ABSTRACT
Objectives: Acute lymphoblastic leukemia (ALL) is the most common cancer before the age of 15 years, seriously endangering the health of children. The main treatment for Childhood ALL was pharmacotherapy. But these drugs have many side effects and some of them could develop drug resistance quickly. Mometasone furoate (MF) is an efficient glucocorticoid for topical treatment of inflammation on the skin, lung and nose.
Methods: In this study, we investigated whether the MF had effects on ALL cells proliferation and migration.
Results: The CCK-8 proliferation test showed that the cell viability was the lowest at 25 nM MF treatment and the increased OD value was time-dependent. In transwell assay, the number of CCRF-CEM cells was reduced in MF treated group. We found the expression of anti-apoptotic protein bcl-2 decreased the expression of pro-apoptotic protein caspase3 and bax increased in CCRF-CEM cell line treated with MF. The expression of p-AKT, p-mTOR, p70S6 K, vascular endothelial growth factor and CyclinD1 were decreased in MF treated group.
Conclusion: This study reveals that MF can inhibit proliferation and invasion/migration and induce apoptosis in Childhood ALL cells, which may be regulated by Phosphatidylinositol 3-kinase signaling pathway. These results suggest MF may be a potential new drug target for clinical ALL treatment.
Introduction
Leukemia is a blood cancer in which hematopoietic stem cells were blocked in a stage of differentiation process and then malignant proliferation [Citation1,Citation2]. Acute leukemia is broadly divided into acute lymphoblastic leukemia (ALL) and acute myeloid leukemia [Citation3]. ALL is the most common cancer before the age of 15 years, seriously endangering the health of children [Citation4]. The main treatment for childhood ALL was pharmacotherapy [Citation5]. Over the past few decades, ALL drugs treatment progress has led to 5-year disease-free survival rate of more than 80% [Citation6]. But after 5 years the survival rate is getting lower and lower. Besides, there are some complications after drugs treatment, such as lack of energy, pain, fatigue, obesity, osteoporosis, cardiomyopathy et al and even death [Citation7,Citation8]. Long-term medications also produce some drug resistance. Therefore, in order to reduce these side effects, it is urgent to develop novel drugs for ALL treatment.
Glucocorticoids are a class of steroid hormones consisting of physiological hormones produced by synthetic analogs and adrenal cortex that bind to glucocorticoid receptor [Citation9]. Glucocorticoids regulate a wide range of key processes, including the regulation of glucose metabolism and the response to physiological stress [Citation10]. Glucocorticoids are the effective immunosuppressive drugs that are extensively used to treat a variety of chronic inflammatory diseases and some malignant tumors [Citation11]. It has been reported that glucocorticoids can be used in prostate cancer [Citation9], rectal cancer [Citation11] and breast cancer [Citation12]. In addition, the main role of glucocorticoids in cancer is used to treat lymphoid malignancies [Citation13]. Studies have indicated that glucocorticoids can be used not only as chemotherapeutic agents, but also as cytotoxic agents in the treatment of ALL [Citation14].
Mometasone Furoate (MF) is an efficient glucocorticoid for topical treatment of inflammation on the skin, lung and nose [Citation15]. Evidence suggests that the using of MF also improves symptoms associated with adenoid hypertrophy [Citation16]. MF contains hexanediol, which has antimicrobial properties [Citation17]. The effects of hexanediol on microorganisms may be potentially beneficial in the treatment of eczema, possibly resulting in better treatment. Hexanediol is also a safe and effective way to treat long-term recurrent diseases [Citation18]. However, so far there were few information published on MF for the treatment of human cancer and childhood ALL.
In our study, we found that MF treatment could inhibit the proliferation and invasion/migration of childhood ALL CCRF-CEM cells, which might be regulated by Phosphatidylinositol 3-kinase (PI3K) signal pathway.
Methods
Cell lines and cell culture
The CCRF-CEM was purchased from U.S.A. ATCC Company. Cells were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) medium (HYCLONE, GE Healthcare Life Sciences, Logan, Utah, U.S.A.) supplemented with 10% serum (Gibco, Mountain View, California, U.S.A.), 100 U/ml penicillin (SIGMA, St. Louis, Missouri, U.S.A.), and 0.1 mg/ml streptomycin (SIGMA), at 37°C in an incubator with 5% CO2. When the cells were cultured to enter the logarithmic growth phase, they were washed three times with PBS and digested with trypsin (Beijing Solarbio Science & Technology, Beijing, China). After the cells turn round, RPMI-1640 was added to end the digestion, and repeatedly blows the cell suspension into a single cell suspension, and then the cells were seeded into 6-well plate for subsequent experiments. When the cell density reached about 80%, the cells were treated with 25 nM MF (MCE, Princeton, New Jersey, U.S.A.) for 24 h, and the negative control group was treated with DMSO (AMRESCO, Olympia, Washington, U.S.A.). The assay was performed in triplicate.
Western blot
After the cells were treated with 0.1% DMSO and MF (25 nM) for 24 h, the protein was extracted with RIPA lysis buffer (including protease inhibitor) (CWBIO, Beijing, China). The concentration was determined by BCA (CWBIO) method. Then the protein was heated at 95°C for 5 min. Approximately 20 μg of protein per group was added to each well in the vertical electrophoresis tank, then it was separated in 10% SDS-PAGE electrophoresis and transferred onto a PVDF membrane. After blocking with 5% nonfat milk for 1 h, the membrane was incubated with primary antibodies at 4°C overnight. On the second day, the membrane was washed three times in 0.1% Tween 20-TBS (TBST) and each time for 5 min, then incubated with secondary antibodies at room temperature for 1 h. After the membrane was washed, an enhanced-chemiluminescence (PTG, Chicago, lllinois, U.S.A.) chromogenic substrate was added to visualize the bands. The gray value was scanned by Quantity One. The GAPDH was recognized as the internal control. The relative expression of each protein was calculated by Objective protein/GAPDH. The assay was performed in triplicate. Western blots were performed with the following antibodies: first antibody rabbit anti-human, AKT (1:1000, CST, Danvers, Massachusetts, U.S.A.), p-AKT (1:1000, CST, Danvers, Massachusetts, U.S.A.), mTOR (1:1000, CST, Danvers, Massachusetts, U.S.A.), p-mTOR (1:1000, CST, Danvers, Massachusetts, U.S.A.), p70S6k (1:1000, PTG, Chicago, lllinois, U.S.A.), Bcl-2 (1:1000, PTG, Chicago, lllinois, U.S.A.), Bax (1:1000, PTG, Chicago, lllinois, U.S.A.), Active Caspase3 (1:1000, PTG, Chicago, lllinois, U.S.A.), GAPDH (1:5000, PTG, Chicago, Illinois, U.S.A.); secondary antibody HRP-labeled goat anti-rabbit/goat anti-mouse (1:5000, PTG, Chicago, Illinois, U.S.A.).
Dose-dependent
CCRF-CEM cells of the conventional culture were digested and counted for preparing cell suspension. 100 µl cells suspension were seeded onto 96-well plates with 1000 cells per well. 0.1% DMSO was added in NC group. The experimental groups were, respectively, supplemented with 0, 1, 2.5, 5, 10, 25, 50, 100, 200 nM MF. Then the cells were cultured in CO2 incubator, detect cell viability once every 24 h. Before detection, 10 μl of CCK8 (CWBIO, Beijing, China) was added to each well and incubated at 37°C for 1.5 h. The OD value was measured with microplate reader in 450 nm excitation light and the results were shown in bar graphs. The assay was performed in triplicate.
Cell counting kit-8 (CCK8) proliferation test
CCRF-CEM cells of the conventional culture were digested and counted for preparing cell suspension. 100 µl cells suspension were seeded onto 96-well plates with 1000 cells per well, and 0.1% DMSO was added in NC group, 25 nM MF was added in the experimental group. Then the cells were cultured in CO2 incubator, detect cell viability once every 24 h. Before detection, 10 μl of CCK8 (CWBIO, Beijing, China) was added to each well and incubated at 37°C for 1.5 h. The OD value was measured with microplate reader in 450 nm excitation light and then plot the multiplication curve. The assay was performed in triplicate.
Cell invasion and migration assessed by transwell assay
Matrigel (BD, Franklin Lakers, New Jersey, U.S.A.) dissolved overnight (serum-free RPMI-1640 medium diluted 1:6), 100 μl was added to the upper chamber of 24-well transwell (Millipore, Billerica, Massachusetts, U.S.A.). After shaking evenly, the matrigel was put into CO2 incubator cultivating for 4–6 h at 37°C until gel formation. Then drying the culture medium, 500 µl serum-free medium was added to the bottom of wells to hydrate basement membrane for half an hour. The cells suspension, treated with MF for 24 h, were prepared using serum-free RPMI-1640 medium. 100 µl (1 × 105) cells suspension were loaded into the upper chamber of the transwell, 500 µl complete culture medium was added to the bottom of transwell for incubation overnight. Next day, the transwell was removed, and cells remaining on the upper chamber were removed with a cotton swab. After washed with PBS, the cells adhering membrane were fixed in 4% paraformaldehyde for 30 min. Then stained with 0.1% crystal violet for 20 min. After washed with PBS, five visual fields were selected random using microscope and took pictures to observe the count. The migration experiment procedure is similar to the invasion assay, and the matrigel was not required to be paved in transwell chamber and the number of cells was 5000. The assay was performed in triplicate.
Cell apoptosis assay
After CCRF-CEM cells were treated with MF for 24 h, the medium was removed and replaced with serum-free medium. After starvation under conventional conditions for 24 h, cells were harvested, digested with trypsin digestion without EDTA, collected in a centrifuge tube, centrifuged at 1000 rpm for 5 min, resuspended in pre-cooling PBS at 4°C, and the cells were centrifuged once again and the supernatant was carefully aspirated. Added 1× binding buffer to resuspend the cells, and regulated the cell density to 1–5 × 106/ml. 100 µl cell suspension was transferred into 5 ml flow tube, first staining with 5 µl Annexin V/FITC (Beijing 4A Biotech, China) for 5 min at room temperature keep in a dark place. Then staining with 10 µl Propidium Iodide (PI) and 400 µl PBS, cells were collected and detected using a flow cytometry. Results are analyzed by Flowjo software. The assay was performed in triplicate.
Statistical analysis
The experimental data were analyzed by SPSS18.0 statistical analysis software. The dates are expressed as the Mean ± SD. The t-test was used to compare between two groups. p < 0.05 was considered as statistically significant difference.
Results
MF inhibits CCRF-CEM cells proliferation
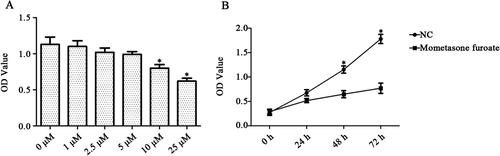
To evaluate the effect of MF on ALL, we selected CCRF-CEM cells and used the concentration of 1, 2.5, 5, 10, 25, 50, 100, 200 nM MF to detect the cell proliferation first. The OD value of CCRF-CEM cells was reduced significantly in various concentrations of MF, compared with the control groups (p < 0.05). And it showed that the cell viability was the lowest at 25 nM MF treatment (p < 0.01, (A)). In this experiment, the cells were cultured for 24, 48 and 72 h. The results indicated that 25 nM MF treatments obviously reduced the number of CCRF-CEM cells at 48 and 72 h (p < 0.05, p < 0.05, (B)). The above results indicated that MF could inhibit CCRF-CEM cells proliferation effectively.
Figure 1. The CCRF-CEM cells treated with different concentrations of MF by the CCK-8 assay. The results indicated that MF could inhibit CCRF-CEM proliferation in dose and time-dependent manner. (A) The cell viability was the lowest at 25 µM MF treatment. (B) 25 µM MF treatment obviously reduced the number of CCRF-CEM cells at 48 h and 72 h. *p < 0.05 compared with control group.
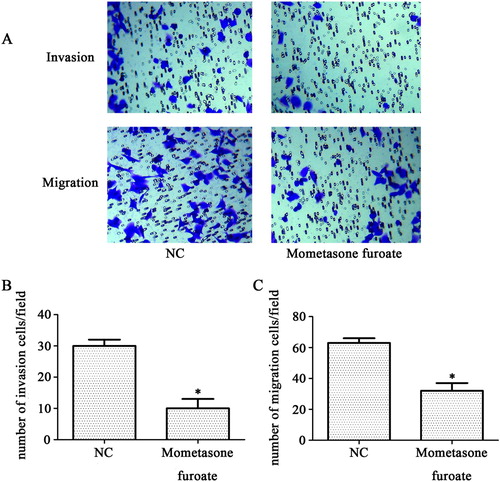
MF inhibits CCRF-CEM cells invasion and migration
We then analyzed the effects of MF on the invasion and migration ability of T-ALL cell CCRF-CEM by a transwell assay. The positive cells of crystal violet staining were reduced by MF treatment in the two experiments. The invaded cell numbers in MF expressing group were less than control group (10 ± 3 < 30 ± 2), showing a negative effect of MF downregulation on the invasion ability of CCRF-CEM cells (p < 0.05, (A,B)). The capacity of migration was also inhibited (32 ± 5 < 63 ± 3), indicating that the experimental results were significantly different (p < 0.05, (A,C)). These results suggested that MF could inhibit invasion and migration of CCRF-CEM cells significantly.
Figure 2. MF inhibits CCRF-CEM cell invasion and migration. (A) The images represent that the invasive and migrated CCRF-CEM cells are stained by crystal violet. Images were captured using an inverted microscope with ×100 magnification. (B,C) The invasion and migration cells are showed by quantification. *p < 0.05 compared with control group.
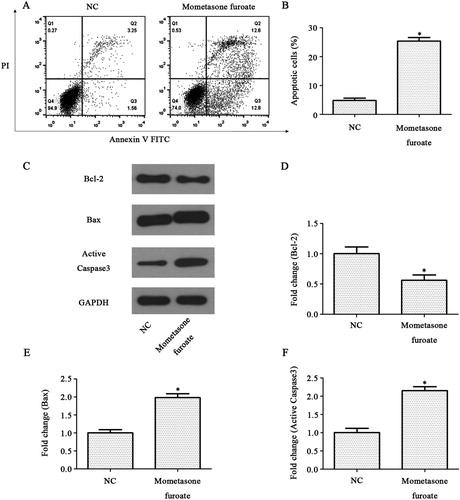
MF promotes CCRF-CEM cells apoptosis
The impact of MF on CCRF-CEM cells apoptosis was determined by an Annexin V-FITC and PI double staining assay. The apoptosis rate in MF treated group was 25.4 ± 0.5% while the apoptosis rate in control group was 4.81 ± 0.3%. These results suggested that the apoptosis rate in the MF treated group was significantly increased compared with the control group (p < 0.05, (A)). Furthermore, the apoptosis regulators such as anti-apoptotic protein Bcl-2, pro-apoptotic protein Active Caspase3 and Bax were analyzed by western blot ((B)). The expression of anti-apoptotic protein Bcl-2 decreased, and the expression of pro-apoptotic protein Active Caspase3 and Bax increased in the MF treated group compared with the control group (p < 0.05, (C–E)). These results indicated that MF could promote CCRF-CEM cell apoptosis.
Figure 3. MF induces CCRF-CEM cell apoptosis. (A,B) Analyze of CCRF-CEM cells (treated with MF 24 h) apoptosis by Annexin V/FITC and PI, showing by representative flow charts (A), and by quantification (B). (C) Western blot analysis of CCRF-CEM cells treated with MF for 24 h. The band intensities were quantified. The results were normalized to the GAPDH loading control. (D–F) Relative protein levels of apoptosis-related protein. *p < 0.05 compared with control group.
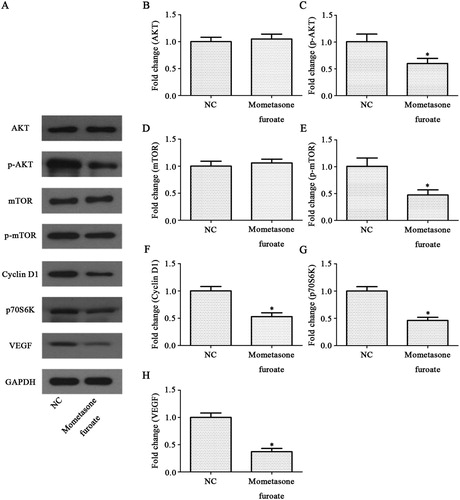
MF suppresses the PI3K pathway in CCRF-CEM cells
PI3K signaling pathway is a very important signal pathway in the tumor. AKT, mTOR, p70S6K, Cyclin D1 and vascular endothelial growth factor (VEGF) proteins, as the indicator to evaluate the activity of PI3K signaling pathway after MF treatment, were analyzed by western blot ((A)). The results showed that the phosphorylation levels of AKT and mTOR decreased significantly in MF treated group (p < 0.05, p < 0.05 (B–E)). Similarly, the expression level of p70S6K, Cyclin D1 and VEGF decreased after MF treatment (p < 0.05, p < 0.05 and p < 0.05, respectively (F–H)). These results suggested MF-induced CCRF-CEM cell growth is suppressed through PI3K pathway.
Figure 4. Effects of MF on the PI3K signaling pathway in CCRF-CEM cells. (A) Expression levels of AKT, p-AKT, mTOR, p-mTOR, p70S6K, Cyclin D1 and VEGF were measured in the CCRF-CEM cells treatment with MF using western blot analysis. (B–H) The relative protein levels of AKT, p-AKT, mTOR, p-mTOR, p70S6 K, Cyclin D1 and VEGF compared with control group. *p < 0.05 compared with control group.
Discussion
In our report, MF, an efficient glucocorticoid, was conducted a preliminary discussion in anti-tumor effect and potential molecular mechanism. Specifically, we identified that MF could inhibit the CCRF-CEM cell proliferation, invasion and migration. Meanwhile, we showed that cell apoptosis was significantly promoted by MF. Finally, we proved that the effects of MF on CCRF-CEM might be related to the inhibition of PI3K signal pathway.
The main role of glucocorticoids in cancer is for the treatment of lymphoid malignancies [Citation13]. Studies have reported that some synthetic glucocorticoids could induce cell cycle arrest and apoptosis in many T-lymphoma cell lines and is therefore included in modern regimens for chemotherapies such as T-ALL lymphoma malignancies [Citation19]. From the treatment of childhood leukemia, most of the glucocorticoids can be understood as chemotherapeutic agents, as well as many other cytotoxic agents [Citation20]. Glucocorticoids can be used in three stages (remission induction, intensification (consolidation) and maintenance) of childhood leukemia, but they are best used during remission induction, with the goal of eliminating more than 99% of the diseased tissue (minimal residual disease) [Citation21]. MF is a synthetic glucocorticoid that induces the synthesis of anti-inflammatory factors as well as the role of immunosuppression [Citation16]. It has minimal side effects. MF can effectively inhibit a variety of inflammatory cell migration, secretion and aggregation, but also can reduce vascular permeability and the sensitivity of receptor stimulation [Citation22]. In recent years, the studies on prednisone steroids are relatively small, probably because of its relatively large side effects [Citation23]. It has been reported in patients with an asthma attack, if taking steroids prednisone excess, they may show weakness in both lower extremity skeletal muscle, increased pain and creatine kinase, and inflammatory changes of gastrocnemius, consistent with the diagnosis of acute steroid myopathy [Citation24]. Recently, it has reported an increase in the adverse reactions of dexamethasone especially in children [Citation25]. Some studies have reported cases of respiratory arrest due to an anaphylactic shock of dexamethasone, died after rescue failed [Citation26].
In the present study, ALL CCRF-CEM cells were treated with MF in concentration gradients of 1, 2.5, 5, 10, 25, 50, 100, 200 nM. The results of CCK8 proliferation test indicated that MF could inhibit CCRF-CEM proliferation in a dose-dependent manner. ALL leukemia cells metastasize to other organs are the main reasons of ALL recurrence and treatment failure [Citation27]. In this study, we also found that MF could inhibit CCRF-CEM cells invasion and migration. MF is a highly potent glucocorticoid used topically to treat perennial allergic rhinitis and the nasal symptoms of seasonal allergic rhinitis [Citation28]. MF seems to have beneficial side effects characteristics and provide long-term practical advantages [Citation29]. MF administered through a dry powder inhaler can be used to maintain inhaled corticosteroid indicated for the treatment of asthma in children older than 4 years of age [Citation30]. MF contains hexylene glycol, which has antibacterial properties [Citation31]. Hexylene glycol is also a safe and effective method for long-term use in the treatment of chronic recurrent disease [Citation32]. But it has not been reported that the MF was applied to treat leukemia.
In addition, our results also found that MF can affect the expression of apoptosis-related proteins. The results of western blot indicated that the expression of Caspase3 and Bax increased and the expression of Bcl-2 decreased in the MF treated group. Bcl-2 is an anti-apoptotic protein, while Bax and Caspase3 are pro-apoptotic proteins, which three are well-characterized regulators of apoptosis [Citation33]. Apoptosis is an important way of anti-tumor, and a lot of anti-tumor drugs play an important role in cancer through the intervention of apoptosis [Citation34,Citation35]. Glucocorticoids play a wide range of physiological roles, including the induction of lymphocyte apoptosis [Citation36]. Endogenous glucocorticoids form T cell profiles by inadvertently induced apoptosis during thymocyte maturation and antagonism of T cell receptor-induced apoptosis during positive selection [Citation37].
The activation of apoptosis is regulated by multiple signaling pathways, while PI3K is one of the more important signaling pathway among them [Citation38]. Our results of western blot showed that the phosphorylation level of AKT and mTOR was decreased significantly in MF treated CCRF-CEM cells. Similarly, the expression level of p70S6k, CyclinD1 and VEGF were reduced after MF treatment. The rapid cytoplasmic effect of glucocorticoids may help to glucocorticoid-induced apoptosis signaling pathway [Citation35]. PI3K, as one member of the lipid kinases family, plays a central role in regulation of angiogenesis, cell cycle, apoptosis, cellular metabolism, DNA repair and senescence [Citation39]. PI3K, as an intermediate signaling molecule, is well known for its role in the PI3K/AKT/mTOR signaling pathway [Citation40]. PI3K activation plays a central role in tumor cell biology, the key proteins AKT and mTOR in which have important implications on the proliferation and metastasis of cancer cells [Citation41]. Studies have found that p70S6K and CyclinD1 are located downstream of the PI3 K/AKT/mTOR pathway, which are closely related to cell proliferation [Citation42,Citation43]. CyclinD1, as a member of the CyclinD family of proteins, plays a key role in facilitating cell cycle [Citation44]. The expression of CyclinD1 is increased in different kinds of tumor tissues [Citation45]. VEGF is one of the most important angiogenic factors in tumor angiogenesis [Citation46]. Studies have found that Matrix metalloproteinase-2 transcriptional inactivation significantly decreased integrin-αVβ3-mediated and PI3K/AKT-induced VEGF expression, which finally reduced tumor cell-induced angiogenesis [Citation47]. Our reports were consistent with these studies, indicating that MF might target key components of the PI3K pathway, which may be important in the successful treatment of ALL.
In summary, we showed that MF could inhibit ALL cells proliferation, invasion and migration and promote cell apoptosis might through PI3K signal pathway. Our study may provide a prospect for further clinical research of MF in ALL treatment. However, for the mechanism of MF in ALL, we conducted a preliminary study. Further verification is considered to perform, such as the in vivo experimental verification and the impact of other signaling pathways et al.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Deqiang Gong http://orcid.org/0000-0001-6086-5610
References
- Luo R, Greenberg A, Stone CD. Outcomes of clostridium difficile infection in hospitalized leukemia patients: a nationwide analysis. Infect Control Hosp Epidemiol. 2015;36(7):794–801. [PubMed: 25801085]. doi: 10.1017/ice.2015.54
- Madhusoodhan PP, Carroll WL, Bhatla T. Progress and prospects in pediatric leukemia. Curr Probl Pediatr Adolesc Health Care. 2016;46(7):229–241. [PubMed: 27283082]. doi: 10.1016/j.cppeds.2016.04.003
- Brethon B, Cave H, Fahd M, et al. [Infant acute leukemia]. Bull Cancer. 2016;103(3):299–311. [PubMed: 26826739]. doi: 10.1016/j.bulcan.2015.11.009
- Rudant J, Lightfoot T, Urayama KY, et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: a childhood leukemia international consortium study. Am J Epidemiol. 2015;181(8):549–562. [PubMed: 25731888]. doi: 10.1093/aje/kwu298
- Lopez-Lopez E, Gutierrez-Camino A, Bilbao-Aldaiturriaga N, et al. Pharmacogenetics of childhood acute lymphoblastic leukemia. Pharmacogenomics. 2014;15(10):1383–1398. [PubMed: 25155938]. doi: 10.2217/pgs.14.106
- Rowe JM. Reasons for optimism in the therapy of acute leukemia. Best Pract Res Clin Haematol. 2015;28(2-3):69–72. PubMed: 26590761]. doi: 10.1016/j.beha.2015.10.002
- Teuffel O, Kuster SP, Hunger SP, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia. 2011;25(8):1232–1238. [PubMed: 21527934]. doi: 10.1038/leu.2011.84
- Lanfranconi F, Pollastri L, Ferri A, et al. Near infrared spectroscopy (NIRS) as a new non-invasive tool to detect oxidative skeletal muscle impairment in children survived to acute lymphoblastic leukaemia. PLoS One. 2014;9(6):e99282. [PubMed: 24956391]. doi: 10.1371/journal.pone.0099282
- Montgomery B, Cheng HH, Drechsler J, et al. Glucocorticoids and prostate cancer treatment: friend or foe? Asian J Androl. 2014;16(3):354–358. [PubMed: 24625881]. doi: 10.4103/1008-682X.125392
- Luhder F, Reichardt HM. Novel drug delivery systems tailored for improved administration of glucocorticoids. Int J Mol Sci. 2017;18(9):1836–1836. [PubMed: 28837059]. doi: 10.3390/ijms18091836
- Ostenfeld EB, Erichsen R, Baron JA, et al. Preadmission glucocorticoid use and anastomotic leakage after colon and rectal cancer resections: a Danish cohort study. BMJ Open. 2015;5(9):e008045. [PubMed: 26408282]. doi: 10.1136/bmjopen-2015-008045
- Pufall MA. Glucocorticoids and cancer. Adv Exp Med Biol. 2015;872:315–333. [PubMed: 5546099]. doi: 10.1007/978-1-4939-2895-8_14
- Jiang L, Xu L, Xie J, et al. Inhibition of autophagy overcomes glucocorticoid resistance in lymphoid malignant cells. Cancer Biol Ther. 2015;16(3):466–476. [PubMed: 4622576]. doi: 10.1080/15384047.2015.1016658
- Gordijn MS, Rensen N, Gemke RJ, et al. Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database Syst Rev. 2015;(8): CD008727. [PubMed: 26282194]
- Valotis A, Hogger P. Significant receptor affinities of metabolites and a degradation product of mometasone furoate. Respir Res. 2004;5:1325. [PubMed: 15285788]. doi: 10.1186/1465-9921-5-7
- Passali D, Spinosi MC, Crisanti A, et al. Mometasone furoate nasal spray: a systematic review. Multidiscip Respir Med. 2016;11:633, [PubMed: 27141307]. doi: 10.1186/s40248-016-0054-3
- Kim DH, Lee HJ, Park CW, et al. The clinical efficacy of mometasone furoate in multi-lamellar emulsion for eczema: A double-blinded crossover study. Ann Dermatol. 2013;25(1):17–22. [PubMed: 23467551]. doi: 10.5021/ad.2013.25.1.17
- Meltzer EO, Baena-Cagnani CE, Gates D, et al. Relieving nasal congestion in children with seasonal and perennial allergic rhinitis: efficacy and safety studies of mometasone furoate nasal spray. World Allergy Organ J. 2013;6(1):1. [PubMed: 23663488]. doi: 10.1186/1939-4551-6-5
- Silveira AB, Laranjeira AB, Rodrigues GO, et al. PI3K inhibition synergizes with glucocorticoids but antagonizes with methotrexate in T-cell acute lymphoblastic leukemia. Oncotarget. 2015;6(15):13105–13118. [PubMed: 25869207]. doi: 10.18632/oncotarget.3524
- Xue L, Li C, Wang Y, et al. Single nucleotide polymorphisms in non-coding region of the glucocorticoid receptor gene and prednisone response in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(6):1704–1709. [PubMed: 25644744]. doi: 10.3109/10428194.2014.951848
- Liang YN, Tang YL, Ke ZY, et al. MiR-124 contributes to glucocorticoid resistance in acute lymphoblastic leukemia by promoting proliferation, inhibiting apoptosis and targeting the glucocorticoid receptor. J Steroid Biochem Mol Biol. 2017;172:62–68. [PubMed: 28578002]. doi: 10.1016/j.jsbmb.2017.05.014
- Rodriguez-Martinez CE, Sossa-Briceno MP, Vladimir Lemos E. Cost-effectiveness analysis of mometasone furoate versus beclomethasone dipropionate for the treatment of pediatric allergic rhinitis in Colombia. Adv Ther. 2015;32(3):254–269. doi: 10.1007/s12325-015-0192-6
- Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842–848. doi: 10.3324/haematol.2014.118471
- Krasselt M, Baerwald C. Efficacy and safety of modified-release prednisone in patients with rheumatoid arthritis. Drug Des Devel Ther. 2016;10:1047–1058. doi: 10.2147/DDDT.S87792
- Ramsey LB, Janke LJ, Payton MAet al. Antileukemic efficacy of continuous vs discontinuous dexamethasone in murine models of acute lymphoblastic leukemia. PloS one. 2015;10(8):e0135134. doi: 10.1371/journal.pone.0135134
- Zeng M, Li ZY, Ma J, et al. Clarithromycin and dexamethasone show similar anti-inflammatory effects on distinct phenotypic chronic rhinosinusitis: an explant model study. BMC Immunol.. 2015;16:1. doi: 10.1186/s12865-015-0096-x
- Deng X, Tu Z, Xiong M, et al. Wnt5a and CCL25 promote adult T-cell acute lymphoblastic leukemia cell migration, invasion and metastasis. Oncotarget. 2017;8(24):39033–39047. [PubMed: 28380463].
- Calverley PM, Rennard S, Nelson HS, et al. One-year treatment with mometasone furoate in chronic obstructive pulmonary disease. Respir Res. 2008;9:765. [PubMed: 19014549]. doi: 10.1186/1465-9921-9-73
- D’Urzo A, Karpel JP, Busse WW, et al. Efficacy and safety of mometasone furoate administered once-daily in the evening in patients with persistent asthma dependent on inhaled corticosteroids. Curr Med Res Opin. 2005;21(8):1281–1289. [PubMed: 16083538]. doi: 10.1185/030079905X56402
- Bijsmans IT, Guercini C, Ramos Pittol JM, et al. The glucocorticoid mometasone furoate is a novel FXR ligand that decreases inflammatory but not metabolic gene expression. Sci Rep. 2015;5:710. [PubMed: 4572934]. doi: 10.1038/srep14086
- Corazza M, Virgili A, Toni G, et al. Mometasone furoate in the treatment of vulvar lichen sclerosus: could its formulation influence efficacy, tolerability and adherence to treatment? J Dermatolog Treat. 2017;4:1–5. [PubMed: 28753097].
- Greive KA, Barnes TM. Bioequivalence of 0.1% mometasone furoate lotion to 0.1% mometasone furoate hydrogel. Australas J Dermatol. 2016;57(2):e39–e45. [PubMed: 25545549]. doi: 10.1111/ajd.12275
- Mei JM, Niu CS. Effects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol Sci. 2014;35(8):1275–1280. [PubMed: 24633814]. doi: 10.1007/s10072-014-1700-1
- Zhang G, Zhang Z, Yang J. DNA tetrahedron delivery enhances doxorubicin-induced apoptosis of HT-29 colon cancer cells. Nanoscale Res Lett. 2017;12(1):717. [PubMed: 28812246].
- Cirillo N, Morgan DJ, Pedicillo MC, et al. Characterisation of the cancer-associated glucocorticoid system: key role of 11beta-hydroxysteroid dehydrogenase type 2. Br J Cancer. 2017;117(7):984–993. [PubMed: 28797028]. doi: 10.1038/bjc.2017.243
- Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1–30. [PubMed: 20541659]. doi: 10.1016/S0079-6123(10)82001-1
- Harr MW, Rong Y, Bootman MD, et al. Glucocorticoid-mediated inhibition of Lck modulates the pattern of T cell receptor-induced calcium signals by down-regulating inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284(46):31860–31871. [PubMed: 19776014]. doi: 10.1074/jbc.M109.005579
- Zhou ZW, Li XX, He ZX, et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des Devel Ther. 2015;9:1511–1554. [PubMed: 25834399]. doi: 10.2147/DDDT.S75976
- Akinleye A, Avvaru P, Furqan M, et al. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6(1):88. [PubMed: 24261963]. doi: 10.1186/1756-8722-6-88
- Khan KH, Wong M, Rihawi K, et al. Hyperglycemia and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) inhibitors in phase I trials: incidence, predictive factors, and management. Oncologist. 2016;21(7):855–860. [PubMed: 27151652]. doi: 10.1634/theoncologist.2015-0248
- Zhang L, Huo X, Liao Y, et al. Zeylenone, a naturally occurring cyclohexene oxide, inhibits proliferation and induces apoptosis in cervical carcinoma cells via PI3K/AKT/mTOR and MAPK/ERK pathways. Sci Rep. 2017;7(1):58. [PubMed: 28490807]. doi: 10.1038/s41598-017-00100-3
- Halacli SO, Dogan AL. FOXP1 regulation via the PI3K/Akt/p70S6K signaling pathway in breast cancer cells. Oncol Lett. 2015;9(3):1482–1488. [PubMed: 25663935]. doi: 10.3892/ol.2015.2885
- Liu W, Ren H, Ren J, et al. The role of EGFR/PI3 K/Akt/cyclinD1 signaling pathway in acquired middle ear cholesteatoma. Mediators Inflamm. 2013;2013:651207. [PubMed: 24311896].
- Zhao S, Yi M, Yuan Y, et al. Expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in esophageal cancer and their association with the clinical and pathological parameters. Int J Clin Exp Med. 2015;8(5):7324–7332. [PubMed: 26221272].
- Cheng R, Liu YJ, Cui JW, et al. Aspirin regulation of c-myc and cyclinD1 proteins to overcome tamoxifen resistance in estrogen receptor-positive breast cancer cells. Oncotarget. 2017;8(18):30252–30264. [PubMed: 28415819].
- Zadeh MA H, Amin EM, Hoareau-Aveilla C, et al. Alternative splicing of TIA-1 in human colon cancer regulates VEGF isoform expression, angiogenesis, tumour growth and bevacizumab resistance. Mol Oncol. 2015;9(1):167–178. [PubMed: 25224594]. doi: 10.1016/j.molonc.2014.07.017
- Chetty C, Lakka SS, Bhoopathi P, et al. MMP-2 alters VEGF expression via αVβ3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127(5):1081–1095. [PubMed: 20027628]. doi: 10.1002/ijc.25134
