (200 and 300 mg/kg) roughly had potential similar to that of α-Toc in reducing the oxidation process. The latter may indicate that PPE had higher bioavailability than PP in broilers tissues.
4. Discussion
4.1. Broiler performance
The inclusion of PP in diets impaired growth performance of chickens. The inclusion of 15 g/kg diet PP caused was decreased of BW and feed intake in broiler (Rajani et al. Citation2011). In the current study, pomegranate pomace contained 19% compound polyphenols of total extracted by the Folin method that HTs and CT constitute 13.7% and 0.6% of total phenols, respectively. HT and CT values diets were showed in . Similarly, the effect of polyphenols has also been studied in chickens using ingredients like grape seed extract, grape pomace, sorghum, and faba bean. In general, relatively high dietary concentrations of polyphenols by the addition of these ingredients reduced performance in chickens as well as other livestock (Goñi et al. Citation2007). Nevertheless, in our study, reduced chickens performance was only observed in birds fed with PP diets. The low concentration of CT present in the PPE diet may be relatively low to produce a growth depression.
Feed intake, growth rate, and feed efficiency of the poultry are impaired by environmental stress, mainly heat stress (Bartlett & Smith Citation2003). Improved feed efficiency of quail was reported by supplementation of vitamin C as an antioxidant that affected the utilization of dietary nutrients (Sahin & Kucuk Citation2003). Previous studies reported that dietary antioxidants, such as vitamin C, E, flavonoids, and phenolics, could reduce oxidative damage in animals which is generated by different stress sources (Brisibe et al. Citation2009, Hu et al. Citation2015). Therefore, the dietary supplementation of PPE, which contained natural antioxidant HTs, may improve feed efficiency by reducing oxidative damage in broilers fed diet fish oil. Supplementation diet with α-Toc no significantly improved growth performance compared with the control diets. The result in the current study is agreement with observed (Zhang et al. Citation2009).
4.2. FA composition
The FA composition of the diets, particularly PUFA LC n-3, was increased by the inclusion of fish oil into the diets. However, the concentrations of SFAs in the breast meat were not influenced by dietary additives. These observations indicated that the tissue of broilers have limited capacity for alteration of their saturated FAs. The SFAs in poultry are mainly derived from the diet and their synthesis in the liver (Ayerza et al. Citation2002).
While increasing the PUFA levels, the concentrations of MUFA in meat samples were decreased (). In agreement with our results, Pinchasov and Nir (Citation1992) reported that increasing the levels of PUFA leads to decreased MUFA synthesis through inhibiting the activity of 9-desaturase complex, which is the key enzyme needed for the conversion of SFAs to MUFAs. Cortinas et al. (Citation2004) documented that the contents of MUFA in breast were decreased linearly as the inclusion of dietary n-3 PUFA increased. Although all diets contained the same level of n-6 FAs, the n-6 FA concentrations in the breast muscle were significantly increased by dietary treatments. Previous studies have shown that the FA profiles of broilers meat reflect the composition of their consumed diet. It has been reported that feeding a diet with sunflower oil containing LA to pigs and broilers was able to increase the LA and AA levels in meat (Crespo & Esteve-Garcia Citation2002). In the present study, the levels of n-6 FAs in breast muscle of broilers fed diets containing antioxidants (PPE, PP, and α-Toc) were the highest and almost the same in the all diets. This indicates the role of antioxidants towards removing and decreasing the free radical in tissues during storage, hence reducing lipid peroxidation.
One of our works showed that the FA composition of the diets, particularly PUFA LC n-3, could be modified and increased by the inclusion of fish oil into the broilers diets (Saleh et al. Citation2010). One of the major results in the present study was the potential of dietary antioxidants towards improvement of the PUFA LC n-3 in the breast meat of broilers. This can be explained by the antioxidant potential of phenolic compounds in experimental additives. Diet-containing antioxidants may inhibit the oxidation of the PUFA LC n-3. Jung et al. (Citation2010) found that the DHA levels in the thigh muscle of broilers fed dietary mixture of gallic acid and linoleic acid were increased. The authors concluded that the aforementioned effect is due to the antioxidant potential of gallic acid in the diet. Dietary α-Toc and sorghum have been shown to increase LC n-3 FAs such as eicosapentaenoic acid and DHA in egg and thigh muscle of broilers (Cherian et al. Citation1996; Citation2002). Our study illustrates a good approach to increase the LC n-3 PUFA contents of broilers meat through dietary supplementation of 200 or 300 PPE mg/kg and 200 mg/kg α-Toc, towards maintaining consumers’ acceptability scores, even after a couple of days of storage. The n-6: n-3 in the breast meat was decreased by dietary supplementation of PP, PPE, and α-Toc. These results can be attractive to the consumers as low n-6: n-3 has a health benefit effects for humans, mainly in protection against cardiovascular disease (Krauss et al. Citation2001).
4.3. Total phenolic content and antioxidant activity
Polyphenolic compounds in PPE are distributed, retained, and remained functional in muscle (Sáyago-Ayerdi et al. Citation2009). Phenolic compounds present in natural plant oils react with lipid and hydroxyl radicals and convert them into stable products (Naveena et al. Citation2008). HT are the most abundant extractable polyphenols and antioxidant compounds in pomegranates and include gallotannins, ellagitannins, and gallagyl esters such as punicalagin and punicalin than CT. Extractable polyphenols appear to be absorbed from the digestive tract and exert systemic antioxidant effects (Madrigal-Carballo et al. Citation2009). Our findings support previous reports.
The DPPH has been used for years as a free radical to evaluate antioxidant activity present in natural sources of organic compounds (Schwarz et al. Citation2009). A solution of DPPH, stable free radical, is mixed with an antioxidant that can donate a hydrogen atom to form a stable DPPH-H molecule. In the current experiment, radical-scavenging capacity was significantly affected by dietary antioxidants. Our results indicate that dietary α-Toc or PPE could exert the DPPH radical-scavenging activity in the breast muscle of broilers. Tannins are known to inhibit lipid peroxidation and lipoxygenases in vitro, and information has been accumulated over the past few years demonstrating their ability to scavenge radicals such as hydroxyl, superoxide, and peroxyl, which are known to be important in cellular prooxidant states (Gyamfi & Aniya Citation2002). Phenolic compounds of PP may act in a similar fashion by donating electrons and reacting with free radicals to convert them to more stable products and terminate free radical chain reactions (Negi & Jayaprakasha Citation2003).
The TBARS values of all meats were gradually increased with storage time. Supplemented diets with dietary antioxidants showed less peroxidation by producing lower amounts of MDA. Breast meat of broilers fed PPE, α-Toc, and PP diets led to lower MDA concentrations in spite of the higher content of PUFA LC n-3 in diets, which can deteriorate the quality of the meat. Long-chain PUFA n-3 family has the potential to generate several types of free radicals and can accelerate lipid peroxidation (Bou et al. Citation2004). Previous studies have shown that the negative outcome of lipid oxidation in chicken meat was diminished by the use of diets containing phenolic compounds such as plant extracts and grape pomace (Sahin & Kucuk Citation2003; Goñi et al. Citation2007; Akbarian et al. Citation2014; Hu et al. Citation2015), which are considered as natural antioxidant substances. These results are in line with the work of Cortinas et al. (Citation2004), who reported that the oxidation process of lipids was interrupted in chickens fed diets containing various PUFA levels and α-Toc acetate. The authors noted that the antioxidant activity of α-Toc acetate could inhibit the lipid peroxidation process. The high levels of phenolic compounds in PP and PPE diets possess strong antioxidant activity, leading to the extension of the shelf life, hence improving the quality of meat products. Sáyago-Ayerdi et al. (Citation2009) indicated that lower TBARS values in meat could be due to the protective effects derived from the polyphenol compounds of grape pomace, which can act in a way similar to that of vitamin E on the lipid bilayers of the cell membrane. Based on observations of Rajani et al. (Citation2011), diet consisting of 15 g/kg PP was able to reduce the MDA levels in the meat of broilers. Vitamin E is a major chain-breaking antioxidant and free radical scavenger, which constitutes a second line of defence for the prevention of lipid peroxidation. Diets supplemented with VE or MVE had a positive influence on stabilizing breast meat quality (Hu et al. Citation2015; Zhang et al. Citation2009).
5. Conclusion
In this experiment, the inclusion of α-Toc and PPE were more effective than PP towards reducing the lipid peroxidation process. In this respect, PPE (200 and 300 mg/kg) roughly had an ability similar to that of α-Toc. This indicates that PPE had higher bioavailability than PP in broiler diets. Results from the present study demonstrate that breast meat in broiler may be successfully enriched with LC PUFA n-3 by the inclusion of fish oil into the diet. Long-chain n-3 FAs (EPA, DPA, and DHA) were higher in broilers fed α-Toc and/or PPE at the rate of 200 or 300 mg/kg diets. The DPPH radical-scavenging activity, TBARS, and total phenolic contents in the breast muscle were improved significantly in the PPE and α-Toc fed birds. All in all, supplementation of diets with 200 and 300 mg/kg PPE could improve the antioxidant potential and quality indices of breast muscle in broilers. The PPE was an effective antioxidant as α-Toc towards enriching the broilers’ meat.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahn DU, Olson DG, Jo C, Love J, Jin SK. 1999. Volatiles production and lipid oxidation on irradiated cooked sausage as related to packaging and storage. J Food Sci. 64(2):226–229. doi: 10.1111/j.1365-2621.1999.tb15870.x
- Akbarian A, Golian A, Kermanshahi H, Raji AR, Farhoosh R, De Smet S, Michiels J. 2013a. Growth performance and gut health parameters of finishing broilers supplemented with plant extracts and exposed to daily increased temperature. Span J Agr Res. 11(1):109–119. doi: 10.5424/sjar/2013111-3392
- Akbarian A, Golian A, Gilani A, Kermanshahi H, Zhaleh S, Akhavan A, De Smet S, Michiels J. 2013b. Effect of feeding citrus peel extracts on growth performance, serum components, and intestinal morphology of broilers exposed to high ambient temperature during the finisher phase. Livest Sci. 157:490–497. doi: 10.1016/j.livsci.2013.08.010
- Akbarian A, Golian A, Kermanshahi H, De Smet S, Michiels J. 2015. Antioxidant enzyme activities, plasma hormone levels and serum metabolites of finishing broiler chickens reared under high ambient temperature and fed lemon and orange peel extracts and Curcuma xanthorrhiza essential oil. J Anim Physiol Anim Nutr. 99(1):150–162. doi: 10.1111/jpn.12188
- Akbarian A, Michiels J, Golian A, Buyse J, Wang Y, De Smet S. 2014. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult Sci. 93(8):1930–1941. doi: 10.3382/ps.2014-03896
- Anonymous. 2013. Iran Statistical Year Book 2003, Area under Cultivation and Production of Selected Perennial Crops. Statistical Center of Iran; [cited 2013 Sep 1]. Available from: http://amar.maj.ir/Portal/Home/Default.aspx?CategoryID=117564e0-507c-4565-9659-fbabfb4acb9b
- Aviagen. 2009. Ross 308 Broiler Management Guide. [cited 2013 Mar 1]. Available from: http://en.aviagen.com/ross-308
- Ayerza R, Coats W, Lauria M. 2002. Chia seed (Salvia hispanica L.) as an omega-3 fatty acid source for broilers: influence on fatty acid composition, cholesterol and fat content of white and dark meats, growth performance, and sensory characteristics. Poult Sci. 81(6):826–837. doi: 10.1093/ps/81.6.826
- Aziza AE, Quezada N, Cherian G. 2010. Antioxidative effect of dietary Camelina meal in fresh, stored, or cooked broiler chicken meat. Poult Sci. 89(12):2711–2718. doi: 10.3382/ps.2009-00548
- Bartlett JR, Smith MO. 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poultry Sci. 82:1580–1588.
- Ben Nasr C, Ayed N, Metche M. 1996. Quantitative determination of the polyphenolic content of pomegranate peel. Z Lebensm Unters Forsch. 203(4):374–378. doi: 10.1007/BF01231077
- Blois MS. 1958. Antioxidant determination by the use of a stable free radical. Nature. 181:1199–1200. doi: 10.1038/1811199a0
- Bou R, Guardiola F, Tres A, Barroeta AC, Codony R. 2004. Effect of dietary fish oil, α-tocopherol acetate, and zinc supplementation on composition and consumer acceptability of chicken meat. Poult Sci. 83(2):282–292. doi: 10.1093/ps/83.2.282
- Brisibe E, Umoren A, Brisibe UE, Magalhäes F, Ferreira PM, Luthria JFS, 2009. Nutritional characterization and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 115:1240–1246.
- Çam M, Hışıl Y. 2010. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 123(3):878–885. doi: 10.1016/j.foodchem.2010.05.011
- Cherian G, Selvaraj RK, Goeger MP, Stitt PA. 2002. Muscle fatty acid composition and thiobarbituric acid-reactive substances of broilers fed different cultivars of sorghum. Poult Sci. 81:1415–1420. doi: 10.1093/ps/81.9.1415
- Cherian GF, Wolfe H, Sim JS. 1996. Dietary oils with added tocopherols: effects on egg or tissue tocopherols, fatty acids, and oxidative stability. Poult Sci. 75:423–431. doi: 10.3382/ps.0750423
- Cortinas L, Villaverde C, Galobart J, Baucells D, Barroeta A. 2004. Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturated level. J Poult Sci. 83(7):1155–1164. doi: 10.1093/ps/83.7.1155
- Crespo N, Esteve-Garcia E. 2002. Nutrient and fatty acid deposition in broilers fed different dietary fatty acid profiles. Poult Sci. 81(10):1533–1542. doi: 10.1093/ps/81.10.1533
- Elfalleh W, Nasri N, Marzougui N, Thabti I, M’rabet A, Yahya Y, Lachiheb B, Guasmi F, Ferchichi A. 2009. Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Int J Food Sci Nutr. 60(2):197–210. doi: 10.1080/09637480903067037
- Flachowsky G, Engelman D, Sunder A, Halle I, Sallmann HP. 2002. Eggs and poultry meat as tocopherol sources in dependence on tocopherol supplementation of poultry diets. Food Res Int. 35:239–243. doi: 10.1016/S0963-9969(01)00191-0
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Goñi I, Brenes A, Centeno C, Viveros A, Saura-Calixtio F, Rebole A. 2007. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation in chickens. Poult Sci. 86(3):508–516. doi: 10.1093/ps/86.3.508
- Gonzalez-Parama’s AM, Esteban-Ruano S, Santos-Buelga C, Pascual-Teresa S, Rivas-Gonzalo JC. 2004. Flavanol content and antioxidant activity in winery products. J Agr Food Chem. 52:234–238. doi: 10.1021/jf0348727
- Gyamfi MA, Aniya Y. 2002. Antioxidant properties of Thonningianin A, isolated from the African medicinal herb, Thonningia sanguinea. Biochem Pharmacol. 63:1725–1737. doi: 10.1016/S0006-2952(02)00915-2
- Hu ZP, Wang T, Ahmad H, Zhang JF, Zhang LL, Zhong X. 2015. Effects of different formulations of α-tocopherol acetate (vitamin E) on growth performance, meat quality and antioxidant capacity in broiler chickens. Brit Poult Sci. 56:687–695. doi: 10.1080/00071668.2015.1080814
- Jung SJH, Kim B, Yun H, Jo C. 2010. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 86(2):520–526. doi: 10.1016/j.meatsci.2010.06.007
- Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ. 2001. Revision 2000: statement for healthcare professionals from the nutrition committee of the American Heart Association. J Nutr. 131(1):132–146.
- Li YF, Guo CJ, Yang JJ, Wei JY, Xu J, Cheng S. 2006. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 96(2):254–260. doi: 10.1016/j.foodchem.2005.02.033
- Lopez-Bote CJ, Gray JK, Gomaa EA, Flegal CJ. 1998. Effect of dietary administration of oil extracts from rosemary and sage on lipid oxidation in broiler meat. Brit Poult Sci. 39(2):235–240. doi: 10.1080/00071669889187
- Madrigal-Carballob S, Rodriguezb G, Kruegera CG, Dreherc M, Reed JD. 2009. Pomegranate (Punica granatum) supplements: authenticity, antioxidant and polyphenol composition. J Funct Foods. 1(24):324–329. doi: 10.1016/j.jff.2009.02.005
- Makkar HPS, Becker K. 1993. Vanillin-HCl method for condensed tannins: effect of organic solvents used for extraction of tannins. J Chem Ecol. 19:613–621. doi: 10.1007/BF00984996
- Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N. 2008. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 80(4):1304–1308. doi: 10.1016/j.meatsci.2008.06.005
- Negi PS, Jayaprakasha GK. 2003. Antioxidant and antibacterial activities of Punicagranitum peel extracts. J Food Sci. 68:1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x
- Pinchasov Y, Nir I. 1992. Effect of dietary polyunsaturated fatty acid concentration on performance, fat deposition, and carcass fatty acid composition in broiler chickens. Poult Sci. 71(9):1502–1504.
- Rajani J, Karimi Torshizi MA, Rahimi S. 2011. Control of ascites mortality and improved performance and meat shelf-life in broilers using feed adjuncts with presumed antioxidant activity. Anim Feed Sci Technol. 170:239–245. doi: 10.1016/j.anifeedsci.2011.09.001
- Reed JD. 1995. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci. 73:1516–1528. doi: 10.2527/1995.7351516x
- Rymer C, Givens DI. 2010. Effects of vitamin E and fish oil inclusion in broiler diets on meat fatty acid composition and on the flavour of a composite sample of breast meat. J Sci Food Agr. 90:1628–1633. doi: 10.1002/jsfa.3991
- Sahin K, Kucuk O. 2003. Heat stress and dietary vitamin supplementation of poultry diets. Feeds Feed. 73:41R–50R.
- SAS Institute Inc. 2003. SAS User’s Guide. Cary, NC: SAS Institute.
- Saleh H, Rahimi Sh, Karimi Torshizi MA, Golian AG. 2010. Omega-3 enrichment broiler of meat using oil. J Anim Vet Adv. 9(22):2877–2882. doi: 10.3923/javaa.2010.2877.2882
- Sáyago-Ayerdi SG, Brenes A, Viveros A, Goñi I. 2009. Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci. 83:528–533. doi: 10.1016/j.meatsci.2009.06.038
- Schwarz M, Rodríguez M, Martínez C, Bosquet V, Guillén D, Barroso CC. 2009. Antioxidant activity of Brandy de Jerez and other aged distillates, and correlation with their polyphenolic content. Food Chem. 116:29–33. doi: 10.1016/j.foodchem.2009.01.096
- Subramanian KN, Padmanaban G, Sarma S. 1965. Folin–Ciocalteu reagent for the estimation of siderochromes. Anal Biochem. 12(1):106–112. doi: 10.1016/0003-2697(65)90147-8
- Terrill TH, Rowa AM, Douglas GB, Barry TN. 1992. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J Sci Food Agr. 58:321–329. doi: 10.1002/jsfa.2740580306
- Yilmaz Y, Toledo RT. 2004. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agr Food Chem. 52:255–260. doi: 10.1021/jf030117h
- Zhang XH, Zhong X, Zhou YM, Du HM, Wang T. 2009. Effect of RRR-α-tocopherol succinate on the growth and immunity in broilers. Poult Sci. 88:959–966. doi: 10.3382/ps.2008-00512