Abstract
Social defeat stress affects behavior and changes the expression of the genes underlying neuronal plasticity in the brain. The circadian clock regulates most neuronal processes in the brain, which results in daily variations of complex behavior, and any disturbance in circadian clock oscillations increases the risk of mood and cognitive disbalance. In this study, we assessed the effect of acute and repeated social defeat stress on Per2 and Nr1d1 expression in prefrontal cortexes, hippocampi, pineal glands, olfactory bulbs, cerebella, and pituitary glands. We also evaluated the effect of our experimental setting on levels of Bdnf and plasma corticosterone, two markers widely used to asses the impact of stress on mammalian physiology. Our data show that single and repeated social defeat stress upregulates the expression of both clock genes and Bdnf in all brain structures, and corticosterone in the blood. While the general pattern of Bdnf upregulation suggests higher sensitivity in the intruder group, the clock genes are induced more significantly in residents, especially by repeated stress sessions. Our work thus suggests that the model of stress-induced anxiety and depression should consider a group of residents because, for some parameters, they may respond more distinctively than intruders.
The resident/intruder experimental paradigm affects the expression of clock genes Per2, Nr1d1and Bdnf in the brain structures and plasma corticosterone level. The induction of clock genes is evident in both experimental groups; however, it is more marked in residents. Together with the significant increase in Bdnf levels in the majority of brain structures and plasma corticosterone in residents, our data suggest that in the model of social defeat stress, the utility of an experimental group of residents could be contributive.
LAY SUMMARY
Introduction
Acute and repeated social defeat stress is a widely used experimental model to study the extent to which stress is involved in the development of anxiety and depression. The pathophysiology of this process is complex, and it includes morphological and functional changes in many brain regions, such as the prefrontal cortex, hippocampus, olfactory bulb, and cerebellum(Moreno-Rius, Citation2019; Qiao et al., Citation2016; Yuan & Slotnick, Citation2014). Stress-related morphologic alterations were observed also in the pituitary gland and pineal gland, two structures involved in the neuroendocrine regulation of physiological stress responses(Cooper et al., Citation2017; Dmitrzak-Weglarz & Reszka, Citation2017; Milin, Citation1998; Milin et al., Citation1996). On a molecular level, the effect of stress is most frequently studied in association with the synaptic structural and functional rearrangements underlying neuronal plasticity, in which local changes in the level of brain-derived neurotrophic factor (Bdnf) play an essential role (Berton et al., Citation2006; Colyn et al., Citation2019; Koo et al., Citation2019).
Most cellular and organismal processes in the body oscillate according to the circadian period originating from the autoregulatory feedback loops of clock genes, which are rhythmically expressed in the majority of the cells in the body, including brain tissue (Albrecht, Citation2012) This cellular clockwork receives time information from the circadian pacemaker in the suprachiasmatic nucleus of the hypothalamus; however, at the local level, it can be affected also by tissue-specific signals (Tahara & Shibata, Citation2018). For example, the clock gene Per2 can be induced by dopaminergic signaling (Natsubori et al., Citation2014)or by glucocorticoids (Cheon et al., Citation2013)and cytokines (Yoshida et al., Citation2013). Clock genes play a significant role in the regulation of complex behavioral responses to stress stimuli (Dong et al., Citation2011; Spencer et al., Citation2013), and individuals with disrupted circadian systems have a higher risk of depression and anxiety (Ben-Hamo et al., Citation2017; Landgraf et al., Citation2016; Roybal et al., Citation2007). Neuronal processes underlying the proper function of neuronal circuitries and synaptic plasticity exhibit circadian rhythmicity on various levels, including the transcriptional control of genes encoding membrane receptors, ion channels, glutamate transporters, vesicle proteins and neurotrophic factors, such as Bdnf (Bendová et al., Citation2009, Citation2012; Bova et al., Citation1998; Chi-Castañeda & Ortega, Citation2018; Pizarro et al., Citation2012).
The goal of the present study was to depict the effect of social defeat stress on the expression of clock genes in the brain. We also evaluated changes in the Bdnf mRNA in the brain and the corticosterone level in plasma, two markers often used to assess the effect of social stress on the brain and systemic physiology (Vasconcelos et al., Citation2015).
Materials and methods
Animals
Male Wistar rats (Velaz, Ltd., Koleč, Czech Republic) were maintained under the 12/12 h light-dark regime, at a temperature of 23 ± 2 °C, with free access to food and water for 2 weeks before the experiment commenced. The illumination was 100 lux on the cage position. All experiments were conducted in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised in 1996), the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, the European Communities Council Directive of 24 November 1986 (86/609/EEC), and the Animal Protection Law of the Czech Republic (license no. MSMT-23852/2014-14).
Experimental design
Rats were subjected to acute (one test) or repeated (four repetitions, one per day) social defeat stress, using a resident-intruder model, adapted from (Vodička et al., Citation2014). Twelve 6-month-old males (residents) were individually housed in standard polycarbonate cages for 2 weeks before the start of the experiment. Twenty four 3-month-old subjects (controls and intruders) were housed in groups of four since their weaning in a different room. For the defeat stress session, 12 intruder rats were individually introduced into the home cage of an isolated resident for 30 min at ZT5 (i.e. 5 h after the lights came on). During this exposure, all intruder rats showed signs of subordination. At the same time, 12 control animals were handled in a different room. After the session, the resident rats remained isolated in their home cages, while the intruders were returned to their respective groups. Six intruders were euthanized 1 h later, together with six residents and six control animals. Another six intruder animals were subjected to repeated defeat stress for four consecutive days (each day in different combinations of resident/intruder pair to avoid social adaptation) and sacrificed 1 h after the last treatment together with residents and control, handled, rats.
Quantitative Real-Time PCR (qRT-PCR)
Prefrontal cortexes, hippocampi, pineal glands, olfactory bulbs, cerebella, and pituitary glands were immediately dissected and homogenized in RNAzol RT (Molecular Research Center). Total RNA was extracted, using Direct-zolTM RNA MiniPrep (Zymo Research), in accordance with the manufacturer’s instructions. Total RNA (1 mg) was converted to cDNA using the one-step SuperScriptTM VILOTM cDNA Synthesis Kit(Invitrogen) according to the manufacturer’s instructions. Samples of cDNA (1 μl) were amplified in 20 μl of PCR reaction mixture, containing 5x HOT FIREPol Probe qPCRMix Plus (Baria) plus TaqMan probes, Per2 (Rn01427704_m1), Nr1d1 (Rn01460662_m1), and Bdnf (Rn02531967_s1) (Life Technologies, Waltham, MA). All qPCR reactions were performed in triplicate in a LightCycler 480 Instrument (Roche Life Science, Indianapolis, IN), as described before (Kubová et al., Citation2018). The mean of the crossing point (Cp) obtained from qPCR was normalized to the level of the housekeeping gene Actb(Rn00667869_m1) and used for the analysis of relative gene expression by the ΔΔCT method.
Measurement of corticosterone levels
Rats were decapitated, and trunk blood samples were treated with heparin and centrifuged for 5 min (5000 rpm at 4 °C). Plasma was stored at −80 °C until use. Levels of corticosterone were assessed with competitive ELISA, using a commercial ELISA kit (ab108821, Abcam, Cambridge, UK), according to the manufacturer’s instructions. All samples were run in duplicate. The assay sensitivity was 0.28 ng/ml, the intra-assay coefficient of variation was 4.1% and inter-assay 10.1%.
Data analysis and statistical procedures
Mean values of gene expression in the controls at each time were considered as a constant, and values of intruders and residents were plotted as percent difference from this constant. For statistics, all control values were counted as percent distribution around the mean and compared with treatment groups. All data were analyzed by two-way ANOVA, followed by Tukey’s post-hoc for single comparisons of mRNA levels and plasma levels of corticosterone. The significance level was set at p < 0.05. Data are expressed as means ± SEM of six animals.
Results
The effect of defeat stress on Per2, Nr1d1, and Bdnf expression
Samples of brain tissues obtained from the intruders, residents, and control rats were subjected to qPCR to detect the levels of Per2, Nr1d1, and Bdnf. The values obtained from controls were considered as zero levels and the expressions in both experimental groups were evaluated in terms of percent difference from this default level. No significant difference has been shown between control groups in single and repeated sessions in any sampled brain tissue.
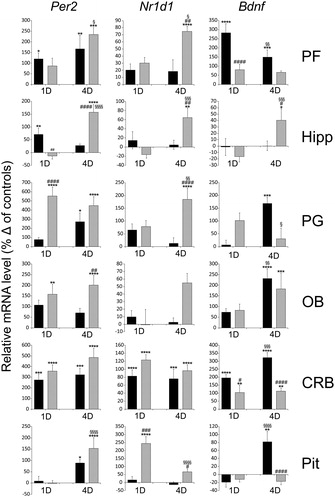
Social defeat stress induced the expression of clock genes and Bdnf in all structures studied (). The difference between groups in Per2 level was significant in all brain structures (Supplemental Table 1), and the difference between single and repeated sessions were significant in prefrontal cortex, hippocampus, cerebellum and pituitary. The difference between groups in Nr1d1 level was significant in prefrontal cortex, pineal gland, olfactory bulbs, cerebellum, pituitary, and the difference between single and repeated sessions were significant in hippocampus and pituitary. The difference between groups in Bdnf level was significant in prefrontal cortex, pineal gland, olfactory bulbs, cerebellum, pituitary, and the difference between single and repeated sessions was significant in all structures. Besides significant difference from controls (, indicated by “*”), multiple comparisons test revealed the difference in the magnitude of the response between intruders and residents (, indicated by “#”), and between single and repeated session either in the intruders or residents (, indicated by “§”).
Figure 1. The effect of acute (1D) and repeated (4D) resident/intruder stress on the Per2, Nr1d1, and Bdnf expression in the prefrontal cortex (PF), hippocampus (Hipp), pineal gland (PG), olfactory bulbs (OB), cerebellum (CRB) and pituitary gland (Pit). mRNA levels were determined using quantitative RT-PCR, and values of intruders (black columns) and residents (gray columns) were converted to a percentage of the control values, which were considered baseline (zero) levels. Two-way ANOVA with Tukey’s multiple comparisons test: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between control and stressed groups; #p < 0.05; ##p < 0.01; ###p < 0.001; ####p < 0.0001, between intruders and residents; §p < 0.05; §§p < 0.01; §§§p < 0.001; §§§§p < 0.0001, between 1D and 4D sessions. The data are expressed as means ± SEM from six animals.
The effect of social stress on plasma corticosterone levels
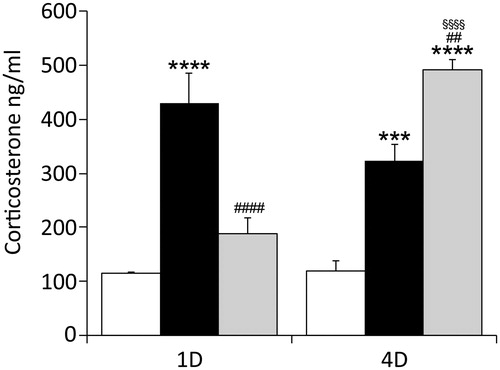
Plasma levels of corticosterone were determined in controls, intruders and residential animals after single sessionsand 4-day sessionsofstress exposure.The two-way ANOVA revealed a significant difference between groups (F(2, 30) = 49.25; p < 0.0001), and sessions (F(1, 30) = 8.265; p = 0.0075), and showed significant group–session interaction (F(2, 30) = 27.80; p < 0.0001). As shown in , Tukey’s multiple comparisons test confirmed that stress significantly increased corticosterone level in intruders after the first and fourth session, and in residents after the fourth session. Furthermore, the magnitude of the response differed significantly between intruders and residents after the first and fourth sessions, as well as between resident after the first and fourth session. No difference has been revealed between controls.
Figure 2. The effect of acute and repeated R/I stress on the plasma corticosterone level. The 30 min exposure to R/I elevated the level of corticosterone within 1 h (1D) in the group of intruders (black columns), compared to controls (white columns). Repeated, 4 day exposure (4D) elevated level in intruders as well as in residents (grey columns). Two-way ANOVA with Tukey’s multiple comparisons test: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between control and stressed groups; #p < 0.05; ##p < 0.01; ###p < 0.001; ####p < 0.0001, between intruders and residents; §p < 0.05; §§p < 0.01; §§§p < 0.001; §§§§p < 0.0001, between 1D and 4D sessions.The data are expressed as means ± SEM from six animals.
Discussion
In the present study, we demonstrate stress-induced changes in the expression of two core clock genes and Bdnf in the rat brain regions implicated in the development of depression and anxiety.From the large set of clock genes, we selected Per2, which can be, in addition to its clock-driven expression, transcriptionally upregulated in the brain by social stress,acting via glucocorticoid signaling (Cheon et al., Citation2013; Christiansen et al., Citation2016). These and our observation are in contrast with the report of Chun et al. (Citation2018), who did not measure any significant Per2 upregulation in the prefrontal cortex after acute restrain stress. A 30 min difference from the onset of the stress session between their and our study, could play a role in the reactivity of Per2 expression. Also, the sensitivity between in situ hybridizations in their study and qPCR may differ. Glucocorticoids canalso upregulate the Nr1d1 gene (Torra et al., Citation2000). This gene encodes nuclear receptor Rev-Erbα, which is thought to link metabolic and immune signals within the cell with the circadian clock (Curtis & Fagundes, Citation2016; Sengupta et al., Citation2016), and,on a systemic level, to be involved in mood and sleep regulation (Chung et al., Citation2014; Mang et al., Citation2016). Both genes are part of the clockwork mechanism and their induction in a phase that is distinct from clock-dictated circadian time may significantly affect the phase of the circadian clock and clock-driven cellular functions.
The transcription of Per2, Nr1d1, and Bdnf genes exhibit circadian oscillations (Bova et al., Citation1998; Schaaf et al., Citation2000; Yi et al., Citation2015), soits monitoring only at a singletime point cannot reveal changes in such parameters as amplitude or circadian rhythm phase. However, our data show that the magnitude of clock gene induction is large, ranging from50 to 500% in various structures. This relatively significant change in mRNA levels suggests that the clockwork mechanism may indeed be affected, clock-driven processes within the cells may also be influenced subsequently, although data on protein levels are needed to confirm this assumption.
With some exceptions (Bohus et al., Citation1992; Manz et al., Citation2018; Vodička et al., Citation2014), the majority of studies focused on the effect of social defeat stress on brain physiology omit the group of aggressors or residents, highlighting only the data obtained from the comparison between intruders and unstressed controls. In our study, we revealed stress-induced upregulation of clock genes expression in most of the intruders’ brain structures. However, our data show thatthe resident’s clock gene transcription is affected as well, and to an even greater extent than that of the intruder. A few publications make referenceto similar results, although only in the context of usingdistinct markers; after social defeat stress, the limbic structures of residents exhibited a higher level of enzymes involved in steroid metabolism (Vodička et al., Citation2014). An interesting observation was published by Bohus et al. (Citation1992), who demonstrated that resident Wistar rats have a significantly shorter survival time after tumor cell inoculation, relative to intruders.
At 3 months of age, rats express fully matured, high-amplitude circadian rhythmicity in clock gene expression as well as in monoamine levels in various brain structures (Asai et al., Citation2001; Kolker et al., Citation2004; Radha et al., Citation1985; Wyse & Coogan, Citation2010). Aging is generally associated with a weakening of the circadian system and amplitude reduction, although between 3 and 12 months of age, the pattern of circadian rhythms is rather similar (Asai et al., Citation2001; Kolker et al., Citation2004). Therefore, we do not expect the induction of the resident’s clock gene transcription to be the result of age differences from the intruders. If the 3-month age difference plays the role, the effect in residents would be somewhat diminished than enhanced.
Bycontrast to clock genes, Bdnf mRNA was induced more in the brains of intruders but was significantly upregulated in most of the brain structures, except for the pituitary and pineal glands, in residents as well. The stress-induced changes in Bdnf expression in intruders’ brains have previously been described, and our results do not contradict previous observations (Bland et al., Citation2007; Colyn et al., Citation2019; Fanous et al., Citation2010; Mallei et al., Citation2018; Murinova et al., Citation2017). The hippocampus is thought to mediate the cognitive aspects of anxiety or depression and is one of the most intensively studied brain structures, with respect to stress-induced behavioral plasticity. We observed no significant change in Bdnf mRNA in the hippocampi of intruders but revealed ∼40% increase in residents after four sessions. Although it may appear to disprove the role of Bdnf in the process of neuroplastic changes, other studies provide similar data and report either a slight decrease of Bdnf transcript in the hippocampus of intruders along with some increase in aggressive individuals (Mallei et al., Citation2018; Patki et al., Citation2013), or no change in Bdnfat the protein level (Razzoli et al., Citation2011). A significant increase of residents’ Bdnf in most of the structures indicates that this group should not be omitted from the assessment of the impact of social stress on molecular processes in the brain.
Corticosterone levels were significantly induced in the intruder group, which is consistent with the findings ofseveral previous studies (Ergang et al., Citation2018; Niraula et al., Citation2018; Norman et al., Citation2015). After the 4-day session, its levels weres ignificantly higher also among residents. The significant change in residents’ corticosterone levels, between single sessions and repeated stress sessions, correlates with the general pattern of clock gene induction in structures, such as that prefrontal cortex, hippocampus,pineal gland or pituitary. This may signify increased sensitivity to the repeated challenges of their social dominance; however, we cannot exclude the possibility that, after 4 days of stress insults, the phase of clock genes and corticosterone rhythms had changed, which would mean that this difference reflects the distinct points in the circadian time between the first and fourth days.
Conclusions
Our data demonstrate that the resident/intruder paradigm affects the expression of clock genes and Bdnf in the brain structures involved in behavioral and endocrine responses to stress. The induction of clock genes is evident in both experimental groups; however, it is more marked in residents. Together with the significant increase in Bdnf levels in the majority of brain structures and plasma corticosterone in residents, our results suggest that, in the model of social defeat stress-induced neuroplasticity, the utility of an experimental group of residents could be contributive.
supplemental_Table_1.docx
Download MS Word (17.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Albrecht, U. (2012). Timing to perfection: The biology of central and peripheral circadian clocks. Neuron, 74(2), 246–260. https://doi.org/10.1016/j.neuron.2012.04.006
- Asai, M., Yoshinobu, Y., Kaneko, S., Mori, A., Nikaido, T., Moriya, T., Akiyama, M., & Shibata, S. (2001). Circadian expression of Per gene m RNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. Journal of Neuroscience Research, 66(6), 1133–1139. https://doi.org/10.1002/jnr.10010
- Bendová, Z., Sládek, M., & Svobodová, I. (2012). The expression of NR2B subunit of NMDA receptor in the suprachiasmatic nucleus of Wistar rats and its role in glutamate-induced CREB and ERK1/2 phosphorylation. Neurochemistry International, 61(1), 43–47. https://doi.org/10.1016/j.neuint.2012.04.016
- Bendová, Z., Sumová, A., & Mikkelsen, J. D. (2009). Circadian and developmental regulation of N- methyl- d- aspartate- receptor 1 m RNA splice variants and N- methyl- d- aspartate- receptor 3 subunit expression within the rat suprachiasmatic nucleus. Neuroscience, 159(2), 599–609. https://doi.org/10.1016/j.neuroscience.2009.01.016
- Ben-Hamo, M., Larson, T. A., Duge, L. S., Sikkema, C., Wilkinson, C. W., de la Iglesia, H. O., & González, M. M. (2017). Circadian forced desynchrony of the master clock leads to phenotypic manifestation of depression in rats. e Neuro, 3(6), 1–13.
- Berton, O., Mc Clung, C. A., Dileone, R. J., Krishnan, V., Renthal, W., Russo, S. J., Graham, D., Tsankova, N. M., Bolanos, C. A., Rios, M., Monteggia, L. M., Self, D. W., & Nestler, E. J. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science, 311(5762), 864–868. https://doi.org/10.1126/science.1120972
- Bland, S. T., Tamlyn, J. P., Barrientos, R. M., Greenwood, B. N., Watkins, L. R., Campeau, S., Day, H. E., & Maier, S. F. (2007). Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor m RNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience, 144(4), 1219–1228. https://doi.org/10.1016/j.neuroscience.2006.11.026
- Bohus, B., Koolhaas, J. M., de Ruiter, A. J. H., & Heijnen, C. J. (1992). Psycho-social stress: Differential alteration in immune system function and tumor growth. In R. Kvietnansky, R. Mc Carty, & J. Axelrod (Eds.), Atress: Neuroedocrine and Molecular Approaches (pp. 607–621). S.A. Publishers.
- Bova, R., Micheli, M. R., Qualadrucci, P., & Zucconi, G. G. (1998). BDNF and trk B m RNAs oscillate in rat brain during the light-dark cycle. Molecular Brain Research, 57(2), 321–324. https://doi.org/10.1016/S0169-328X(98)00092-8
- Cheon, S., Park, N., Cho, S., & Kim, K. (2013). Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Research, 41(12), 6161–6174. https://doi.org/10.1093/nar/gkt307
- Chi-Castañeda, D., & Ortega, A. (2018). Circadian regulation of glutamate transporters. Frontiers in Endocrinology, 9, 340. https://doi.org/10.3389/fendo.2018.00340
- Christiansen, S. L., Bouzinova, E. V., Fahrenkrug, J., & Wiborg, O. (2016). Altered expression pattern of clock genes in a rat model of depression. International Journal of Neuropsychopharmacology, 19(11), pyw061. https://doi.org/10.1093/ijnp/pyw061
- Chun, L. E., Christensen, J., Woodruff, E. R., Morton, S. J., Hinds, L. R., & Spencer, R. L. (2018). Adrenal-dependent and -independent stress-induced Per1 m RNA in hypothalamic paraventricular nucleus and prefrontal cortex of male and female rats. Stress, 21(1), 69–83. https://doi.org/10.1080/10253890.2017.1404571
- Chung, S., Lee, E. J., Yun, S., Choe, H. K., Park, S. B., Son, H. J., Kim, K. S., Dluzen, D. E., Lee, I., Hwang, O., Son, G. H., & Kim, K. (2014). Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell, 157(4), 858–868. https://doi.org/10.1016/j.cell.2014.03.039
- Colyn, L., Venzala, E., Marco, S., Perez-Otaño, I., & Tordera, R. M. (2019). Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behavioural Brain Research, 373, 112079. https://doi.org/10.1016/j.bbr.2019.112079
- Cooper, O., Bonert, V., Moser, F., Mirocha, J., & Melmed, S. (2017). Altered pituitary gland structure and function in posttraumatic stress disorder. Journal of the Endocrine Society, 1(6), 577–587. https://doi.org/10.1210/js.2017-00069
- Curtis, A. M., & Fagundes, C. T. (2016). Understanding the role of cellular molecular clocks in controlling the innate immune response. Methods in Molecular Biology, 1390, 301–316. https://doi.org/10.1007/978-1-4939-3335-8_19
- Dmitrzak-Weglarz, M., & Reszka, E. (2017). Pathophysiology of depression: Molecular regulation of melatonin homeostasis - Current status. Neuropsychobiology, 76(3), 117–129. https://doi.org/10.1159/000489470
- Dong, L., Bilbao, A., Laucht, M., Henriksson, R., Yakovleva, T., Ridinger, M., Desrivieres, S., Clarke, T. K., Lourdusamy, A., Smolka, M. N., Cichon, S., Blomeyer, D., Treutlein, J., Perreau-Lenz, S., Witt, S., Leonardi-Essmann, F., Wodarz, N., Zill, P., Soyka, M., … Schumann, G. (2011). Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. The American Journal of Psychiatry, 168(10), 1090–1098. https://doi.org/10.1176/appi.ajp.2011.10111579
- Ergang, P., Mikulecka, A., Vodicka, M., Vagnerova, K., Miksik, I., & Pacha, J. (2018). Social defeat stimulates local glucocorticoid regeneration in lymphoid organs. Endocrine Connections, 7(12), 1389–1396.
- Fanous, S., Hammer, R. P., Jr., & Nikulina, E. M. (2010). Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience, 167(3), 598–607. https://doi.org/10.1016/j.neuroscience.2010.02.064
- Kolker, D. E., Vitaterna, M. H., Fruechte, E. M., Takahashi, J. S., & Turek, F. W. (2004). Effects of age on circadian rhythms are similar in wild-type and heterozygous clock mutant mice. Neurobiology of Aging, 25(4), 517–523. https://doi.org/10.1016/j.neurobiolaging.2003.06.007
- Koo, J. W., Chaudhury, D., Han, M. H., & Nestler, E. J. (2019). Role of mesolimbic brain-derived neurotrophic factor in depression. Biological Psychiatry, 86(10), 738–748. https://doi.org/10.1016/j.biopsych.2019.05.020
- Kubová, H., Bendová, Z., Moravcová, S., Pačesová, D., Rocha, L. L., & Mareš, P. (2018). Neonatal Clonazepam administration induces long-lasting changes in glutamate receptors. Frontiers in Molecular Neuroscience, 11, 382. https://doi.org/10.3389/fnmol.2018.00382
- Landgraf, D., Long, J. E., Proulx, C. D., Barandas, R., Malinow, R., & Welsh, D. K. (2016). Genetic disruption of Circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biological Psychiatry, 80(11), 827–835. https://doi.org/10.1016/j.biopsych.2016.03.1050
- Mallei, A., Ieraci, A., & Popoli, M. (2018). Chronic social defeat stress differentially regulates the expression of BDNF transcripts and epigenetic modifying enzymes in susceptible and resilient mice. World Journal of Biological Psychiatry., 19, 1–12.
- Mang, G. M., La Spada, F., Emmenegger, Y., Chappuis, S., Ripperger, J. A., Albrecht, U., & Franken, P. (2016). Altered sleep homeostasis in Rev-erbα knockout mice. Sleep, 39(3), 589–601. https://doi.org/10.5665/sleep.5534
- Manz, K. M., Levine, W. A., Seckler, J. C., Iskander, A. N., & Reich, C. G. (2018). A novel adolescent chronic social defeat model: Reverse-Resident-Intruder Paradigm (r RIP) in male rats. Stress, 21(2), 169–178. https://doi.org/10.1080/10253890.2017.1423285
- Milin, J. (1998). Stress-reactive response of the gerbil pineal gland: Concretion genesis. General and Comparative Endocrinology, 110(3), 237–251. https://doi.org/10.1006/gcen.1998.7069
- Milin, J., Demajo, M., & Todorovic, V. (1996). Rat pinealocyte reactive response to a long-term stress inducement. Neuroscience, 73(3), 845–854. https://doi.org/10.1016/0306-4522(96)00014-0
- Moreno-Rius, J. (2019). The cerebellum under stress. Frontiers in Neuroendocrinology, 54, 100774. https://doi.org/10.1016/j.yfrne.2019.100774
- Murinova, J., Hlavacova, N., Chmelova, M., & Riecansky, I. (2017). The evidence for altered BDNF expression in the brain of rats reared or housed in social isolation: A systematic review. Frontiers in Behavioral Neuroscience., 11, 101. https://doi.org/10.3389/fnbeh.2017.00101
- Natsubori, A., Honma, K., & Honma, S. (2014). Dual regulation of clock gene Per2 expression in discrete brain areas by the circadian pacemaker and methamphetamine-induced oscillator in rats. The European Journal of Neuroscience, 39(2), 229–240. https://doi.org/10.1111/ejn.12400
- Niraula, A., Wang, Y., Godbout, J. P., & Sheridan, J. F. (2018). Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. The Journal of Neuroscience : The Oscience, 38(9), 2328–2340. https://doi.org/10.1523/JNEUROSCI.2568-17.2018
- Norman, K. J., Seiden, J. A., Klickstein, J. A., Han, X., Hwa, L. S., De Bold, J. F., & Miczek, K. A. (2015). Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology, 232(6), 991–1001. https://doi.org/10.1007/s00213-014-3733-9
- Patki, G., Solanki, N., Atrooz, F., Allam, F., & Salim, S. (2013). Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Research., 1539, 73–86. https://doi.org/10.1016/j.brainres.2013.09.033
- Pizarro, A., Hayer, K., Lahens, N. F., Hogenesch, J. B., & Circa, D. B. (2012). A database of mammalian circadian gene expression pro-files. Nucleic Acids Research, 41(D1), D1009–D1013. https://doi.org/10.1093/nar/gks1161
- Qiao, H., Li, M. X., Xu, C., Chen, H. B., An, S. C., & Ma, X. M. (2016). Dendritic spines in depression: What we learned from animal models. Neural Plasticity, 2016, 8056370. https://doi.org/10.1155/2016/8056370
- Radha, E., Shankaraiah, K., Halberg, F., & Bhaskaran, D. (1985). Developmental, circadian and aging aspects of dopamine, norepinephrine and 5-HT in rat brain regions. In P. H. Redfern, I. Campbell, J. A. Xavier, & K. F. Martin (Eds.), Circadian Rhythms in the Central Nervous System, Proceedings of the IX Conference IUPHAR, SAT Symposium (pp. 199–209). Macmillan.
- Razzoli, M., Domenici, E., Carboni, L., Rantamaki, T., Lindholm, J., Castrén, E., & Arban, R. (2011). A role for BDNF/Trk B signaling in behavioral and physiological consequences of social defeat stress. Genes, Brain and Behavior, 10(4), 424–433. https://doi.org/10.1111/j.1601-183X.2011.00681.x
- Roybal, K., Theobold, D., Graham, A., Di Nieri, J. A., Russo, S. J., Krishnan, V., Chakravarty, S., Peevey, J., Oehrlein, N., Birnbaum, S., Vitaterna, M. H., Orsulak, P., Takahashi, J. S., Nestler, E. J., Carlezon, W. A., Jr., & Mc Clung, C. A. (2007). Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America, 104(15), 6406–6411. https://doi.org/10.1073/pnas.0609625104
- Schaaf, M. J., Duurland, R., de Kloet, E. R., & Vreugdenhil, E. (2000). Circadian variation in BDNF m RNA expression in the rat hippocampus. Molecular Brain Research, 75(2), 342–344. https://doi.org/10.1016/S0169-328X(99)00314-9
- Sengupta, S., Yang, G., O’Donnell, J. C., Hinson, M. D., McCormack, S. E., Falk, M. J., La, P., Robinson, M. B., Williams, M. L., Yohannes, M. T., Polyak, E., Nakamaru-Ogiso, E., & Dennery, P. A. (2016). The circadian gene Rev-erbα improves cellular bioenergetics and provides preconditioning for protection against oxidative stress. Free Radical Biology & Medicine, 93, 177–189. https://doi.org/10.1016/j.freeradbiomed.2016.02.004
- Spencer, S., Falcon, E., Kumar, J., Krishnan, V., Mukherjee, S., Birnbaum, S. G., & Mc Clung, C. A. (2013). Circadian genes period 1 and period 2 in the nucleus accumbens regulate anxiety-related behavior. The European Journal of Neuroscience, 37(2), 242–250. https://doi.org/10.1111/ejn.12010
- Tahara, Y., & Shibata, S. (2018). Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition, Free Radic. Free Radical Biology & Medicine, 119, 129–138. https://doi.org/10.1016/j.freeradbiomed.2017.12.026
- Torra, I. P., Tsibulsky, V., Delaunay, F., Saladin, R., Laudet, V., Fruchart, J. C., Kosykh, V., & Staels, B. (2000). Circadian and glucocorticoid regulation of Rev-erbalpha expression in liver. Endocrinology, 141(10), 3799–3806. https://doi.org/10.1210/endo.141.10.7708
- Vasconcelos, M., Stein, D. J., & de Almeida, R. M. (2015). Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: A systematic review of the last decade. Trends in Psychiatry and Psychotherapy, 37(2), 51–66. https://doi.org/10.1590/2237-6089-2014-0034
- Vodička, M., Ergang, P., Mikulecká, A., Řeháková, L., Klusoňová, P., Makal, J., Soták, M., Musílková, J., Zach, P., & Pácha, J. (2014). Regulation of 11β-hydroxysteroid dehydrogenase type 1 and 7α-hydroxylase CYP7B1 during social stress. PLoS One, 9(2), e89421. https://doi.org/10.1371/journal.pone.0089421
- Wyse, C. A., & Coogan, A. N. (2010). Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Research, 1337, 21–31. https://doi.org/10.1016/j.brainres.2010.03.113
- Yi, L. T., Luo, L., Wu, Y. J., Liu, B. B., Liu, X. L., Geng, D., & Liu, Q. (2015). Circadian variations in behaviors, BDNF and cell proliferation in depressive mice. Metabolic Brain Disease, 30(6), 1495–1503. https://doi.org/10.1007/s11011-015-9710-0
- Yoshida, K., Hashiramoto, A., Okano, T., Yamane, T., Shibanuma, N., & Shiozawa, S. (2013). TNF-α modulates expression of the circadian clock gene Per2 in rheumatoid synovial cells. Scandinavian Journal of Rheumatology, 42(4), 276–280. https://doi.org/10.3109/03009742.2013.765031
- Yuan, T. F., & Slotnick, B. M. (2014). Roles of olfactory system dysfunction in depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 54, 26–30. https://doi.org/10.1016/j.pnpbp.2014.05.013
