ABSTRACT
The brain aging process triggers cognitive function impairment, such as memory loss and compromised quality of life. Cognitive impairment is based on bioenergetic status, with reduced glucose uptake and metabolism in aged brains. Anaplerotic substrates are reported to promote mitochondrial ATP generation, having been tested in clinical trials for the treatment of neurological disorders and metabolic diseases.
Objectives and Methods: To assess whether the improvement in oxidative capacity ameliorates cognitive function in adults (12 weeks), and aged (22-month-old) C57/6BJ mice, they received (1) a ketogenic diet, (2) a ketogenic diet supplemented with the anaplerotic substance, triheptanoin, or (3) a control diet for 12 weeks. Spontaneous alternation and time spent in a previously closed arm in the Y-maze test and time interacting with an unknown object in the novel object recognition test (NORT) were used to evaluate working memory. Acetylcholinesterase (AChE) activity in the prefrontal lobe, brain left hemisphere, and cerebellum was also evaluated. Glucose transporter 3 (GLUT3) expression in the prefrontal lobe was analyzed by western blotting.
Results: The ketogenic diet (KD) reduced spontaneous alternation in aged mice, leading to lower AChE activity in the aged prefrontal lobe and cerebellum, and in the parieto-temporal-occipital lobe of adult mice. Furthermore, KD decreased GLUT3 protein expression in the frontal lobe of the adults.
Discussion: Supplementation of KD with triheptanoin prevented memory impairment and showed similar values of AChE activity and GLUT3 expression compared to the controls. Our data suggest that triheptanoin has a potential role in the bioenergetic capacity of the brain, improving cognitive function.
Introduction
The aging process establishes functional loss in the central nervous system (CNS), that impairs memory consolidation and neuronal activity [Citation1]. Besides this loss there is a bioenergetic crisis, with a decreased capacity of the aged brain to oxidase substrates and provide energy for proper metabolism [Citation2]. Glucose is the major substrate for the brain; however, aged brains display reduced glucose uptake along with reduced glucose transporter expression and glycolysis within neuronal cells, known as hypometabolism, triggering an ATP deficiency [Citation3].
Hypometabolism of glucose contributes to cognitive loss during aging [Citation2], which increases the risk of developing neurodegenerative diseases. This energetic impairment in aged brains occurs years or decades before the onset of any disease [Citation4]. The most prevalent type of dementia worldwide is Alzheimer's disease (AD) [Citation5].
In contrast to glucose uptake, ketone body (KB) uptake remains similar in young and old brains. An approach that provides an energetic substrate to the brain bypassing the glucose uptake in the membrane cell is likely to minimize the bioenergetic crisis and ameliorate ATP synthesis [Citation6]. Restoring ATP levels can decelerate cognitive loss and the aging process in the brain. Clinical studies have shown improvement in memory tasks in patients with Alzheimer's disease after acute intake of ketogenic compounds [Citation7].
The ketogenic diet (KD) comprises a food regimen carbohydrate-restricted (<20 g/day) and very high fat (>90% calories), able to provide acetyl-CoA from ketone bodies to the brain [Citation8]. KD improved a visuospatial learning and memory in aged mice [Citation9]; but accelerated apoptosis and inhibited neurogenesis was reported in the rat hippocampus [Citation10]. The role of KD in cognitive functioning is yet to be understood. Furthermore, KD could compromise bioenergetics by limiting the refilling of intermediary molecules to the tricarboxylic acid cycle (TAC), a process known as anaplerosis; [Citation11]. Limited anaplerosis leads to impaired oxidative capacity in the brain, which can reduce neurotransmitter synthesis, such as acetylcholine [Citation12]. Here, we suggest that the addition of an anaplerotic and ketogenic compound to a ketogenic diet could bypass this metabolic problem.
Triheptanoin is an odd-medium-chain triglyceride with seven carbons in each fatty acid that provides the KB with five carbons (β-ketopentanoate and β-hydroxypentanoate). Triheptanoin is anaplerotic, given that five-carbon-KB can enter the cycle as succinyl-CoA and replenish TAC [Citation11]. The anaplerotic capacity of triheptanoin has been tested in metabolic disorder models, including GLUT1 deficiency [Citation13] and Alzheimer's disease [Citation14], with positive results. This study aimed to evaluate the effect of a triheptanoin-supplemented ketogenic diet on memory, cholinergic function and, brain metabolism in adult and aged mice.
Material and methods
Animal husbandry
Male C57/6BJ mice (Mus musculus) were obtained from the Central Vivarium of the University at two different ages: adults (12 weeks old) weighing an average of 24 g and aged mice (22-month-old) weighing approximately 32 g (n = 12 per group). The mice were acclimatized for five days before the experiments and maintained in a temperature-controlled (20 ± 2°C) room with a 12:12 h light–dark cycle (lights on at 6:00 am). Animals were housed in two-three mice per cage in the sectorial bioterium and fed a commercial chow diet (Nuvilab; Nuvital Nutrientes S/A) until the initiation of the experimental period. The use of animals in this study was approved by the University Animal Care and Use Committee of the UFAL (71/2018) and was guided by the European Communities Council Directives (86/609/EEC) regarding the care and use of animals for experimental procedures.
Experimental design
Mice were divided into six experimental groups: (1) adult control, (2) adults receiving ketogenic diet (KD), (3) adults receiving ketogenic diet supplemented with triheptanoin (257 g/Kg diet) (KD + T7), (4) aged controls, (5) aged ketogenic (KD), and (6) aged ketogenic with triheptanoin (KD + T7). Mice received the experimental or control diets for 12 weeks starting at the 12th week for the adults and at the 22nd for the aged animals. The experimental design is described as follows.
To monitor adaptation to diets, we measured body weight weekly until euthanasia, and food intake was measured for ten consecutive days from the beginning of the experimental period.
Dietetic treatments
shows the diets used in this study based on previous study [Citation15]. Except for the carbohydrate and lipid proportions, all other nutrients were similar to those of the control diet. The ketogenic diet was provided ready for use by Rhoster (São Paulo, Brazil). The ketogenic diet supplemented with triheptanoin was partially supplied by Rhoster, except for triheptanoin oil, which was kindly donated by Ultragenyx Pharmaceutical Inc (California, USA).
Table 1. Composition of control, ketogenic (KD), and ketogenic supplement with triheptanoin (KDT7) diets.
Blood biochemistry
To assess ketogenic status, glycemia and β-hydroxybutyrate levels were measured at baseline and at three, six, nine, and 12 weeks during the experimental period. Animals were briefly restrained, and blood was obtained from the lateral tail vein for biochemical measurement with glucose test strips (Accu-Chek Active) and β-hydroxybutyrate strips (FreeStyle Abbott). We conducted the measurements during the same period of the day (from 2 to 3 p.m.) for all groups and at all time points.
Y maze test
The Y-maze test evaluates animal spatial working memory based on the innate preference of animals to explore an arm that was not available (intentionally closed) or the arm last seemed the longest. The Y-maze had three identical arms (arm width, 5 cm; arm length, 35 cm; arm height, 10 cm). On the first day, each individual mouse was placed in the apparatus for 5 min to evaluate spontaneous alternation, calculated as [(number of alternations)/(total number of arm entries − 2)] × 100 [Citation16]. On the second day, each animal was placed in the apparatus with one arm closed for 10 min. Four hours later, the animal was returned to the apparatus with the three arms opened for 5 min of exploration. After each trial, the apparatus was cleaned with 70% (v/v) alcohol to minimize olfactory cues. The time spent (seconds) in the previously closed arm was recorded for further comparison between groups. The mean obtained by three blinded subjects was used for the analysis. In each group, 8–12 mice were analyzed.
Novel object recognition test (NORT)
To assess short-term memory involving multiple brain regions, we performed a novel object recognition test. First, animals (n = 8–12 per group) were placed with a muzzle turned towards the apparatus wall without any object for adaptation for 3 min to the field. Subsequently, two identical objects were placed on opposite sides along with the apparatus, and animals were allowed to explore for more than 3 min. An hour later, we replaced one object with a novel one. The animals were given 3 min to explore the objects. Working memory was evaluated by the exploration index (time exploring the novel object/time exploring both objects × 100) in the last trial. The apparatus and the objects were cleaned after each trial. The test was performed at the baseline and after 12 weeks. The mean of three blinded subjects were used for analysis.
Acetylcholinesterase (AChE) activity
AChE activity was measured using Ellman's assay method [Citation17]. Briefly, aliquots of the prefrontal lobe, brain left hemisphere, and cerebellum homogenates (1 mL saline [0.9%] for each 80 mg tissue) from the pool of three animals per group were sonicated for 15 s (five times) and then diluted in saline [0.9%] in a one:five. 50 microliters of the diluted aliquot were added to 1.39 mL of phosphate buffer (0.1 M, pH 8.0) and 50 µL of 0.01 M 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB). Next, 10 µL acetylthiocholine (ATCh) iodide solution (0.075 M) was added to the reaction mixture. For the blank, ATCh was replaced with ultrapure water. The absorbance was read in triplicate using a UV/VIS spectrophotometer (Perkin Elmer UV/VIS Lambda 2) at a wavelength of 412 nm, and the change in absorbance was recorded for 3 min. The rate of AChE activity was measured following an increase in the color produced from thiocholine when it reacted with DTNB. The cholinesterase activity unit (U) represents the amount of enzyme catalyzing 1 µmol of substrate hydroxylation per minute (U = µmol.min−1) and was calculated as previously described [Citation17]. AChE activity was expressed in U/mL, and the specific activity of AChE was expressed in U/mg of protein.
Protein quantification was performed using the Bradford method (1976). Briefly, the calibration curve was determined using bovine serum albumin protein for standardization. A diluted aliquot (100 µL) was added to 2.5 mL Bradford reagent, and the absorbance was read at 595 nm. The assays were conducted in triplicate.
Brain sections
For immunohistochemistry and Hematoxylin-Eosine (H&E) staining, the animals (n = three per group) were anesthetized with thiopental (i.p. 20–40 mg/kg), perfused with saline solution (0.9% NaCl), and 4% paraformaldehyde, and dissected for brain collection. The brains were post-fixed in 10% paraformaldehyde at 4°C for 24 h, then dehydrated in increasing concentrations of alcohol followed by xylene and embedded in paraffin. Brain coronal sections (7 µm) containing the prefrontal cortex (2 mm Bregma), medial parietal-temporal cortex (from −1.6 to −2.5 mm Bregma), and cerebellum (−5.6 mm Bregma) were obtained.
Immunohistochemistry
For immunohistochemistry, the deparaffinized brain sections were subjected to antigen retrieval with citrate buffer (0.01 M, pH = 6.4) for 10 min at 80–90°C and 20 min at room temperature [Citation18], incubated for 10 min in 1% hydrogen peroxide diluted in phosphate-buffered saline 0.1 M (PBS), washed twice in PBS for 5 min and then incubated in 1.5% blocking serum (ImmunoCruz®) for one hour. After that, the slides were incubated for two hours at room temperature with primary antibodies diluted in 1.5% blocking serum (anti-GLUT3 1:100, Invitrogen), washed with PBS for 5 min (three times), and incubated with biotinylated secondary antibody (ImmunoCruz®) for one hour and thirty min. After washing with PBS for 5 min (three times), the slides were incubated with AB enzyme reagent for 30 min and then incubated with peroxidase substrate for 10 min. The slides were washed in deionized water for 5 min and counterstained with hematoxylin. The sections were then observed under an optical microscope (Nikon).
Hematoxylin-Eosine staining
To access if the experimental diets affected the cell density in the brain, the H&E staining was performed with slides containing 7 µm coronal sections from the pre-frontal lobe (primary and secondary motor areas), the parietal and temporal lobes (primary somatosensory and auditory area), and the cerebellum (paraflocculus, molecular layer). Four to five slices of three animals per group were evaluated. Briefly, after deparaffinization and rehydration, sections were stained with hematoxylin solution for 15 s (twice) and then rinsed in distilled water. Then the sections were stained with an eosin solution for one min, followed by dehydration with increasing concentrations of alcohols followed by xylene. The mounted slides were then examined and photographed using an optical microscope (NikonTM), objective lens 20x. The quantification of number of hematoxylin-stained nuclei and nuclei size in the selected areas was made by using ImageJ image software version 1.51p and expressed in absolute value.
Western blot
For the GLUT-3 protein expression analysis, three animals per group were anesthetized with thiopental and decapitated. Each brain was rapidly dissected on ice and divided into the prefrontal lobe, right hemisphere, and cerebellum. The tissues were homogenized by mechanical processing in protein extraction buffer PEB (Tris 1 M pH 7.6; EDTA 0.5 M; NaCl 1.5 M, 10% triton x-100; NP40, protease inhibitor cocktail; Sigma Aldrich). After homogenization, the tissue was centrifuged for 20 min at 12,000 rpm (14,000 RCF) and 4°C, and the supernatant was aliquoted and stored at −80°C until further analysis. The lysates were then used to measure glucose membrane transporter GLUT-3 protein levels by western blotting. The total protein levels were quantified using the Bradford assay (1976).
Briefly, 30 µg of protein were loaded in triplicate and separated using 12% gel SDS-PAGE at a constant voltage. The gel was then transferred by electrophoresis in Tris-glycine buffer (pH 7.4) buffer (350 mA constant at 4°C for one hour and thirty min) onto a nitrocellulose membrane (Bio-Rad ®). Non-specific binding sites were blocked in a solution containing 5% bovine serum albumin (BSA) for 1 h at room temperature (RT) and then incubated with rabbit polyclonal anti-GLUT-3 (1:1000, Invitrogen) primary antibody solution containing 3% BSA overnight at 4°C. After rinsing four times in tris-buffer saline plus 0.3% tween 20 (TBST), the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (1:1000) for 2 h at RT. Signal detection and quantification of the optical density were respectively performed using ChemiDoc and Image Lab software (Bio-Rad ®).
Statistical analysis
For all the variables analyzed in the present experiment, the difference between the conditions was determined using one-way, two-way, or mixed ANOVA. A Bonferroni post-test was used to compare the groups if significance was reached using analysis of variance. A value of p ≤ 0.05 was considered significant. Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, United States).
Results
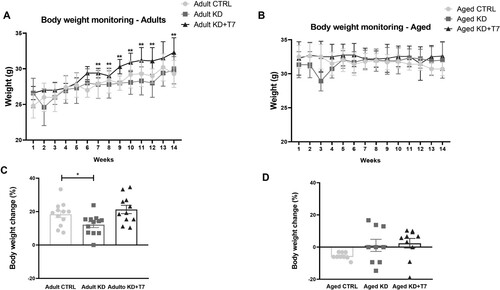
Triheptanoin determined higher weight gain in adult mice
During the 12-week-period, three mice from aged CTRL, four mice from aged KD and, three mice from aged KD+T7 groups died from unknown causes. Adults receiving KD supplemented with triheptanoin ingested more calories than those receiving KD only. Both experimental diet groups ingested more than the control adult group (CTRL = 13.6; KD = 17.1; KD+T7 = 20.6 kcal/animal/day; p < 0.001). In the aged groups, only mice on the supplemented diet ingested more calories than the other groups (CTRL = 13.2; KD = 16.2; KD+T7 = 26.3 kcal/animal/day; p < 0.001). Both adults and aged KD lost weight during the first week of the experimental period when adapting to the diet, although the means did not reach significance compared to the other groups. Weight loss was not observed during the first week in the supplemented groups (). After six weeks of the experimental diet, the supplemented group remained heavier than other groups between adults (p = 0.03) with no difference in aged groups (p = 0.14; F = 2.071) . Considering the weight change from the baseline value, the supplemented adults gained more weight than the KD group. In the aged groups, weight change varied, and no intergroup difference was found: all animals of control group lost weight; in the KD group, half of animals lost and half gained weight; and in the supplemented group, seven gained, two lost, and one animal did not change in weight ().
Figure 1. Body weight monitoring of the adults (A) and aged (B) groups. Body weight (g) was measured weekly before and during all the experimental periods. Body weight change of adults (C) and aged (D) considering the baseline and 12-week weight, in percentual. (A and B) Each dot represents the mean of the group (n = 8–12 per group). (C) Bars represent mean and SEM. (D) Each dot represents the result of an animal and horizontal lines represent the mean of the group. *p < 0.05; **p < 0.01 KD×KD+T7.
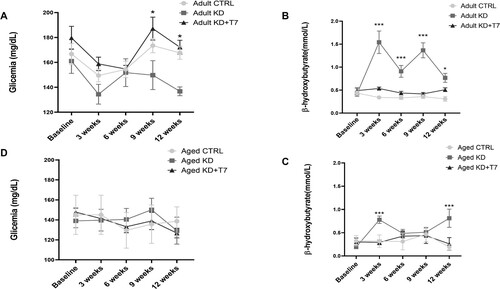
Ketogenic diet produced ketosis in both adult and aged mice
Nine weeks of the KD diet reduced glycemia in adults (reduction of 8%, p = 0.02), which was maintained at lower levels for the rest of the 12-week-analysis. No changes were observed in the aged group (). Adult KD mice showed higher levels of beta-hydroxybutyrate (BHB) than other groups at all time points during the experimental period. In the aged groups, the KD diet increased BHB levels at the three and 12-week time points ().
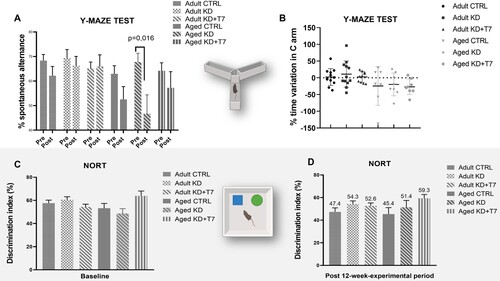
KD, but not KD supplemented with triheptanoin reduced spontaneous alternation in aged mice
To determine whether KD and triheptanoin supplementation interfered with spontaneous alternation or spatial reference memory in mice, we conducted the Y-maze test. KD decreased spontaneous alternation in aged mice (diet x time F = 9.8806, p = 0.0027), with no effect in the other groups ((A)). The number of entries was similar between the aged groups in the baseline and post-diet tests (data not shown), suggesting that the difference in spontaneous alternation could not be due to the changes in locomotor activity. Diets did not influence the time spent in arm C in either adult or aged mice (diet × time F = 0.8216, p = 0.5398). We observed an important variation in the percentage of time spent in the C arm by all groups, with some mice reducing the time after the experimental period, and others increasing it ((B)). Ketogenic diets did not change the mean discrimination index in the novel object recognition test (diet × time F = 1.79, p = 0.13) ((C)).
Figure 3. Behavioral tests to access spatial memory (Y maze, A and B) and short-term working memory (NORT; C, and D). Trials were conducted at baseline and after the 12-weeks-experimental period. (A, C, D) Bars represent the mean of each group (n = 8–12 per group). (B) Each dot represents the variation of an animal, and horizontal lines represent the mean of the group. (D) Numbers represents the means of discrimination index.
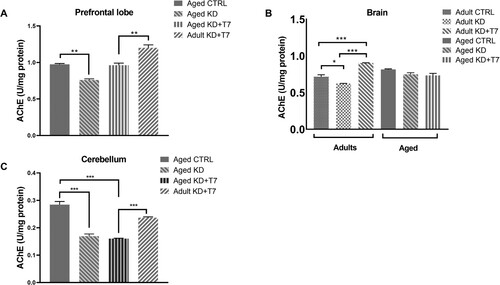
Triheptanoin preserved AChE activity in the prefrontal cortex of aged mice
AChE activity has been used as a marker of the status of the cholinergic system, which is known as the basis of cognitive performance. The aged KD group had a lower level of AChE activity in the prefrontal lobe than the aged control group (p < 0.01). Triheptanoin KD led to higher AChE activity in adults than in aged animals compared to the control group ((A)). Considering the brain left hemisphere, KD led to lower levels and supplemented KD led to higher AChE activity in the adult groups, with no difference in the aged groups ((B)). In the cerebellum, both experimental diets led to lower enzymatic activities in the aged groups ((C)).
Figure 4. Acetylcholinesterase (AChE) activity of the prefrontal lobe (A), the brain left hemisphere (B), and the cerebellum (C). The unit of cholinesterase activity (U) was measured using a spectrophotometric assay in triplicate and normalized by the total amount of protein, calculated by the Bradford method. Bars represent the group mean and horizontal lines represent the standard error (n = 3 per group). **p < 0.01; ***p < 0.001.
H&E staining of the aged mice brain
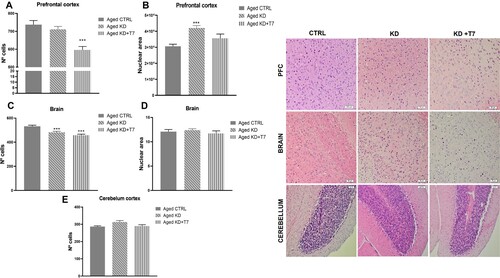
H&E staining was conducted to verify whether the experimental diets modified brain histology during aging. We found a lower number of cells (counted by hematoxylin-stained nuclei) in the triheptanoin group, but greater nuclei size in the ketogenic group in the prefrontal cortex ((A,B)). Comparing the cortex near the CA3 in the temporal lobe, the ketogenic groups showed fewer cells per area evaluated, with no difference in the nuclei size ((C,D)). Furthermore, the number of cells in the cerebellum was similar among the aged groups ((E)).
Figure 5. Cell and/or nuclear area counting of the prefrontal cortex (A and B), temporal lobe cortex (C and D), and cerebellum (E) slices. The counts were made in triplicate using Image J software. Bars represent the group mean and horizontal lines represent the standard error (n = 3 per group). ***p < 0.001. Representative slices H&E stained of each aged group (F). White rectangles represent 50 µm (H&E, ×20).
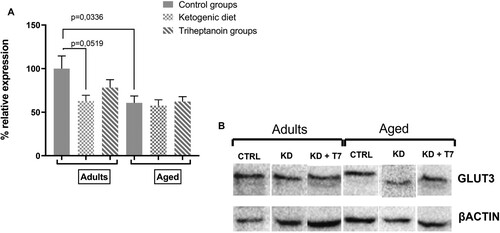
KD decreased GLUT3 expression in adult mice
GLUT3 is the main glucose transporter expressed in the neurons. In adult mice, KD decreased GLUT3 protein levels in the frontal lobe by 40% of that observed in the control expression. This decrease indicated a tendency but did not reach the statistical significance (p = 0.05190519) The KD group supplemented with triheptanoin showed GLUT3 protein expression similar to the control group. In aged mice, In the experimental diets did not modify GLUT3 protein levels (p = 0.087; F = 0.12). Nevertheless, GLUT3 protein levels were lower in the aged control than in the respective adult control group (p = 0.03; F = 3.34) ().
Figure 6. GLUT3 protein expression in the prefrontal lobe (A) was quantified by the intensity of the GLUT3 bands using the western blot method in triplicate. The b-actin bands normalized the GLUT3 expression in the same protein sample. Bars represent the mean of each group and horizontal lines represent SEM (n = 3 per group). (B) Representative immunostaining with anti-GLUT3 of prefrontal lobe slice.
Discussion
We aimed to evaluate whether a ketogenic diet supplemented with triheptanoin influences memory, cholinergic function in the brain, and metabolism in adult and aged mice. Our rationale was to improve brain energy metabolism by providing an alternative source of energy for the aged brain, which is known to be affected by glucose hypometabolism. The experimental diets altered body weight, glucose and β-hydroxybutyrate levels, performance in short-term working memory tests, and molecular parameters of cognitive function and energy metabolism. Our main finding is that adding triheptanoin to the ketogenic diet reverted some injuries detected by working behavioral and molecular tests, in brains of mice subjected to this high-fat and ketogenic approach.
The aged control group lost weight; this data is supported by the previous finding that physiological aging determines the loss of body weight [Citation19]. Although the aged KD groups consumed more calories than the control group, higher food intake did not trigger a higher mean body weight. This was probably due to the high variance between the KD and KD+T7 groups. The diets differentially changed mice glycemia levels. KD+T7 increased glycemia at two-time points in adults and did not influence BHB levels in either age group. A higher rate of triheptanoin metabolism [Citation20] could explain this effect, with the conversion of glycerol providing glucose more rapidly. T7 provides ketone bodies with five carbon atoms [Citation21], which were not detected by the BHB strips. As expected, KD increased BHB levels in both adult and aged mice.
Spontaneous alternation in the Y-maze test measures short-term spatial memory and is based on the natural curiosity of rodents to explore the arm visited last. The more spontaneous alternation the mice present, the more intact their prefrontal cortex function [Citation22]. The experimental diets did not interfere with spontaneous alternation in adults, suggesting that the ketogenic approach has no influence in working memory when factors such as age or disease do not compromise cognitive function. Huang et al. [Citation23] found no difference in spontaneous alternation in adult mice after three weeks of KD or control diets. However, KD reduced the spontaneous alternation in aged mice, an effect not observed in aged mice supplemented with triheptanoin. In contrast to our data, Hernandez et al. [Citation19] found beneficial effects of KD in aged mice using 8-shaped maze to test the spatial alternation. The maintenance of physiological prefrontal function by the triheptanoin found here could provide a basis for this anaplerotic property [Citation24]. Anaplerotic compounds, such as triheptanoin, replenish the tricarboxylic acid cycle with intermediary molecules [Citation11]. The energetic improvement could ameliorate brain oxidative capacity and function. The experimental diets did not influence the discrimination index evaluated in NORT. Limitations of aging, such as reduced vision, could have limited the interaction with objects by the animals.
Besides behavioral experiments, the status of the cholinergic neuronal system in the brain provides evidence that the underlying process of cognitive impairment in aged animals rises [Citation25]. AChE enzyme hydrolyzes acetylcholine to choline and acetate in the synaptic left. AChE activity has been used as a parameter of acetylcholine levels in preclinical brain studies [Citation26]; reduced AChE activity was found in neurodegenerative disease models [Citation27]. In the present study, KD led to lower AChE activity in the aged prefrontal cortex. However, KD supplemented with triheptanoin determined similar activity levels of AChE in aged animals compared to the control group. In the brain left hemisphere, AChE activity was lower in adults with KD but higher when KD was supplemented with triheptanoin, compared to adult controls. Our enzymatic data corroborated the findings of behavioral analysis since KD compromised the cholinergic system in aged rats and supplementation with triheptanoin showed a preventive role. To our knowledge, our study is the first to evaluate the effect of a ketogenic diet on brain AChE activity.
Isoform 3 of the glucose passive transporter (GLUT3) is mainly expressed in neurons, with a higher glucose affinity among GLUTs [Citation28]. GLUT3 correlates with regional brain glucose utilization; regions with more electrochemical activity express more membrane GLUT3 [Citation29]. In the present study, triheptanoin prevented the reduction in GLUT3 expression in the prefrontal cortex of adult mice. This finding suggests a potential role of triheptanoin in improving the energetic status within the prefrontal cortex, which could have an impact on neuronal function and neurotransmission. A previous study found lower GLUT3 expression in the hippocampus of young and aged (20-month-old) rats after 12 weeks of KD enriched with medium-chain triglycerides [Citation30], without specifying whether it was an even-or odd-chain TCM.
Our study has methodological limitations. We did not quantify the ketone bodies with five carbons (C5) levels for the groups supplemented with triheptanoin. However, we strongly believe the C5 was synthesized in mice liver and delivered to plasma. The interindividual variability of the manual evaluation in the NORT is another limitation and could explain why we did not find a statistical difference between the aged groups. Finally, acetylcholine levels and GLUT 1 brain expression were not evaluated. However, we believe that the power of our findings is maintained.
Several preclinical studies have shown the beneficial effects of a ketogenic diet on brain molecular pathways [Citation31]. Ingestion of high amounts of fat can trigger neuroinflammation [Citation32] and impair cognitive function in the long term. On the other hand, ketone bodies, primarily beta-hydroxybutyrate [Citation33] have shown beneficial effects on the brain. Given the potential injury role of the KD demonstrated in our research, future studies will determine whether the beneficial effects of BHB are obtained without the induction of ketosis by severely restricting carbohydrates. This is possible because the brain consumes ketone bodies at normal levels of glycemia [Citation34]. Furthermore, novel experiments will demonstrate whether dietary supplementation with triheptanoin has a positive role in the brain aging process by ameliorating the energetic function in the absence of ketosis.
In summary, the ketogenic diet impaired memory in aged mice, reduced acetylcholinesterase activity in aged and adult mice, and reduced GLUT3 expression in the frontal lobe of adult mice. This impairment was prevented with the addition of the odd-medium-chain triglyceride triheptanoin, suggesting the beneficial role of triheptanoin in ameliorating memory and cholinergic function.
Acknowledgements
The authors are grateful to Ultragenyx Pharmaceutical Inc (California, USA) for triheptanoin oil donation and Dr. Vivaldo Moura-Neto for his scientific advice and material support. The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Notes on contributors
L. S. da Rocha
L. S. da Rocha Dietitian. Master's in Nutrition at Federal University of Pernambuco and PhD in Health Science at Federal University of Alagoas, Brazil. Her research focus on ketogenic diet aging-associated effects in memory and brain glucose transport. Dr Rocha is currently a dietitian at Federal University of Alagoas.
C. B. Mendes
C. B. Mendes Nurse. Master's degree in Health Sciences. Her studies are focused on neuro-oncology and bioinformatic of brain tumors of glial origin and water transporters (aquaporins 1 and 4) in the central nervous system. She is currently a member of the Laboratory of Electrophysiology and Brain Metabolism at Federal University of Alagoas, Brazil.
J. S. Silva
J. S. Silva Pharmacist from the Federal University of Alagoas, Brazil. Former member of the Laboratory of Electrophysiology and Brain Metabolism at the Federal University of Alagoas.
R. L. G. F. Alcides
R. L. G. F. Alcides Biologist. master's in philosophy student. Former member of the Laboratory of Electrophysiology and Brain Metabolism at the Federal University of Alagoas.
I. P. Mendonça
I. P. Mendonça Biologist. Master's degree and doctoral student in Biological Sciences at Federal University of Pernambuco, Brazil. Current research study is related to the benefits of the drug metformin and the use of prebiotic saccatossis) and galacto-oligosaccharides in experimental Parkinson's disease.
B. L. S. Andrade-da-Costa
B. L. S. Andrade-da-Costa PhD in Biological Sciences (Biophysics) from the Federal University of Rio de Janeiro. She is currently a full professor of physiology at the Federal University of Pernambuco, Brazil. Currently, her main interests in neurophysiology are the retina functions, development of the nervous system, essential fatty acids, malnutrition and neurodegeneration.
S. S. Machado
S. S. Machado Chemical Engineer, master in Biochemical, PhD in Enzymology from Delft University of Technology-TUD (Holland). Postdoctoral fellow at the Department of Molecular Medicine at The Scripps Research Institute-TSRI (San Diego-USA). She is currently a full professor (Biochemistry) at the Federal University of Alagoas (Brazil). Her studies in enzymology integrate purification and characterization of proteins of biotechnological/toxicological interest mainly employing cholinesterases from animals (fish and mice) for environmental monitoring toxicological and neurophysiological studies.
A. Ximenes-da-Silva
A. Ximenes-da-Silva Dietitian. Associate Professor of Physiology at Federal University of Alagoas, Brazil. PhD in Neurosciences, University of Paris VI – France, post-doctorate in Neurosciences at University of Lausanne, Switzerland. My research work consists in studying the expression of glucose transporters and aquaporins in different developmental stages of the central nervous system in health and disease.
References
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–6. Available from: http://www.nature.com/articles/nature20411
- Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19:609–33.
- Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimer’s Dis. 2014;43:1343–53.
- Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, et al. Can ketones help rescue brain fuel supply in later life? implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci. 2016;9:1–21.
- Li XY, Men WW, Zhu H, Lei J-F, Zuo F-X, Wang Z-J, et al. Age- and brain region- specific changes of glucose metabolic disorder, learning, and memory dysfunction in early Alzheimer’s disease assessed in APP/PS1 transgenic mice using 18F-FDG-PET. Int J Mol Sci. 2016;17:1–15.
- Nugent S, Tremblay S, Chen KW, et al. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging. 2014;35:1386–95. doi:10.1016/j.neurobiolaging.2013.11.027
- Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:28–36. doi:10.1016/j.trci.2017.11.002
- Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar S J. Ketogenic diet in Alzheimer’s disease. Int J Mol Sci. 2019;20:3892. Available from: https://www.mdpi.com/1422-0067/20/16/3892
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng C-P, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26:547–57.e8. doi:10.1016/j.cmet.2017.08.004
- Irfannuddin I, Sarahdeaz SFP, Murti K, Santoso B, Koibuchi N. The effect of ketogenic diets on neurogenesis and apoptosis in the dentate gyrus of the male rat hippocampus. J Physiol Sci. 2021;71. doi:10.1186/s12576-020-00786-7
- Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab. 2013;33:175–82. doi:10.1038/jcbfm.2012.151
- Szutowicz A, Bielarczyk H, Jankowska-Kulawy A, Pawełczyk T, Ronowska A. Acetyl-CoA the key factor for survival or death of cholinergic neurons in course of neurodegenerative diseases. Neurochem Res. 2013;38(8):1523–42. doi:10.1007/s11064-013-1060-x
- Kass HR, Winesett SP, Bessone SK, Turner Z, Kossoff EH. Use of dietary therapies amongst patients with GLUT1 deficiency syndrome. Seizure. 2016;35:83–7. doi:10.1016/j.seizure.2016.01.011
- Xiaodong Y, Wang L, Tandon N, Sun H, Tian J, Du H, et al. Triheptanoin mitigates brain ATP depletion and mitochondrial dysfunction in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2020;78(1):425–37.
- de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, de Melo Lucena AL, de Lira CEPR, Soares AA, et al. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett. 2008;434:66–70.
- Ghafouri S, Fathollahi Y, Javan M, Shojaei A, Asgari A, Mirnajafi-Zadeh J. Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Res. 2016;126:37–44. doi:10.1016/j.eplepsyres.2016.06.010
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. Available from: https://linkinghub.elsevier.com/retrieve/pii/0006295261901459
- Namimatsu S, Ghazizadeh M, Sugisaki Y. Reversing the effects of formalin fixation with Citraconic Anhydride and heat: a universal antigen retrieval method. J Histochem Cytochem. 2005;53:3–11.
- Hernandez AR, Hernandez CM, Campos KT, Truckenbrod LM, Sakarya Y, McQuail JA, et al. The antiepileptic ketogenic diet alters hippocampal transporter levels and reduces adiposity in aged rats. J Gerontol - Ser A Biol Sci Med Sci. 2018;73:450–8.
- Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–69.
- Tefera TW, Tan KN, McDonald TS, Borges K. Alternative fuels in epilepsy and amyotrophic lateral sclerosis. Neurochem Res. 2017;42:1610–20.
- Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–11.
- Huang, J. et al. The effect of ketogenic diet on behaviors and synaptic functions of naive mice. Brain Behav. 2019;9(4):1–12.
- McDonald T, Hodson MP, Bederman I, Puchowicz M, Borges K. Triheptanoin alters [U-13C6]-glucose incorporation into glycolytic intermediates and increases TCA cycling by normalizing the activities of pyruvate dehydrogenase and oxoglutarate dehydrogenase in a chronic epilepsy mouse model. J Cereb Blood Flow Metab. 2020;40:678–91. doi:10.1177/0271678X19837380
- Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–17. doi:10.1016/j.mcn.2010.08.001
- Paul R, Borah A. Global loss of acetylcholinesterase activity with mitochondrial complexes inhibition and inflammation in brain of hypercholesterolemic mice. Sci Rep. 2017;7:1–13. doi:10.1038/s41598-017-17911-z
- Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–18. Available from: http://www.tandfonline.com/doi/full/10.158614737175.8.11.1703
- Iancu CV, Bocci G, Ishtikhar M, Khamrai M, Oreb M, Oprea TI, et al. GLUT3 inhibitor discovery through in silico ligand screening and in vivo validation in eukaryotic expression systems. Sci Rep. 2022;12:1–13. doi:10.1038/s41598-022-05383-9
- Shah K, DeSilva S, Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer’s disease. Int J Mol Sci. 2012;13:12629–55.
- Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, et al. A ketogenic diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from hippocampus. Front Aging Neurosci. 2018;10:1–16.
- Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer’s disease. Antioxidants. 2018;7:1–16.
- Valsamakis G, Arapaki A, Balafoutas D, Charmandari E, Vlahos NF. Diet-induced hypothalamic inflammation, phoenixin, and subsequent precocious puberty. Nutrients. 2021;13:1–9.
- Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol. 2014;88:584–93. doi:10.1016/j.bcp.2013.11.010
- Suissa L, Kotchetkov P, Guigonis JM, Doche E, Osman O, Pourcher T, et al. Ingested ketone ester leads to a rapid rise of acetyl-coa and competes with glucose metabolism in the brain of non-fasted mice. Int J Mol Sci. 2021;22:1–17.