ABSTRACT
Objective: The intestinal microbiota is acknowledged to be essential in brain development and behaviour. Their composition can be modulated by prebiotics such as short-chain galacto-oligosaccharides (scGOS) and long-chain fructo-oligosaccharide (lcFOS). Several studies reported potential health benefit of prebiotics on behaviour. As the prebiotic mixture of scGOS and lcFOS is included in infant formula, we investigated the effects of dietary supplementation with this specific mixture from the day of birth onwards on behaviour and intestinal microbiota development in mice.
Method: Healthy male BALB/cByJ mice received, from day of birth, a dietary supplement with or without 3% scGOS:lcFOS (9:1). Behavioural tests were performed pre-weaning, in adolescence, early adulthood and adulthood. We assessed faecal microbiota compositions over time, caecal short-chain fatty acids as well as brain mRNA expression of Htr1a, Htr1b and Tph2 and monoamine levels.
Results: Compared to control fed mice, scGOS:lcFOS fed mice showed reduced anxiety-like and repetitive behaviour over time and improved social behaviour in adulthood. The serotonergic system in the prefrontal cortex (PFC) and somatosensory cortex (SSC) was affected by the scGOS:lcFOS. In the PFC, mRNA expression of brain-derived neurotrophic factor (Bdnf) was enhanced in scGOS:lcFOS fed mice. Although the bacterial diversity of the intestinal microbiota was unaffected by the scGOS:lcFOS diet, microbiota composition differed between the scGOS:lcFOS and the control fed mice over time. Moreover, an increased saccharolytic and decreased proteolytic fermentation activity were observed in caecum content.
Discussion: Supplementing the diet with scGOS:lcFOS from the day of birth is associated with reduced anxiety-like and improved social behaviour during the developmental period and later in life, and modulates the composition and activity of the intestinal microbiota in healthy male BALB/c mice. These data provide further evidence of the potential impact of scGOS:lcFOS on behaviour at several developmental stages throughout life and strengthen the insights in the interplay between the developing intestine and brain.
Introduction
The trillions of microbes colonizing our intestine, collectively referred to as the intestinal microbiota, have an essential role in health and disease throughout life [Citation1–3]. The establishment of the intestinal microbiota is influenced by several factors, such as gestational age, delivery-mode and nutrition (e.g. breast- or formula-feeding) and early life antibiotic exposure [Citation4]. These factors critically influence the optimal maturation of the metabolic, immune and neurological systems [Citation4], i.e. alterations of intestinal microbiota composition (e.g. due to antibiotic-exposure and cesarean section) can increase the risk of developing metabolic-related (e.g. obesity), immune-related (e.g. allergies), but also behaviour-related brain disorders (e.g. autism spectrum disorder) [Citation3,Citation5].
In both human and mice, microbial compositional changes can lead to behavioural changes [Citation1,Citation2,Citation6,Citation7]. The importance of the intestinal microbiota for brain development and behaviour has been demonstrated in animals treated with antibiotics or in germ-free animals. Compared with conventional colonized mice, germ-free mice show social deficits and reduced anxiety [Citation1]. In addition, germ-free mice also have altered neurochemical messenger systems [Citation8]. Antibiotic-treated mice show cognitive impairments, reduced anxiety and reduced sociability [Citation7]. Interestingly, behavioural impairments caused by depletion of the microbiota either with antibiotics or in germ-free mice were ameliorated by bacterial re-colonization or treatment with probiotics, indicating that changes in the microbiota are closely connected to behaviour [Citation1,Citation7].
Prebiotics modulate the growth and activity of the intestinal microbiota and several studies reported the capability of prebiotics to also modulate behaviour. The prebiotic mixture of galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS) (1:1) reduced anxiety-like and depression-like behaviour in adult mice [Citation2]. Moreover, a prebiotic mixture of polydextrose and GOS administered during early life, diminished stress-induced behaviour in juvenile rats, improved recognition memory and increased explorative behaviour in young pigs [Citation9,Citation10].
Human milk is known to contain substrates with prebiotic function, known as human milk oligosaccharides, to specifically shape the intestinal microbiota of new-borns [Citation11]. Also, the human milk oligosaccharide 2′-fucosyllactose (2′FL) possess cognitive modulatory capacities; rat pups exposed to 2′FL during lactation showed improved cognition in adulthood [Citation12].
The specific prebiotic mixture of 90% of the low molecular mass short-chain GOS and 10% of the high molecular mass long-chain FOS (scGOS:lcFOS, 9:1) similar to the molecular size distribution of human milk oligosaccharides is included in infant formula and have been shown to exert similar functionalities as human milk oligosaccharides [Citation11]. These prebiotic carbohydrates can be digested by specific bacteria, such as Bifidobacterium and Bacteroides spp. [Citation4], and subsequently fermented into short-chain fatty acids (SCFAs), which are reported to have neuroactive properties locally and systemically [Citation5,Citation11]. As the behavioural modulatory effects of this specific mixture of scGOS:lcFOS (9:1) is not yet known, we investigated, from the day of birth, in healthy male BALB/c mice, the effects of this specific prebiotics mixture scGOS:lcFOS (9:1) on behaviour and intestinal microbiota development, both in taxonomic and in SCFA-composition.
We showed that dietary supplementation with scGOS:lcFOS from birth in healthy male BALB/c mice reduced anxiety-like and stereotypic behaviour over time and improved social behaviour in adulthood. These behavioural improvements were associated with marked differences in microbiota composition and microbiota metabolite composition. In addition, the improved behaviour induced by scGOS:lcFOS was accompanied by altered monoamine levels, mRNA expression of serotonergic markers and BDNF mainly in the prefrontal cortex (PFC).
Material and methods
Animals
Sixteen days pregnant BALB/cByJ mice were purchased from Charles River Laboratories (Maastricht, The Netherlands). From the day of birth of the litter (post-natal day zero, PND0) the dams were allocated to either the control (n = 6, pups n = 17) or the 3% scGOS:lcFOS (n = 5, pups n = 14) enriched diet. After weaning on PND21, the male offspring (n = 10 per group, n = 1–3 from each litter) continued the same diet as allocated to their mother. Additional male offspring (n = 9) from control fed dams were used as interaction mice in the social interaction test (more details in the supplement). All animal experimental procedures were carried out in compliance with national legislation following the EU-Directive for the protection of animals used for scientific purposes, and were approved by the Ethical Committee for Animal Research.
Diets
Based on the standard AIN-93G control diet, the enriched diet consisted of a 3% (w/w) mixture of short-chain galacto-oligosaccharides (scGOS) (degree of polymerization 2-8) and long-chain fructo-oligosaccharides (lcFOS) (degree of polymerization on average ≥ 23) in a 9:1 (w/w) ratio added in an isocaloric manner (table S1). Both diets were obtained from Research Diet Services (Wijk bij Duurstede, The Netherlands).
Experimental design
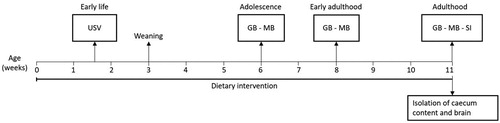
shows the experimental design. At several ages across lifespan, behavioural tests for anxiety, stereotypic and social behaviour were conducted. In the pre-weaning period, ultrasonic distress vocalization of the male offspring was measured (1.5 weeks old). During adolescence (6 weeks old) and early adulthood (8 weeks old), the marble burying test and self-grooming behaviour were assessed. During adulthood (11 weeks old) the social interaction test was conducted in addition to the marble burying and the self-grooming test. Faecal pellets were collected at 4, 6, 8 and 11 weeks of age and from the dams before starting dietary supplementation. The mice were euthanized by decapitation to collect caecum and brain. Due to limited brain material of the PFC, amygdala and hippocampus n = 5 was used to measure monoamine levels and n = 5 for qPCR.
Figure 1. Schematic overview of the experimental protocol and the conducted behavioural tests. The mice received either a control diet or a diet enriched with 3% scGOS:lcFOS (9:1) from the day of birth. The ultrasonic distress vocalization test was conducted during infancy. At 3 weeks of age mice were weaned and the male mice continued the allocated diet of the dam. Grooming behaviour and the marble burying test were performed during adolescence, early adulthood and adulthood and the social interaction test was performed during adulthood. After the last behavioural test organs were collected. USV: ultrasonic distress vocalisation. GB: Grooming behaviour. MB: Marble burying test. SI: Social interaction test.
Behavioural tests
Ultrasonic distress vocalization (USV) of male offspring was measured to determine anxiety-like behaviour as described previously [Citation13]. The marble burying test assesses anxiety-like and stereotypic/repetitive behaviour [Citation14]. Mice were scored for spontaneous self-grooming behaviour as previously described [Citation15]. The sociability of the mice was assessed with a previously described social interaction test [Citation15]. Time spent in the interaction zone near the cage of the unfamiliar target mouse; latency to first approach of the interaction zone and total distance moved were analysed (more details in the supplement).
Measurement of monoamine levels
The pre-frontal cortex (PFC), amygdala (AM), dorsal hippocampus (DH) and the somatosensory cortex (SSC) were, with the previously described HPLC method [Citation16], measured for levels of tryptophan, serotonin (5-hydroxytryptamin, 5-HT) and its metabolite 5-hydroxyindolacetic acid (5-HIAA), noradrenalin (NA), dopamine (DA) and its metabolites dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine (3-MT) and homovanillic acid (HVA) (details in the supplement). The concentrations of each compound were calculated by comparison with both external and internal standards.
RNA isolation, cDNA synthesis and qRT-PCR
RNA was isolated from PFC, SSC, amygdala and hippocampus using the RNeasy isolation kit (Qiagen) and cDNA was synthesised using iScript cDNA synthesis kit (BioRad) following manufacturer’s protocol. Quantitative real-time PCR was performed on a CFX96 real-time PCR detection system using iQ SYBR green supermix (BioRad) and primers (Qiagen) for Rps13 (house-keeping gene), 5-hydroxytryptamin 1a receptor (Htr1a), Htr1b, brain-derived neurotrophic factor (Bdnf) and tryptophan hydroxylase 2 (Tph2) (details in the supplement). The mRNA expression of the gene of interest was normalized to the housekeeping gene and data are presented as fold change in expression compared to control mice.
Short-chain fatty acids (SCFAs) levels in caecum content
The levels of short-chain fatty acids (SCFAs): acetic (AA), propionic (PA), butyric (BA), and valeric acid (VA), as well as the branched short-chain fatty acids; isobutyric (iBA) and isovaleric (iVA) acid in the caecum content were quantified with gas chromatography, as described previously [Citation17] (details in the supplement).
Bacterial DNA extraction from faecal pellets
Total DNA was extracted from faecal pellets. The pellets were mixed with 350 μL S.T.A.R. buffer (Roche, Basel, Switzerland), followed by three 1-minute rounds of bead beating (25 g of 0.1 mm zirconia beads plus five 2.5 mm glass beads) on a FastPrep instrument (MP Biomedicals, Santa Ana, California, USA) at a power level of 5.5. The sample was heated to 95°C and mixed by shaking at 100 rpm for 15 min. The homogenate was centrifuged at 4°C for 5 min at 14,000 g to pellet stool particles. The supernatant, containing DNA, was collected and the pellet was subjected to another extraction round with half the volume of S.T.A.R. buffer. Supernatants were pooled per sample and mixed, and 250 μL was further purified on the Maxwell 16 MDx instrument using the cartridge preparation from the Maxwell 16 tissue LEV total RNA purification kit as described by the manufacturer (Promega, Madison, WI, USA). DNA quality and quantity were assessed with the NanoDrop 2000 spectrophotometer (Thermo Fisher, Waltham, Massachusetts, USA). DNA extracts were diluted to the same concentration before preparation for 16S rRNA-gene sequencing.
Gut microbiota profiling by 16S rRNA-gene sequencing
Extracted faecal DNA was used for microbiota profiling by 16S rRNA-gene sequencing. The V3-V4 region of the 16S rRNA-gene was PCR-amplified with universal primers S-D-Bact-0341-b-S-17 primer (forward 5′-CCTACGGGNGGCWGCAG-3′) and S-D-Bact-0785-a-A-21 primer (reverse 5′-GACTACHVGGGTATCTAATCC-3’) [Citation18] and barcoded using a two-step PCR protocol as described by Van den Bogert et al. [Citation19]. Sequencing was performed on an Illumina HiSeq sequencing platform (Illumina, USA) in a 300 bp paired-end mode.
Bioinformatic and analysis of 16S rRNA sequencing data
The ‘Quantitative Insights Into Microbial Ecology’ (QIIME) v1.9.0 package was used to analyse sequence data [Citation20]. Settings for demultiplexing and merging of paired-end sequences were as recommended by QIIME (according http://qiime.org/tutorials/processing_illumina_data.html). Sequences were clustered into Operational Taxonomic Units (OTUs) based on 97% sequence identity using VSEARCH v2.03 with chimera checking against the RDP gold database [Citation21,Citation22]. Taxonomic assignment was performed using the RDP classifier against the SILVA119 database [Citation23]. The species diversity (α-diversity) was calculated using the Chao1 and Shannon index for diversity [Citation24] with correction for the differences in sequencing depths (number of reads per sample) by rarefaction.
Statistical analysis
Statistical analyses were performed comparing the control diet group and the scGOS:lcFOS diet group. Fisher’s exact test was performed to determine whether the number of pups emitting calls during the USV test differed between the control diet group and the scGOS:lcFOS diet group. The USV, grooming, marble burying and social interaction tests were analysed with linear mixed models, controlled for litter effect and post hoc Sidak’s multiple comparison test, repeated measures were added in the analysis of grooming and marble burying tests. All other data were statistically analysed with an unpaired, two-tailed Student’s t-test. When not normally distributed or unequal variances, data were transformed taking the common logarithm before statistical analysis. The Mann–Whitney test was used to analyse differences between the dietary groups for latency of first approach to the target mouse during the social interaction test as the variance was still unequal after transformation. Correlations were analysed using the Spearman correlation. Results were considered statistically significant when P < 0.05. Analyses were performed using SPSS version 24 and GraphPad Prism Software version 7.03 for Windows (GraphPad Prism software, La Jolla, CA. USA).
16S rRNA-gene sequencing
The species richness and diversity (α-diversity) indexes calculated in QIIME from the 16S rRNA-gene sequencing data were analysed at one single rarefied sequencing depth. Differences between treatment groups over time were tested by two-way ANOVA with Sidak’s multiple comparison test using GraphPad Prism version 7.03 for Windows.
The non-rarefied OTU tables obtained from QIIME were aggregated at the bacterial genus level. Genera present in less than 30% of the samples or with an average relative abundance less than 0.005% were omitted from the analysis. Statistical comparisons were performed by applying a combination of multivariate analysis with Canoco 5 software [Citation25], followed by differential abundance testing using the R-package MetagenomeSeq [Citation26]. Firstly, the constrained ordination method, principal response curves (PRC), was used to test time-dependent treatment effects [Citation27]. The Monte Carlo Permutation test (MCPT), with 1000 permutations, was used to evaluate statistical significance (P < 0.05) of the resulting model. Next, the top 10 responding bacterial taxa identified from the model were evaluated on differential abundances at the different timepoints by metagenomeSeq using the zero-inflation log-normal distribution (FitFeatureModel) as recommended by the developers of the package [Citation28]. The Benjamini–Hochberg false-discovery rate (FDR) was used to correct for multiple comparisons in the differential abundance tests [Citation29], and statistical significance was considered for FDR < 0.1 when observed for at least two repeated measurements.
Results
Ultrasonic distress vocalization
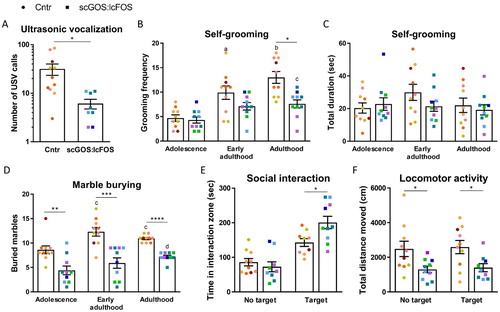
Ultrasonic distress vocalization was measured 10 days after birth. The number of male pups emitting at least one call was not significantly different between the dietary groups (table S2, P > 0.05). The pups emitting at least one call were used for further analysis of the USV. The total number of calls was significantly reduced in pups from lactating dams receiving the scGOS:lcFOS diet (P < 0.05; (A)). The average frequency (Hz) showed a trend towards a decrease in the pups from scGOS:lcFOS diet fed dams (P < 0.06) and the average time of a call was not significantly different, figure S1.
Figure 2. Anxiety-like, repetitive and social behavioural effects of scGOS:lcFOS. (A) scGOS:lcFOS receiving mice emitted significantly less ultrasonic vocalisation calls (USV). (B) Grooming frequency significantly increased over time in both control and scGOS:lcFOS receiving mice (diet: P = 0.08, age: P < 0.000001, interaction: P < 0.05). In adulthood the grooming frequency was significantly lower in the scGOS:lcFOS receiving mice compared with the control mice. (C) The grooming duration was unaffected over time and also unaffected by scGOS:lcFOS (diet: ns, age: ns, interaction: ns). (D) The number of buried marbles was increased across age independent of the dietary supplementation (diet: P < 0.0001, age: P < 0.001, interaction: P > 0.05). In early adulthood and adulthood the scGOS:lcFOS receiving mice buried significantly less marbles compared with control. (E) In the presence of a target mouse the scGOS:lcFOS receiving mice spent more time in the interaction zone compared with control. No differences between the groups in time the mice spent in the zone in absence of target. One scGOS:lcFOS receiving mouse in absence of target and one control mouse in presence of target were excluded as significant outliers (Grubbs test 293 s and 0 s, respectively). (F) The locomotion activity measured by distance moved was decreased in the scGOS:lcFOS receiving mice in both absence and presence of a target mouse. One scGOS:lcFOS receiving mouse in presence of target was excluded as significant outlier (Grubbs test 6200 cm).The data are shown as individually data points, colour indicating the litter, and mean +/− SEM. A-F were analysed with linear mixed models followed by Sidak’s multiple comparison post-hoc test. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, a=*** compared with adolescence within diet group, b=****** compared with adolescence within diet group, c=* compared with adolescence within diet group, d=** compared with adolescence within diet group, ns = not significant. A: control group n = 11, scGOS:lcFOS group n = 7, B-F: n = 9–10 per group.
Self-grooming and marble burying
The grooming frequency trended towards an overall effect by diet (control vs scGOS:lcFOS) (F(1, 7.241) = 4.055, P = 0.083) and overall age was significantly affected (F(2, 18) = 32.667, P < 0.000001) ((B)). The change in grooming frequency over time was dependent on the interaction effect between diet and age (F(2, 18) = 4.347, P < 0.05). The grooming frequency increased over time from adolescence to adulthood in control diet fed mice (adolescence vs early adulthood P < 0.001, adolescence vs. adulthood P < 0.000001). A significant increase in grooming frequency was only observed between adolescence and adulthood in the scGOS:lcFOS fed mice (P < 0.01). Although no significant differences were observed between the diets in grooming frequency in adolescence and early adulthood, in adulthood the mice receiving the scGOS:lcFOS diet showed a significantly lower grooming frequency compared with mice receiving the control diet (P < 0.05). However, the scGOS:lcFOS diet displayed no effect on self-grooming duration neither over time nor at any age ((C)).
The number of buried marbles was significantly affected by diet (control vs scGOS:lcFOS) (F(1, 13.337) = 39.208, P < 0.0001) and age (F(2, 18) = 9.64, P < 0.001). The number of marbles buried over time was independent on the diet (interaction effect between diet and age (F(2, 18) = 2.505, P > 0.05). Mice receiving the control diet buried significantly more marbles in early adulthood and adulthood compared to adolescence (P < 0.05). The scGOS:lcFOS fed mice buried significantly more marbles in adulthood compared with adolescence (P < 0.01). From adolescence and onwards the mice exposed to the scGOS:lcFOS diet demonstrated a significant decrease in the number of buried marbles compared to control mice (adolescence P < 0.01, early adulthood P < 0.001, adulthood P < 0.0001) ((D)).
Social interaction
The scGOS:lcFOS fed adult mice spent as much time in the interaction zone as the control mice in absence of a target mouse (P > 0.05). However, in presence of a target mouse, mice exposed to the scGOS:lcFOS diet spent significantly more time in the interaction zone than did the control mice (P < 0.05) ((E)). The latency of first approach to the target mouse and the frequency a mouse entered the interaction zone did not significantly differ between the two dietary groups with either the absence or presence of a target mouse (P > 0.05) (figure S1). The locomotion activity measured as total distance moved through the arena was significantly less in the scGOS:lcFOS receiving mice in both absence and presence of a target mouse (P < 0.05) ((F)).
Monoamine levels in several brain regions
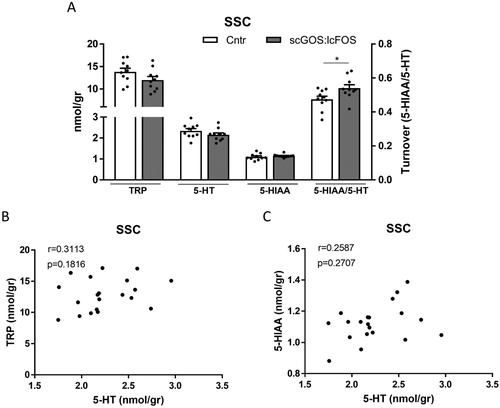
In the PFC, tryptophan (P < 0.05) and 5-HT (P < 0.05) levels were significantly decreased in the scGOS:lcFOS dietary group compared with the control group ((A)) and the NA, DA and DA metabolites levels were unchanged (figure S2). In the SSC the individual monoamines were unchanged; however, the 5-HT turnover indicated by 5-HIAA/5-HT was significantly higher in the scGOS:lcFOS fed group (P < 0.05) ((A) and figure S2). The tryptophan and 5-HT levels as well as the 5-HT and the 5HIAA levels correlated significantly in the PFC (Spearman correlation were respectively r = 0.782, P < 0.05 and r = 0.673, P < 0.05) ((B,C)), whereas no significant correlations between these monoamines were present in the SCC ((B,C)). In the AM and the DH no significant differences in the monoamine levels and their metabolites were observed between the control and scGOS:lcFOS diet groups (figure S3).
Figure 3. The PFC levels of tryptophan, 5-HT, the 5-HT metabolite 5-HIAA and the serotonin turnover (5-HIAA/5-HT). (A) The tryptophan level was significantly decreased in the scGOS:lcFOS receiving mice. The serotonin level was significantly decreased in the scGOS:lcFOS receiving mice. The 5-HIAA level and the serotonin turnover were not significantly different. The tryptophan levels and the serotonin levels (B) as well as the 5-HT and the 5-HIAA levels (C) correlated significantly. (A) Data shown as individually data points and mean +/− SEM. A: Analysed by student t-test; (B,C) analysed by spearman correlation. * P < 0.05. A–C: n = 5 per group. TRP: tryptophan, 5-HT: 5-hydroxytryptamine (serotonin), 5-HIAA: 5-hydroxyindolacetic acid.
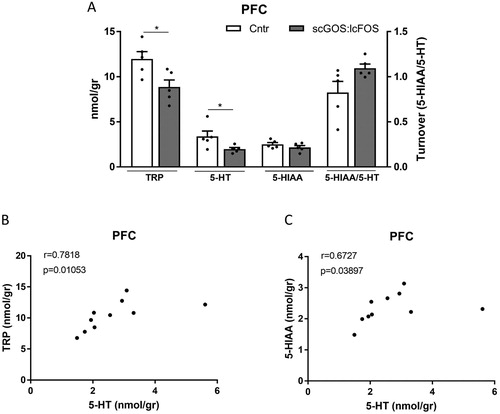
Figure 4. The SSC levels of tryptophan, 5-HT, the 5-HT metabolite 5-HIAA and the serotonin turnover (5-HIAA/5-HT). (A) The tryptophan, 5-HT and 5-HIAA levels were not significantly different between the scGOS:lcFOS and control diet receiving mice. The serotonin turnover was significantly increased in the scGOS:lcFOS receiving mice. The tryptophan and the 5-HT levels (B) as well as the 5-HT and the 5-HIAA levels (C) did not correlate. (A) Data shown as individually data points and mean +/− SEM. A: Analysed by student t-test; (B,C): Analysed by spearman correlation. * P < 0.05. A: n = 10 per group; (B, C) n = 10. TRP: tryptophan, 5-HT: 5-hydroxytryptamine (serotonin), 5-HIAA: 5-hydroxyindolacetic acid.
Serotonergic system
The mRNA expression of Htr1a was significant decreased in the PFC (P < 0.01) in the scGOS:lcFOS receiving mice, whereas the expression of Htr1b was unchanged. As the tryptophan and the 5-HT levels in the PFC were decreased in the mice receiving the scGOS:lcFOS diet, scGOS:lcFOS might influence the 5-HT synthesis from tryptophan. This is regulated by tryptophan hydroxylase 2 (TPH2). However, the mRNA expression of Tph2 was unaffected by the dietary intervention ((A–C)). In the SSC the mRNA expression of Htr1a trended towards a decrease (P < 0.1) in the scGOS:lcFOS receiving mice, whereas the expression of Htr1b and Tph2 were unchanged ((E–G)). In the AM and the DH, the mRNA expression of the tested markers was unchanged between the dietary groups (figure S4). These data are preliminary as the sample size was small.
Figure 5. The mRNA expression of Htr1a, Htr1b, Tph2 and Bdnf in the PFC and SSC. (A) In the PFC the mRNA expression of 5ht1a was significantly decreased in the scGOS:lcFOS receiving mice. The Htr1b (B) and Tph2 (C) mRNA expressions were unchanged. The mRNA expression of Bdnf was significantly increased in the scGOS:lcFOS receiving mice (D). In the SSC the mRNA expression of Htr1a trended towards a decrease in the scGOS:lcFOS group (E). The Htr1b (F), Tph2 (G) and Bdnf (H) mRNA expression was not significantly changed between the groups. A-H: Data shown as individually data points and mean +/− SEM. A-H: Analysed by student t-test. * P < 0.05, ** P < 0.01. (A–H) n = 3–5 per group. PFC: prefrontal cortex, SSC: somatosensory cortex, Htr1a: serotonin receptor 1a, Htr1b: serotonin 1b receptor, Tph2: tryptophan hydroxylase 2, Bdnf: Brain derived neurotrophic factor.
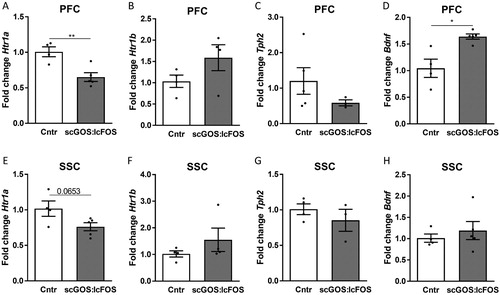
Brain-derived neurotrophic factor (Bdnf)
The mRNA expression of Bdnf significantly increased in the PFC (P < 0.05) ((D)), but not in the SSC ((H)) or AM (figure S5) of the scGOS:lcFOS mice.
Caecal SCFAs and branched SCFAs levels
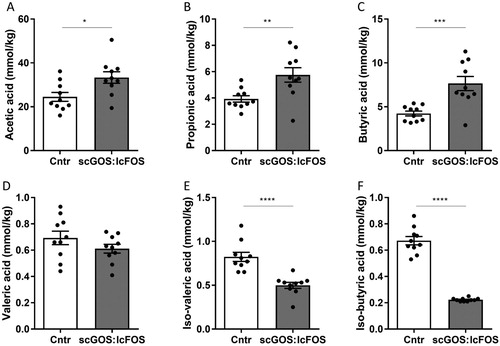
The absolute levels of the bacterial fermentation end-products, AA (P < 0.05), PA (P < 0.01) and BA (P < 0.01) were increased in the caecum content of the mice fed the scGOS:lcFOS diet ((A–C)). In contrast, compared to the control group, the levels of protein-derived fermentation end-products VA (not significant, (D)), and the branched SCFAs iBA and iVA all decreased in the scGOS:lcFOS group (P < 0.0001 for both, (E,F)).
Figure 6. Caecal SCFA. The levels of the SCFAs acetic (A), propionic (B) and butyric (C) acids were significantly increased in the scGOS:lcFOS group. (D) The level of valeric acid was unaffected by scGOS:lcFOS and the levels of the branched SCFA iso-valeric acid (E) and iso-butyric acid (F) were significantly decreased in the scGOS:lcFOS group. (A–F) Data shown as individually data points and mean +/− SEM. A-F: Analysed by student t-test. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. (A–F): n = 10 per group. SCFA: short-chain fatty acids.
Faecal microbiota composition
The 16S rRNA-gene sequencing of the collected faecal pellets resulted in an average sequence depth of 351,548 (SD = 220,402) reads per sample (table S3). Species richness by Chao1 index was significantly decreased in scGOS:lcFOS vs control at week 4 (P < 0.01). The species diversity (based on Shannon index) was not significantly different between the dietary groups at the different ages ((B)). The PRC ordination method [Citation27] was used to assess the age dependent effects on the intestinal microbiota composition in scGOS:lcFOS fed mice compared to control fed mice. The microbial composition over time was significantly dependent on the diet (MCPT interaction, P = 0.001). The top 10 associated bacterial genera from this PRC analysis were further evaluated by differential abundance testing, which confirmed that 9 of the 10 identified genera were differentially abundant for at least 2 repeated measurements ((C) and table S4). In sum, an increase of 3 genera within the order of Bacteroidales was observed for scGOS:lcFOS compared to control, as well as an increase of the genus Dorea within the family of Lachnospiraceae and an unknown genus of the family Erysipelotrichaeae. In contrast, a decrease was observed for 3 unknown genera within the family of Peptostreptococcaceae, and the genus Anaerovorax within the Clostridiales family XIII ((A,C)).
Figure 7. Faecal microbiota. (A) Taxa composition of each mouse per group and age at the bacterial phylum and order level. Only taxa with a relative abundance > 0.1% on average (based on number of reads per total reads) are plotted in the stacked area chart. Sorted from largest to smallest relative abundance. (B) Chao1 richness and Shannon species diversity plotted as box plots with whiskers showing the range, boxes the interquartile range and the line at median. The ‘+’ shows the average Chao1 and Shannon index per group and age. Statistics performed with two-way ANOVA with Sidak’s multiple comparisons test comparing scGOS:lcFOS with control per timepoint (** P < 0.01). Rarefaction depth used for summary of diversity: 27,242 reads per sample. (C) PRC with the top 10 responding bacterial genera for treatment over time with adjustment for age. The treatments are presented as a single response curves over time (on the horizontal axis) with the control-group as reference with zero PRC values (on the vertical axis) and so its curve lays over the horizontal axis. The top 10 bacterial genera are plotted on the separate vertical (one-dimensional) plot based on best fit with the first PRC axis. Monte Carlo Permutation Test (MCPT) with 1,000 permutations showed a significant interaction (P = 0.001) of treatment with time on the 1st PRC axis (78.1% explained variation). Differential abundance testing was performed with the metagenomeSeq-package on the top 10 identified bacterial taxa. The taxa that were confirmed to be significantly different at 2 or more timepoints (with correction for multiple testing by false discovery rate < 0.1) are shown in bold. The bacterial genera are detailed at the phylum level (Ba = Bacteroidetes, Fi = Firmicutes), the order, family and genus level. (D) PCA tri-plot of faecal microbiota composition of all mice in adulthood coloured by membership to the scGOS:lcFOS diet group and control group, respectively. Individual samples (dots) are plotted on the first two axes of the PCA-analysis with more similar compositions closer to each other. The bacterial genera that were identified from the PRC analysis (the top 10) are plotted as the black biplot arrows. The behavioural readouts and the SCFAs are supplemented as blue biplot arrows to visualize the correlations in adulthood. The bacterial genera are detailed at the phylum level (Ba = Bacteroidetes, Fi = Firmicutes), the order, family and genus level. PRC: Principal Response Curve, PCA: Principal Component Analysis, SI: Social Interaction, seconds in interaction zone in presence of target, MB: Number of buried marbles, GB: Grooming frequency.
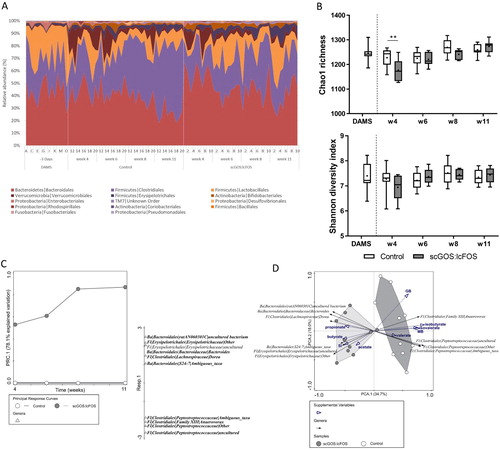
A principal component analysis (PCA) was used to evaluate the microbiota compositional differences in adulthood between the dietary groups and to correlate these with the adulthood behavioural assessments as well as the caecal concentrations of SCFAs ((D)). The PCA analysis confirmed the distinct microbiota composition of the two dietary groups. The compositional differences between scGOS:lcFOS and control in adulthood correlated with increased sociability, lower number of marbles buried and lower frequency in grooming behaviour. Moreover, as measured by the caecal levels of SCFAs, the microbial composition also correlated positively with the changes in microbial activity.
Discussion
Prebiotic fibres are known to be effective modulators of the intestinal microbiota composition and activity and may thereby positively influence health through immune- and neuromodulation [Citation30]. However, little is known about the effect of dietary prebiotic fibres on the development of the nervous system [Citation31]. Here, we demonstrated that dietary supplementation with scGOS:lcFOS in healthy male BALB/cByJ, started on the day of birth, was associated with altered intestinal microbiota composition and increased saccharolytic fermentation and decreased proteolytic fermentation activity. These microbial changes were accompanied with reduced stereotypic and anxiety-like behaviour throughout life and improved social behaviour in adulthood.
The first effect of scGOS:lcFOS on behaviour in our study was already observed in early life in the ultrasonic distress vocalization test. The pups from dams fed the scGOS:lcFOS diet, emitted fewer USV calls compared to control pups. This indicates reduced anxiety-like behaviour of the pups [Citation32] and might be a result of modulation of beneficial intestinal bacteria by scGOS:lcFOS leading to improved behaviour. On the other hand, a decreased number of USV calls by pups might indicate better maternal responsiveness [Citation33], which may lead to improved behaviour in the pups later in life. Possibly, the maternal effects of scGOS:lcFOS on the neurodevelopment of the pups could be additive to the direct effects of scGOS:lcFOS on the brain of the pups or more relevant. To confirm this, maternal behaviour has to be measured in future studies.
During adolescence, early adulthood and adulthood, anxiety-like behaviour was reduced in mice exposed to dietary scGOS:lcFOS. This is in line with the observation that a lower number of neonatal USV calls is related to reduced anxiety-like behaviour later in life [Citation34]. In adulthood, the reduced anxiety-like behaviour was accompanied by improved social interaction in the scGOS:lcFOS receiving mice. During the social interaction test, locomotion activity was reduced in mice fed the scGOS:lcFOS diet in presence and absence of target mouse. The reduced locomotion did not confound the time spent in the interaction zone. We used male BALB/c mice because behaviour-related brain disorders like autism spectrum disorder is more prevalent in males [Citation35] and the BALB/c behavioural phenotype is represented by low sociability and high levels of anxiety-like behaviour [Citation36]. Our findings that scGOS:lcFOS reduced anxiety-like behaviour and improved sociability is in agreement with a recently published study by Burokas et al. using a similar prebiotic combination [Citation2]. In their research, the prebiotic combination GOS:FOS (1:1) given during adulthood for 10 weeks reduced the anxiety-like behaviour in healthy mice [Citation2]. In contrast to our study, prebiotic supplementation had no effect on social interaction, however, the GOS:FOS receiving mice showed significantly increased number of prosocial events [Citation2]. Other prebiotic mixtures also have behavioural modulatory capacities; juvenile rats and young pigs exposed to a prebiotic mixture of polydextrose and GOS showed reduced stress-induced behaviour and improved explorative behaviour, respectively [Citation9,Citation10]. These behavioural changes were accompanied with increased Lactobacillus spp. [Citation9]. Together these results suggest the capability of prebiotic fibres to modulate behaviour.
In addition to the behavioural changes, scGOS:lcFOS also influenced the neurochemistry of the brain in adulthood. It significantly affected the serotonergic system mainly in the PFC indicated by reduced tryptophan and 5-HT levels and lower mRNA expression of the serotonin receptor 1A, however these are preliminary data due to small sample size. In the GOS:FOS study by Burokas et al. [Citation2], the plasma level of tryptophan was lowered after GOS:FOS administration. Together with our data this may indicate that the combination of GOS and FOS may be able to stimulate the growth and/or function of tryptophan utilizing intestinal bacteria. Several intestinal bacteria, for example Eschericheria coli, Lactobaccillus and Bacteroides [Citation37] possess tryptophanase, an enzyme that converts tryptophan into metabolites like indole [Citation38]. The scGOS:lcFOS induced changes of the microbiota could lead to lower availability of tryptophan for the brain leading to lower levels of 5-HT. The decreased level of 5-HT is in contrast with the monoamine theory stating that higher levels of 5-HT and tryptophan reduce depressive and anxiety symptoms [Citation39]. However, the monoamine theory is based on the available monoamine levels in the synaptic cleft and in this study, we measured total 5-HT levels including unreleased 5-HT in the presynaptic neurons. In other murine prebiotic studies, the total 5-HT level was either increased or unaffected in the PFC [Citation2] or the frontal cortex [Citation40]. However, in a comprehensive meta-study by Ruhé et al. [Citation41] systemic depletion of tryptophan and 5-HT did not lead to decreased mood in healthy human controls. Considering the available preliminary data, the causal role of changed 5-HT and tryptophan levels on behaviour in a healthy situation is inconclusive.
The 5-HT1A receptor has an essential role in anxiety [Citation42]. Activating the receptor with a 5-HT1A agonist induces anxiogenic effects which are inhibited by a 5-HT1A antagonist [Citation43]. In our study preliminary data showed that decreased (mRNA) Htr1a expression in the PFC was associated with reduced anxiety-like behaviour. Due to limited brain material the mRNA levels were measured in a small sample size and therefore a limitation of this study. During adolescence, signalling of 5-HT through the 5-HT1A receptor is important and its disruption can lead to an increase in anxiety later in life [Citation44]. The serotonin signalling during adolescence might be stimulated by scGOS:lcFOS supplementation and consequently lead to reduced anxiety-like behaviour later in life.
The neurotrophin BDNF is important in neural circuit development and in regulation of mature neural circuits [Citation45]. Higher BDNF levels in the hippocampus, striatum and hypothalamus are associated with improved social interaction in mice [Citation46, Citation47]. Bdnf mRNA and protein expression were enhanced in the hippocampus of adult mice and rats after prebiotic supplementation [Citation2, Citation48]. Although the hippocampal (mRNA) Bdnf level was unavailable in our study due to insufficient material, the (mRNA) Bdnf level in the PFC was increased in scGOS:lcFOS fed mice. This is in line with a study in healthy rats, in which a mix of prebiotics including GOS resulted in an increase of (mRNA) Bdnf in the PFC [Citation9], indicating a beneficial effect of specific prebiotics in behaviour development.
The prebiotics modulated the intestinal microbiota in the mice and led to differences in bacterial richness and marked differences in taxonomic composition. These included increased relative abundances of the genus Dorea and several genera of Bacteroidales and Erysipelotrichales, and decreased relative abundances of the genus Anaerovorax and several genera of Peptostreptococcacae (all within the order of Clostridiales). The prebiotic effects observed in these mice differ markedly from observations in human infants, in which prebiotics predominantly leads to increased levels of bifidobacteria [Citation11]. Although the mice receiving scGOS:lcFOS showed enhanced levels of bifidobacteria compared to control, the differences were not significant (figure S5). The modulatory effects of scGOS:lcFOS likely depends on the already resident mouse microbiota. This is supported by the findings in another mouse study, in which only the combination of scGOS:lcFOS with the probiotic Bifidobacterium breve M-16 V showed a bifidogenic effect in contrast to the intervention containing the prebiotic components only [Citation49]. Possibly, the mice specific bifidobacterial species are inefficient in utilizing scGOS:lcFOS or are outcompeted by other bacterial species. Indeed, we observed that the supplementation with scGOS:lcFOS mainly led to increased relative abundances of the more dominant Bacteroidales spp., which are known to degrade similar complex carbohydrates as used in this experiment [Citation50, Citation51]. Similarly to our study, Burokas et al. found increased levels of the Bacteroidales S24-7 group, which is a prominent but yet uncultured group in the murine gut that may have the machinery to degrade scGOS:lcFOS [Citation52]. However, the other bacterial groups reported to respond to the scGOS:lcFOS intervention were distinct from our observations. This could be due to several factors such as the different genetic background of the mice (BALB/c vs C57BL/6J), supplier, housing (group vs individual), diets and ratio of used prebiotic mixture, and location of the laboratory. Moreover, the selective effects of the prebiotics on the Bacteroidales spp. may have stimulated the putative butyrate-producing microbial groups of Erysipelotrichales [Citation53] through cross-feeding mechanisms [Citation4]. Therefore, investigating the composition of intestinal microbiota as a whole in relation to in depth metabolomics is important to obtain functional information on precisely how the intestinal microbiota is influenced by scGOS:lcFOS and influences brain and behaviour.
In contrast to the distinct bacterial composition between our and the Burokas study, the bacterial activity assessed by caecal SCFAs were similar. The acetate and propionate levels were increased, and the iso-butyrate level was decreased, and this was also observed by Burokas et al. [Citation2]. Additionally, in our study, the butyrate level was increased and the iso-valerate level was decreased. Overall, the decrease of branched SCFAs (iso-butyrate and iso-valerate), which are fermentation products from degradation of proteins and amino acids [Citation54, Citation55], indicates that the scGOS:lcFOS diet shifted the intestinal microbiota from a more proteolytic profile to a more saccharolytic profile. The latter is typically associated with higher levels of acetate, propionate and butyrate, and metabolite profiles considered to be more beneficial for colonic health [Citation56].
Changes in intestinal microbiota can affect brain and behaviour through the gut-brain axis through several mechanisms, including microbe-derived molecules like SCFAs, and tryptophan metabolites neuroactive molecules like neurotransmitters and neuronal signalling by stimulation of the vagus nerve [Citation57]. As SCFAs are fermentation products from scGOS:lcFOS and SCFAs have been shown to reduce anxiety-like and depressive-like behaviour in mice [Citation58], these mediators might play a role in the behavioural changes observed in our study. How this communication between the SCFAs and the brain occurs is unknown, however, signalling through the vagus nerve [Citation10] or improved integrity of the blood brain barrier could play a role [Citation59]. In the central nervous system, SCFAs (among others) play a role in the homeostasis of microglia cells, macrophage-like cells in the brain. Studies in germ-free mice indicated that microglia cells are affected by the intestinal microbiota [Citation60] indicating that microglial function might be influenced by scGOS:lcFOS in an immunomodulatory way.
In conclusion, scGOS:lcFOS from the day of birth modulates the composition and activity of the intestinal microbiota, and is associated with reduced anxiety-like and improved social behaviour in healthy male BALB/c mice. To evaluate the exact effects in early life, it is essential to investigate brain neurochemistry at several time points throughout phases of development. Here, we provide further evidence of the potential beneficial effects of scGOS:lcFOS on behaviour at several developmental stages throughout life and strengthen the insights in the interplay between the developing intestine and brain.
Supplemental Material
Download Zip (2.4 MB)Acknowledgement
We thank Raish Oozeer from Danone Nutricia Research for his advice on experimental design, Heleen de Weerd from Danone Nutricia Research for the support in the bio-informatics analysis of sequencing data and Rachid El Galta from Danone Nutricia Research for the support in the mixed models analysis.
Disclosure statement
Johan Garssen, Leon Knippels, Kees van Limpt, Harm Wopereis and Jan Knol are employees of Danone Nutricia Research. Cindy de Waard is owner of CAM practice specialised in gut health.
Notes on contributors
Kirsten Szklany is a PhD candidate in the Pharmacology group at the Utrecht Institute of Pharmaceutical Sciences, Utrecht University, The Netherlands, studying the role of dietary fibres and omega-3 unsaturated fatty acids in the gut-immune-brain-axis in healthy as well as allergic conditions. Kirsten completed a BSc in Pharmacy at Utrecht University and a MSc in Drug Innovation at the same University. She worked at Crucell in Leiden before she started her PhD.
Harm Wopereis obtained his PhD in microbiology in 2019. In 2006 he joined the Gut Biology and Microbiology department of Danone Nutricia Research headed by Professor Jan Knol. During his career, Harm Wopereis has been involved in preclinical and clinical research regarding the effects of nutritional concepts on the gastrointestinal microbiota. His current research focuses on bringing health through nutrition by supporting the gut microbiota in early life to positively influence the developing gastrointestinal, immune and metabolic systems.
Cindy de Waard obtained a BSc in Biomedical Sciences and a MSc in Pharmaceutical Sciences. Owner of a Complementary and Additive Medicine (CAM) practice specialised in gut health.
Thecla van Wageningen is currently a PhD candidate at the department of Anatomy & Neurosciences at the Amsterdam UMC (location VUmc) studying the role of glial cells in Multiple Sclerosis. Previously she has done two research internships at Utrecht University and McMaster University investigating the gut-brain axis.
Ran An is a PhD candidate working in the lab of Microbiology at Wageningen University and Research, The Netherlands. Her work focuses on the gastrointestinal microbiota and its interactions with different fibres. In 2018, she published her first article, Age-dependent changes in GI physiology and microbiota: time to reconsider?, in Gut.
Kees van Limpt, BSc in Microbiology. Scientist in the Gut Biology and Microbiology department at Danone Nutricia Research in Utrecht since 2001. Main research area concerns the early life microbiota development in relation to nutrition and health.
Jan Knol is Director of the Gut Biology & Microbiology Platform at Danone Nutricia Research, Utrecht (The Netherlands) and Singapore since 2010. He is responsible for the gut and microbiology related research programs focussing on nutrition and health. The platform brings together unique expertises of microbiology and human (gut) physiology to substantiate nutritional concepts and to develop innovative products for the Baby Nutrition and Medical Nutrition Divisions of Danone. Since April 2012, Jan is Special Professor of Intestinal Microbiology of Early Life at the Laboratory of Microbiology, Wageningen University focusing on early colonisation of the intestinal tract of newborn infants linked also to potential health outcomes. Jan studied biology/biochemistry with specialisations in molecular biology and microbiology at University of Groningen, The Netherlands. He completed his PhD thesis in 1999 at the University of Groningen on sugar transport mechanisms in lactic acid bacteria. Part of this work was performed at the University of Leeds (United Kingdom) and the “Laboratoire J. Maetz” in Villefranche sur mer (France). After his PhD he started as a scientist at Nutricia Research in Wageningen. Jan Knol published more than 125 peer reviewed papers in the field of microbiology and gut biology (h-index 44), and is inventor of more than 25 patent applications.
Johan Garssen studied medicine and biology at the Free University, Amsterdam, Netherlands. He specialized in immunology and pharmacology and finished both studies in 1987 cum laude. He finished his PhD thesis at the University of Utrecht in 1991 on the role of T cells in respiratory allergy. This PhD program and a postdoc period was partly performed at Yale University, New Haven, USA. After the postdoc period he became senior scientist at the National Institute of Public Health in the Netherlands. In 2002 he became head of the section immunology at Numico-Research, Wageningen, Netherlands and in October 2008 he was appointed director of the immunology platform of Nutricia Research. Since 2005 he was also appointed at the Utrecht University in order to startup a strategic research alliance between Utrecht University and Nutricia research. Since 2007 he is Professor Immunopharmacology at the Utrecht Institute for Pharmaceutical Sciences and still involved in managing a strategic alliance with Nutricia research. He is managing and coaching research programs at the interface between food and pharma. Johan Garssen published over 700 peer reviewed papers/book chapters/patents in the field of “immunomodulation” with strong focus on inflammation.
Leon M. J. Knippels studied biology and he received a Ph.D. in 1998 in immunology/allergy at the University of Utrecht, Utrecht, the Netherlands. He continued his research as a postdoc at TNO Nutrition and Food Research Institute, Zeist, in the Netherlands and became Senior Scientist/Study Director and later Product Manager. There he supervised research projects as well as contract research for both Food and Pharma companies, in the field of immunotoxicology, immunology with a specialism in (food) allergy. In 2006 he joined Danone/Nutricia Research as a Senior Scientist Immunology and became Group Leader Immunology & Allergy. He was responsible for several research projects in which food ingredients are characterized for their immunomodulating properties, with a focus on allergy. Léon is currently Allergy & Immunology Director at Nutricia Research in the Netherlands and affiliate Associate Professor at the Utrecht Institute of Pharmaceutical Sciences at the University of Utrecht in the Netherlands. Research within his team focuses on the immunomodulating properties of food ingredients in both pre-clinical and clinical studies. He is (co)author on several patents, has published 94 peer reviewed publications and several book chapters in the field of food allergy and immunotoxicology.
Aletta D. Kraneveld (MSc Pharmacy & pharmacologist) was appointed as full professor Interdisciplinary Translational Pharmacology at the Faculty of Science and the Faculty of Veterinary Medicine of the Utrecht University in 2016. She has published >115 papers (H-index: 39). Besides science leading the neuroimmunopharmacology group, she is an active member of several boards of (inter)national scientific and societal organizations (Dutch Society of Pharmacology, EPHAR, IUPHAR, Netherlands Federation of Innovative Drug research; Diversity committee UU; Food Lives-NL). Kraneveld’s current research interests involve targeting the interaction between innate and adaptive immunity as well as host-microbiome interactions in chronic (inflammatory) diseases with pharmaceutical as well as nutritional interventions. The Kraneveld group is focusing to in depth study the role of the gut-immune-brain-axis in neurodevelopmental and neurodegenerative disorders. Results will further enhance the knowledge of the interaction of microbiome with the immune and nervous systems in chronic conditions in the gut and CNS. Aletta Kraneveld has set up a program that is a (inter)national neuro-immune platform where academia, patient organisations and industry meet for research on the gut-immune-brain axis as target for medicine and medical food concepts.
Additional information
Funding
References
- Desbonnet L, Clarke G, Shanahan F, et al. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–8.
- Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82(7):472–87.
- Borre YE, O'Keeffe GW, Clarke G, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–18.
- Wopereis H, Oozeer R, Knipping K, et al. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25(5):428–38.
- de Theije CG, Wu J, da Silva SL, Kamphuis PJ, Garssen J, Korte SM, et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668(Suppl 1):S70–80.
- Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
- Leclercq S, Mian FM, Stanisz AM, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062.
- Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–73.
- Mika A, Day HE, Martinez A, et al. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. 2017;45(3):342–57.
- Fleming SA, Monaikul S, Patsavas AJ, et al. Dietary polydextrose and galactooligosaccharide increase exploratory behavior, improve recognition memory, and alter neurochemistry in the young pig. Nutr Neurosci. 2017: 1–14.
- Oozeer R, van Limpt K, Ludwig T, et al. Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr. 2013; 98(2 Suppl):S561–71.
- Oliveros E, Ramirez M, Vazquez E, et al. Oral supplementation of 2'-fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem. 2016;31:20–7.
- Verdouw PM, van Esterik JC, Peeters BW, et al. CRF1 but not glucocorticoid receptor antagonists reduce separation-induced distress vocalizations in Guinea pig pups and CRF overexpressing mouse pups. A combination study with paroxetine. Pharmacol Biochem Behav. 2017;154:11–9.
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1(1):122–4.
- de Theije CG, Wu J, Koelink PJ, et al. Autistic-like behavioural and neurochemical changes in a mouse model of food allergy. Behav Brain Res. 2014;261:265–74.
- Olivier JD, Jans LA, Korte-Bouws GA, et al. Acute tryptophan depletion dose dependently impairs object memory in serotonin transporter knockout rats. Psychopharmacology (Berl). 2008;200(2):243–54.
- Bakker-Zierikzee AM, Alles MS, Knol J, et al. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94(5):783–90.
- Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1.
- van den BB, Erkus O, Boekhorst J, et al. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol. 2013;85(2):376–88.
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6.
- Rognes T, Flouri T, Nichols B, et al. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584.
- Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21(3):494–504.
- Pruesse E, Quast C, Knittel K, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35(21):7188–96.
- Shannon CE. A mathematical theory of communication (parts I and II). Bell Syst Techn J. 1948;27:379–423.
- Šmilauer P, Lepš J. Multivariate analysis of ecological data using CANOCO 5. 2nd ed. Cambridge: Cambridge University Press; 2014.
- Paulson JN, Stine OC, Bravo HC, et al. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10(12):1200–2.
- Van den BP, Braak CJFT. Principal response curves: analysis of time-dependent multivariate responses of biological community to stress. Environ Toxicol Chem. 1999;18(2):138–48.
- Paulson JN, Stine OC, Bravo HC, et al. Differential abundance analysis for microbial marker-gene surveys. Nat Meth. 2013;10(12):1200–2.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodological). 1995;57(1):289–300.
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–12.
- Groenink L, Verdouw PM, van Oorschot R, Olivier B. Models of anxiety: ultrasonic vocalizations of isolated rat pups. Curr Protoc Pharmacol. 2008. Chapter 5:Unit 5 18.
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125(1–2):49–56.
- Kromer SA, Kessler MS, Milfay D, et al. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci. 2005;25(17):4375–84.
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(Suppl 10):S3–8.
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176(1):53–65.
- DeMoss RD, Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969;98(1):167–71.
- Lee JH, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34(4):426–44.
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509–22.
- Savignac HM, Couch Y, Stratford M, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-beta levels in male mice. Brain Behav Immun. 2016;52:120–31.
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–59.
- Kalynchuk LE, Pinel JP, Meaney MJ. Serotonin receptor binding and mRNA expression in the hippocampus of fearful amygdala-kindled rats. Neurosci Lett. 2006;396(1):38–43.
- Solati J, Salari AA, Bakhtiari A. 5HT(1A) and 5HT(1B) receptors of medial prefrontal cortex modulate anxiogenic-like behaviors in rats. Neurosci Lett. 2011;504(3):325–9.
- Garcia-Garcia AL, Meng Q, Richardson-Jones J, Dranovsky A, Leonardo ED. Disruption of 5-HT1A function in adolescence but not early adulthood leads to sustained increases of anxiety. Neuroscience. 2016;321:210–21.
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23.
- Branchi I, D'Andrea I, Fiore M, et al. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60(7):690–6.
- Neufeld KM, Kang N, Bienenstock J, et al. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–64.
- Savignac HM, Corona G, Mills H, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int. 2013;63(8):756–64.
- Mischke M, Arora T, Tims S, et al. Specific synbiotics in early life protect against diet-induced obesity in adult mice. Diabet Obes Metab. 2018;20(6):1408–18.
- Marcobal A, Barboza M, Froehlich JW, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agri Food Chem. 2010;58(9):5334–40.
- Ose R, Hirano K, Maeno S, et al. The ability of human intestinal anaerobes to metabolize different oligosaccharides: novel means for microbiota modulation? Anaerobe. 2018;51:110–9.
- Ormerod KL, Wood DL, Lachner N, et al. Genomic characterization of the uncultured bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4(1):36.
- Vital M, Karch A, Pieper DH. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems. 2017;2(6):e00130–17.
- Rasmussen HS, Holtug K, Mortensen PB. Degradation of amino acids to short-chain fatty acids in humans. An in vitro study. Scand J Gastroenterol. 1988;23(2):178–82.
- Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132(6):1647–56.
- Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–72.
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55.
- van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–44.
- Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158.
- Erny D, de Angelis AL H, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77.
