 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effect of heat treatment (raw or micronization) and vitamin E supplementation (0 or 200 mg/kg DM of RRR-α-tocopherol) on ruminal biohydrogenation (BH) kinetic of whole flaked rapeseed was studied using in vitro batch culture in a 2 × 2 factorial design. Experimental treatments were: whole flaked raw rapeseed without vitamin E (RR0), whole flaked raw rapeseed supplemented with 200 mg/kg DM of vitamin E (RR200), micronized whole flaked rapeseed without vitamin E (MR0) and micronized whole flaked rapeseed supplemented with 200 mg/kg DM of vitamin E (MR200). The disappearance of linoleic acid (LA), alpha-linolenic acid (LnA), oleic acid (OA), and appearance of stearic acid (SA), and C18:1 trans-11 (VA) were significantly decreased by micronization (P < 0.0001 for all). The appearance of cis-9, trans-11 conjugated linoleic acid (CLA; P < 0.0001) and C18:1 trans-10 (P = 0.01) was significantly increased by micronization. Biohydrogenation of LA (P = 0.03) and OA (P = 0.02) was significantly decreased by vitamin E. There was a tendency to reduce disappearance of LnA by vitamin E (P = 0.06). In conclusion, micronization is an effective method to protect unsaturated fatty acids (UFA) from ruminal BH. Vitamin E supplementation can be an advantageous strategy to decrease LA, LnA and OA biohydrogenation.
1. Introduction
Depending on the type of oilseeds, the fat content can vary from 18 to 45%. Therefore, oilseeds are considered an important energy source that may supply certain beneficial fatty acids (FA) for animals (Nogueira et al. Citation2020). Rapeseed is the second most abundant source of oilseeds in the world and has potential as an alternative to soybeans and cottonseed in the dairy ration (Wang et al. Citation1997). It is known for its desirable UFA profile with a high proportion of OA, LA and LnA (Cieslak et al. Citation2010). It is well documented that UFA play a number of key roles in health (Ian Givens et al. Citation2003), immune system (Yang et al. Citation2015) and reproductive function (Dewanckele et al. Citation2019) of animals. Unsaturated long-chain fatty acid (LCFA) can have toxic effects on rumen microbiota by creating oxidative stress and raising a load of peroxides in the rumen environment (Nogueira et al. Citation2020). Excess unsaturated LCFA can also disrupt the growth and function of fibre digesting bacteria (Harvatine and Allen Citation2006).
The BH is considered a mechanism where rumen bacteria regulate the concentration and type of UFA in the rumen and thereby cope with the presence of UFA (Pottier et al. Citation2006; Gurbuz Citation2007). Because of the BH, the profile of ingested FA in ruminant species is not the same as that reaching the small intestine (Freitas et al. Citation2018). On one side, it is crucial to protect the FA profile of oilseeds in the rumen, and on the other side, it is important to avoid the detrimental effects of UFA on rumen microbes.
Vitamin E is one of the most important antioxidants that can neutralize the peroxides released by lipids (Bloomberg et al. Citation2011). Supplementing α-tocopherol as an antioxidant can reduce the detrimental effects of dietary lipids on the efficiency and growth of rumen microflora, and it also stimulates the growth of cellulolytic bacteria responsible for the C18:1 trans-11 BH pathway (Hino et al. Citation1993), which finally can increase cis-9, trans-11 CLA synthesis in the mammary gland (Hou et al. Citation2013). Vitamin E can prevent milk fat depression and milk fat oxidation induced by unsaturated high-fat diets, and increase milk yield (Pottier et al. Citation2006). However, the mechanisms of vitamin E are still unclear, but it might alter rumen microflora/dynamics and be involved in FA hydrogenation (Hou et al. Citation2013).
The application of heat treatment to oilseeds has been proven to protect dietary poly-unsaturated fatty acids (PUFA) from ruminal BH. Micronization is a heat processing in which feeds are exposed to infrared radiation (IR) at wavelength of ≥2.5 µm within a very short period (30–90 s). Penetration of IR into the feeds increased internal temperature (Sajjadi et al. Citation2022), resulting in protein denaturation. Denaturation reduces protein solubility and decreases ruminal protein degradability (McAllister and Sultana Citation2011). In addition, the protein barrier reduces the digestion of starch and fat in the rumen (McAllister and Sultana Citation2011).
The effect of micronization on ruminal BH of unsaturated LCFA of flaxseeds (Petit et al. Citation2002; Gonthier et al. Citation2004; Gurbuz Citation2007; Leduc et al. Citation2017) and soybeans (Chouinard et al. Citation2001) was investigated in some in vivo and in vitro studies. However, to our knowledge, the effectiveness of micronizing for protecting UFA in rapeseed and estimating rate of ruminal BH in micronized rapeseed has not been studied so far. Further, it is still not clear how a combination of micronized rapeseed and vitamin E can influence the ruminal BH of UFA. We hypothesized that micronization of rapeseed will decrease the BH rate of UFA, and vitamin E will increase the formation of desirable intermediates FA and thereby influence the rumen bypass of FA. Therefore, the aim of the present work was to study the effect of micronization and supplementing vitamin E on ruminal BH kinetic of whole flaked rapeseed using in vitro ruminal batch fermentation technique.
2. Material and methods
2.1. Samples preparation
Winter rapeseed (Brassica napus), Neptune variety (harvested in June 2021), was used in the present study. The full-fat rapeseeds were divided into two batches. One of which was first soaked by 5.0% water (w/w) an hour prior to processing with mixing every 15 min and subsequently micronized using a gas-fired ceramic micronizer (Faravardaneh Ferdowsi Mashhad, Mashhad, Iran) at a wavelength of 2.8 µ. Monolayers of seeds were moved on the vibrating conveyor 12 cm below the infrared radiation emission source at speed of 6.25 cm/s to achieve a surface temperature of 130°C at exiting. Micronized seeds were immediately flacked by passing between two rotating rollers with a 0.50 mm distance gap. The non-micronized batch was flaked similarly without micronization.
Two levels (0 and 200 mg/kg DM) of vitamin E (67% (+)-α-Tocopherol; from vegetable oil, type V, ∼1000 IU/g, Sigma-Aldrich, Merck) were used to supplement raw and micronized whole flaked rapeseeds. To obtain a uniform mixture of feed samples and vitamin E, all samples were milled through a 0.5 mm screen. Accurately, 300 mg of vitamin E was well dissolved in 100 mL of ethanol, and then 30 µL of the prepared solution was added to cultured tubes containing 300 mg of the substrates. The ethanol was evaporated before the start of the in vitro incubation. The experimental treatments were as follows: whole flaked raw rapeseed without vitamin E (RR0), whole flaked raw rapeseed supplemented with 200 mg/kg DM vitamin E (RR200), micronized whole flaked rapeseed without vitamin E (MR0), and micronized whole flaked rapeseed supplemented with 200 mg/kg DM vitamin E (MR200).
2.2. Chemical analysis
Raw and micronized whole flaked rapeseeds were milled through one mm screen prior to chemical analysis. Dry matter was determined by oven-drying at 60°C for 48 h (AOAC Citation2000). Ash content was determined by combustion samples at 500°C for 6 h in muffle furnace (AOAC Citation1990). Total nitrogen (N) content was analyzed by the Kjeldahl procedure and crude protein (CP) as N × 6.25 (AOAC Citation1990). Fat content was extracted with hexane for 6 h according to the AOAC (Citation1990) procedure. Buffer soluble nitrogen was measured according to the procedure described by Norfor (Citation2011).
2.3. In vitro incubation
2.3.1. Donor cows and basal diet
The animals used in the current study were kept in accordance with the Danish Ministry of Justice Law No. 1306 (23 November 2007) regarding animal experiments and the care of experimental animals. Three ruminally cannulated heifers were used as the donors for the collection of rumen fluid. The heifers were fed a total mixed ration diet composed of 4.0 kg grass hay, 2.0 kg barley straw, and 2.8 kg concentrate on a DM basis twice a day. The concentrate mixture consisted of barley, soybean meal, oat, rapeseed meal, sugar beet molasses, and vitamin and mineral premix at levels of 400, 100, 400, 30, 30, and 40 g/kg DM, respectively.
2.3.2. In vitro incubation procedure
Rumen fluid was taken from each heifer by hand before morning feeding and separately strained through double-layered cheesecloth. The rumen fluids were immediately transferred to the sealed and pre-warmed (39°C) vacuum flasks and transported to the laboratory. In vitro incubation was accomplished as described by Petersen and Jensen (Citation2014) in triplicate, and rumen fluid taken from each heifer created a replicate. Rumen fluids were placed separately in the water bath at 39°C prior to being added to the fermentation tubes. Accurately, 18 mL of strained rumen fluid and 18 mL buffer solution (McDougall Citation1947) were added to 50.0 mL falcon tubes containing experimental substrates. The screw cap of the tubes was equipped with an injection needle (1.2 × 40 mm) to remove the gas produced during fermentation from the culture tubes while maintaining the anaerobic environment. The tubes were placed in a water bath at 39°C for 0, 2, 4, 8, 12, 24 and 48 h. Fermentation was stopped by transferring the tubes to an ice slurry and subsequently frozen at −20°C. All samples were freeze-dried and then stored at −20°C until fatty acids analysis.
2.4. Vitamin E analysis
Total α-tocopherol of raw and micronized whole flaked rapeseeds and prepared vitamin E solution were analyzed by HPLC according to Jensen et al. (Citation2006). Briefly, 2.00 mL of ethanol (96% v/v), 0.50 mL of methanol (100%), 1.00 mL of ascorbic acid (20% w/v), 0.30 mL of KOH-water (1:1, w/v), and 0.70 of mL water were used to suspend 1.000 g DM of finely ground whole flaked rapeseed samples and 1.00 mL of vitamin E solution. Samples were saponified at 80°C for 20 min and then cooled in the dark. Tocopherol extraction was done in two volumes of 5 mL of heptane and 100 µL of the pooled heptane phase was injected into the HPLC equipped with a Perkin-Elmer HS-5 silica column (4.0 × 125 mm; Perkin-Elmer GmbH, D-7770 Überlingen, Germany). The solvents used had HPLC grades. Heptane containing 2-propanol (3.0 mL/L) constituted the mobile phase and degassed with helium at a flow rate of 3.0 mL/min. Fluorescence detection was adjusted to wavelengths of 290 and 327 nm for excitation and emission, respectively. Identification and quantification of the tocopherols were obtained by comparison of retention time as well as peak areas with external standards. The following extinction coefficients in ethanol (96% v/v) were used: α-tocopherol, A1%1cm = 71.0 at 294 nm; γ-tocopherol, A1%1cm = 92.8 at 298 nm and δ-tocopherol, A1%1cm = 91.2 at 298 nm (Merck; D-6100 Damstadt, Germany).
2.5. Fatty acid analysis
Two hundred and fifty mg of freeze-dried samples were weighed out in culture tubes and acidified in 1.5 mL of 3.0 mol/L HCL for 1 h at 80°C in a water bath according to recommendations reported by Jensen (Citation2008). Lipids were extracted with 3.0 mL of chloroform, 3.0 mL methanol, 1.5 mL of distilled water (Bligh and Dyer Citation1959) and 5.0 mg of C19:0 (nonadecanoic acid, Sigma-Aldrich, St. Louis, MO, USA) as internal standard. The extracts were centrifuged for 10 min at 3000 rpm. One mL of chloroform phase was transferred to a new tube, evaporated under a nitrogen stream, and then methylated with 0.8 mL of NaOH (2%) in methanol according to Petersen and Jensen (Citation2014). The tubes were filled with argon and transferred to an oven for 20 min at 100°C. After cooling, 1.0 mL of boron trifluoride reagent was added, filled with argon, and placed in an oven for 45 min at 100°C. Finally, fatty acid methyl esters were extracted with 2.0 mL of heptane and 4 mL saturated NaCl solution and then centrifuged for 10 min at 3000 rpm. A gas chromatograph (Hewlett Packard 6890, Agilent Technologies, Palo Alto, CA, USA) equipped with an auto-column injector (HP 7673), a capillary column of 60 m × 0.32 mm inner diameter and film thickness of 0.25 µm (OmegawaxTM 320; Supelco 4-293-415, Sigma-Aldrich), and a flame ionization detector were used for quantifying the fatty acids as fatty acid methyl esters. The initial temperature was adjusted to 86°C and increased to 200°C at a rate of 2°C/min. This temperature was kept for 5 min and then increased to the final temperature of 220°C. Each peak was identified through a comparison of retention time with external standard (GLC 68C, Nu-Prep- Check, Elysian, MN, USA).
2.6. Kinetic calculations
The disappearance rate of LA, LnA and OA, and the appearance rate of VA and SA were estimated using the nonlinear model. To calculate the net amount of BH products at the given times, the initial concentrations of LA, LnA and OA (concentration at time = 0 h) were subtracted from their concentration at the time when the incubation was stopped.
The data of each replicate were fitted using the PROC NLIN in SAS (9.4 version, SAS Institute Inc.) using the equation Qt = b × [1 – exp (−(C × (t − L)))], where Qt is the percentage of fatty acid at time t (h), b is a potentially degradable fraction, C is the disappearance rate of LA, LnA and OA (h−1) and appearance rate of VA and SA (h−1), L is the lag time (h), and t is the incubation time (h).
2.7. Statistical analysis
Chemical composition, dissolvable nitrogen, tocopherol content and initial FA content were analyzed in a completely randomized design using the GLM procedure of SAS software (9.4 version, SAS Institute Inc.).
Kinetic parameters were analyzed in a 2 × 2 factorial design using PROC MIXED procedures of SAS software (9.4 version, SAS Institute Inc.), and the model was as follow:
Where µ is the overall mean, Mi is the fixed effect of micronization, Vj is the fixed effect of vitamin E, Mi × Vj is the interaction between micronization and vitamin E, cf is the random effect of cowand eijf is the residual error.
Changes in the FA concentration and crude fat were analyzed in a randomized 2 × 2 factorial design using PROC MIXED procedures of SAS software (9.4 version, SAS Institute Inc.), and the model was as follows:
Where µ is the overall mean, Mi is the fixed effect of micronization, Vj is the fixed effect of vitamin E, Mi × Vj is the interaction between micronization and vitamin E, Tk is the fixed effect of time (k = 2, 4, 8, 12, 24, 48), Tk × Mi is the interaction between time and micronization, Tk × Vj is the interaction between time and vitamin E, Tk × Mi × Vj is the interaction between time, micronization and vitamin E, cf is the random effect of cow, and eijfk is the residual error. The values presented in the text, tables and figures are Ls-means, unless otherwise noted.
3. Results
3.1. Chemical composition, dissolvable nitrogen, tocopherol content and initial FA content of whole flaked rapeseed
The chemical composition of raw and micronized whole flaked rapeseeds is presented in . Nitrogen solubility of the whole flaked rapeseed was significantly reduced by micronization (P < 0.0001). The content of α-tocopherol (P < 0.0001) and γ-tocopherol (P < 0.0001) was significantly decreased by micronization. γ-Tocopherol was the major tocopherol in whole flaked rapeseed followed by α-tocopherol and δ-tocopherol, respectively.
Table 1. Chemical composition, dissolvable nitrogen, tocopherol content (mg/kg DM), total FA content (g/kg DM) and FA composition (g FA/kg FA) of raw and micronized rapeseeds.
Except for C16:0 (P = 0.01), we observed no effect of micronization on total FA content and FA composition of rapeseed (). Among the saturated FA, C16:0 had the highest content in both raw and micronized whole flaked rapeseeds (47 and 48 g FA/kg FA, respectively). Oleic acid was the predominant UFA in raw and micronized whole flaked rapeseed followed by LA and LnA, respectively.
3.2. In vitro crude fat disappearance
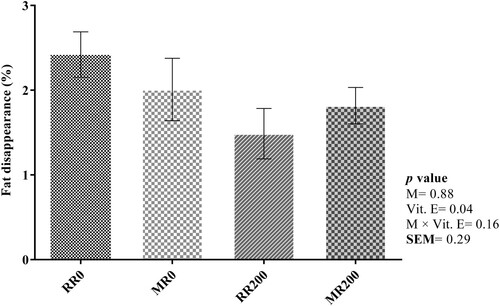
The percentage of fat disappearance after 48 h in vitro incubation is shown in . The percentage of crude fat decreased significantly by supplementing whole flaked rapeseed with vitamin E (2.22 vs. 1.65%; Ls-means of un-supplemented vs. supplemented with vitamin E, the values not shown in tables; P = 0.04). No significant effect of micronization was observed on the percentage of fat disappearance (P = 0.88).
Figure 1. Effect of micronization and vitamin E on crude fat disappearance. Whole flaked raw rapeseed without vitamin E (RR0), micronized whole flaked rapeseed without vitamin E (MR0), whole flaked raw rapeseed supplemented with 200 mg/kg DM vitamin E (RR200), micronized whole flaked rapeseed supplemented with 200 mg/kg DM vitamin E (MR200). Error bars represent the standard error of the mean. The interaction between micronization and incubation time and the interaction between vitamin E and incubation time were not significant.
3.3. In vitro biohydrogenation
As shown in (a), the disappearance of LA was significantly decreased by micronization (1.59 vs. 1.75 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.0001) and vitamin E (1.64 vs. 1.71 g/100 g DM; Ls-means of un-supplemented vs. supplemented with vitamin E, ; P = 0.03). There was an interaction between micronization and incubation time (P = 0.0003) for disappearance of LA. At 2, 4, 8 and 12 h after incubation, the disappearance of LA was decreased in micronized compared to non-micronized whole flaked rapeseed. However, at 48 h after incubation, the micronized whole flaked rapeseed had the higher disappearance of LA compared to non-micronized whole flaked rapeseed. The disappearance of LnA ((b)) was significantly decreased by micronization (0.68 vs. 0.77 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.001). Vitamin E supplementation tended to decrease the disappearance of LnA (0.71 vs. 0.74 g/100 g DM; Ls-means of un-supplemented vs. supplemented with vitamin E, ; P = 0.06). There was an interaction between micronization and incubation time (P = 0.0001) for disappearance of LnA. At 2, 4, 8 and 12 h after incubation, the disappearance of LnA was decreased in micronized whole flaked rapeseed compared to non-micronized whole flaked rapeseed. The disappearance of OA was significantly decreased by micronization (6.56 vs. 7.38 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.0001) and vitamin E (6.81 vs. 7.13 g/100 g DM; Ls-means of un-supplemented vs. supplemented with vitamin E, ; P = 0.02).
Figure 2. Changes in linoleic acid (a), α-linolenic acid (b) and stearic acid (c) concentration during incubation. Presented valuse are Ls-means for whole flaked raw rapeseed (RR) and micronized whole flaked rapeseed (MR). Error bars represent the standard error of mean.
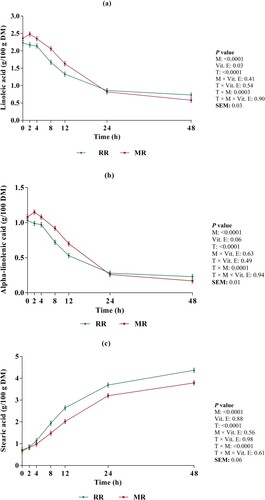
Table 2. Effects of micronization and vitamin E on FA biohydrogenation(g/100 g DM).
As presented in (c), the appearance of SA was significantly affected by micronization (2.18 vs. 1.86 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.0001). We observed an interaction between micronization and incubation time for appearance of SA (P < 0.0001). At 8, 12, 24 and 48 h of incubation, the appearance of SA was decreased in micronized whole flaked rapeseed compared to non-micronized whole flaked rapeseed, which was more apparent by increasing incubation time.
The appearance of cis-9, trans-11 CLA ((a)) was significantly increased by micronization (0.020 vs 0.028 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.0001). We also observed an interaction between micronization and incubation time (P < 0.0001) for appearance of cis-9, trans-11 CLA. At 4 h after incubation, the appearance of cis-9, trans-11 CLA was decreased in micronized whole flaked rapeseed compared to non-micronized whole flaked rapeseed, whereas at 12, 24 and 48 h after incubation, micronized whole flaked rapeseed had the higher appearance of cis-9, trans-11 CLA compared to non-micronized whole flaked rapeseed. The appearance of trans-10, cis-12 CLA was significantly influenced by incubation time (; P < 0.0001). There was no effect of micronization (P = 0.19) and supplementation of vitamin E (P = 0.84) on appearance of trans-10, cis-12 CLA ().
Figure 3. Changes in cis-9, trans-11 CLA (a), C18:1 trans-11 (b) and C18:1 trans-10 (c) concentration during incubation. Presented valuse are Ls-means for whole flaked raw rapeseed (RR) and micronized whole flaked rapeseed (MR). Error bars represent the standard error of mean.
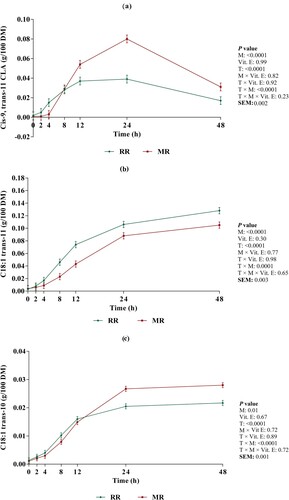
The appearance of VA ((b)) was significantly affected by micronization (0.055 vs. 0.039 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P < 0.0001). We observed an interaction between micronization and incubation time for appearance of VA (P = 0.0001). At 8, 12, 24 and 48 h after incubation, the appearance of VA was decreased in micronized whole flaked rapeseed compared to non-micronized whole flaked rapeseed, which was more obvious by increasing incubation time.
The appearance of C18:1 trans-10 ((c)) was significantly increased by micronization (0.011 vs. 0.012 g/100 g DM; Ls-means of non-micronized vs. micronized, ; P = 0.01). There was an interaction between micronization and incubation time (P < 0.0001) for appearance of C18:1 trans-10. At 8 h after incubation, the appearance of C18:1 trans-10 was decreased in micronized whole flaked rapeseed compared to non-micronized whole flaked rapeseed, whereas at 24 and 48 h after incubation, micronized whole flaked rapeseed had the higher appearance of C18:1 trans-10 compared to non-micronized whole flaked rapeseed.
3.4. Biohydrogenation kinetic parameters
As shown in , micronization significantly decreased the BH rate of LA (0.09 vs. 0.06%/h; P = 0.004), LnA (0.09 vs. 0.06%/h; P = 0.005) and OA (0.09 vs. 0.05%/h; P = 0.04) (values represent Ls-means of non-micronized vs. micronized). The lag phase of LA (P = 0.16) and OA (P = 0.22) was not affected by micronization. Micronization tended to increase the lag phase of LnA (2.10 vs. 3.74 h; Ls-means of non-micronized vs. micronized, ; P = 0.07). There was no effect of vitamin E on the BH rate and lag phase of LA (P = 0.14 and P = 0.66, respectively), LnA (P = 0.16 and P = 0.66, respectively) and OA (P = 0.39 and P = 0.59, respectively).
Table 3. Effect of micronization and vitamin E on biohydrogenation rate (%/h) and lag time (h) of LA, LnA, OA, SA and VA.
As seen in , the appearance rate of VA (0.07 vs. 0.05%/h; P = 0.004), and SA (0.07 vs. 0.05%/h; P = 0.02) was significantly decreased by the micronization (values represent Ls-means of non-micronized vs. micronized). The lag phase for the appearance of VA (1.95 vs. 2.74 h; P = 0.0003) and SA (1.55 vs. 2.14 h; P = 0.01) was significantly increased by micronzation (values represent Ls-means of non-micronized vs. micronized). There was no effect of vitamin E on the BH rate and lag phase of VA (P = 0.25 and P = 0.73, respectively) and SA (P = 0.18 and P = 0.46, respectively). We observed no interaction between micronizing and vitamin E for the BH rate and lag phase of all FA, except for the lag phase of SA. The highest lag phase for appearance of SA was observed in MR0, whereas the lowest lag phase was found in RR0 (2.41 and 1.41 h, respectively).
4. Discussion
4.1. Chemical composition, dissolvable nitrogen, tocopherol content and initial FA content of whole flaked rapeseed
Heat treatment could reduce the nitrogen solubility, probably due to denaturation and transformation of protein to a structure more resistant to ruminal digestion, as stated by Lashkari et al. (Citation2017). Thus, the lower nitrogen solubility in response to micronization might be due to the denaturation of protein content of whole flaked rapeseed. Our findings suggest that micronization could be a feasible method to protect nitrogen content against rumen degradation and thereby improve the protein bypass. In agreement with our results, Wang et al. (Citation1997) and Petit et al. (Citation2002) stated that micronization reduced the amount of soluble nitrogen.
A low content of α- and γ-tocopherols in micronized flaked rapeseed indicated a high degradation of tocopherols by micronization. The differences in the degradation of individual tocopherols might be related to their bond dissociation energy of the phenolic hydrogen. In line with our results, Kmiecik et al. (Citation2019) stated that the α-tocopherol with the lower bond dissociation energy compared to γ- and δ-tocopherols, respectively, had the highest degradation during heat processing. Despite the lower content of δ-tocopherol in the whole flaked rapeseed, it was completely stable against micronization.
Rapeseed is known for its valuable content of UFA with the highest amount of OA, LA and LnA, respectively (Thacker Citation1998). Although the FA composition may change differently with various heat treatments (Ishrat et al. Citation2014), our findings revealed that micronization could be a reliable method for UFA preservation. In agreement with our results, Anwar et al. (Citation2015) reported that gamma irradiation did not change the fatty acid profile of canola seed.
4.2. In vitro crude fat disappearance
Triglycerides and most other FA esters are hydrolyzed extensively by microbial lipases in the rumen, releasing the free FA (Jenkins Citation1993). The key role of vitamin E in reducing microbial lipase activity is clearly proved (El-Essawy et al. Citation2019); therefore, the reduction of crude fat disappearance in supplemented treatments with vitamin E could be correlated with the inhibitory effect of vitamin E on microbial lipase.
4.3. In vitro biohydrogenation
Ruminal biohydrogenation is a part of the rumen microorganisms’ lipid metabolism and includes a series of isomerization and hydrogenation of double bonds (Baldin et al. Citation2022). The lower disappearance of LA, LnA and OA in micronized treatments could be explained by reduced protein solubility. A decrease in the rate of FA release due to protein denaturation may have been responsible for decreasing the BH of LA, LnA and OA in micronized whole flaked rapeseed. Khorasani et al. (Citation1992) reported that FA may be physically surrounded by the denatured protein-rich matrix, decreasing the rate of FA release, and subsequently decreasing the BH of UFA. Gonthier et al. (Citation2004) stated that heat processing of oilseeds can denature the protein-rich matrix surrounding the fat droplets, and therefore, protect UFA against ruminal BH. In agreement with our results, Gonthier et al. (Citation2004) observed a reduction in BH of LA and LnA by micronization of flaxseed.
Vitamin E can reduce the free FA release by inhibition of microbial lipase (Jenkins Citation1993). Therefore, decreased crude fat disappearance by vitamin E might have been responsible for decreasing the BH of LA, LnA and OA in supplemented treatments, suggesting that vitamin E could increase ruminal LA, LnA and OA bypass. It has been shown that vitamin E can modify the ruminal BH of UFA in dairy cow (Kay et al. Citation2005; Pottier et al. Citation2006), beef cattle (Juárez et al. Citation2011) and lamb (De Almeida et al. Citation2015). Consistent with our results, Juárez et al. (Citation2011) showed that vitamin E (1051 IU dl- α-tocopheryl acetate/head/day) could decrease the ruminal BH of monounsaturated fatty acid (MUFA) and PUFA when flaxseed was included in steers diet. An in vitro study by Hou et al. (Citation2013) showed that vitamin E supplementation (2.0 mg/80 mL of culture solution) decreased the BH of LA and LnA in the rumen fluid of goats fed a diet containing soybean oil. However, De Almeida et al. (Citation2015) demonstrated that vitamin E supplementation at 1000 mg/kg DM had no effect on the BH of UFA when sunflower seed was included in the diet of lambs.
In the ruminal BH pathway, LA and LnA are initially isomerized to several cis or trans FA followed by hydrogenation to di- and/or monoenoic acids and then hydrogenated to SA (Jenkins et al. Citation2008). The BH pathways of OA also include isomerization to trans FA or hydrogenation directly to SA (Baldin et al. Citation2022). The lower appearance of SA and VA were observed in micronized treatments, which had the lower BH of LA, LnA and OA. In agreement with our result, Lashkari et al. (Citation2017) reported that heat treatment of partly defatted flaxseed decreased the BH of LA and LnA, and the appearance of SA and VA.
Disappearance of LA, LnA and OA was reduced by micronization, and appearance of cis-9, trans-11 CLA and trans-10, cis-12 CLA followed a different pattern ((a,b)), which indicated that the effect of micronization on the formation of intermediates was different. As stated by Lashkari et al. (Citation2017), various heat treatments may cause the formation of intermediates that act as inhibitors of bacteria and/or enzymes involved in BH steps. Micronization can reduce the accessibility of MUFA and PUFA to ruminal BH, resulting in decreasing BH end products and the increasing BH intermediates in the rumen. Consistent with our results, Whitlock et al. (Citation2002) and Ward et al. (Citation2003) reported an increase in CLA concentrations in heat treated seed (i.e. extruded, micronized or roasted full-fat soybean). It has been suggested that the decrease in the rate of LA biohydrogenation is the main factor that contributes to an increase in CLA isomers concentration in the rumen (Karri et al. Citation2014). Troegeler-Meynadier et al. (Citation2006) found an increase in the appearance of cis-9, trans-11 CLA by extruding soybean, linked to decrease in the disappearance of LA. Therefore, the increased appearance of cis-9, trans-11 CLA in micronized compared to non-micronized treatments might be related to reduced rate of LA biohydrogenation by micronization. Privé et al. (Citation2009) observed the heated oilseeds decreased cis-9, trans-11 CLA and increased trans-10, cis-12 CLA. In the study of Kaleem et al. (Citation2013), roasted soybean had no effect on the cis-9, trans-11 CLA production. Consistent with Troegeler-Meynadier et al. (Citation2014), who observed no effect of extrusion on the appearance of trans-10, cis-12 CLA in soybean, micronization did not affect the appearance of trans-10, cis-12 CLA in our study.
Lashkari et al. (Citation2019) observed that LA produced more cis-9, trans-11 CLA than trans-10, cis-12 CLA compared with LnA. As stated earlier, LA is the second predominant FA in the rapeseed; therefore, the higher concentration of cis-9, trans-11 CLA, and C18:1 trans-11 than trans-10, cis-12 CLA, and C18:1 trans-10 in the present study reflected that ruminal BH of LA plays a major part in the synthesis of cis-9, trans-11 CLA. In addition, enzyme-catalyzed reactions leading to the appearance of CLA isomers may response differently to the different UFA (Lashkari et al. Citation2019).
The effect of micronization was not the same in the appearance of individual trans C18:1 isomers. Heat processing could lead to the formation of some products that inhibit the growth and/or activity of bacteria producing trans-11 isomers and favour bacteria producing trans-10 isomers (Privé et al. Citation2009). Consistent with our findings, Vázquez-Añón et al. (Citation2008) and Kaleem et al. (Citation2013) showed that heat treated oilseeds decreased trans-11 isomers and increased trans-10 isomers. In the present study, an inhibition might have occurred at the saturation of second and/or third reactions of LA biohydrogenation by micronization, because only the appearance of C18:1 trans-11 and SA were decreased. The inhibition of LA biohydrogenation to CLA coupled with decreased disappearance rate of LA, could also explain the increased accumulation of cis-9, trans-11 CLA. Hino et al. (Citation1993) observed that addition of α-tocopherol and β-carotene to safflower oil stimulated the growth of Butyrivibrio fibrisolvens (B. fibrisolvens), which were supposed to be primarily responsible for the C18:1 trans-11 BH. The high degradation of tocopherols by micronization also seems to stimulate the growth of C18:1 trans-11- producing bacteria.
As previously suggested (Juárez et al. Citation2011), vitamin E can change the rumen BH toward formation of cis-9, trans-11 CLA. The mechanism by which vitamin E may alter the BH is unclear, but the modification of rumen microorganism population/dynamic may be involved (Pottier et al. Citation2006). Hou et al. (Citation2013) reported that α-tocopherol and its derivatives could alter the rumen microbial activity and reduce the precursors of trans-10, cis-12 CLA formation. Pottier et al. (Citation2006) hypothesized that vitamin E might act either as electron donor for B. fibrisolvens or as an inhibitor of C18:1 trans-10 producing bacteria. Despite the significant effect of vitamin E on disappearance of LA, LnA and OA, the reason why the appearance of cis-9, trans-11 CLA, trans-10, cis-12 CLA, C18:1 trans-11 and C18:1 trans-10 was not affected by the supplementation of vitamin E is controversial. An in vitro study by Hou et al. (Citation2013) showed that dietary vitamin E supplementation (0.5, 1.0 and 2.0 mg/80 mL culture solution) decreased the appearance of cis-9, trans-11 CLA, C18:1 trans-10 and increased the appearance of C18:1 trans-11, while had no effect on the appearance of trans-10, cis-12 CLA when soybean oil was included in the diet of goats. Pottier et al. (Citation2006) observed that supplementation of dietary vitamin E at 12,000 IU/d increased the appearance of cis-9, trans-11 CLA, C18:1 trans-11 and decreased the appearance of trans-10, cis-12 CLA, C18:1 trans-10 in the rumen when linseed was included in the diet of dairy cows. As stated by O’Donnell-Megaro et al. (Citation2012) the effect of vitamin E supplementation on the rumen BH may vary depending on diet components and vitamin E doses.
4.4. The biohydrogenation kinetic parameters
It is well documented that the high content of available UFA is toxic to the function of rumen microbiota and may be more toxic than their BH intermediates (Choi et al. Citation2009). Lashkari et al. (Citation2017) stated that heat treatment protected the lipid droplets from rumen lipolysis. Therefore, to decrease the toxic effect of LA, LnA and OA, BH took place to a greater extent in whole flaked raw rapeseed than micronized ones. The lag phase is assumed to be the time needed for lipolysis and/or for a proliferation of rumen microbiota to have enough microbiota to lipolyze and hydrogenate UFA, and is dependent on fat source, and might be correlated with dry matter digestibility and incubation conditions (Ribeiro et al. Citation2007; Lashkari et al. Citation2017). A decrease in disappearance rate and increase in lag phase observed for LA, LnA and OA of micronized compared to non-micronized treatments could potentially increase the amount of UFA escaping ruminal BH. Subsequently, an increase in UFA absorption from the small intestine can enhance the concentration of UFA in dairy products, as stated by Jones et al. (Citation2001) and Lashkari et al. (Citation2017).
The lowest appearance rate of SA and VA occurred in the micronized treatments, which also had the lowest BH rate of LA, LnA and OA. The highest lag phase observed for appearance of SA and VA in the micronized rapeseed followed the same trend as disappearance of LA, LnA and OA. The alternation of the microbiota ecosystem and/or the inhibition of the reductase activity of ruminal microbes might be responsible for the higher lag phase in appearance of VA (Lashkari et al. Citation2017) in micronized compared to non-micronized treatment.
5. Conclusion
The present study showed that micronization can be an effective method to protect MUFA and PUFA supplied through feeding micronized whole flaked rapeseed from ruminal BH and has the potential of increasing beneficial intermediate FA such as cis-9, trans-11 CLA. Since rapeseed is a main source of OA, if it is aimed to feed a particular amount of OA to ruminants, using micronized whole flaked rapeseed would supply OA or other PUFA, especially in high-producing animals that have a shorter retention time of feed particles in the rumen. Our study also suggests that vitamin E supplementation could be an advantageous strategy to decrease LA, LnA and particularly OA biohydrogenation. However, in our experiment condition, vitamin E supplementation had no influence on the appearance of intermediate FA and SA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anwar MM, Ali SE, Nasr EH. 2015. Improving the nutritional value of canola seed by gamma irradiation. J Radiat Res Appl Sci. 8(3):328–333. http://dx.doi.org/10.1016/j.jrras.2015.05.007.
- Baldin M, Adeniji YA, Souza JG, Green MH, Harvatine KJ. 2022. In vivo kinetics of oleic, linoleic, and α-linolenic acid biohydrogenation in the rumen of dairy cows. J Dairy Sci. 105:7373–7385. doi:10.3168/jds.2022-21831.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37:911–917. doi:10.1139/y59-099.
- Bloomberg BD, Hilton GG, Hanger KG, Richards CJ, Morgan JB, Van Overbeke DL. 2011. Effects of vitamin E on color stability and palatability of strip loin steaks from cattle fed distillers grains. J Anim Sci. 89:3769–3782. doi:10.2527/jas.2011-3843.
- Choi NJ, Park HG, Kim JH, Hwang HJ, Kwon KH, Yoon JA, Kwon EG, Chang J, Hwang IH, Kim YJ. 2009. Characterizations of environmental factors in conjugated linoleic acid production by mixed rumen bacteria. J Agric Food Chem. 57:9263–9267. doi:10.1021/jf9021465.
- Chouinard PY, Corneau L, Butler WR, Chilliard Y, Drackley JK, Bauman DE. 2001. Effect of dietary lipid source on conjugated linoleic acid concentrations in milk fat. J Dairy Sci. 84:680–690. doi:10.3168/jds.S0022-0302(01)74522-5.
- Cieslak A, Kowalczyk J, Czauderna M, Potkanski A, Szumacher-Strabel M. 2010. Enhancing unsaturated fatty acids in ewe’s milk by feeding rapeseed or linseed oil. Czech J Anim Sci. 55:496–504. doi:10.17221/874-cjas.
- De Almeida FA, Da Silva Sobrinho AG, Manzi GM, Lima NLL, Endo V, Zeola NMBL. 2015. Dietary supplementation with sunflower seeds and vitamin E for fattening lambs improves the fatty acid profile and oxidative stability of the Longissimus lumborum. Anim Prod Sci. 55:1030–1036. doi:10.1071/AN13383.
- Dewanckele L, Jing L, Stefańska B, Vlaeminck B, Jeyanathan J, Van Straalen WM, Koopmans A, Fievez V. 2019. Distinct blood and milk 18-carbon fatty acid proportions and buccal bacterial populations in dairy cows differing in reticulorumen pH response to dietary supplementation of rapidly fermentable carbohydrates. J Dairy Sci. 102:4025–4040. doi:10.3168/jds.2018-15823.
- El-Essawy AM, Khattab IM, Abdou AR, Abdel-Wahed AM. 2019. Effect of linseed oil beads addition With vitamin E on performance, blood metabolites and milk yield of lactating goats. Egypt J Nutr Feed. 22:283–296. doi:10.21608/ejnf.2019.79405.
- Freitas JE, Takiya CS, Del Valle TA, Barletta RV, Venturelli BC, Vendramini THA, Mingoti RD, Calomeni GD, Gardinal R, Gandra JR, et al. 2018. Ruminal biohydrogenation and abomasal flow of fatty acids in lactating cows fed diets supplemented with soybean oil, whole soybeans, or calcium salts of fatty acids. J Dairy Sci. 101:7881–7891. doi:10.3168/jds.2017-13666.
- Gonthier C, Mustafa AF, Berthiaume R, Petit HV, Martineau R, Ouellet DR. 2004. Effects of feeding micronized and extruded flaxseed on ruminal fermentation and nutrient utilization by dairy cows. J Dairy Sci. 87:1854–1863. doi:10.3168/jds.S0022-0302(04)73343-3.
- Gurbuz Y. 2007. Determination of nutritive value of leaves of several Vitis vinifera varieties as a source of alternative feedstuff for sheep using in vitro and in situ measurements. Small Rumin Res. 71(1-3):59–66. http://dx.doi.org/10.1016/j.smallrumres.2006.04.009.
- Harvatine KJ, Allen MS. 2006. Effects of fatty acid supplements on ruminal and total tract nutrient digestion in lactating dairy cows. J Dairy Sci. 89:1092–1103. doi:10.3168/jds.S0022-0302(06)72177-4.
- Hino T, Andoh N, Ohgi H. 1993. Effects of β-carotene and α-tocopherol on rumen bacteria in the utilization of long-chain fatty acids and cellulose. J Dairy Sci. 76:600–605. doi:10.3168/jds.S0022-0302(93)77380-4.
- Hou J, Wang F, Wang Y, Liu F. 2013. Effects of vitamin E on the concentration of conjugated linoleic acids and accumulation of intermediates of ruminal biohydrogenation in vitro. Small Rumin Res. 111:63–70. doi:10.1016/j.smallrumres.2012.09.015.
- Ian Givens D, Allison R, Blake J. 2003. Enhancement of oleic acid and vitamin E concentrations of bovine milk using dietary supplements of whole rapeseed and vitamin E. Anim Res EDP Sci. 52:531–542. doi:10.1051/animres.
- Ishrat M, Ashraf SA, Khan AF, Azad AZR. 2014. Effect of conventional heat treatment on fatty acid profile of different edible oils using gas chromatography. Int J Biosci (IJB). 4:238–243.
- Jenkins TC. 1993. Lipid metabolism in the rumen. J Dairy Sci. 76:3851–3863. doi:10.3168/jds.S0022-0302(93)77727-9.
- Jenkins TC, Wallace RJ, Moate PJ, Mosley EE. 2008. Board-invited review: recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim Sci. 86:397–412. doi:10.2527/jas.2007-0588.
- Jensen SK. 2008. Improved Bligh and Dyer extraction procedure. Lipid Technol. 20:280–281. doi:10.1002/lite.200800074.
- Jensen SK, Nørgaard JV, Lauridsen C. 2006. Bioavailability of α-tocopherol stereoisomers in rats depends on dietary doses of all-rac - or RRR-α-tocopheryl acetate. Br J Nutr. 95:477–487. doi:10.1079/BJN20051667.
- Jones RA, Mustafa AF, Christensen DA, McKinnon JJ. 2001. Effects of untreated and heat-treated canola presscake on milk yield and composition of dairy cows. Anim Feed Sci Technol. 89:97–111. doi:10.1016/S0377-8401(00)00219-4.
- Juárez M, Dugan MER, Aalhus JL, Aldai N, Basarab JA, Baron VS, McAllister TA. 2011. Effects of vitamin E and flaxseed on rumen-derived fatty acid intermediates in beef intramuscular fat. Meat Sci. 88:434–440. doi:10.1016/j.meatsci.2011.01.023.
- Kaleem A, Enjalbert F, Farizon Y, Troegeler-Meynadier A. 2013. Effect of chemical form, heating, and oxidation products of linoleic acid on rumen bacterial population and activities of biohydrogenating enzymes. J Dairy Sci. 96:7167–7180. doi:10.3168/jds.2013-6768.
- Karri S, Talla SG, Tyagi AK. 2014. An over view on conjugated linoleic acid (CLA): a dairy product derivative and its applications. Indian J Dairy Sci. 67:99–106.
- Kay JK, Roche JR, Kolver ES, Thomson NA, Baumgard LH. 2005. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J Dairy Res. 72:322–332. doi:10.1017/S0022029905000944.
- Khorasani GR, de Boer G, Robinson PH, Kennelly JJ. 1992. Effect of canola fat on ruminal and total tract digestion, plasma hormones, and metabolites in lactating dairy cows. JDairy Sci. 75:492–501. doi:10.3168/jds.S0022-0302(92)77786-8.
- Kmiecik D, Fedko M, Siger A, Kulczyński B. 2019. Degradation of Tocopherol Molecules and Its Impact on the Polymerization of Triacylglycerols during Heat Treatment of Oil. Molecules. 24(24):4555. http://dx.doi.org/10.3390/molecules24244555.
- Lashkari S, Hymøller L, Jensen SK. 2017. Ruminal biohydrogenation kinetics of defatted flaxseed and sunflower is affected by heat treatment. J Agric Food Chem. 65:8839–8846. doi:10.1021/acs.jafc.7b03008.
- Lashkari S, Krogh Jensen S, Bernes G. 2019. Biodiscrimination of α-tocopherol stereoisomers in plasma and tissues of lambs fed different proportions of all-rac-α-tocopheryl acetate and RRR-α-tocopheryl acetate. J Anim Sci. 97:1222–1233. doi:10.1093/jas/skz011.
- Leduc M, Létourneau-Montminy MP, Gervais R, Chouinard PY. 2017. Effect of dietary flax seed and oil on milk yield, gross composition, and fatty acid profile in dairy cows: a meta-analysis and meta-regression. J Dairy Sci. 100:8906–8927. doi:10.3168/jds.2017-12637.
- McAllister TA, Sultana H. 2011. Effects of micronization on the in situ and in vitro digestion of cereal grains. Asian-Aust J Anim Sci. 24:929–939. doi:10.5713/ajas.2011.10387.
- McDougall EI. 1947. Studies on ruminant saliva. Biochem J. 43:99–109. doi:10.1042/bj0430099.
- Nogueira RGS, Perna Junior F, Pereira ASC, Cassiano ECO, Carvalho RF, Rodrigues PHM. 2020. Methane mitigation and ruminal fermentation changes in cows fed cottonseed and vitamin E. Sci Agric. 77:1–10. doi:10.1590/1678-992x-2018-0247.
- O’Donnell-Megaro AM, Capper JL, Weiss WP, Bauman DE. 2012. Effect of linoleic acid and dietary vitamin E supplementation on sustained conjugated linoleic acid production in milk fat from dairy cows. J Dairy Sci. 95:7299–7307. doi:10.3168/jds.2012-5802.
- Official Methods of Analysis of AOAC International. 1990. 15th ed. Association of Official Analytical Chemists: Arlington, V. Kenneth. 1. doi:10.7312/seir17116-004.
- Official methods of Analysis of AOAC International. 2000. 17th ed., The Association of Official Analytical Chemists, Gaithersburg, MD, USA.
- Petersen MB, Jensen SK. 2014. Biohydrogenation of fatty acids is dependent on plant species and feeding regimen of dairy cows. J Agric Food Chem. 62:3570–3576. doi:10.1021/jf405552m.
- Petit HV, Tremblay GF, Tremblay E, Nadeau P. 2002. Ruminal biohydrogenation of fatty acids, protein degradability, and dry matter digestibility of flaxseed treated with different sugar and heat combinations. Can J Anim Sci. 82:241–250. doi:10.4141/A01-083.
- Pottier J, Focant M, Debier C, De Buysser G, Goffe C, Mignolet E, Froidmont E, Larondelle Y. 2006. Effect of dietary vitamin E on rumen biohydrogenation pathways and milk fat depression in dairy cows fed high-fat diets. J. Dairy Sci. 89:685–692. doi:10.3168/jds.S0022-0302(06)72131-2.
- Privé F, Combes S, Cauquil L, Farizon Y, Enjalbert F, Troegeler-Meynadier A. 2009. Temperature and duration of heating of sunflower oil affect ruminal biohydrogenation of linoleic acid in vitro. J Dairy Sci. 93:711–722. doi:10.3168/jds.2009-2534.
- Ribeiro CVDM, Eastridge ML, Firkins JL, St-Pierre NR, Palmquist DL. 2007. Kinetics of fatty acid biohydrogenation in vitro. J Dairy Sci. 90:1405–1416. doi:10.3168/jds.S0022-0302(07)71626-0.
- Sajjadi H, Ebrahimi SH, Vakili SA, Rohani A, Golzarian MR, Heidarian Miri V. 2022. Operational conditions and potential benefits of grains micronization for ruminant: a review. Anim Feed Sci Technol. 287:115285. doi:10.1016/j.anifeedsci.2022.115285.
- Thacker PA. 1998. Effect of micronization of full-fat canola seed on performance and carcass characteristics of growing–finishing pigs. Anim Feed Sci Technol. 71(1-2):89–97. http://dx.doi.org/10.1016/S0377-8401(97)00133-8.
- Troegeler-Meynadier A, Nicot MC, Enjalbert F. 2006. Effects of heating process of soybeans on ruminal production of conjugated linoleic acids and trans-octadecenoic acids in situ. Rev Med Vet (Toulouse). 157:509–514.
- Troegeler-Meynadier A, Puaut S, Farizon Y, Enjalbert F. 2014. Effects of the heating process of soybean oil and seeds on fatty acid biohydrogenation in vitro. J Dairy Sci. 97:5657–5667. doi:10.3168/jds.2013-7783.
- Vázquez-Añón M, Nocek J, Bowman G, Hampton T, Atwell C, Vázquez P, Jenkins T. 2008. Effects of feeding a dietary antioxidant in diets with oxidized fat on lactation performance and antioxidant status of the cow. J Dairy Sci. 91:3165–3172. doi:10.3168/jds.2007-0737.
- Volden H, editor. 2011. NorFor - the nordic feed evaluation system. Wageningen Academic Publishers, The Netherlands. https://www.wageningenacademic.com/doi/book/10.3920978-90-8686-718-9
- Wang Y, McAllister TA, Zobell DR, Pickard MD, Rode LM, Mir Z, Cheng KJ. 1997. The effect of micronization of full-fat canola seed on digestion in the rumen and total tract of dairy cows. Can J Anim Sci. 77:431–440. doi:10.4141/A96-113.
- Ward AT, Wittenberg KM, Froebe HM, Przybylski R, Malcolmson L. 2003. Fresh forage and solin supplementation on conjugated linoleic acid levels in plasma and milk. J Dairy Sci. 86:1742–1750. doi:10.3168/jds.S0022-0302(03)73760-6.
- Whitlock LA, Schingoethe DJ, Hippen AR, Kalscheur KF, Baer RJ, Ramaswamy N, Kasperson KM. 2002. Fish oil and extruded soybeans fed in combination increase conjugated linoleic acids in milk of dairy cows more than when fed separately. J Dairy Sci. 85:234–243. doi:10.3168/jds.S0022-0302(02)74072-1.
- Yang B, Chen H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen W. 2015. Review of the roles of conjugated linoleic acid in health and disease. J Funct Foods. 15:314–325. doi:10.1016/j.jff.2015.03.050.
