Abstract
Chronic unpredictable mild stress (CUMS) is a widely used model to study stress-coping strategies in rodents. Different factors have been shown to influence whether animals adopt passive or active coping responses to CUMS. Individual adaptation and susceptibility to the environment seem to play a critical role in this process. To further investigate this relationship, we examined the effects of CUMS on Carioca high- and low-conditioned freezing rats (CHF and CLF, respectively), bidirectional lines of animals selected for high and low freezing in response to contextual cues that were previously associated with footshocks. For this purpose, the behavior of CHF and CLF animals was evaluated in the contextual fear conditioning, open field, elevated T maze, and forced swimming tests before and after 21 days of CUMS. For all tests, CHF rats were more susceptible to the effects of CUMS compared to CLF. CHF animals exposed to CUMS displayed a reduction in freezing behavior, decreased number of entries and time spent in the center of the open field, greater latencies to become immobile, and increased avoidance and escaping behaviors in the elevated T maze. Overall, these findings support the hypothesis that a heightened susceptibility to the environment exerts a strong influence on coping responses to chronic stress.
Introduction
According to a generalist definition, stress refers to nonspecific aspects of coping with environmental changes, demands, and threats, and it can be either characterized as a stimulus or described only as responses (Cannon, Citation1929; Mason, Citation1975; Selye, Citation1976). More recently, Cohen et al. (Citation2016), argued that this generalist definition can then unfold in at least three perspectives depending on the choice of research approach (i.e. methodological traditions and levels of analysis). They propose an integrative model, in which stress is viewed as a set of stages that direct overloading environmental demands toward psychological, behavioral, and biological responses that may put the organism at risk for disease. Indeed, when stress is intense and persistent, it can greatly impact the quality of life, affecting problem-solving and task performance, causing dysfunctional social relationships and leading to mental disorders and somatic diseases (American Psychological Association, Citation2016; Mental Health Foundation, Citation2016). However, stress constitutes a vital mechanism as it promotes responses that increase the chances of survival. In other words, the stages of the stress response mediate the achievement of two goals: to motivate organisms to manipulate or accommodate stressors and to promote activities that seek to reduce or eliminate them through physiological and behavioral changes (Baum & Posluszny, Citation1999; Mcfetridge & Yarandi, Citation1997; Santagostino et al., Citation1996). As pointed out before the line between the protective and deleterious effects of stress may lie in the magnitude and the chronicity of the stimuli (American Psychiatric Association, Citation2013; Aschbacher et al., Citation2013). However, it might also depend on the coping mechanisms employed by each individual.
Coping mechanisms (i.e. a set of cognitive, behavioral, and physiological resources that are deployed by an individual to mitigate situations that they perceive as stressful; (Folkman, Citation2013; Folkman & Lazarus, Citation1980; Ghanem et al., Citation2020) can be as diverse as the stressors themselves and be classified as active or passive coping (Nielsen & Knardahl, Citation2014). Active coping is often associated with focused problem-solving or fleeing strategies, and passive coping is related to self-targeting and avoidance behaviors (Folkman & Lazarus, Citation1980; Wood & Bhatnagar, Citation2015). By tracing coping strategies to nonhuman animals, various studies have identified inner and outer common characteristics that might influence the active/passive switch, such as neurogenesis and neural plasticity, monoamine balance, cortisol production, plasma catecholamine levels, cytokine levels, threat recognition, social dominance, and feeding behavior (Bulos et al., Citation2015; Hijzen et al., Citation1984; Øverli et al., Citation2007; Perez-Tejada et al., Citation2019). However, different stressors have been applied to various populations and animal species, and extensive inter-individual variation is observed both between and within studies (Øverli et al., Citation2007). Moreover, differences between acute and chronic stress can also contribute to the heterogeneity of results across animal models (Dragoş & Tănăsescu, Citation2010; Harris, Citation2015; Herman et al., Citation2016; Rohleder, Citation2019). Taking this into account, the chronic unpredictable mild stress (CUMS) protocol tries to circumvent this problem by varying the type and intensity of the stressor over time. The CUMS protocol is one of the most reliable animal stress models used to mimic the effects of long-term expositions to environmental stress in humans (Willner, Citation2017).
The present study applied the chronic unpredictable mild stress (CUMS) protocol in outbred strains of rats that were selected according to their freezing responses to contextual conditioned aversive cues: Carioca High-conditioned Freezing (CHF) and Carioca Low-conditioned freezing (CLF). We sought to identify in genetically related individuals the influence of chronic stress on divergent coping responses according to multiple aversive stimuli and environmental settings.
Methods
Animals and housing
A total of 105 male Wistar rats, 110 days old, were obtained from the Laboratory of Behavioral Neuroscience (LANEC) of the Pontifical Catholic University of Rio de Janeiro. We chose to use only male rats because CHF males display a stronger response to context fear conditioning than females (Gomes et al., Citation2011). Two outbred strains were used, CHF (n = 52) and CLF (n = 53), that were selectively bred for high- and low-conditioned freezing responses to contextual cues as previously described (Gomes & Landeira-Fernandez, Citation2008). Bidirectional selective breeding is based on the animals’ freezing response to contextual cues. After the third generation (Gomes et al., Citation2011), strains possessing markedly opposed freezing responses could be identified (i.e. CHF and CLF lines). The protocol used for contextual fear conditioning is described in detail below. Except as specified below, animals were kept on a 12 h/12 h light/dark cycle (lights on at 7:00 AM, lights off at 7:00 PM) at a controlled temperature (24 °C ± 1 °C) with free access to food and water. The behavioral experiments were conducted between 9:00 AM and 1:00 PM and performed in accordance with the Declaration of Helsinki and Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Chronic unpredictable mild stress
Stress was applied for a total of 21 days based on procedures that were previously described and reviewed by Willner (Citation2017). Briefly, the weekly stress regimen consisted of a period of some of the most commonly used stressors (Lages et al., Citation2021): restraint (4 h), immersion in cold water (18 °C, 5 min), soiled cage (24 h), low-intensity stroboscopic lighting (150 flashes/min, 12 h), light/dark cycle inversion (24 h), uninterrupted lighting (24 h), water deprivation (24 h), tilted cage (45°, 24 h), low temperature (4 °C, 1 h), food deprivation (24 h), and excessive grouping (24 h). There was also a rest period. Before the behavioral tests, the animals in the CUMS group remained stress-free for 24 h. A control group (CON), comprising half of the total number of subjects, was kept under standard maintenance procedures and handling occurred once a week.
Behavioral tests
Before the initiation of any behavioral testing, animals were individually handled for 2 min for five consecutive days by male and female researchers blind to the experimental condition of the animal. Initially, 36 rats from each line were randomly selected and equally divided into CON and CUMS groups and tested in the contextual fear conditioning, open field, and forced swimming paradigms. Contextual-conditioned fear testing and forced swimming were conducted on consecutive days before and after the 21-day CUMS protocol. The open field test was conducted at the end of the CUMS period and 24 h before contextual fear conditioning and forced swimming tests. Another set of animals (CHF, n = 16; CLF, n = 17) underwent the same stress protocol and were only tested in the T-maze.
Contextual fear conditioning
Contextual fear conditioning was carried out in conditioning chambers (25 × 20 × 20 cm, Insight, Ribeirão Preto, SP, Brazil) under red-light illumination (25 W) and a continuous background sound (white noise generator, 76 dB). Each chamber was located inside a sound-attenuating box. The floor of the chambers was made of 15 stainless-steel rods (1.5 cm apart) connected to an electric shock generator and scrambler (AVS SCR04, São Paulo, SP, Brazil). Before and after each use, the chamber was cleaned with an ammonia solution (5%). Contextual fear conditioning was conducted on two consecutive days. On the first day, animals were individually placed inside conditioning chambers (total of 4) and left unperturbed for 8 min (baseline, pre-shock period). Immediately after the pre-shock period, animals were exposed to three unavoidable electric footshocks (0.6 mA, 1 s) at fixed 20-s intervals, which were then followed by an additional 3 min period without any aversive stimulation (post-shock period). At the end of the post-shock period, animals were returned to their home cages. Twenty-four hours later, animals were returned to the same conditioning chamber to evaluate fear conditioning to contextual cues (testing session–Before). No footshocks were administered during this period. Freezing was scored for 8 min, and after that animals were returned to their home cages and taken back to the animal facility. A trained observer, blind to the experimental group of the animal, quantified freezing episodes every 2 s. Freezing, a classic measure of fear (Fanselow, Citation1980), was defined as a complete absence of movements except those related to breathing. A retest session (After) was conducted by the end of the CUMS 21-day period.
Open field test
After the experimental protocol, all of the animals were tested in an open field arena that was flanked by walls (60 cm × 60 cm). For the analysis, the area was delimited into nine isometric squares. An area entry was defined as the rat having entered a square with all four legs. The following parameters were evaluated over a total of 300 s: number of crossings between the eight peripheral squares, number of entries into the central square, and percentage of time spent in the central square.
Forced swim test
An adaptation of the forced swimming test described by Mezadri et al. (Citation2011) was performed before and after the 21-day CUMS protocol. Briefly, a forced swim trial consisted of placing the animal inside a cylindrical plastic container (60 cm) filled with water (18 °C) for 300 s. The container was filled up to 80% of its total volume so that the rat could not easily touch the bottom or escape over the edges (Porsolt et al., Citation1978). The animal’s behavior was continuously recorded by an overhead video camera and the container was cleaned and the water exchanged across testing sessions. Later, the latency to immobility (i.e. when exploration stopped) and the total immobility time were measured by an observer blind to the animal’s experimental group. Immobility was considered when the animal remained passively floating making only small movements necessary to keep its head above the water.
Elevated T-maze
The elevated T-maze was an apparatus that was similar to the elevated plus maze, with the exception that one of the closed arms was obstructed at its interface to the central area. The procedure, as described previously (Teixeira et al., Citation2000), consisted of a series of three trials to measure avoidance or escape behaviors. In each of these trials, the animal was positioned at the end of the closed (avoidance) or open (escape) arms, facing the center of the apparatus. The time taken for the rat to leave the initial arm and enter the central area was recorded. A time limit of 300 s was set for each trial. To enhance the efficiency of escape behavior, all of the animals underwent a habituation phase 24 h before the test phase that consisted of 30 min exposure to the open arm that was blocked at its interface with the central platform.
Statistical analysis
The data analysis was conducted using Prism 8 software (GraphPad, La Jolla, CA, USA). A three-way repeated-measures analysis of variance (rANOVA) was carried out for the following tests: conditioned contextual fear, forced swim test, and elevated T-maze. Two-way ANOVA was applied to analyze the open field test results. Significant results in the ANOVAs were followed by pairwise comparisons with the Bonferroni correction. For all of the statistical tests, significance was set at a two-tailed p < .05.
Results
Contextual fear conditioning
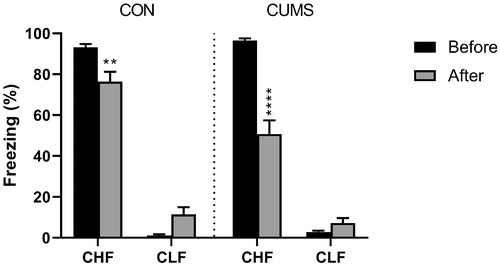
The animals were tested for the frequency of freezing in response to previously conditioned contextual cues at two time-points: 1 week before the stress procedure and immediately after the end of the stress procedure. Consistent with their original selective breeding, an inherent difference was found between strains (strain: F1,68 = 910.6, p < .0001). The retrial alone had a modest effect on behavior in CHF animals (trial × strain: F1,68 = 4.08, p = .0472). Chronic unpredictable mild stress led to a more pronounced decrease in freezing behavior in CHF rats but did not affect CLF rats (stress × strain: F2,102 = 69.48, p < .0001; ).
Figure 1. Fear conditioning in Carioca High-conditioned Freezing (CHF) and Carioca Low-conditioned Freezing (CLF) rats. The animals were tested before and 21 days after the CUMS protocol (gray bars) or ordinary handling (CON; black bars). Bars and symbols show the mean ± SEM. **p < .01, ****p < .0001.
Open field test
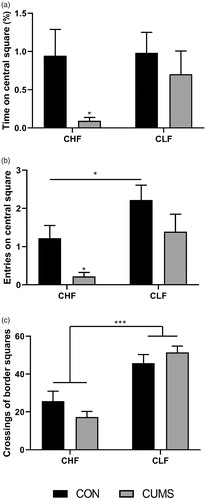
Innate fear was assessed in the open field test () after 21 days. The results indicated that CUMS led to a reduction of the number of entries into the central area (stress: F1,68 = 7.06, p = .0098) and time spent in the central area (stress: F1,68 = 4.48, p = .0380) only in CHF animals. Although stress did not alter motor performance in either strain of animals, CLF rats exhibited higher mobility compared with CHF rats in both the CON and CUMS groups (strain: F1,68 = 43.39, p < .0001).
Figure 2. Behavioral patterns in the open field test, showing entries into (a) and the time spent in (b) the central area and crossings in the periphery (c). The experiments were performed 21 days after of CUMS protocol (gray bars) or ordinary handling (CON; black bars). Bars and symbols show the mean ± SEM. *p < .05, ***p < .001.
Forced swim test
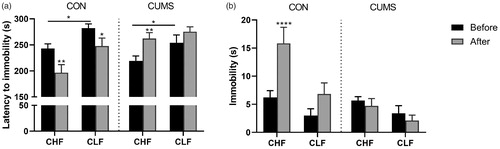
The forced swim test was conducted before and after the 21-day CUMS protocol to assess the response to acute stress. The data showed an effect of repeated exposure to the cold bath in the CON group, in which latency to immobility decreased in both strains (trial × stress: F1,68 = 24.47, p < .0001; ). Chronic unpredictable mild stress hindered the effects of reexposure to the forced swim test and had an opposite effect on latency in CHF rats, in which these animals exhibited a longer latency in the second trial of the forced swim test (). Moreover, an effect of strain on latency (strain: F1,68 = 13.49, p = .0005) and immobility (strain: F1,68 = 13.25, p = .0005) was observed, regardless of trial and the CUMS protocol.
Figure 3. Activity in the forced swim test. (a) Latency to immobility. (b) Total immobility time during the 300 s test. Sessions were performed before and 21 days after of CUMS protocol (gray bars) or ordinary handling (CON; black bars). Bars and symbols show the mean ± SEM. *p < .05, **p < .01, ****p < .0001, comparison between sessions 1 and 2 in groups of the same strain and the same stress protocol (i.e. CON or CUMS).
Elevated T-maze
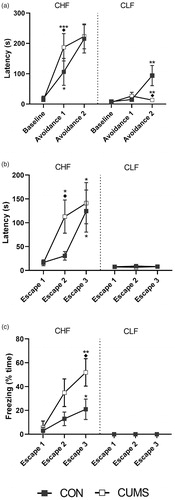
An additional set of animals that were different from the ones used previously was tested in the elevated T-maze after 21 days of either CON or CUMS. The latency for the animals to leave the closed arm and enter the central area was recorded during three trials (baseline, avoidance 1, and avoidance 2; ). Although baseline latencies were similar, a strain effect was observed in the avoidance 1 and 2 trials (strain × trial: F2,58) = 9.781, p = .0002). Moreover, CUMS increased the avoidance latency in CHF rats in trial 2 (avoidance 1) and decreased the avoidance latency in CLF rats in trial 3 (avoidance 2). After the avoidance trials, each animal was exposed to one of the open arms, and the time to leave this platform and enter the central area was recorded (). Different responses were observed between strains throughout the three trials (strain × trial: F2,57 = 8.32, p = .0007), with different responses to stress. The response was null in CLF animals, whereas CHF animals exhibited an increase in the escape latency in trial 2 (escape 2). Moreover, the longer escape latency in CHF rats compared with CLF rats (strain: F1,29 = 24.18, p < .0001) was attributable to higher freezing times in CHF rats after CUMS (strain: F1,29 = 19.56, p = .0001; ).
Figure 4. Elevated T-maze assessment of (a) avoidance behavior, (b) escape from open arms and (c) freezing during escape trials. Experiments were performed 21 days after CUMS protocol (white squares) or ordinary handling (CON, black squares). Lines and squares are means ± SEM. Data points without noticeable error bars indicate that the ± SEM was smaller than the square. *p < .05, **p < .01, ***p < .001 are displayed for comparison of same trials between strains within each stress protocol (i.e. CON or CUMS). ◆p < .01 is shown for comparison between stress protocols of groups of the same strain and trial.
Discussion
Stress coping strategies in humans can be as diverse as the stressors themselves. Among these are cultural and self-motivated behaviors, such as physical activity, engaging in self-help literature, accommodation, and self-punishment (Brougham et al., Citation2009; Herbert et al., Citation2020; Kim & Duda, Citation2003). However, many stress coping strategies are rooted in adaptive, evolutionary, and emotion-focused mechanisms that can be traced back to less complex animals (Øverli et al., Citation2007). Distinct responses are species-specific (Anderson & Adolphs, Citation2014), but the selection of an appropriate behavioral repertoire depends on evolutionarily conserved inner and outer cues, such as physiological needs, the presence of a predator, the proximity of a threat, and the existence of an escape route (Barrett, Citation2017; Bulos et al., Citation2015; Fanselow & Lester, Citation1988; Perusini & Fanselow, Citation2015; Viana et al., Citation1994). In the present study, we found that responses to CUMS depended on the context and the inherent predisposition of outbred rat strains. Specifically, CHF rats exhibited a combination of passive and active behavioral responses, depending on the aversive situation to which they were exposed. Conversely, CLF rats exhibited low or no responses to stress.
The CHF and CLF strains were selected based on the frequency of their freezing responses to conditioned aversive contextual cues (Gomes & Landeira-Fernandez, Citation2008). Therefore, in the present study, experiments were first conducted to determine the maintenance of this innate characteristic after 21 days of CUMS. The results showed a sharper decrease in freezing in CHF rats, whereas freezing responses in CLF animals were unaltered. A lower frequency of freezing behavior is often interpreted as a diminished fear or anxiety state (Macedo-Souza et al., 2020; Sun et al., Citation2020). However, an alternative hypothesis suggests an increase in motor function or a shift to active defensive behaviors (e.g. fleeing or panic; (Fanselow & Wassum, Citation2015; Roelofs, Citation2017). Previous studies have reported a potentially anxiogenic effect of CUMS (Sequeira-Cordero et al., Citation2019). Thus, we investigated defensive responses in these animals in the open field test. The data showed an increase in avoidance behavior in CHF animals, in which the number of entries into the more aversive central area and time spent in the central area were lower after CUMS exposure. In contrast, these responses in CLF rats remained unchanged. Moreover, CUMS did not increase motor activity, which supports the hypothesis of an anxiogenic effect of CUMS rather than an increase in mobility. However, one should keep in mind that the current findings were obtained from male rats and there is a vast literature on sex differences and the susceptibility to CUMS (for a review, please see Franceschelli et al., Citation2014), which might be influenced by genomic (Barko et al., Citation2019) and hormonal variations (Guo et al., Citation2018). Moreover, women experience higher rates of anxiety disorders and posttraumatic stress disorder (PTSD) than men (Hu et al., Citation2017; McLean et al., Citation2011; Olff, Citation2017). Interestingly, however, CHF males display a stronger response to context fear conditioning than females (Gomes et al., Citation2011). Therefore, future studies are needed to systematically explore differences in the susceptibility to chronic stress between male and female CHL and CLF rats.
To further investigate the possible shift between passive and active behavioral responses to stress, we applied the forced swim test. This test is classically considered a standard procedure to assess depressive-like behavior in rodents (Porsolt et al., Citation1977). The forced swim test was also recently suggested to reveal important coping strategies in response to stress. Commons et al. (Citation2017) reported that this test is based on the observation that when rodents face an inescapable aversive situation, initial active coping strategies (e.g. climbing and swimming) are typically replaced with a passive strategy (i.e. floating) that conserves energy rather than reflects a coping failure. These authors emphasized, “coping strategy is measured in the forced swimming test, “depression-like” is an inference that may or may not be correct.” Moreover, the predictive validity of the forced swim test for evaluating existing and potential antidepressants relies on overlapping neural networks that control coping strategies in response to acute stress and are impacted by depression, such as the hypothalamic-pituitary-adrenal axis and hippocampus (Gold et al., Citation1988; Naughton et al., Citation2014). Importantly, such coping strategies might rely on learning mechanisms. For example, it has been suggested that a forced swimming test protocol with two-trials generally induces the development of passive coping strategies aiming at the minimization of energy expenditure (Enginar et al., Citation2016; Vinader-Caerols et al., Citation1999; West, Citation1990). However, others have also proposed that revisiting the aversive environment might promote escape-directed attempts driven by fear, which can be interpreted as anxiety-like behavior (Anyan & Amir, Citation2018). The present results showed that repeated exposure to the forced swim test led to an active-passive response shift. However, stress altered the learning of this behavior by promoting the maintenance of, or even an increase in, active responses, such that the latency to immobility decreased in CHF rats but not in CLF rats. Chronic unpredictable mild stress is classically used to elicit depression-like states in animals and is often described to enhance immobility in the forced swim test as a reflection of despair or hopelessness (Aricioglu et al., Citation2020; Du et al., Citation2020; Kudryashov et al., Citation2020; Porsolt et al., Citation1978). However, this is not always the case as some studies also reported the reinforcement of active responses (Haidkind et al., Citation2003; Harro et al., Citation2001; Stepanichev et al., Citation2018). Possible sources of such discrepant results have been investigated, such as housing conditions, sex and age of the animals (Bogdanova et al., Citation2013; Bourke & Neigh, Citation2011) as well as the water temperature, which by itself can be a stressor (Arai et al., Citation2000; Colom-Lapetina et al., Citation2017; Jefferys & Funder, Citation1994). Variables in the CUMS protocol should always be considered, such as the duration of stressor exposure, the regimen of exposure, and specific stressors, because they can cause variability in the results (Antoniuk et al., Citation2019; Strekalova et al., Citation2011).
Based on the hypothesis that a stress-induced shift in defensive behaviors occurs, we employed the elevated T-maze test to assess alternating avoidance and escape behaviors. Consistent with the open field test results, data from the avoidance trials of the elevated T-maze indicated an increase in passive defensive behaviors, in which the latency to exit the closed arms was higher after CUMS exposure in CHF rats. In contrast, CLF rats exhibited a decrease in avoidance after stress exposure. Moreover, the latency to escape the open arms was increased in CHF rats. A reduction of escape times might reflect a panic-related behavior in response to certain treatments (Bulos et al., Citation2015; Gobira et al., Citation2013). Our results indicate that the longer latency was accompanied by higher immobility rather than exploratory behavior, suggesting an exacerbation of passive coping strategies over active coping strategies (e.g. escape).
Altogether, our data indicate that the shift of passive/active coping responses to chronic stress relies on contextual cues that are inherent to the test environment and on an innate predisposition of the subjects. The CHF and CLF strains were selectively outbred for their freezing responses to contextual conditioned aversive cues, and these two strains had markedly different responses to stress. The alterations that were observed in CLF rats were subtle, whereas CHF rats responded differently to each behavioral test, alternating between active and passive coping responses. Compared to CLF, CHF rats exhibited an increase in immobility and/or freezing in the absence of CUMS. In other words, their passive behavioral response seems to be their preferred choice when coping with a stressful event, either during the open field and T-maze tests or in the first exposure to the contextual fear conditioning and forced swimming tests. Other studies from our group reinforce this hypothesis. It has been shown that CHF rats display diminished exploration of the open arms in the elevated plus maze (Cavaliere et al., Citation2020), as well as an increased freezing response to cued (tone) fear conditioning (Macedo-Souza et al., 2020). Taken together these results reinforce the use of CHF rats as a model of generalized anxiety disorder. During stress, however, two different responses were observed for CHF animals. In the open field and the T-maze tests, the stress produced an increase in passive responses (i.e. CHF rats exposed to chronic stress froze more and explored less than their control counterparts). On the other hand, in the contextual fear conditioning and the forced swimming tests, CHF animals subjected to chronic stress engaged in more active responses. Stressed CHF rats displayed slightly less freezing when re-exposed to the conditioning chamber compared to CHF controls. Similarly, stressed CHF animals showed an increase in the latency to immobility as well as less immobility compared to controls. Thus, similarities and differences between the behavioral tests need to be more thoroughly investigated.
Previous studies assessed the influence of specific contextual characteristics on the decision to engage appropriate behavioral repertoires (Fucich & Morilak, Citation2018; Grafe et al., Citation2020). Some studies indicated that restriction or an inability to escape from the apparatus might result in active responses, which may indeed occur in a conditioning chamber or the forced swim test (Commons et al., Citation2017; Faraji et al., Citation2020; West, Citation1990). Due to the inescapability of a situation and the magnitude of the aversive stimulus, changes in behavioral responses occur, as the animal shifts from avoidance, to freezing and finally to escaping and/or fighting strategies (Perusini & Fanselow, Citation2015). While early passive responses conserve energy and prepare the animal for possible threatening encounters, active responses enhance the chances of survival once the threat is close or the encounter is inevitable (Fanselow, Citation2018). Notably, we did not observe darting responses after stress in the contextual fear conditioning experiment, an active coping behavior mostly observed in females (Gruene et al., Citation2015). However, in the forced swimming test we observed a decrease in immobility times, which is often interpreted as attempts to escape the tank (Commons et al., Citation2017). Thus, instead of developing depressive-like symptoms in response to chronic stress, CHF animals in the forced swimming test display a behavior response coherent with the strengthening of anxiety-like symptoms (Anyan & Amir, Citation2018).
Other studies reported that alterations of memory and neuroplasticity that are caused by chronic stress can account for variable responses during repeated trials (Benatti et al., Citation2019; Fee et al., Citation2020; Widman et al., Citation2019). Indeed, innate and learned fear behavioral responses rely on different neural circuitries (Fanselow, Citation2018). For instance, the behavioral response to an acute threat, such as the freezing response evocated right after a footshock, is mediated by a circuitry involving the basolateral amygdala (BLA), the central amygdala (CeA), and the ventromedial periaqueductal gray (PAG). However, if we consider sustained fear responses, as the freezing observed in the re-testing session of the contextual fear conditioning, there is the involvement of BLA activation that projects to the bed nuclei of the stria terminalis (BNST), which then communicates to the ventromedial PAG (Isosaka et al., Citation2015; Maren, Citation2003; Motta et al., Citation2017; Perusini & Fanselow, Citation2015). On the other hand, active behavioral responses are mediated by the dorsal PAG, a circuitry that may or may not involve the basolateral and medial nuclei of the amygdala, but likely does not entail its central part (Silva et al., Citation2016). Common to both systems, there is the regulation from the dorsal raphe nuclei that act on the amygdala and the PAG through serotoninergic projections, which are involved in learning mechanisms (Berg et al., Citation2014; Maier et al., Citation1993). Notably, reports have been made that amygdaloid lesions impair freezing responses of CHF rats (Gomes & Landeira-Fernandez, Citation2008) and that injections of a 5HT-2a antagonist either systemically or locally in the infralimbic or prelimbic cortices decreased anxiety-like responses (Leon et al., Citation2017). Moreover, differences in hippocampal activity have been identified between CHF and CLF rats, which indicate possible alterations in the learning and processing of aversive memories (Bannerman et al., Citation2014; Leon et al., Citation2020). More studies are needed to evaluate which circuitry is involved in the shift of coping strategies observed in CHF stressed rats as well as whether mechanisms of learning or threat perception are involved in this process.
Other rodent models of anxiety-like behavioral phenotypes have been investigated for their stress response. The LAL mice and HAB rats have been proposed as animal models for several characteristics of human stress-related mood disorders such as anxiety and depression (Landgraf & Wigger, Citation2002; Veenema et al., Citation2004). These lines have been selected based on the latency to attack a non-aggressive opponent male placed on their home cage (van Oortmerssen & Bakker, Citation1981) and on their behavior on the elevated plus maze (Liebsch et al., Citation1998), respectively. As the CHF rats, the LAL and HAB animals a reactive coping style to acute stress, instead of a proactive one, in several behavioral tests, such as the forced swimming, the exploration in the modified hole board, the open field, the two-way avoidance, and the shock-probe defensive burying tests (Koolhaas et al., Citation1999; Landgraf & Wigger, Citation2002). Also, these three lines share altered functions of the amygdala, the hippocampus, and the 5-HT neurotransmission (for a review of the LAL and HAB rodents, see Veenema & Neumann, Citation2007). Human studies addressing the relationship between anxiety and stress have reported shrinking and loss of connectivity of the amygdala, damage or atrophy of the hippocampus and defects in the serotoninergic connectivity (Craske et al., Citation2017; Di Giovanni & De Deurwaerdere, Citation2020; Liu et al., Citation2020; Mah et al., Citation2016; Price & Drevets, Citation2012; Santos et al., Citation2019). Furthermore, our results support the association between anxiety and chronic stress found in humans (Mah et al., Citation2016; McEwen & Stellar, Citation1993). Individuals facing increased levels of daily stress display more severe anxiety (American Psychological Association, Citation2017; Health & Safety Executive, Citation2020). Specifically, our findings with an animal model of trait anxiety, the CHF (Gomes et al., Citation2011; Macedo-Souza et al., Citation2019), demonstrate that individuals with a preestablished increased anxiety-related behaviors are more susceptible to the effects of chronic stress. Moreover, the CUMS impacts the choice of behavior repertoire in response to aversive stimuli–a process that can be influenced by both environmental and innate factors. Thus, the CHF model can pave the way for a better understanding of the neurobiological factors underlying the connection between stress and anxiety as well as for testing novel approaches to the treatment of chronic stress disorders.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yury V. Lages
Yury V. Lages Ph.D. Graduate in Psychology at PUC-Rio
Silvia S. Maisonnette
Silvia S. Maisonnette Ph.D in Neurosciences and Post-doctorate at PUC-Rio
Beatriz Marinho
Beatriz Marinho Undergraduate student at PUC-Rio
Flávia P. Rosseti
Flávia P. Rosseti Biologist and technician at PUC-Rio
Thomas E. Krahe
Thomas E. Krahe Ph.D. in Biology and Associate Professor at PUC-Rio
J. Landeira-Fernandez
J. Landeira-Fernandez Ph.D. in Behavioral Neurosciences and Full Professor at PUC-Rio
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. https://doi.org/10.1176/appi.books.9780890425596
- American Psychological Association. (2016). Stress in America: The impact of discrimination (Stress in AmericaTM Survey, Issue. https://www.apa.org/news/press/releases/stress/2015/impact-of-discrimination.pdf
- American Psychological Association. (2017). Stress in America: The State of Our Nation (Stress in AmericaTM Survey Issue. https://www.apa.org/news/press/releases/stress/2017/state-nation.pdf
- Anderson, D. J., & Adolphs, R. (2014). A framework for studying emotions across species. Cell, 157(1), 187–200. https://doi.org/10.1016/j.cell.2014.03.003
- Antoniuk, S., Bijata, M., Ponimaskin, E., & Wlodarczyk, J. (2019). Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neuroscience and Biobehavioral Reviews, 99, 101–116. https://doi.org/10.1016/j.neubiorev.2018.12.002
- Anyan, J., & Amir, S. (2018). Too depressed to swim or too afraid to stop? A reinterpretation of the forced swim test as a measure of anxiety-like behavior. Neuropsychopharmacology, 43(5), 931–933. https://doi.org/10.1038/npp.2017.260
- Arai, I., Tsuyuki, Y., Shiomoto, H., Satoh, M., & Otomo, S. (2000). Decreased body temperature dependent appearance of behavioral despair in the forced swimming test in mice. Pharmacological Research, 42(2), 171–176. https://doi.org/10.1006/phrs.2000.0672
- Aricioğlu, F., Yalcinkaya, C., Ozkartal, C. S., Tuzun, E., Sirvanci, S., Kucukali, C. I., & Utkan, T. (2020). NLRP1-mediated antidepressant effect of ketamine in chronic unpredictable mild stress model in rats. Psychiatry Investigation, 17(4), 283–291. https://doi.org/10.30773/pi.2019.0189
- Aschbacher, K., O’Donovan, A., Wolkowitz, O. M., Dhabhar, F. S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38(9), 1698–1708. https://doi.org/10.1016/j.psyneuen.2013.02.004
- Bannerman, D. M., Sprengel, R., Sanderson, D. J., McHugh, S. B., Rawlins, J. N., Monyer, H., & Seeburg, P. H. (2014). Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews. Neuroscience, 15(3), 181–192. https://doi.org/10.1038/nrn3677
- Barko, K., Paden, W., Cahill, K. M., Seney, M. L., & Logan, R. W. (2019). Sex-specific effects of stress on mood-related gene expression. Molecular Neuropsychiatry, 5(3), 162–175. https://doi.org/10.1159/000499105
- Barrett, L. F. (2017). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience, 12(11), 1833–1833. https://doi.org/10.1093/scan/nsx060
- Baum, A., & Posluszny, D. M. (1999). Health psychology: Mapping biobehavioral contributions to health and illness. Annual Review of Psychology, 50, 137–163. https://doi.org/10.1146/annurev.psych.50.1.137
- Benatti, C., Radighieri, G., Alboni, S., Blom, J. M. C., Brunello, N., & Tascedda, F. (2019). Modulation of neuroplasticity-related targets following stress-induced acute escape deficit. Behavioural Brain Research, 364, 140–148. https://doi.org/10.1016/j.bbr.2019.02.023
- Berg, B. A., Schoenbaum, G., & McDannald, M. A. (2014). The dorsal raphe nucleus is integral to negative prediction errors in Pavlovian fear. The European Journal of Neuroscience, 40(7), 3096–3101. https://doi.org/10.1111/ejn.12676
- Bogdanova, O. V., Kanekar, S., D’Anci, K. E., & Renshaw, P. F. (2013). Factors influencing behavior in the forced swim test. Physiology & Behavior, 118, 227–239. https://doi.org/10.1016/j.physbeh.2013.05.012
- Bourke, C. H., & Neigh, G. N. (2011). Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60(1), 112–120. https://doi.org/10.1016/j.yhbeh.2011.03.011
- Brougham, R. R., Zail, C. M., Mendoza, C. M., & Miller, J. R. (2009). Stress, sex differences, and coping strategies among college students. Current Psychology, 28(2), 85–97. https://doi.org/10.1007/s12144-009-9047-0
- Bulos, E. M., Pobbe, R. L., & Zangrossi, H. Jr.(2015). Behavioral consequences of predator stress in the rat elevated T-maze. Physiology & Behavior, 146, 28–35. https://doi.org/10.1016/j.physbeh.2015.04.019
- Cannon, W. B. (1929). Organization for physiological homeostasis. Physiological Reviews, 9(3), 399–431. https://doi.org/10.1152/physrev.1929.9.3.399
- Cavaliere, D. R., Maisonnette, S., Krahe, T. E., Landeira-Fernandez, J., & Cruz, A. P. M. (2020). High- and low-conditioned behavioral effects of midazolam in carioca high- and low-conditioned freezing rats in an ethologically based test. Neuroscience Letters, 715, 134632. https://doi.org/10.1016/j.neulet.2019.134632
- Cohen, S., Gianaros, P. J., & Manuck, S. B. (2016). A stage model of stress and disease. Perspect Psychological Science, 11(4), 456–463. https://doi.org/10.1177/1745691616646305
- Colom-Lapetina, J., Begley, S. L., Johnson, M. E., Bean, K. J., Kuwamoto, W. N., & Shansky, R. M. (2017). Strain-dependent sex differences in a long-term forced swim paradigm. Behavioral Neuroscience, 131(5), 428–436. https://doi.org/10.1037/bne0000215
- Commons, K. G., Cholanians, A. B., Babb, J. A., & Ehlinger, D. G. (2017). The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chemical Neuroscience, 8(5), 955–960. https://doi.org/10.1021/acschemneuro.7b00042
- Craske, M. G., Stein, M. B., Eley, T. C., Milad, M. R., Holmes, A., Rapee, R. M., & Wittchen, H. U. (2017). Anxiety disorders. Nature Reviews. Disease Primers, 3, 17024. https://doi.org/10.1038/nrdp.2017.24
- Di Giovanni, G., & De Deurwaerdere, P. (2020). Serotonin research: Crossing scales and boundaries. Neuropharmacology, 181, 108340. https://doi.org/10.1016/j.neuropharm.2020.108340
- Dragoş, D., & Tănăsescu, M. D. (2010). The effect of stress on the defense systems. Journal of Medicine and Life, 3(1), 10–18.
- Du, X., Yin, M., Yuan, L., Zhang, G., Fan, Y., Li, Z., Yuan, N., Lv, X., Zhao, X., Zou, S., Deng, W., Kosten, T. R., & Zhang, X. Y. (2020). Reduction of depression-like behavior in rat model induced by ShRNA targeting norepinephrine transporter in locus coeruleus. Translational Psychiatry, 10(1), 130. https://doi.org/10.1038/s41398-020-0808-8
- Enginar, N., Yamantürk-Çelik, P., Nurten, A., & Güney, D. B. (2016). Learning and memory in the forced swimming test: Effects of antidepressants having varying degrees of anticholinergic activity. Naunyn-Schmiedeberg’s Archives of Pharmacology, 389(7), 739–745. https://doi.org/10.1007/s00210-016-1236-4
- Fanselow, M. S. (1980). Conditioned and unconditional components of post-shock freezing in rats. Pavlovian Journal of Biological Science, 15(4), 177–82. https://doi.org/10.1007/BF03001163
- Fanselow, M. S. (2018). The role of learning in threat imminence and defensive behaviors. Current Opinion in Behavioral Sciences, 24, 44–49. https://doi.org/10.1016/j.cobeha.2018.03.003
- Fanselow, M. S., & Lester, L. S. (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In Evolution and learning (pp. 185–212). Lawrence Erlbaum Associates, Inc.
- Fanselow, M. S., & Wassum, K. M. (2015). The origins and organization of vertebrate pavlovian conditioning. Cold Spring Harbor Perspectives in Biology, 8(1), a021717. https://doi.org/10.1101/cshperspect.a021717
- Faraji, J., Singh, S., Soltanpour, N., Sutherland, R. J., & Metz, G. A. S. (2020). Environmental determinants of behavioural responses to short-term stress in rats: Evidence for inhibitory effect of ambient landmarks. Behavioural Brain Research, 379, 112332. https://doi.org/10.1016/j.bbr.2019.112332
- Fee, C., Prevot, T., Misquitta, K., Banasr, M., & Sibille, E. (2020). Chronic stress-induced behaviors correlate with exacerbated acute stress-induced cingulate cortex and ventral hippocampus activation. Neuroscience, 440, 113–129. https://doi.org/10.1016/j.neuroscience.2020.05.034
- Folkman, S. (2013). Stress: Appraisal and Coping. In M. D. Gellman, J. R. Turner (Eds.), Encyclopedia of Behavioral Medicine. New York, NY: Springer. https://doi.org/10.1007/978-1-4419-1005-9_215.
- Folkman, S., & Lazarus, R. S. (1980). An analysis of coping in a middle-aged community sample. Journal of Health and Social Behavior, 21(3), 219–239. https://doi.org/10.2307/2136617
- Franceschelli, A., Herchick, S., Thelen, C., Papadopoulou-Daifoti, Z., & Pitychoutis, P. M. (2014). Sex differences in the chronic mild stress model of depression. Behavioural Pharmacology, 25(5–6), 372–383. https://doi.org/10.1097/FBP.0000000000000062
- Fucich, E. A., & Morilak, D. A. (2018). Shock-probe defensive burying test to measure active versus passive coping style in response to an aversive stimulus in rats. Bio Protoc, 8(17), 2998. https://doi.org/10.21769/BioProtoc.2998
- Ghanem, I., Castelo, B., Jimenez-Fonseca, P., Carmona-Bayonas, A., Higuera, O., Beato, C., García, T., Hernández, R., & Calderon, C. (2020). Coping strategies and depressive symptoms in cancer patients. Clinical and Translational Oncology, 22(3), 330–336. https://doi.org/10.1007/s12094-019-02123-w
- Gobira, P. H., Aguiar, D. C., & Moreira, F. A. (2013). Effects of compounds that interfere with the endocannabinoid system on behaviors predictive of anxiolytic and panicolytic activities in the elevated T-maze. Pharmacology, Biochemistry, and Behavior, 110, 33–39. https://doi.org/10.1016/j.pbb.2013.05.013
- Gold, P. W., Goodwin, F. K., & Chrousos, G. P. (1988). Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress. The New England Journal of Medicine, 319(7), 413–420. https://doi.org/10.1056/NEJM198808183190706
- Gomes, V. d C., & Landeira-Fernandez, J. (2008). Amygdaloid lesions produced similar contextual fear conditioning disruption in the Carioca high- and low-conditioned freezing rats. Brain Research, 1233, 137–145. https://doi.org/10.1016/j.brainres.2008.07.044
- Gomes V. D. C., Silva, C. E. B., & Landeira-Fernandez, J. (2011). The carioca high and low conditioned freezing lines: A new animal model of generalized anxiety disorder. In V. Kalinin (Ed.), Anxiety Disorders. IntechOpen.
- Grafe, L. A., Mara, L., Branch, A., Dobkin, J., Luz, S., Vigderman, A., Shingala, A., Kubin, L., Ross, R., & Bhatnagar, S. (2020). Passive coping strategies during repeated social defeat are associated with long-lasting changes in sleep in rats. Frontiers in Systems Neuroscience, 14, 6. https://doi.org/10.3389/fnsys.2020.00006
- Gruene, T. M., Flick, K., Stefano, A., Shea, S. D., & Shansky, R. M. (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife, 4, e11352. https://doi.org/10.7554/eLife.11352
- Guo, L., Chen, Y. X., Hu, Y. T., Wu, X. Y., He, Y., Wu, J. L., Huang, M. L., Mason, M., & Bao, A. M. (2018). Sex hormones affect acute and chronic stress responses in sexually dimorphic patterns: Consequences for depression models. Psychoneuroendocrinology, 95, 34–42. https://doi.org/10.1016/j.psyneuen.2018.05.016
- Haidkind, R., Eller, M., Harro, M., Kask, A., Rinken, A., Oreland, L., & Harro, J. (2003). Effects of partial locus coeruleus denervation and chronic mild stress on behaviour and monoamine neurochemistry in the rat. European Neuropsychopharmacology, 13(1), 19–28. https://doi.org/10.1016/S0924-977X(02)00076-7
- Harris, R. B. (2015). Chronic and acute effects of stress on energy balance: Are there appropriate animal models? American Journal of Physiology-Regulatory Integrative and Comparative Physiology, 308(4), R250–R265. https://doi.org/10.1152/ajpregu.00361.2014
- Harro, J., Tonissaar, M., Eller, M., Kask, A., & Oreland, L. (2001). Chronic variable stress and partial 5-HT denervation by parachloroamphetamine treatment in the rat: Effects on behavior and monoamine neurochemistry. Brain Research, 899(1–2), 227–239. https://doi.org/10.1016/S0006-8993(01)02256-9
- Health and Safety Executive. (2020). Work-related stress, anxiety or depression statistics in Great Britain. https://www.hse.gov.uk/statistics/causdis/stress.pdf
- Herbert, C., Meixner, F., Wiebking, C., & Gilg, V. (2020). Regular physical activity, short-term exercise, mental health, and well-being among university students: The results of an online and a laboratory study. Frontiers in Psychology., 11, 509. https://doi.org/10.3389/fpsyg.2020.00509
- Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. https://doi.org/10.1002/cphy.c150015
- Hijzen, T. H., Van Der Gugten, J., & Bouter, L. (1984). Active and passive coping under different degrees of stress; Effects on urinary and plasma catecholamines and ECG T-wave. Biological Psychology, 18(1), 23–32. https://doi.org/10.1016/0301-0511(84)90023. 1 https://doi.org/10.1016/0301-0511(84)90023-1
- Hu, J., Feng, B., Zhu, Y., Wang, W., Xie, J., & Zheng, X. (2017). Gender differences in PTSD: Susceptibility and resilience. In A. Alvinius (Ed.), Gender differences in different contexts. IntechOpen.
- Isosaka, T., Matsuo, T., Yamaguchi, T., Funabiki, K., Nakanishi, S., Kobayakawa, R., & Kobayakawa, K. (2015). Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell, 163(5), 1153–1164. https://doi.org/10.1016/j.cell.2015.10.047
- Jefferys, D., & Funder, J. (1994). The effect of water temperature on immobility in the forced swimming test in rats. European Journal of Pharmacology, 253(1–2), 91–94. https://doi.org/10.1016/0014-2999(94)90761-7
- Kim, M.-S., & Duda, J. L. (2003). The coping process: Cognitive appraisals of stress. The Sport Psychologist, 17(4), 406–425. https://doi.org/10.1123/tsp.17.4.406
- Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., De Jong, I. C., Ruis, M. A., & Blokhuis, H. J. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews, 23(7), 925–935. https://doi.org/10.1016/S0149-7634(99)00026-3
- Kudryashov, N. V., Kalinina, T. S., Shimshirt, A. A., Volkova, A. V., Narkevich, V. B., Naplekova, P. L., Kasabov, K. A., Kudrin, V. S., Voronina, T. A., & Fisenko, V. P. (2020). The behavioral and neurochemical aspects of the interaction between antidepressants and unpredictable chronic mild stress. Acta Naturae, 12(1), 63–72. https://doi.org/10.32607/actanaturae.10942
- Lages, Y. V. M., Rossi, A. D., Krahe, T. E., & Landeira-Fernandez, J. (2021). Effect of chronic unpredictable mild stress on the expression profile of serotonin receptors in rats and mice: A meta-analysis. Neuroscience and Biobehavioral Reviews, 124, 78–88. https://doi.org/10.1016/j.neubiorev.2021.01.020
- Landgraf, R., & Wigger, A. (2002). High vs low anxiety-related behavior rats: An animal model of extremes in trait anxiety. Behavior Genetics, 32(5), 301–314. https://doi.org/10.1023/a:1020258104318
- Leon, L. A., Brandao, M. L., Cardenas, F. P., Parra, D., Krahe, T. E., Cruz, A. P. M., & Landeira-Fernandez, J. (2020). Distinct patterns of brain Fos expression in Carioca High- and Low-conditioned Freezing Rats. PLoS One, 15(7), e0236039. https://doi.org/10.1371/journal.pone.0236039
- Leon, L. A., Castro-Gomes, V., Zarate-Guerrero, S., Corredor, K., Mello Cruz, A. P., Brandao, M. L., Cardenas, F. P., & Landeira-Fernandez, J. (2017). Behavioral effects of systemic, infralimbic and prelimbic injections of a serotonin 5-HT2A antagonist in Carioca High- and Low-Conditioned Freezing rats. Frontiers in Behavioral Neuroscience, 11, 117. https://doi.org/10.3389/fnbeh.2017.00117
- Liebsch, G., Montkowski, A., Holsboer, F., & Landgraf, R. (1998). Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behavioural Brain Research, 94(2), 301–310. https://doi.org/10.1016/S0166-4328(97)00198-8
- Liu, W. Z., Zhang, W. H., Zheng, Z. H., Zou, J. X., Liu, X. X., Huang, S. H., You, W. J., He, Y., Zhang, J. Y., Wang, X. D., & Pan, B. X. (2020). Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nature Communications, 11(1), 2221. https://doi.org/10.1038/s41467-020-15920-7
- Macedo-Souza, C., Maisonnette, S. S., Filgueiras, C. C., Landeira-Fernandez, J., & Krahe, T. E. (2019). Cued fear conditioning in carioca high- and low-conditioned freezing rats. Frontiers in Behavioral Neuroscience, 13, 285. https://doi.org/10.3389/fnbeh.2019.00285
- Mah, L., Szabuniewicz, C., & Fiocco, A. J. (2016). Can anxiety damage the brain? Current Opinion in Psychiatry, 29(1), 56–63. https://doi.org/10.1097/YCO.0000000000000223
- Maier, S. F., Grahn, R. E., Kalman, B. A., Sutton, L. C., Wiertelak, E. P., & Watkins, L. R. (1993). The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behavioral Neuroscience, 107(2), 377–388. https://doi.org/10.1037//0735-7044.107.2.377
- Maren, S. (2003). The amygdala, synaptic plasticity, and fear memory. Annals of the New York Academy of Sciences, 985, 106–113. https://doi.org/10.1111/j.1749-6632.2003.tb07075.x
- Mason, J. W. (1975). A historical view of the stress field. Journal of Human Stress, 1(1), 6–12 contd. https://doi.org/10.1080/0097840X.1975.9940399
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. https://doi.org/10.1001/archinte.1993.00410180039004
- Mcfetridge, J. A., & Yarandi, H. (1997). Cardiovascular function during cognitive stress in men before and after coronary artery bypass grafts. Journal of Nursing Research. 46(4), 188–194. https://doi.org/10.1097/00006199-199707000-00002
- McLean, C. P., Asnaani, A., Litz, B. T., & Hofmann, S. G. (2011). Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research, 45(8), 1027–1035. https://doi.org/10.1016/j.jpsychires.2011.03.006
- Mental Health Foundation. (2016). Fundamental facts about mental health 2016. https://www.mentalhealth.org.uk/sites/default/files/fundamental-facts-about-mental-health-2016.pdf
- Mezadri, T. J., Batista, G. M., Portes, A. C., Marino-Neto, J., & Lino-de-Oliveira, C. (2011). Repeated rat-forced swim test: Reducing the number of animals to evaluate gradual effects of antidepressants. Journal of Neuroscience Methods, 195(2), 200–205. https://doi.org/10.1016/j.jneumeth.2010.12.015
- Motta, S. C., Carobrez, A. P., & Canteras, N. S. (2017). The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neuroscience and Biobehavioral Reviews, 76(Pt A), 39–47. https://doi.org/10.1016/j.neubiorev.2016.10.012
- Naughton, M., Dinan, T. G., & Scott, L. V. (2014). Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handbook of Clinical Neurology, 124, 69–91. https://doi.org/10.1016/b978-0-444-59602-4.00005-8
- Nielsen, M. B., & Knardahl, S. (2014). Coping strategies: A prospective study of patterns, stability, and relationships with psychological distress. Scandinavian Journal of Psychology, 55(2), 142–150. https://doi.org/10.1111/sjop.12103
- Olff, M. (2017). Sex and gender differences in post-traumatic stress disorder: An update. European Journal of Psychotraumatology, 8(sup4), 1351204. https://doi.org/10.1080/20008198.2017.1351204
- Øverli, Ø., Sørensen, C., Pulman, K. G., Pottinger, T. G., Korzan, W., Summers, C. H., & Nilsson, G. E. (2007). Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neuroscience and Biobehavioral Reviews, 31(3), 396–412. https://doi.org/10.1016/j.neubiorev.2006.10.006
- Perez-Tejada, J., Garmendia, L., Labaka, A., Vegas, O., Gómez-Lazaro, E., & Arregi, A. (2019). Active and passive coping strategies: Comparing psychological distress, cortisol, and proinflammatory cytokine levels in breast cancer survivors. Clinical Journal of Oncology Nursing, 23(6), 583–590. https://doi.org/10.1188/19.cjon.583-590
- Perusini, J. N., & Fanselow, M. S. (2015). Neurobehavioral perspectives on the distinction between fear and anxiety. Learning & Memory, 22(9), 417–425. https://doi.org/10.1101/lm.039180.115
- Porsolt, R. D., Anton, G., Blavet, N., & Jalfre, M. (1978). Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology, 47(4), 379–391. https://doi.org/10.1016/0014-2999(78)90118-8
- Porsolt, R. D., Le Pichon, M., & Jalfre, M. (1977). Depression: A new animal model sensitive to antidepressant treatments. Nature, 266(5604), 730–732. https://doi.org/10.1038/266730a0
- Price, J. L., & Drevets, W. C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences, 16(1), 61–71. https://doi.org/10.1016/j.tics.2011.12.011
- Roelofs, K. (2017). Freeze for action: Neurobiological mechanisms in animal and human freezing. Philosophical Transactions of the Royal Society B, 372, 206. https://doi.org/10.1098/rstb.2016.0206
- Rohleder, N. (2019). Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology, 105, 164–171. https://doi.org/10.1016/j.psyneuen.2019.02.021
- Santagostino, G., Amoretti, G., Frattini, P., Zerbi, F., Cucchi, M., Preda, S., & Corona, G. (1996). Catecholaminergic, neuroendocrine and anxiety responses to acute psychological stress in healthy subjects: Influence of alprazolam administration. Neuropsychobiology, 34(1), 36–43. https://doi.org/10.1159/000119289
- Santos, V. A., Carvalho, D. D., Van Ameringen, M., Nardi, A. E., & Freire, R. C. (2019). Neuroimaging findings as predictors of treatment outcome of psychotherapy in anxiety disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 91, 60–71. https://doi.org/10.1016/j.pnpbp.2018.04.001
- Selye, H. (1976). Stress in health and disease. Butterworths.
- Sequeira-Cordero, A., Salas-Bastos, A., Fornaguera, J., & Brenes, J. C. (2019). Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Scientific Reports, 9(1), 17403. https://doi.org/10.1038/s41598-019-53624-1
- Silva, B. A., Gross, C. T., & Graff, J. (2016). The neural circuits of innate fear: Detection, integration, action, and memorization. Learning & Memory, 23(10), 544–555. https://doi.org/10.1101/lm.042812.116
- Stepanichev, M., Manolova, A., Peregud, D., Onufriev, M., Freiman, S., Aniol, V., Moiseeva, Y., Novikova, M., Lazareva, N., & Gulyaeva, N. (2018). Specific activity features in the forced swim test: Brain neurotrophins and development of stress-induced depressive-like behavior in rats. Neuroscience, 375, 49–61. https://doi.org/10.1016/j.neuroscience.2018.02.007
- Strekalova, T., Couch, Y., Kholod, N., Boyks, M., Malin, D., Leprince, P., & Steinbusch, H. M. (2011). Update in the methodology of the chronic stress paradigm: Internal control matters. Behavioral and Brain Functions, 7, 9. https://doi.org/10.1186/1744-9081-7-9
- Sun, Y., Gooch, H., & Sah, P. (2020). Fear conditioning and the basolateral amygdala. F1000Research, 9, 53. https://doi.org/10.12688/f1000research.21201.1
- Teixeira, R. C., Zangrossi, H., & Graeff, F. G. (2000). Behavioral effects of acute and chronic imipramine in the elevated T-maze model of anxiety. Pharmacology, Biochemistry, and Behavior, 65(4), 571–576. https://doi.org/10.1016/S0091-3057(99)00261-0
- van Oortmerssen, G. A., & Bakker, T. C. (1981). Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behavior Genetics, 11(2), 115–126. https://doi.org/10.1007/BF01065622
- Veenema, A. H., & Neumann, I. D. (2007). Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol, 70(4), 274–285. https://doi.org/10.1159/000105491
- Veenema, A. H., Koolhaas, J. M., & de Kloet, E. R. (2004). Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Annals of the New York Academy of Sciences, 1018, 255–265. https://doi.org/10.1196/annals.1296.030
- Viana, M. B., Tomaz, C., & Graeff, F. G. (1994). The elevated T-maze: A new animal model of anxiety and memory. Pharmacology, Biochemistry, and Behavior, 49(3), 549–554. https://doi.org/10.1016/0091-3057(94)90067-1
- Vinader-Caerols, C., Monleón, S., Simon, V., & Parra, A. (1999). Learned immobility is also involved in the forced swimming test in mice. Psicothema, ISSN 0214-9915, 11, N(2), 239–246.
- West, A. P. (1990). Neurobehavioral studies of forced swimming: The role of learning and memory in the forced swim test. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 14(6), 863–877. https://doi.org/10.1016/0278-5846(90)90073-P
- Widman, A. J., Cohen, J. L., McCoy, C. R., Unroe, K. A., Glover, M. E., Khan, A. U., Bredemann, T., McMahon, L. L., & Clinton, S. M. (2019). Rats bred for high anxiety exhibit distinct fear-related coping behavior, hippocampal physiology, and synaptic plasticity-related gene expression. Hippocampus, 29(10), 939–956. https://doi.org/10.1002/hipo.23092
- Willner, P. (2017). The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiology of Stress, 6, 78–93. https://doi.org/10.1016/j.ynstr.2016.08.002
- Wood, S. K., & Bhatnagar, S. (2015). Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiology of Stress, 1, 164–173. https://doi.org/10.1016/j.ynstr.2014.11.002
