Abstract
Background: Glucocorticoids (GCs) play an important role in the treatment of several hematological malignancies, such as immune thrombocytopenia (ITP). The glucocorticoid receptor (GR) mediates the effects of GCs. Five isoforms of GR mRNA were described: GRα, GRβ, GRγ, GRP, and GRA. GR levels are regulated by alternative splicing of GR mRNA. Several studies demonstrated that a lower GR expression was associated with poor GC response.
Purpose: This study investigated the expression of GR isoforms and the relationship between GC resistance in ITP.
Methods: This study determined GRα/β/γ/P mRNA and GRα/β protein expression levels using SYBR Green Real-time PCR and Western blot, respectively, in 49 newly diagnosed ITP patients and 31 controls.
Results: The expression of GR isoform mRNA in ITP and controls showed the following trend: GRα > GRP > GRγ > GRβ. The expression of GRα, β mRNA and the total frequency of the four GR isoforms in ITP was significantly higher than in controls (P < 0.05). The expression of GRα mRNA and protein in the GC-resistant group was significantly lower than that in the GC-sensitive group and controls (P < 0.05). GRβ could not be detected at the protein level in our experimental conditions.
Conclusion: GRα and GRP were the main GR isoforms responsible for the effects of GC, and GRα and GRP exhibited synergistic effects. The down-regulation of GRα levels may play an important role in GC resistance in ITP. The effects of GCs in ITP were not associated with changes in GRβ and GRγ.
Idiopathic thrombocytopenic purpura (ITP) is a thrombocytopenic hemorrhagic disease that is caused by a weakening in platelet immunity, and it has been renamed immune thrombocytopenia.Citation1 Glucocorticoids (GCs) are the first choice for first-line therapy. Patient sensitivity to GCs is an important factor that determines whether GCs are chosen to cure ITP.
Most GC effects are mediated through the GC receptor (GR), which is a member of the nuclear hormone receptor superfamily, and GRs participate in numerous signaling pathways. The mechanisms that regulate the GR response to GC remain controversial, but the influence of GR splice variant expression is one mechanistic component. GR mRNA produces five isoforms, i.e., GRα, GRβ, GRγ, GRP, and GRA, after alternative splicing.
Steroid hormone receptors, such as the GC, are members of the nuclear receptor subfamily 3. These receptors contain three distinct functional regions: the N-terminal transactivation domain, the central zinc-finger DNA-binding domain (DBD), and the C-terminal ligand-binding domain (LBD). The GR gene includes nine exons: exon 1 is the 5′ non-translating region, exon 2 codes for the aminoterminal portion of GR, exons 3 and 4 code for the DBD, and exons 5–9 code for the LBD. Alternative slicing of exon 9 produces two highly homologous GR isoforms: GRα and GRβ. GRα is the classic receptor that binds GCs and mediates most known GC actions. In contrast, GRβ does not bind GCs, but it functions as a dominant-negative inhibitor of GRα-induced transactivation of GC response element-containing genes. However, it has recently been reported that GRβ has an inhibitory effect on GRα-mediated genetic transcription.Citation2 The LBDs of GRA and GRP are incomplete because of the deficiency of exons 5–7 and 8 and 9, respectively.Citation3 GRγ is produced because three groups of bases that encode arginine are inserted in the region between two zinc-finger motifs of the DBD in the area between exons 3 and 4,Citation4 which may lower GRα-mediated transcriptional activity.Citation5
The regulation of GR expression is complex and not fully understood. There are many previous studies on GC sensitivity and GR isoforms. However, these studies primarily focused on the GRα and GRβ isoforms and emphasized bronchial asthma, inflammatory bowel disease, acute leukemia, dermatosis, and systemic lupus erythematosus. There are few studies on the relationship between GR isoforms and GC resistance in ITP in China or around the world. Our study investigated the expression of GRα, GRβ, GRγ, and GRP and the relationship between GR expression and GC therapeutic effects in ITP patients.
Patients and methods
Patient samples
Forty-nine newly diagnosed patients with ITP were recruited in the Hematology/Oncology Department of Tai'an Traditional Chinese Medical Hospital from July 2012 to March 2014. All patients were diagnosed using ITP standards.Citation6 Thirty-two ITP patients received GC treatment of 1.0 mg/kg/day prednisone for 4 weeks. These 32 patients were further divided into two groups according to PLT counts after treatment: a GC-sensitive group (PLT >30 × 109/l) and a GC-resistant group (PLT <30 × 109/l). Thirty-one healthy volunteers were selected from blood donors at blood stations in the Tai'an City center. Volunteers with hypertension, diabetes, cardiovascular diseases, acute or chronic infections, or autoimmune disease were excluded. Volunteers who were pregnant were also excluded. None of the subjects suffered infectious diseases or received GC or other immunosuppressants within 2 weeks before the examinations (Tables and ).
Table 1 Clinicopathological features of ITP patients and controls
Table 2 Primers of GRα, GRβ, GRγ, GRP, and GAPDH
Extraction of total RNA
A volume of 6 ml venous blood was collected from ITP patients and healthy volunteers in the morning after an overnight fast, using EDTA as the anticoagulant. PBMCs were collected using lymphocyte separating medium density gradient centrifugation and preserved at a temperature of −80°C. Total RNA was isolated from PBMCs using RNAiso plus (D9108A, TaKaRa Biotechnology (Takara Biotechnology, Dalian, China) Co., Ltd), according to the manufacturer's instructions, and was quantified photometrically. RNA was reverse transcribed to complementary DNA. Reverse transcription was performed using the PrimeScript® RT reagent Kit (DRR037, TaKaRa Biotechnology (Dalian) Co., Ltd). The reaction system of real-time PCR was prepared according to SYBR® Premix Ex TaqTM (DRR041, TaKaRa Biotechnology (Dalian) Co., Ltd). Primers for GRα, GRβ, GRγ, GRP, and GAPDH were designed using GenBank (Table ). Initial denaturation was performed at 95°C for 30 seconds for one cycle, and PCR was performed at 95°C for 5 seconds and 60°C for 20 seconds for 40 cycles. The reactions for melting curve analysis were performed at 95°C for 10 second, 65°C for 60 seconds, and 95°C for 10 seconds. A LightCycler® 480 instrument (Roche Diagnostics Ltd, Roche Bio Inc., Rotkreuz, Switzerland) was used for real-time RT-PCR analysis, according to the manufacturer's instructions.
Table 3 Expression of four GR isoform mRNAs in ITP patients and controls
Western blot
Protein was extracted from protein lysates using RIPA buffer. A BCA Protein Assay Kit measured protein concentration, and proteins were preserved at −20°C. An SDS–PAGE gel was prepared, and 50–100 μg protein samples were injected into each well. Proteins were separated using electrophoresis and transferred to a membrane. Membrane-bound proteins were identified using immunoreactions, development, and fixation. The film was developed after exposure and dried at room temperature.
Statistics
Statistical analyses were performed using SPSS 17.0 and Prism 5. Values are given as medians (p25–p75) when the data were abnormally distributed. Statistically significant differences and correlations between groups were analyzed using the Mann–Whitney U-test and Spearman's rank correlation test, respectively. Data in normal distributions are expressed as the means ± SE, and statistically significant differences between groups were analyzed using the t test. All significance levels were two-tailed (P < 0.05).
Results
Expression of GR isoform mRNAs in ITP patients and controls
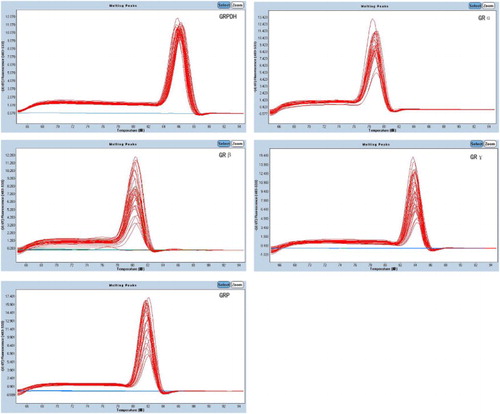
The amplification and melting curves were analyzed using the LightCycler® 480 (Roche Diagnostics Ltd) (Fig. ). The ratio of the target gene content to housekeeping gene content is presented using ,
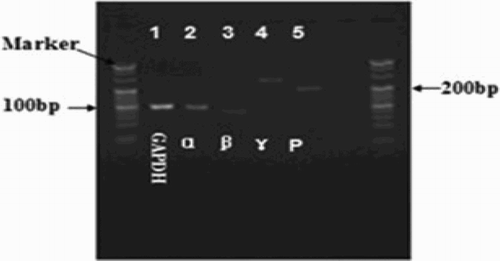
PCR product purity was verified using agarose gel electrophoresis (Fig. ).
Figure 2 Image of an electrophoresis gel of PCR products of GRα, β, γ, and P. Single band of PCR products showed the absence of other non-specific products among the PCR products.
The mRNA expression of the four isoforms in the two groups showed the following trend: GRα > GRP > GRγ > GRβ (Table , Fig. A). The frequency of α, P, γ, and β expression in ITP was 58.91, 23.76, 13.37, and 3.96%, respectively, and 47.32, 33.04, 18.69, and 1.79%, respectively, in the control group. The mRNA expression of GRα, β and GR in ITP patients was significantly higher than that in controls (P < 0.05, Table , Fig. ). GRα and GRP were positively correlated in ITP and controls, with correlation coefficients of 0.48 and 0.69, respectively (P < 0.05, Fig. B). The GC-resistant group exhibited increased expression of GRγ mRNA compared to the GC-sensitive group, but GRα, GRP, and GRβ mRNA expression was decreased. However, no significant differences in GR isoform expression were observed between the two groups (Table , Fig. ).
Figure 3 (A) Expression of GRα, β, γ, and P mRNA in the ITP and control groups. (B) Correlations between GRα and GRP in ITP patients and controls.
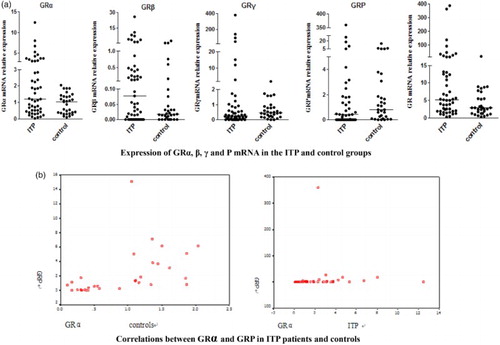
Figure 4 Expression of GRα, β, γ, and P mRNA in the GC-sensitive group, GC-resistant group, and the control group.
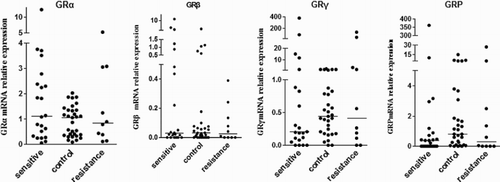
Table 4 Expression of four GR isoform mRNAs in GC-sensitive, GC-resistant, and control groups
Expression of GRα and GRβ protein
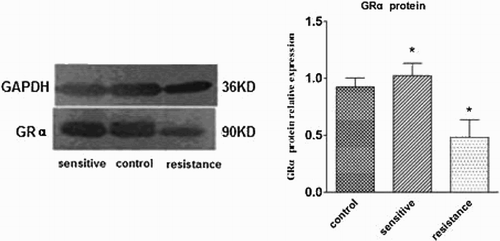
The expression of GRα protein was significantly lower in the GC-resistant group (0.48 ± 0.15) than in the GC-sensitive group (1.02 ± 0.11) and controls (0.93 ± 0.08) (P < 0.05, Fig. ). Western blotting did not detect GRβ protein in our experimental conditions.
Discussion
Using real-time RT PCR, our study detected the expression of GRα, GRβ, GRγ, and GRP mRNA in PBMCs of 49 ITP patients and 31 healthy volunteers. We also detected the expression of GRα and GRβ protein in PBMCs from 12 ITP patients and 6 healthy volunteers to preliminarily investigate the relationship between GC resistance and GR isoforms in ITP patients and provide a theoretical basis to study the pathogenetic mechanisms of GC resistance and improve clinical treatment for ITP patients.
ITP is an autoimmune disease. GCs are the first choice for first-line therapy. GC can inhibit macrophage to process antigen, and promote lymphocyte damage and collapse, reduce the number of lymphocytes in the circulation of the blood, to suppress the cellular immunity and humoral immunity.
This study demonstrated that the amounts of GRα and GRβ mRNA and the summed amounts of mRNA of the four GR isoforms in the ITP group were significantly higher than those in the control group (P < 0.05). LiCitation7 demonstrated that total GR expression in initially treated ITP patients was higher than that in normal subjects, which is consistent with our experimental results. However, Li did not detect various GR isoforms. ITP is an autoimmune disease, and humoral immunity and cellular immunity contribute to the damaging of platelets. The increase in GR expression in ITP patients may be related to immune functional changes and various cell factor immune modulations during the disease attack. The amount of GRβ mRNA was extremely low. GRβ in the ITP group was approximately 0.07 times GRα, and GRβ in the control group was approximately 0.04 times GRα. These results indicated that GRβ was not the main isoform that contributed to the efficacy of GC therapy.
A recent report demonstrated that GRP augmented GRα-mediated hexadecadrol in multiple myeloma (RPMI 8226-S) and UM3 cells, and de Lange et al.Citation8 inferred that GRP exhibited a conformation that was conducive to integration with ligand-bound GRα, which synthesized a dimer. The GRα and GRP dimer translocated to the nucleus and stimulated transcription of target genes in a more effective manner than ordinary GRα dimers. GRα and GRP may also regulate GC reactivity. GRα and GRP accounted for a large proportion of GRs, approximately 80% in both groups, in our study, and these GR isoforms were positively correlated with GC resistance (P < 0.05), with r values of 0.48 and 0.69, respectively, compared to the GC-sensitive and control groups. GRα and GRP mRNAs were decreased, and GRα protein expression was significantly decreased in the GC-resistant group (P < 0.05), which indicated that GRα and GRP were the primary isoforms responsible for the efficacy of GC. GRα and GRP also exhibited synergistic effects.
Inui et al.Citation9 found that GC-resistant patients had significantly lower GRα than GC-sensitive and control groups in a study of GC resistance in atopic dermatitis (AD) patients. This author only detected GRβ in one patient in the GC-sensitive group. Therefore, he inferred that the decrease in GRα mRNA may play an important role in GC resistance, and GRβ would not affect the sensitivity of AD patients to GCs. The expression of GRγ mRNA in GC-resistant ITP patients was increased compared with the GC-sensitive and control groups, and GRα/GRγ expression was lower (2.02-fold) in the GC-resistant group than in the GC-sensitive group (5.55-fold) and the control group (2.65-fold). These results indicated that increased GRγ mRNA in ITP patients may be related to GC resistance. Haarman et al.Citation10 detected GRα and GRβ mRNA and GRγ and GRα protein expression in mononuclear cells in peripheral blood and marrow in children diagnosed with ALL and found that GC resistance was related to an increase in GRγ mRNA, but it was not related to the mRNA expression of GRα, GRβ, or GRα/GRβ. BegerCitation11 divided 10 ALL patients into two groups, GC-sensitive and GC-resistant, extracted PBMCs, and employed Dex for 0, 1.5, 4, and 10 hours. The expression of GR and GRγ, GRγ/GR mRNA was detected at each time point. Changes in GRγ/GR, total GR, and GRγ mRNA participated in GC resistance, and increasing GC resistance was produced with increasing GRγ expression. These studies are consistent with our experimental results.
The last 15 amino acids in the GRβ LBD are not homologous with GRα, which results in an incomplete LBD and disintegration with GC. Therefore, GRβ is considered as a non-functional isoform. Some scholars demonstrated that the endogenous inhibitory effect of GRβ arose from 15 amino acids in the carboxyl terminal,Citation12 and GRβ also had inherent, gene-specific transcriptional activityCitation13 to regulate target gene expression, in addition to the negative regulation of GRα-mediated gene transcription. However, the results of many studies of the relationship between GC resistance and GRβ expression are not always consistent.
Some domestic research reported that GC resistance in ITP patients was related to an increased expression of GRβ mRNA,Citation14,Citation15 but our experimental results are consistent with the following findings. Choi et al.Citation16 reported that the average expression of GRβ mRNA was lower than that of GRα in GC-sensitive and GC-resistant nasal polyp patients and control subjects, but there was no significant difference between these three groups. This result indicated that the expression of GRβ was not related to GC sensitivity in patients with nasal polyps. Fujishima et al.Citation17 found that GC-resistant ulcerative colitis patients exhibited more GRβ-positive cells in colonic mucosa than did GC-sensitive patients, but GRβ expression was only 10−2–10−3 times that of GRα. Therefore, he inferred that the change in GRβ expression was not related to GC resistance. Our study demonstrated that GRβ mRNA expression in all groups was rather low, and there was no significant difference between groups. Our results suggest that the change in GRβ mRNA does not participate in GC resistance in ITP patients.
There were significantly larger differences in GR isoform mRNA expression in ITP patients than controls, but the fluctuation range of these values varied widely, and there were four cases with especially large changes. The clinical data demonstrated that two of these patients were sensitive to GC, and GC therapy was not adopted in the other two patients. No other differences were found, which indicated that the expression difference in GR isoforms may have resulted from individual differences in ITP patients.
Other members of our research team are hoping to make an important discovery by comparing the expression levels of GR isoforms between acute ITP and chronic ITP and in other diseases of the blood system, such as acute lymphoblastic leukemia and multiple myeloma. The role that GCs play in physiology is influenced by many factors, including transcription factors, genetic polymorphisms, and partner hormone receptor proteins. Other members of our team are examining the differences in the nuclear transcription factor (NF-kB), activated protein-1 (AP-1), and heat shock protein 90 (HSP90) between the GC-sensitive group and GC-resistant group. Therefore, we are committed to providing updates to our discoveries.
We look forward to the development of a new method of regulating GR expression levels to overcome hormone resistance. At the same time, we encourage GC-resistant ITP patients to combine traditional Chinese and Western medical treatments, such as oral Chinese medicine and acupuncture point injection, to improve their therapeutic outcomes. This may be a new breakthrough for the treatment of ITP patients with GC resistance. Traditional Chinese medical therapy provides a new direction for the study of GC resistance.
In summary, the down-regulation of GRα level may play an important role in GC resistance in ITP patients, but the effects of GCs in ITP patients are not associated with the changes in GRβ, GRγ, or GRP. The treatment of ITP should be individualized to overcome GC resistance.
Project Source: Tai'an City Shandong Province Science and Technology Development Program (201440774-29B).
Disclaimer statements
Contributors Y.L. performed the data analyses and wrote the manuscript; M.M.S. contributed to the conception of the study. S.Y.L. contributed significantly to analysis and manuscript preparation; L.L.M. helped perform the analysis with constructive discussions. Y.L. and M.M.S. are the joint first authors.
Funding None.
Conflicts of interest None.
Ethics approval This paper received ethics approval.
References
- Hou M, Qin P. Chinese specialists’ consensus on diagnosis and treatment of adults’ primary immune thrombocytopenia (revised version). Chin J Hematol. 2011;32(3):214–6.
- Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform b: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435–48. doi: 10.1007/s00018-009-0098-z
- Moalli PA, Pillay S, Krett NL, Rosen ST. Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid resistant human multiple myeloma cells. Cancer Res. 1993;53:3877–9.
- Rivers C, Levy A, Hancock J, Lightman S, Norman M. Insertion of an amino acid in the DNA-binding domain of the glucocorticoid receptor as a result of alternative splicing. J Clin Endocrinol Metab. 1999;84:4283–6. doi: 10.1210/jcem.84.11.6235
- Ray DW, Davis JR, White A, Clark AJL. Glucocorticoid receptor structure and function in glucocorticoid-resistant small cell lung carcinoma cells. Cancer Res. 1996;56:3276–80.
- Zhang Z, Shen T. Diagnosis and therapeutic effect criterion of hematopathy. The second version, Beijing: Science Press; 1998. p. 168–218.
- Li Q, Ou YX, Wu ZD, Gao GY, Zeng WQ, Gu MJ, et al. Study of glucocorticoid receptors in adult diopathic thrombocytopenic purpura. Chin J Clin. 2010;4(12):82–6.
- de Lange P, Segeren CM, Koper JW, Wiemer E, Sonneveld P, Brinkmann AO, et al. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res. 2001 May 15;61(10):3937–41.
- Inui S, Sumikawa Y, Asada H, Itami S. Glucocorticoid resistance in atopic dermatitis associated with decreased expression of glucocorticoid receptor-alpha in peripheral blood mononuclear cells. J Dermatol. 2010;37:496–9. doi: 10.1111/j.1346-8138.2010.00866.x
- Haarman EG, Kaspers GJ, Pieters R, Rottier MM, Veerman AJ. Glucocorticoid receptor alpha,beta and gamma expression vs in vitro glucocorticoid resistance in childhood leukemia. Leukemia. 2004;18(3):530–7. doi: 10.1038/sj.leu.2403225
- Beger C, Gerdes K, Lauten M, Tissing WJ, Fernandez-Munoz I, Schrappe M, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122(2):245–52. doi: 10.1046/j.1365-2141.2003.04426.x
- Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform b: recent understanding of its potential implications in physiology and pathophysiology. Cell.Mol. Life Sci. 2009;66(21):3435–48. doi: 10.1007/s00018-009-0098-z
- Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem Biophys Res Commun. 2009;381(4):671–5. doi: 10.1016/j.bbrc.2009.02.110
- Wang Y, Mai HR, Lin l, Yuan XL, Shi HS, Liu SX, et al. Relationship between glucocorticoid receptors and glucocorticoid resistance in children with idiopathic thrombocytopenia purpura. Chin J Contemp Pediatr. 2009;11(9):714–6.
- Zhao YH, Zhou J, Li XX. A study on the relationship between α-and β-isoform of glucocorticoid receptors and glucocorticoid resistant idiopathic thrombocytopenia purpura. Chin J Intern Med. 2005;44(5):363–5.
- Choi BR, Kwon JH, Gong SJ, Kwon MS, Cho JH, Kim JH, et al. Expression of glucocorticoid receptor mRNAs in glucocorticoid-resistant nasal polyps. Exp Mol Med. 2006;38:466–73. doi: 10.1038/emm.2006.55
- Fujishima S, Takeda H, Kawata S, Yamakawa M. The relationship between the expression of the glucocorticoid receptor in biopsied colonic mucosa and the glucocorticoid responsiveness of ulcerative colitis patients. Clin Immunol. 2009;133:208–17. doi: 10.1016/j.clim.2009.07.006