Abstract
We systematically reviewed interventions substituting sucrose for other macronutrients in apparently healthy adults to assess impact on cardiometabolic risk indicators. Multiple databases were searched to January 2012 and abstracts assessed by 2 reviewers. Twenty-five studies (29 papers) met inclusion criteria but varied in quality and duration. Weaknesses included small subject numbers, unclear reporting of allocation, unusual dietary regimes, differences in energy intake, fat composition or fibre between conditions, unhealthy subjects, heterogeneity of results, and selective reporting. Insufficient data were available to draw reliable conclusions except with regard to the substitution of sucrose for starch, where effects on plasma lipids were inconsistent, mostly explicable by other factors, or nonsignificant. Based on fewer studies, there was little evidence for significant effects on plasma glucose or insulin. Sucrose substitution for starch up to 25% energy does not appear to have adverse effects on cardiometabolic risk indicators in apparently healthy adults. Furthermore, there is no consistent evidence that restricting sucrose in an isoenergetic diet would affect risk indicators, except perhaps in people with certain preexisting metabolic abnormalities. Larger, high-quality studies, lasting several months and studying a wider range of outcomes, are needed in order to provide evidence on which to base public health initiatives.
INTRODUCTION
Current dietary guidelines for improving cardiometabolic risk indicators and reducing the risk of cardiovascular disease (CVD) have tended to focus on restricting saturated fat consumption and replacing fat with carbohydrate (Krauss et al., Citation2000). However, there is increasing interest in the role of dietary carbohydrate type (and by implication sugars intake) in preventing or promoting disorders of lipid and carbohydrate metabolism, including metabolic syndrome and type 2 diabetes (noninsulin-dependent diabetes mellitus (NIDDM)) as well as CVD (Parks and Hellerstein, Citation2000; Hellerstein, Citation2002; Fried and Rao, Citation2003; Stanhope and Havel, Citation2010; Tappy et al., Citation2010). This review focuses on the potential adverse metabolic effects of sucrose, the main contributor to total sugar intakes worldwide.
Most dietary recommendations define sugars in terms of their origin (added sugars, free sugars, or simple sugars) rather than their chemical structure (sucrose, fructose, glucose, etc.). In the United States, the Institute of Medicine suggested a maximum intake level of 25% energy from added sugars in the 2002 Dietary Reference Intakes, on the ground of preventing nutritional deficiencies (Institute of Medicine, Citation2002). The 2005 Dietary Guidelines for Americans recommended a much more conservative level, limiting discretionary calories (including both sugars and solid fat) to 13% of energy requirement (US Department of Health and Human Services, Citation2005), while the American Heart Association proposed limiting sugar calories to 140 kcal/day for men or 100 kcal/day for women, which equates to a mere 5% of energy requirement (Johnson et al., Citation2009). In Europe, the European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) (2010) concluded that there are insufficient data to set an upper limit for (added) sugar intake, although most European countries aspire to follow the recommendations of the World Health Organisation (<10% of energy from “free sugars”; WHO/FAO, Citation2003). Dietary reference values for the UK adult population relate to so-called “nonmilk extrinsic sugars” (NMES; comprising added sugars, fruit juice, and 50% proportion of dried and cooked fruit) and suggest that these should provide no more than 10% of total energy intake (Department of Health, Citation1991). Currently, mean NMES intake in Britain is 12% of energy among adults and 15% among children (Department of Health, Citation2011) and two-thirds is sucrose (8–10% of energy; Gibson, unpublished). Across Europe, estimated average intakes of sucrose range from 8% in Nordic countries to 15–17% of energy in southern countries (Elmadfa, Citation2009). In the United States, an estimated 15% of total energy intake is derived from “added sugars” (Marriott et al., Citation2010) with a higher proportion among adolescents (21.4%; Welsh et al., Citation2011). However, according to food supply statistics, sucrose contributes only around 44% of total caloric sweetener volume in the United States, due to the widespread use of high fructose corn syrup (HFCS; Marriott et al., Citation2009).
Sucrose is a disaccharide that is efficiently hydrolyzed (by sucrase) in the intestinal mucosa to its constituent monosaccharides. It has been established that glucose stimulates fructose uptake in a dose-dependent manner (Rumessen and Gudmand-Hoyer, Citation1986) and that monosaccharides derived from sucrose are essentially absorbed at a similar rate to glucose:fructose mixtures (Tappy et al., Citation2010). However, whereas glucose elicits a glycaemic and insulinaemic response that stimulates its uptake into cells, fructose is mainly metabolized in the liver via an insulin-independent pathway not regulated by energy supply. There it may be converted into trioses that can be used for the de novo synthesis of triglycerides (TG) and cholesterol (Frayn and Kingman, Citation1995; Hellerstein, Citation2002). Thus, sucrose has the potential to influence both insulin sensitivity and lipid metabolism and there may be interconnectivity between the two. For example, according to Daly, hypertriglyceridemia (HTG) has long been known to be associated with insulin resistance (IR) in metabolic syndrome, although it is not clear whether HTG is caused by IR, or IR caused by HTG; there are plausible mechanisms for both (Daly et al., Citation1997). However, much of the evidence for adverse effects of sucrose comes from studies on animals fed excessive amounts of sugars, or else relates to human subjects with existing metabolic disorders such as type 1 or type 2 diabetes, or hyperlipidemia. Regarding the animal data, Daly has commented “overall, there is no conclusive or consensual evidence to show that humans respond similarly (to rodents) to sucrose- or fructose-rich diets at the doses used in human studies” (Daly, Citation2003). Moreover, to date there has been much less evidence that diet-induced HTG occurs in normal healthy subjects or with amounts of sucrose more typical of Western diets (<20% energy from sucrose; Frayn and Kingman, Citation1995; Fried and Rao, Citation2003).
Among the current uncertainties, therefore, is the dose limit, that is, “What amount of sucrose can be included in diets without adverse effects on lipid profile and carbohydrate metabolism?” Length of study is also important in interpreting outcome data. If the observed effect is transient, it may be less important; on the other hand, if it is cumulative, it may be missed in short-term studies. Alteration in levels of other dietary constituents as a result of the intervention may be contributing to adverse effects (e.g., a high intake of saturated fatty acids (SFAs) or low intake of essential fatty acids, fibre, antioxidants, or an increase in energy intake). Finally, as noted above, adverse effects may be strongly influenced by individual factors, such as IR, obesity, genetic factors, and lifestyle (Hellerstein, Citation2002). While analysis of these factors is beyond the scope of the present review, we have sought to interpret studies in their context.
The most robust type of evidence addressing the research question consists of studies in which there was a deliberate change in the sucrose component of diet under controlled or semicontrolled conditions in normal adults. Relevant outcomes were cardiovascular risk factors relating to lipid and carbohydrate metabolism, in particular TG, LDL, HDL cholesterol, and glucose and insulin responses. Effects on uric acid, gut hormones, appetite, and satiety were considered beyond the scope, as was the vast literature on sugars and bodyweight. Very short term studies lasting less than 3 days and mechanistic studies involving acute effects were also excluded unless they followed a period of adaptation.
METHODS
We conducted a systematic search using Medline and EMBASE and the Cochrane Library of systematic reviews to build the bibliographic database. Search strategy and terms were as follows:
Sucrose$ AND (starch OR fat OR protein) AND (lipid$5 OR lipoprotein$1 OR triglyceride$1 OR insulin OR glucose OR glucose tolerance OR HOMA OR blood pressure OR hypertension OR cardiac OR cardiovascular OR coronary OR metabolic syndrome) AND (replace$4 OR substitut$3 OR isocaloric$4).
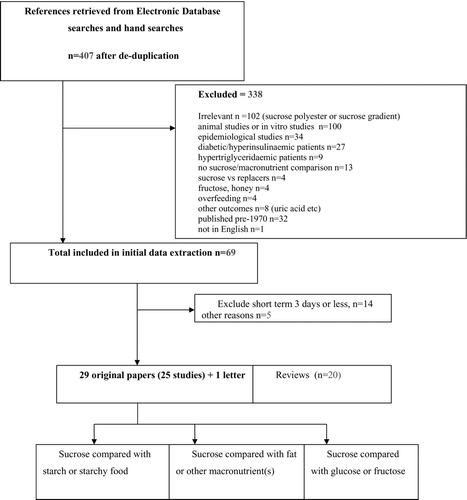
The electronic search was complemented by a hand search of relevant cross-references. Search results were imported into a bibliographic database (Endnote © Thomson Reuters; www.endnote.com) and then sifted for eligibility; 2 reviewers independently read abstracts using inclusion/exclusion criteria in . Copies of potentially relevant papers were obtained from the web, requested from authors, or purchased from libraries. The process of identifying pertinent articles and major reasons for exclusion are shown in the flowchart ().
Table 1 Inclusion and exclusion criteria
Details of each study were entered into a spreadsheet based on PICOS principles for research questions (Liberati et al., Citation2009) with fields for Population, Intake/exposure (sucrose), Comparators (e.g., starch, fat), Outcome measures, Study design, and in addition Results, Conclusions, and Comments (including confounders). gives a summary for the area with the most comprehensive evidence, namely, studies where sucrose was substituted for starch. This table is ordered by sucrose dose to make it easier for the reader to see an effect. summarize the studies and describe the population, dose, comparison, and design of each study. These tables are ordered alphabetically by first author for ease of referencing. Tables 6–8 give the main study results and the authors’ conclusions.
Table 2 Studies on the metabolic effects of isocaloric substitution of sucrose for starch (ordered by sucrose dose)
Table 3 Details of studies comparing sucrose with starch (in alphabetical order by first author)
Table 4 Details of studies comparing sucrose with glucose or fructose (in alphabetical order by first author)
RESULTS
Twenty-nine papers on 25 different studies were identified as potentially relevant and data were extracted. Of these, 7 (5 studies) included both sexes, 17 were on men only, and 5 (3 studies) were on women only. Study descriptions are in and the results are in .
Table 5 Details of studies comparing sucrose with fat or mixed/control diet (in alphabetical order by first author)
Table 6 Results of studies comparing sucrose with starch (in alphabetical order by first author)
All studies intended to involve healthy subjects, although some subsequently identified individuals with high fasting TG or who were “carbohydrate-sensitive” (Nestel et al., Citation1970; Reiser et al., 1979a,b, 1980). One included healthy subjects with one or more CVD risk factors (Brynes et al., Citation2003) and several included overweight, obese, or post-obese individuals. These are included in the review as they represent approximately two- thirds of the general adult population; however, it is likely that some would have some degree of IR and/or mild hyperlipidemia.
The duration of each experimental period ranged from 4 days to 1 year with most studies being around 14–28 days. Four were carried out in a metabolic ward or hospital environment, but most were on free-living subjects given prepared diets of known composition that were isocaloric and intended to be weight maintaining (eucaloric). Two noneucaloric studies in women are included in this review but discussed separately: one was isocaloric but hypocaloric (designed to achieve weight loss; Surwit et al., Citation1997), while the Danish study allowed the prescribed diets ad libitum (Raben et al., Citation1997; Marckmann et al., Citation2000; Raben et al., Citation2001).
Results of all pertinent studies are described in the following section, with effects on lipids distinguished from effects on carbohydrate metabolism. The latter section includes only studies in which carbohydrate content was held constant as this is the major determinant of blood glucose and insulin responses.
Effects of Sucrose on Blood Lipids (Isocaloric Studies)
Most studies compared sucrose versus starch/starchy foods ( and ), while a few compared sucrose versus glucose/corn syrup or fructose ( and ). The remainder involved substitutions of mixed or unspecified composition, where the control was a normal Western diet ( and ).
Table 7 Results of studies comparing sucrose with glucose/fructose (in alphabetical order by first author)
Table 8 Results of studies comparing sucrose with fat or other mixed diet (in alphabetical order by first author)
Studies Involving Sucrose Substitution for Starch
Studies where sucrose was substituted for starch provided the majority of studies and allowed some insight as to the effect of sucrose dose on metabolic end points ().
Studies Using Sucrose Intakes Above the Normal Range (>30% Energy)
A high level of sucrose in the context of a high carbohydrate diet was shown in a number of early studies to modestly increase TG responses, although frequently with considerable variation between subjects. For example, when eucaloric 70% carbohydrate diets either very high in sucrose (52–64% sucrose energy) or starch (14–19% sucrose) were given to 6 men for 7 days each, all subjects showed a greater rise in (fasting) TGs with sucrose but large increments were seen only in 4 men with hyperlipidemia, while the effects in the 2 normal subjects were modest (Nestel et al., Citation1970). TG concentration was also significantly related to body mass index (BMI) and the insulin response.
Using slightly lower levels of total carbohydrate, Wu et al. also found significantly raised TG (+12.6%) and VLDL-TG (+22%) among 17 overweight subjects fed a high sucrose diet (45%) compared with 5% sucrose for 10–14 days, although there was no effect on cholesterol (Wu and Shreeve, Citation1975). By contrast, among 23 free-living healthy young men consuming a high sucrose diet (39% or 300 g sucrose) for 14 days and then returning to their normal diet for another 14 days (13% sucrose or 105 g), there were significant increases in total cholesterol (TC) (8%), TG (13%), and phospholipids (10%) on the sucrose diet (all p < 0.01), which returned to baseline on resumption of the normal diet (Naismith et al., Citation1974). Adopting a similar protocol, 14 young male volunteers were asked to double their normal sucrose consumption for 14 days and then return to their normal diet for another 14 days (Yudkin et al., Citation1986). Sucrose intakes in the 2 periods averaged 37% and 17% energy, respectively. In this study, the change in TG (+10%) with higher sucrose intake was not significant but there was a significant fall (−16%) in HDL-C. However, a second experiment intended to demonstrate that HDL levels rose when high consumers of sucrose reduced intake from approximately 22% sucrose to 9% was unsuccessful in that subjects did not compensate for the sucrose and energy reduction. As a result it is not possible to determine whether the null result for HDL (and fall in TG) in the second experiment was attributable to the reduction in sucrose or an approximately 10% energy reduction, or both.
In the only study investigating the impact of a range of levels of sucrose, Albrink et al. (Citation1986) compared the effect of providing 0, 18, 36, or 52% of energy as sucrose (in very low fat/ high carbohydrate diet) for 10 days after a normal Western high fat diet (39% fat, 40% carbohydrate; Albrink and Ullrich, Citation1986). This design enabled comparison of sucrose/starch substitution at different doses and also substitution of fat by carbohydrate mixtures (see later section). There were 6 men in each group and the design included a crossover high/low fibre condition within each level of sucrose. In terms of the sucrose-for-starch substitution, TG concentration at day 11 was higher on the 3 sucrose-containing diets (when combined) than on the 0% sucrose diet (p < 0.01) and higher on the high sucrose diets (36% and 52% sucrose combined) compared with the 18% sucrose diet. The authors estimated, from regression analysis, that for every 10% increase in sucrose energy there was a 11 mg/dl TG increase at the end of the study. Compared with baseline TG values of 100–120 mg/dl, this is equivalent to a 10% rise. Total and LDL-C was higher on the 36% and 52% sucrose than on the 0 and 18% sucrose diets (Albrink and Ullrich, Citation1986).
Finally, in the only study on young women included in this review, Behall et al. investigated the metabolic effects of a 43% energy from sucrose diet (compared with an 8% sucrose, 43% wheat starch diet) and whether there was an interaction with oral contraceptive use among 12 university students (Behall et al., Citation1980). Both diets supplied protein, fat, and carbohydrate in the ratio 13:36:51 (percentage energy) and each diet was fed for 4 weeks in a crossover design with a 4-week washout period between. There was no effect of diet type on TG, TC, or individual lipoproteins (HDL, LDL, VLDL). However, post-prandial free fatty acid (FFA) levels measured after a high sucrose meal peaked faster and declined sooner with the sucrose diet condition.
Thus, while these studies provide some evidence for an increase in plasma lipids, especially TG in men over the short term (1–2 weeks), the effect is modest and not consistent across studies. Furthermore, intake in these protocols is at least 3 times the mean estimated intake of normal adults.
Studies using sucrose intakes within normal range (30% energy or less)
In a longer-term study, Dunnigan et al. found no significant effects overall after a 4-week moderately high sucrose diet (31% of total energy) on plasma lipids (TC or serum TG) compared with a sucrose-free, high starch diet (Dunnigan et al., Citation1970). Both diets in this crossover study contained 45% of energy from CHO; 40% from fat; and 15% from protein, and subjects were 9 middle-aged men and women hospitalized for neurological disability. Overall, this study suggests that serum lipids are not altered (over a period of weeks) by the substitution of starch for sucrose and vice versa within the normal range of sucrose intakes in a Western diet of modest total carbohydrate content.
Similarly, Mann and Truswell (Citation1972) investigated the effects of isocalorically replacing starch with a physiologic amount of sucrose (23% energy) among 9 men on a metabolic ward fed a diet with 55% energy from CHO (protein:fat:CHO of 15:30:55). After 14 days on each diet, there were no differences in fatty acids, cholesterol, or TG (Mann and Truswell, Citation1972). However, in 5 subjects studied post-prandially, there was a higher lipaemic response after the sucrose, compared with the starch meal (rice and potatoes). The authors suggested that the higher insulin response to starch (see later section) improved lipid clearance from the circulation. Overall this study suggests that normal sucrose intake may increase post-prandial TG levels, possibly due to reduced clearance, while it does not alter basal lipid profiles.
Two studies in the review (Grande et al., Citation1974; Fraser et al., Citation1981) compared supplementary sucrose with other mixed carbohydrate foods, including legumes and vegetables. A randomized crossover study in 12 healthy young men found that 500 kcal of sucrose in a background diet (36% fat) had a similar effect on serum cholesterol, phospholipids, and TG to isocaloric supplements of refined wheat flour, fruits, and leguminous seeds, over 2 weeks (Grande et al., Citation1974). Only the vegetable diet resulted in lower lipid levels. The amounts of sucrose in this experiment were moderately high but not excessive (19–23% sucrose) whereas the comparison diets contained 2–6% sucrose (a 16% difference). Similarly, Fraser et al. (Citation1981) investigated the effects of sucrose versus wholegrain starchy food added as 400 kcal supplement (12.5% energy) to a standard controlled diet (42% fat) for 3 weeks each to 16 healthy young men (Fraser et al., Citation1981). There were also 2 other conditions involving partial substitution of sucrose with root vegetables or leafy vegetables. The sucrose diet was associated with a small elevation in TC and LDL (9 mg/dl and 8 mg/dl; p < 0.01) compared with the wholegrain diet but no significant change in TG or HDL, while leafy vegetables plus 200 kcal sucrose condition resulted in lower total and VLDL cholesterol. However, higher fibre intakes in these conditions may have confounded the association. Taken together, these studies fail to provide good evidence of a significant detrimental effect of sucrose on lipid metabolism, at least over 2–3 weeks, although they do raise the possibility of small post-prandial changes of uncertain significance.
In contrast to the studies quoted above, a series of papers in the late 70s by Reiser and colleagues from the Carbohydrate Nutrition Laboratory, Beltsville/University of Maryland, USA suggested that high sucrose diets could produce deleterious changes in lipid profiles over 6 weeks. Effects of a controlled diet rich in sucrose (30% energy) compared with a low sucrose diet (<3% energy) for 6 weeks each were studied in 19 men and women. The diets were identical in protein, fat, and total carbohydrate content (15:42:43). During the high sucrose period, plasma TGs were 33% higher, total lipids were 10% higher, and TC were 7.4% higher than in the starch period. There was no difference in levels of FFA or other lipoproteins (alpha or HDL-C, and beta or LDL-C) between diet types (Reiser et al., 1979a).
However, one major problem with the interpretation of these studies is that 9 out of 19 subjects (7 of whom were men) were discovered to have high fasting TG levels (>150 mg/100 ml); these same individuals had markedly higher lipid and insulin responses, skewing the results. Using data given in the paper (Reiser et al., 1979a), it can be estimated that the rise in TG levels for the normal individuals was of the order of 22% (compared with 45% for the HTG subjects) and the rise in TC was approximately 4% (compared with 11% in TG subjects) after 6 weeks.
Two other factors may have contributed to the magnitude of the sucrose effect on metabolism. These are the gorging pattern (90% of the sucrose was administered at dinner after an 8 hr fast since breakfast) and the high intake of dietary cholesterol (562 mg/day). Together with the heterogeneous responses between healthy and less healthy individuals identified above this may limit the extent to which these studies are generalizable to normal dietary patterns and healthy people. However, they do suggest that diets including high levels of sucrose (30% energy) may be associated with adverse changes in blood lipid levels that are accentuated in adults with underlying metabolic abnormalities.
More recently, another 6 week randomized controlled study using levels of sucrose consistent with modern European diets, Black et al. (Citation2006) fed a group of 14 healthy young men a diet containing 25% or 10% sucrose energy (starch replacing sucrose). The high sucrose diet resulted in no significant differences in HDL or TG, or measures of vascular compliance (blood pressure, arterial stiffness). IR, as measured by the gold standard method, was also unaffected (see the next section). Although total and LDL cholesterol were significantly higher on the 25% sucrose diet (p < 0.01), this was in comparison to the unexpected fall in LDL cholesterol on the 10% sucrose diet. Furthermore, the high sucrose diet was significantly higher in SFAs (15% energy vs. 11%) and lower in polyunsaturated fatty acids (PUFA; 5% vs. 7%). Overall, this study suggests that a diet of 25% sucrose under controlled conditions has little impact on metabolism of healthy young men who were mildly overweight (mean BMI 26 kg/m2).
The above studies fed under controlled isocaloric conditions fail to demonstrate a consistent or meaningful adverse effect of sucrose at a level up to 25% energy in healthy young adults, although this cannot be extrapolated to adults with dyslipidemia or diabetes. Nor can it be assumed to be the same in ad libitum conditions, or in the context of underfeeding or overfeeding, discussed later.
Studies Involving Sucrose Replacing Fat or a Combination of Nutrients
Some studies involved manipulating the sucrose content of the diet without controlling which other macronutrients were substituted ( and ). For example, in a study primarily designed to look at glycaemic effects, Anderson et al. (Citation1973) tested the effects of increasing sucrose to 40% of energy for 4–5 days and then to 80% of energy up to 65 days among 9 normal young men whose normal diet contained 43% fat, 40% carbohydrate energy. On the 80% sucrose diet (substituting both fat and carbohydrate), TG levels rose by 50–100%, although no lipid data were given at the 40% sucrose level. The unphysiological level of sucrose at which effects were observed means that results from this study are of limited value to the research question.
In a parallel control study of much longer duration, the impact of reducing sucrose intake (from about 13% to about 2%) over 22 weeks was explored among 51 free-living men aged 36–55 years (Mann et al., Citation1972). Fasting TG levels fell by 22% overall, but this was mainly in those with high baseline TG; there was a minimal drop among those with normal values. There was no significant effect on serum cholesterol (−4%). Weight loss occurred due to a significantly reduced energy intake and was correlated with the reduction in lipids, begging the question as to whether the negative energy balance or sucrose reduction caused improvement in fasting lipids. The authors took the view that negative calorie balance was the likely cause and speculated that weight loss from restriction of other constituents may have a similar effect.
Most recently, a crossover study to assess the effects of added glucose, fructose, and sucrose in the form of sweetened beverages, additional sucrose for 3 weeks (19% sucrose in total diet) was not associated with any significant change in energy intake or in the traditional lipid profile compared with baseline (10% sucrose). The authors reported a decrease in LDL particle size, attributable to a decrease in the large LDL1 subclass but no significant increase in small dense LDL particles (Aeberli et al., Citation2011).
Studies Substituting Sucrose for Other Sugars
Five studies in the review compared sucrose with glucose or corn syrup (variable length glucose polymers), one compared sucrose with fructose and one compared fructose, sucrose, and glucose drinks ( and ).
When sucrose was replaced with corn syrup at either 45% or 65% energy for 10 days, there was no effect of carbohydrate type on fasting TG. However, 24 hr plasma TG concentrations measured by continuous blood withdrawal were significantly higher on both sucrose diets (Hayford et al., Citation1979). In a longer study among young men in the British Antarctic survey team, Roberts examined the effect of substituting virtually all dietary sucrose with glucose for 14 weeks and then restoring sucrose for another 24 weeks (Roberts, Citation1973). Initial sucrose intake during the run-in period was at the level of 10–13% energy. Weight did not change throughout the study and there was no change in TC overall. TG levels did not change significantly in the men whose initial levels were low (<120 mg/l00 ml) but a 10% reduction was reported among 5 men who had initially high TG levels (>120 mg/100 ml). The author interpreted this as evidence for increased sucrose sensitivity although it might be attributable to regression to the mean (Mann and Truswell, Citation1973). During the sucrose-restored period, TG initially rose but then slowly subsided toward normal (preintervention) levels. Bearing in mind the small effects observed, this study does not suggest that sucrose produced lipid changes that were either harmful or beneficial.
However, the longest study by far included in this review was a 2-year study of the effect on plasma lipids of replacing table sugar with dried glucose syrup (each diet followed for 1 year; Lock et al., Citation1980). The normal diet of these 18 men contained approximately 100 g sucrose (16% energy), which was reduced to 40 g (6%). Fasting blood samples were taken every 4 weeks and dietary questionnaires administered every 3 months to monitor compliance in macronutrient composition. In subjects whose weight remained unchanged (n = 8) and in those who lost weight (n = 5), there was a significant (8%) fall in TC (p < 0.025) and phospholipid P (p < 0.025), compared with the 16% sucrose period. However, there was no change in fasting TG except in those who gained weight. This study suggests that the elevated fasting TG levels seen in shorter studies may be a transient phenomenon. It also raises the possibility that carbohydrate type may modify some aspects of lipid metabolism (e.g., cholesterol level) over the longer term, although this lacks confirmation from other studies.
In a study comparing sucrose with fructose, Bosetti et al., compared isocaloric diets at a realistic level of consumption (15% energy) using typical American foods for 14 days in a crossover study. There was no change in total TG, TC, LDL cholesterol, or HDL cholesterol concentrations between diet periods. They concluded that there is no difference between sucrose or fructose on various lipid components in the “real world” in normal subjects (Bossetti et al., Citation1984). Similarly, in a crossover study using fructose, sucrose, and glucose drinks for 3 weeks, lipoprotein concentrations were unchanged on all conditions, and sucrose and fructose results were similar with regard to LDL subclass distribution (Aeberli et al., Citation2011).
Effects of Sucrose on Glucose and Insulin Levels (Isocaloric Studies)
Elevated fasting plasma insulin is regarded as an early sign of IR and results of this basic measurement correlate moderately well with the euglycaemic clamp technique. Homeostatic model assessment (HOMA), derived from the ratio of fasting insulin and glucose concentrations, is better than fasting insulin per se, but this too reflects the basal state. Most of insulin action occurs in the post-prandial state (Daly, Citation2003), therefore studies that provide evidence on response to a glucose or sucrose load after habituation to high sucrose diets are also valuable. In comparison to blood lipids, relatively few studies examined the effect of sucrose substitution on blood glucose or insulin.
Studies involving sucrose substitution for starch
An early study using a diet containing >50% sucrose energy for 7 days found that insulin responses (to intravenous glucose) were higher than on the high starch diet (Nestel et al., Citation1970). However, studies using less extreme intakes have not shown the same results. For example, Dunnigan et al. (Citation1970) found a small but significantly higher fasting blood glucose during the 4-week sucrose period (28% of total energy) compared with the 4-week “sucrose-free,” high starch diet (45% carbohydrate; Dunnigan et al., Citation1970). However, no significant differences were found for fasting plasma insulin or area under curve (AUC) for blood glucose or insulin using the glucose tolerance test. Overall, this study suggests that except for small changes in fasting blood glucose, carbohydrate tolerance was not altered.
When sucrose (at 23% energy) was replaced by starch in a crossover study, there were no differences in fasting insulin after 14 days (Mann and Truswell, Citation1972). However, post-prandial differences were studied in 5 out of the 9 subjects. On the sucrose diet, there was a significantly lower glycaemic and insulin response compared with the starch diet (provided by rice and potatoes), so this well-controlled study suggests that ingestion of sucrose at a normal physiological level may reduce post-prandial insulin secretion compared with starch. This may be a consequence of the lower glycaemic index (GI) of sucrose compared with the starches used in the intervention.
More recent studies have tended to confirm that diets high in sucrose do not cause greater insulinemia than starch, which is not unexpected given that the fructose component of sucrose is a poor insulin stimulator. In fact on the basis of GI, the effect of sucrose would be predicted to be intermediate between low GI and high GI diets. Indeed, compared with a high GI diet, which appeared to increase IR (PP-HOMA +31%), a diet containing 22% sucrose energy slightly reduced post-prandial HOMA over 24 days (−20%) though not as much as a low GI diet (−43%; Brynes et al., Citation2003). In the study by Kiens and Richter (Citation1996) in which the high sucrose diet was actually a high GI diet (GI = 90; 25% sucrose), insulin sensitivity was not adversely affected compared with the low GI diet (GI = 66; 1% sucrose). By the end of the 30-day period, fasting blood glucose and plasma insulin were not significantly higher on the high sucrose diet. Post-prandially the high GI/sucrose diet showed higher glucose and insulin responses, while the low GI diets were associated with an increased plasma fatty acid concentration, suggesting that low GI diets may impair whole body insulin action, perhaps because of higher FFAs. However, there was also some evidence of adaptation resulting in a more rapid and larger insulin response over time.
In agreement with these general findings are the Danish studies of Raben, Marckmann, and colleagues who examined diurnal metabolic profiles on day 15, after 2 weeks of (ad libitum) diets high in sucrose or starch. Possibly due to the lower GI of sucrose relative to starch, the sucrose-rich diet appeared to improve glucose metabolism (glucose AUCs were lower and there was no significant difference in insulin AUCs; Raben et al., Citation2001). These findings are in agreement with other work by Daly et al. on post-prandial metabolism (Daly et al., Citation1998). Lastly, in the controlled study by Black et al. (Citation2006), the 25% sucrose diet had no effect on glucose tolerance measured by a euglycaemic-hyperinsulinaemic clamp, nor on fasting blood glucose and insulin. Taken together these results suggest that insulin sensitivity is not reduced with 25% sucrose ingestion in place of starch in normal healthy adults. However, variation between studies may be partly explained by the form of starch and by total dietary GI, fibre, and fat content.
In contrast to the above, the only studies suggesting adverse effects of sucrose on glucose and insulin levels are those of Nestel, who found higher insulin response (statistics not given) on a very high (>50%) sucrose diet, and the series of studies from Reiser and colleagues from the Beltsville Laboratory. The latter reported fasting insulin levels to be 23% higher after 6 weeks on a 30% versus 3% sucrose diet, accompanied by a much smaller change in fasting glucose (+2.4%), and therefore a higher insulin to glucose ratio (Reiser et al., 1979b). While the study did not examine insulinaemic or glycaemic responses to the diets directly, it did assess the response to a sucrose load (2 g/kg) after adaptation to the diets. Subjects required more insulin to achieve equivalent levels of blood glucose when they consumed sucrose than when they consumed starch, suggesting that they were less insulin-sensitive on the sucrose diet. However, data presented show that this was only apparent among the 9 subjects who were hypertriglyceridaemic or potentially carbohydrate-sensitive, while the normal subjects showed no significant effect of diet on their insulin response to the sucrose load. A subsequent paper (Reiser et al., Citation1980) reported evidence for a possible mechanism for the hyperinsulinaemic response to a sucrose load in sucrose-adapted individuals, showing that secretion of the enteric hormone glucose-dependent insulinotropic polypeptide (GIP; an insulin secretagogue) was higher in the sucrose condition. However, as the paper did not distinguish between carbohydrate-sensitive and normal subjects, it is not clear to what extent this applied to the latter. As noted in the previous section on lipid outcomes, the gorging pattern of sucrose delivery in this study may have exaggerated the metabolic effects.
Studies on Substituting Sucrose for Other Sugars
Only 3 studies in the review include data on the glycaemic responses of sucrose compared with other sugars and none indicated significant differences in metabolic response. In an early study of sucrose versus glucose among men living on an Antarctic Base camp (Fry, Citation1972), the AUC for blood glucose was the same for both diets although the pattern of response was slightly different; blood glucose levels were slightly higher at 30 min for sucrose but declined more rapidly. Bosetti et al. (1984) comparing sucrose with fructose found no differences in fasting glucose or insulin, or the ratio of insulin to glucose, at 7 or 14 days at the modest levels used (15% energy), while the study of Aeberli found that fasting glucose was similar for the sucrose, fructose, and glucose conditions.
Studies Involving Sucrose Replacing Fat or a Combination of Nutrients
Three studies examined outcomes related to glucose and/or insulin response ( and ). One metabolic ward study in healthy young men aged 18–22 years found that a supra-physiologic level of sucrose over a relatively short time period resulted in an improvement of glucose tolerance. Increasing sucrose content to 40% for 4–5 days improved both oral and intravenous glucose tolerance compared with a control/normal diet (17:43:40 protein:fat:CHO; Anderson et al., Citation1973). Glucose tolerance continued to improve with the 80% sucrose diet administered for up to 65 days, an effect accompanied by a reduction in fasting plasma insulin. The authors suggested that high CHO diets may increase insulin sensitivity in these normal individuals, possibly by enhancing activity of the important glycolytic and pentose phosphate pathway enzymes in various tissues. They contrasted their results in normal men with those in HTG patients, in whom abnormalities of lipid and glucose metabolism frequently occur together, suggesting that these may not be causally related but rather separate manifestations of a more basic underlying abnormality (Anderson et al., Citation1973). Another study using a much more modest increase in sucrose intake (by 40 g/day) coupled with a reduced fat intake to maintain energy balance had no effect on fasting plasma glucose, serum insulin, or insulin responses over 8 weeks (Erkkilä et al., Citation2007). The third study (Brynes et al., Citation2003) is described in the section “ad libitum studies.”
Overall, the studies included in our review do not provide evidence of any consistent difference between sucrose and starch or other carbohydrates in respect of the basic markers of insulin sensitivity. Short-term studies of post-prandial and diurnal metabolism offer a more precise means of assessing metabolic effects using more sophisticated assessment methods such as the hyperinsulinaemic, euglycaemic clamp. These also suggest that 24 hr AUC for insulin may be comparable but patterns of response may differ (Daly et al., Citation1998). As yet there is no evidence to determine which feature (post-prandial elevation or 24 hr AUC) has more influence on metabolic health over the longer term (Daly, Citation2003).
Ad libitum studies
One of the questions raised by ad libitum and noneucaloric studies is whether the outcomes observed are attributable to altered energy intake. In a series of papers from the same Danish study of 20 women, Raben et al. (Citation1997, Citation2001) and Marckmann et al. (Citation2000) investigated the effects of 3 diets (high fat, high sucrose, high starch) each fed for 2 weeks in a crossover design with 2 or more weeks washout period in between. The sucrose diet (23% sucrose energy) and starch diets (2.5% sucrose energy) were matched for total carbohydrate (59%) and fat (29%) but the high sucrose diet was higher in SFA and dietary cholesterol and lower in fibre (20 g vs. 31 g). Energy intake was lower (−1.2 MJ/day) on the starch diet resulting in a slight (0.7 kg) weight loss overall, although whether this (and the differences in fatty acid composition) are consequences of the switch in carbohydrate source is a moot point. The high sucrose diet resulted in higher TG levels (20% higher fasting and 50% higher nonfasting) and 10% higher LDL-cholesterol levels compared with the starch diet (Marckmann et al., Citation2000). However, the difference in fasting TG levels was found to be nonsignificant after correction for the unintended differences in body weight and energy and nutrient intake between the groups (Raben et al., Citation2001). The authors commented that a high sucrose diet may lead to an undesirable lipid profile possibly due to fructose effects on TG production in the liver, on VLDL production and clearance. This study is one of few to investigate effects on clotting factors and found a 10% increase in nonfasting Factor VIIc but no significant effect on fibrinogen. After 14 days on the high sucrose diet, 24 hr energy expenditure as well as post-prandial plasma adrenaline and noradrenaline concentrations were significantly increased compared with the other two diets, although with no untoward effect on blood pressure (Raben et al., Citation1997). In this case, the subjects were in energy balance on the sucrose diet and the improved lipid profiles on the starch diet could be due at least in part to a (spontaneous) reduction in energy intake in that phase.
By contrast, the ad libitum crossover study of Brynes et al. (Citation2003) found no difference in fasting glucose or HOMA between low GI/High GI or high sucrose diets at day 24, although there were post-prandial differences. Subjects in this study were middle-aged men who were healthy but at slightly increased risk because of overweight, high waist circumference, or high cholesterol levels and the high sucrose diet contained approximately 22% energy from sucrose. The high sucrose diet had effects intermediate between that of the low GI and high GI diets but only the high GI diet appeared to increase post-prandial IR. Although instructed to maintain weight, men tended to lose weight on the low GI diet (−0.27 kg) and gain weight on the other diets and this may have influenced the results.
Hypocaloric Diets
Surwit et al. (Citation1997) studied the effect on metabolic parameters of a high sucrose (43%) diet compared with an isocaloric low sucrose (4%) diet in a hypocaloric context for 6 weeks among 60 overweight women. The diets were very low in fat and high in carbohydrate (protein, fat, CHO ratios = 19:11:71) and both provided approximately 4606 kJ/day. Both groups had similar fasting plasma glucose levels, achieved a similar weight loss, and showed a decrease in percentage body fat, blood pressure, resting energy expenditure, and plasma lipids. Thus, as long as a low fat, low calorie diet can be adhered to, a high sucrose content (>40%) does not appear to adversely affect weight loss, metabolic rate, or plasma lipids. The findings of this study and the ad libitum studies above would suggest that metabolic effects may be more strongly related to energy balance than carbohydrate type and in the eucaloric (weight maintaining) or hypocaloric situation there are few adverse effects of sucrose.
DISCUSSION
Limitations of the Data
Studies demonstrated a high degree of heterogeneity in design and quality. Most were on small numbers of subjects (only 7 studies used more than 20 adults) and, given the variation in response between individuals, may have been underpowered. Thus, for example, most studies involving both men and women did not distinguish results by gender. Second, there were few studies (3) reporting specifically on women, and only one was both isocaloric (between diet groups) and eucaloric (weight maintaining) and this found no effect on plasma lipids (Behall et al., Citation1980). The ad libitum and hypocaloric studies have been included because they offer insights into the possible impact of changing dietary composition in the real world, although confounding effects have to be borne in mind in interpreting the findings. Lack of control over diet composition is also a more general issue for studies involving real foods. Thus in some cases, manipulation of sucrose content also allowed carbohydrate, protein, or fat content to differ between treatment groups, while in others diets were matched in regard to macronutrient composition (fat:protein:carbohydrate) but differed in regard to SFA, PUFA, or fibre content. Thus, some of the observed effects could be due to confounding by other dietary constituents. In our view the evidence is too poorly defined and heterogeneous to derive reliable conclusions on any sucrose substitution except that with starch.
Limitations of the Review Process
By excluding studies lasting less than 3 days, the protocol omitted studies of post-prandial and 24 hr metabolism, except where these were investigated as part of a longer study (i.e., adapted individuals). Nevertheless, results from included studies suggest that there may be subtle differences in the time course of post-prandial response to sucrose compared with other macronutrients. The clinical significance of transient elevations in plasma lipids and glycemia/insulinemia and the levels associated with increased risk of metabolic disease remain uncertain and their discussion beyond the scope of the current review.
Studies specifically on fructose were also outside the scope of the search strategy, but it is apparent from the large number of recent reviews on fructose that these studies may have implications for sucrose, which is the major source of dietary fructose worldwide. Some groups have hypothesized that high fructose consumption results in increased visceral adiposity, lipid dysregulation, and also IR (Stanhope and Havel, Citation2010) and that fructose has similar effects to sucrose (Stanhope et al., Citation2009), while others conclude that evidence of adverse effects is lacking at normal dietary fructose levels (Livesey, Citation2009) and that excess calories are more important (Tappy et al., Citation2010). It has been suggested that there is limited evidence for adverse effects of fructose at intakes <50 g/day or 10% of energy for a 2000 kcal diet (Livesey and Taylor, Citation2008), equivalent to 100 g sucrose or sucrose at 20% of energy intake.
Validity
By focusing on human intervention studies in adults without diagnosed CVD, diabetes, or NIDDM, we have tried to avoid what we consider a major threat to validity, that of drawing conclusions based on subjects with preexisting metabolic abnormality. However, in a number of studies subjects had elevated blood lipids at the start of the study (Dunnigan et al., Citation1970; Nestel et al., Citation1970; Mann et al., Citation1972; Wu and Shreeve, Citation1975; Reiser et al., Citation1979a, 1979b, 1980; Behall et al., Citation1980), were overweight or obese (Nestel et al., Citation1970; Wu and Shreeve, Citation1975; Behall et al., Citation1980; Black et al., Citation2006), or were moderately insulin resistant (Black et al., Citation2006). Hence, whether the conclusions of this review are indeed applicable to the “normal” disease-free population of young- and middle-aged adults with a range of body weights is debatable. Such factors and others such as genetics and lifestyle may modify the response to dietary change (Hellerstein, Citation2002). Nevertheless, there appeared to be heterogeneity in subject response even among apparently healthy individuals. One of the most reproducible findings was that increases in plasma TGs tended to be correlated with baseline levels. This raises the question as to whether subjects with high baseline levels should be excluded from analysis in studies or whether they should be considered as part of the normal distribution. It is our view that results for all subgroups should be reported separately where power permits. This was mostly followed, although in a few studies (and also in some reviews) there was evidence of possible reporting bias where conclusions were based on selected subgroups.
A further cause of confounding that affects interpretation and generalizability is weight loss or gain and energy deficit or excess during the study. Energy balance may be as (or even more) important than diet composition in promoting hyperlipidemia and IR, but whether diets high in sugar always result in higher energy intakes compared with diets high in starch or fat is not established; thus we have considered it legitimate to discuss ad libitum studies, with caveats.
Probably the major consideration in evaluating the generalizability of findings is the realism of the sucrose levels used in interventions. Very high sucrose intakes (exceeding 30% energy) were associated with elevations in plasma TG and/or VLDL in most studies (Nestel et al., Citation1970; Anderson et al., Citation1973; Naismith et al., Citation1974; Wu and Shreeve, Citation1975; Reiser et al., 1979a; Hayford et al., Citation1979; Albrink and Ullrich, Citation1986; Yudkin et al., Citation1986). However, two studies in women showed no effect (Behall et al., Citation1980; Surwit et al., Citation1997). Protocols involving diets with less than 30% energy from sucrose tended to show no significant effect on fasting TGs (Dunnigan et al., Citation1970; Mann and Truswell, Citation1972; Roberts, Citation1973; Grande et al., Citation1974; Lock et al., Citation1980; Fraser et al., Citation1981; Bossetti et al., Citation1984; Albrink and Ullrich, Citation1986; Brynes et al., Citation2003; Black et al., Citation2006; Aeberli et al., Citation2011). Those that did report adverse effects included a 22-week study on men (Mann et al., Citation1972) and the ad libitum studies of women by Marckmann et al. (Citation2000) and Raben et al. (Citation2001) in which confounding due to weight change and differences in diet composition make it difficult to draw conclusions.
Evidence for TC and LDL were similarly conflicting: 4 studies using >30% sucrose suggested adverse effects (Naismith et al., Citation1974; Reiser et al., 1979a,b; Albrink and Ullrich, Citation1986; Yudkin et al., Citation1986), while 3 did not (Wu and Shreeve, Citation1975; Behall et al., Citation1980; Surwit et al., Citation1997). More moderate levels of sucrose (<30%) were associated with small effects on cholesterol in 5 studies (Roberts, Citation1973; Lock et al., Citation1980; Fraser et al., Citation1981; Marckmann et al., Citation2000; Black et al., Citation2006) but not in 7 others (Dunnigan et al., Citation1970; Mann and Truswell, Citation1972; Grande et al., Citation1974; Bossetti et al., Citation1984; Albrink and Ullrich, Citation1986; Brynes et al., Citation2003; Aeberli et al., Citation2011). Effects on HDL were purportedly adverse in 2 studies at high levels of sucrose (>30%) versus starch (Albrink and Ullrich, Citation1986; Yudkin et al., Citation1986) although others found no significant effect (Behall et al., Citation1980; Marckmann et al., Citation2000; Black et al., Citation2006). One study reported changes in components of the atherogenic lipoprotein profile, including LDL particle size, not accompanied by increases in other CVD risk factors (Aeberli et al., Citation2011). Confirmation of these findings and their significance is awaited as these proposed markers have yet to be shown to be associated with CVD risk by prospective studies in a general population cohort.
In the United States, an estimated 15% of total energy intake is derived from “added sugars” (Marriott et al., Citation2010). However, intakes are higher among adolescents (mean 21.4%) and, according to NHANES data (1999–2004), 32% of adolescents’ diets exceed 25% energy from added sugars. By comparison, population mean intake of sucrose (the main form of added sugars across the world) ranges from 8% to 17% of total energy in Europe (Elmadfa, Citation2009). In the UK, mean sucrose intake, calculated from National Diet and Nutrition Survey data for 2008–2010, was approximately 8% of energy for adults and 10% of energy for children, or 17% at 95th percentile and 19% at the 97.5th percentile for both age groups. Only 4 out of 2126 individuals consumed >25% energy from sucrose (S. Gibson, unpublished). Thus, current mean intakes are well below the level at which adverse effects on metabolic health were observed in this review. Nevertheless, adults with sucrose intakes >25% will likely have added sugar intakes of >30% and might benefit from reduction, especially if they have preexisting metabolic conditions or central obesity. However, it would be unwise, on the basis of current evidence, to place more emphasis on dietary sugars intake than on physical fitness, weight control, and a healthy balanced diet, which have well-established benefits on cardiometabolic risk factors.
Although relatively few studies examined the effects on blood glucose and insulin, there was little evidence of adverse effects on plasma glucose and some evidence of improved glucose tolerance on diets high in sucrose compared with starch (Kiens and Richter, Citation1996; Raben et al., Citation2001; Black et al., Citation2006). Fasting insulin, which is a better risk indicator than plasma glucose for impaired insulin action or reduced insulin sensitivity, was only found to be higher with the sucrose diet in one study whose subjects were potentially carbohydrate-sensitive (Reiser et al., 1979b), while other studies found no effect or even a beneficial effect (Dunnigan et al., Citation1970; Mann and Truswell, Citation1972; Kiens and Richter, Citation1996; Brynes et al., Citation2003). Moreover, the large bolus of sucrose (90% of the total given at one meal) in the studies by Reiser et al. is not representative of normal meal patterns.
Strength of Effect
It is important to distinguish between statistical significance and clinical significance. As noted above only around half the studies reported effects on TG to be statistically significant and for those in healthy adults consuming <30% sucrose, the actual elevation was typically of the order of 10–20%, although not always quantified. This is similar to normal day-to-day variation but the increment indicative of increased risk remains a matter of debate (Parks and Hellerstein, Citation2000). For cholesterol (total or LDL), differences ranged from nil to 25%, but in some cases this was confounded by higher SFA and cholesterol content in the high sucrose diet.
Conclusions and Further Research Needs
From the studies reviewed, it would appear that a moderate dietary sucrose intake at levels up to 25% of energy appears to have no significant adverse effects on lipid or carbohydrate metabolism in normal healthy adults when substituted for starch, at least in the medium term (several weeks). This conclusion is slightly more conservative than that of Truswell (Citation1994), who concluded that the evidence then available allowed a limit of 30% before effects on lipid parameters became apparent in some studies. However, there is a paucity of evidence for dose levels between 25% and 30% from sucrose and we have been reluctant to dismiss entirely the studies by Reiser et al., despite reservations as to their generalizability to the normal healthy population's eating habits. Insufficient data are available to draw reliable conclusions on the effect of substitution of sucrose for other macronutrients, although evidence of any detrimental effect is limited.
While mean intakes of sucrose in most populations are well below the 25% energy level, data are needed on the distribution of sucrose (and fructose) intakes in order to identify vulnerable groups. On our estimates, less than 0.5% of the UK population are consuming sucrose at this level, but the proportion may be higher in other groups.
New research appears to be focusing on the metabolic effects of fructose as the component purported to be responsible for adverse effects of sucrose. Fructose at high doses has been observed to increase de novo lipogenesis and reduce VLDL-C/TG clearance and more recently to increase ectopic fat accumulation (Stanhope et al., Citation2009). More studies are needed that directly address possible effects of moderately high intake of sucrose on atherogenic lipoprotein phenotype and other indicators of risk of CVD and metabolic syndrome, including inflammatory markers and endothelial function. Longer-term interventions are needed to establish whether any changes attenuate, persist, or worsen over time and how they are related to IR and visceral adiposity. There is a need to differentiate between subjects at high and low risk and to use healthy adults rather than those in whom metabolism already shows evidence of dysregulation. Studies are especially needed among women of different ages, hormonal status, and body fat distribution. The physical form of sucrose consumed (liquid beverages vs. solid sugary foods), meal size and rate of consumption may be important in relation to post-prandial metabolism, which has not been studied in this review. Finally, intervention studies are desirable to compare the relative impact on CVD risk factors of dietary change with that of changes in body weight and exercise, as these are the two modifiable physiological factors that appear to have greatest impact on response to dietary carbohydrate (Hellerstein, Citation2002).
Acknowledgments
Sigrid Gibson is Director of Sig-Nurture Ltd., an independent nutrition research consultancy to industry, government, and nonprofit organizations. This study followed ILSI guidelines relating to financial conflicts and scientific integrity (Rowe, 2009). It was funded by the World Sugar Research Organisation.
REFERENCES
- Aeberli , I. , Gerber , P. A. , Hochuli , M. , Kohler , S. , Haile , S. R. , Gouni-Berthold , I. , Berthold , H. K. , Spinas , G. A. and Berneis , K. 2011 . Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial . Am. J. Clin. Nutr. , 94 : 479 – 485 .
- Albrink , M. J. and Ullrich , I. H. 1986 . Interaction of dietary sucrose and fiber on serum lipids in healthy young men fed high carbohydrate diets . Am. J. Clin. Nutr. , 43 : 419 – 428 .
- Anderson , J. W. , Herman , R. H. and Zakim , D. 1973 . Effect of high glucose and high sucrose diets on glucose tolerance of normal men . Am. J. Clin. Nutr. , 26 : 600 – 607 .
- Bates , B. , Lennox , A. , Prentice , A. , Bates , C. and Swan , G. 2008/09–2009/10 . National Diet and Nutrition Survey: Headline Results from Years 1 and 2 (combined) of the Rolling Programme . Department of Health, available online at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsStatistics/DH_128166
- Behall , K. M. , Moser , P. B. , Kelsay , J. L. and Prather , E. S. 1980 . The effect of kind of carbohydrate in the diet and use of oral contraceptives on metabolism of young women II Serum lipid levels . Am. J. Clin. Nutr. , 33 : 825 – 831 .
- Black , R. N. , Spence , M. , McMahon , R. O. , Cuskelly , G. J. , Ennis , C. N. , McCance , D. R. , Young , I. S. , Bell , P. M. and Hunter , S. J. 2006 . Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: A randomized controlled trial . Diabetes , 55 : 3566 – 3572 .
- Bossetti , B. M. , Kocher , L. M. , Moranz , J. F. and Falko , J. M. 1984 . The effects of physiologic amounts of simple sugars on lipoprotein, glucose, and insulin levels in normal subjects . Diabetes Care , 7 : 309 – 312 .
- Brynes , A. E. , Edwards , M. C. , Ghatei , M. A. , Dornhorst , A. , Morgan , L. M. , Bloom , S. R. and Frost , G. S. 2003 . A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men . Br. J. Nutr. , 89 : 207 – 218 .
- Daly , M. 2003 . Sugars, insulin sensitivity, and the postprandial state . Am. J. Clin. Nutr. , 78 : 865S – 872S .
- Daly , M. , Vale , C. , Walker , M. , Alberti , K. G. and Mathers , J. C. 1997 . Dietary carbohydrates and insulin sensitivity: A review of the evidence and clinical implications . Am. J. Clin. Nutr. , 66 : 1072 – 1085 .
- Daly , M. , Vale , C. , Walker , M. , Littlefield , A. , Alberti , K. G. and Mathers , J. C. 1998 . Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet . Am. J. Clin. Nutr , 67 : 1186 – 1196 .
- Department of Health . Dietary Reference Values for Food Energy and Nutrients for the United Kingdom Chapter 5, pp. 72–74. HMSO, London ,
- Department of Health . 2011 . “ Headline results of Years 1 and 2 (combined) of the rolling programme (2008/2009 – 2009/10) ” . In National Diet and Nutrition Survey , Edited by: Bates , B. , Lennox , A. , Bates , C. and Swan , G. London : NatCen, UCL, MRC .
- Dunnigan , M. G. , Fyfe , T. , McKiddie , M. T. and Crosbie , S. M. 1970 . The effects of isocaloric exchange of dietary starch and sucrose on glucose tolerance, plasma insulin and serum lipids in man . Clin. Sci. , 38 : 1 – 9 .
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) . 2010 . Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre . EFSA J. , 83 ( 3 ) : 1462, 1–77
- Elmadfa , I. 2009 . European Nutrition and Health report 2009 . Forum Nutr. , 62 : I – XIII .
- Erkkilä , A. T. , Schwab , U. S. , Agren , J. J. , Hallikainen , M. , Gylling , H. and Uusitupa , M. I. J. 2007 . Moderate increase in dietary sucrose does not influence fasting or postprandial serum lipids regardless of the presence of apolipoprotein E2 allele in healthy subjects . Eur. J. Clin. Nutr. , 61 : 1094 – 1101 .
- Food and Nutrition Board, Institute of Medicine, National Academy of Sciences Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein and amino acids (Macronutrients) National Academic Press. USA . 2002 . (Republished in 2005)
- Fraser , G. E. , Jacobs , D. R. Jr. , Anderson , J. T. , Foster , N. , Palta , M. and Blackburn , H. 1981 . The effect of various vegetable supplements on serum cholesterol . Am. J. Clin. Nutr. , 34 : 1272 – 1277 .
- Frayn , K. N. and Kingman , S. M. 1995 . Dietary sugars and lipid metabolism in humans . Am. J. Clin. Nutr. , 62 : 250S – 261S . discussion 61S–63S
- Fried , S. K. and Rao , S. P. 2003 . Sugars, hypertriglyceridemia, and cardiovascular disease . Am. J. Clin. Nutr. , 78 : 873S – 880S .
- Fry , A. J. 1972 . The effect of a ‘sucrose-free’ diet on oral glucose tolerance in man . Nutr. Metab. , 14 : 313 – 323 .
- Gibson , S. Sucrose intake calculated from National Diet and Nutrition Survey data, available via http://www.data-archive.ac.uk/sign-up
- Grande , F. , Anderson , J. T. and Keys , A. 1974 . Sucrose and various carbohydrate containing foods and serum lipids in man . Am. J. Clin. Nutr. , 27 : 1043 – 1051 .
- Hayford , J. T. , Danney , M. M. , Wiebe , D. , Roberts , S. and Thompson , R. G. 1979 . Triglyceride integrated concentrations: Effect of variation of source and amount of dietary carbohydrate . Am. J. Clin. Nutr. , 32 : 1670 – 1678 .
- Hellerstein , M. K. 2002 . Carbohydrate-induced hypertriglyceridemia: Modifying factors and implications for cardiovascular risk . Curr. Opin. Lipidol. , 13 : 33 – 40 .
- Institute of Medicine . 2002 . Dietary Reference Intakes for Energy, Carbohydrate, Fat, Fibre, Fatty Acids, Cholesterol, Protein and Amino Acids . J. Am. Diet. Assoc. , 102 : 1621 – 1630 .
- Johnson , R. K. , Appel , L. J. , Brands , M. , Howard , B. V. , Lefevre , M. , Lustig , R. H. , Sacks , F. , Steffen , L. M. and Wylie-Rosett , J. 2009 . Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association . Circulation , 120 : 1011 – 1020 .
- Kiens , B. and Richter , E. A. 1996 . Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans . Am. J. Clin. Nutr. , 63 : 47 – 53 .
- Krauss , R. M. , Eckel , R. H. , Howard , B. , Appel , L. J. , Daniels , S. R. , Deckelbaum , R. J. , Erdman , J. W. Jr. , Kris-Etherton , P. , Goldberg , I. J. , Kotchen , T. A. , Lichtenstein , A. H. , Mitch , W. E. , Mullis , R. , Robinson , K. , Wylie-Rosett , J. , St Jeor , S. , Suttie , J. , Tribble , D. L. and Bazzarre , T. L. 2000 . AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association . Circulation , 102 : 2284 – 2299 .
- Liberati , A. , Altman , D. G. , Tetzlaff , J. , Mulrow , C. , Gøtzsche , P. C. , Ioannidis , J. P. A. , Clarke , M. , Devereaux , P. J. , Kleijnen , J. and Moher , D. 2009 . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration . PLoS Med. , 6 : e1000100
- Livesey , G. 2009 . Fructose ingestion: Dose-dependent responses in health research . J. Nutr. , 139 : 1246S – 1252S .
- Livesey , G. and Taylor , R. 2008 . Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies . Am. J. Cli. Nutr. , 88 : 1419 – 1437 .
- Lock , S. , Ford , M. A. , Bagley , R. and Green , L. F. 1980 . The effect on plasma lipids of the isoenergetic replacement of table sucrose by dried glucose syrup maize-syrup solids in the normal diet of adult men over a period of 1 year . Br. J. Nutr. , 43 : 251 – 256 .
- Mann , J. I. and Truswell , A. S. 1972 . Effects of isocaloric exchange of dietary sucrose and starch on fasting serum lipids, postprandial insulin secretion and alimentary lipaemia in human subjects . Br. J. Nutr. , 27 : 395 – 405 .
- Mann , J. I. and Truswell , A. S. 1973 . Sucrose-free diet and serum-lipid levels . Lancet , 2 : 153 – 154 .
- Mann , J. I. , Truswell , A. S. and Manning , E. B. 1972 . Effects on serum lipids of reducing dietary sucrose or starch for 22 weeks in normal men . S. Afr. Med. J. , 46 : 827 – 834 .
- Marckmann , P. , Raben , A. and Astrup , A. 2000 . Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: Effects on blood lipids, factor VII coagulant activity, and fibrinogen . Metab.: Clin. Exp. , 49 : 731 – 735 .
- Marriott , B. P. , Cole , N. and Lee , E. 2009 . National estimates of dietary fructose intake increased from 1977 to 2004 in the United States . J. Nutr. , 139 : 1228S – 1235S .
- Marriott , B. P. , Olsho , L. , Hadden , L. and Connor , P. 2010 . Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006 . Crit. Rev. Food Sci. Nutr. , 50 : 228 – 258 .
- Naismith , D. J. , Stock , A. L. and Yudkin , J. 1974 . Effect of changes in the proportions of the dietary carbohydrates and in energy intake on the plasma lipid concentrations in healthy young men . Nutr. Metab. , 16 : 295 – 304 .
- Nestel , P. J. , Carroll , K. F. and Havenstein , N. 1970 . Plasma triglyceride response to carbohydrates, fats and caloric intake . Metabolism , 19 : 1 – 18 .
- Parks , E. J. and Hellerstein , M. K. 2000 . Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms . Am. J. Clin. Nutr. , 71 : 412 – 433 .
- Raben , A. , Holst , J. J. , Madsen , J. and Astrup , A. 2001 . Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women . Am. J. Clin. Nutr. , 73 : 177 – 189 .
- Raben , A. , Macdonald , I. and Astrup , A. 1997 . Replacement of dietary fat by sucrose or starch: Effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects . Int. J. Obesity , 21 : 846 – 859 .
- Reiser , S. , Hallfrisch , J. , Michaelis , O. E. 4th , Lazar , F. L. , Martin , R. E. and Prather , E. S. 1979a . Isocaloric exchange of dietary starch and sucrose in humans I Effects on levels of fasting blood lipids . Am. J. Clin. Nutr. , 32 : 1659 – 1669 .
- Reiser , S. , Handler , H. B. , Gardner , L. B. , Hallfrisch , J. G. , Michaelis , O. E. t. and Prather , E. S. 1979b . Isocaloric exchange of dietary starch and sucrose in humans II Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load . Am. J. Clin. Nutr. , 32 : 2206 – 2216 .
- Reiser , S. , Michaelis , O. E. 4th , Cataland , S. and O’Dorisio , T. M. 1980 . Effect of isocaloric exchange of dietary starch and sucrose in humans on the gastric inhibitory polypeptide response to a sucrose load . Am. J. Clin. Nutr. , 33 : 1907 – 1911 .
- Roberts , A. M. 1973 . Effects of a sucrose-free diet on the serum-lipid levels of men in Antarctica . Lancet , 1 : 1201 – 1204 .
- Rowe , S. , Alexander , N. , Clydesdale , F. M. , Applebaum , R. S. , Atkinson , S. , Black , R. M. , Dwyer , J. T. , Hentges , E. , Higley , N. A. , Lefevre , M. , Lupton , J. R. , Miller , S.A. , Tancredi , D. L. , Weaver , C. M. , Woteki , C. E. and Wedral , E. 2009 . for the International Life Sciences Institute North America Working Group on Guiding Principles . Am. J. Clin. Nutr. , 89 : 1285 – 1291 . Funding food science and nutrition research: financial conflicts and scientific integrity
- Rumessen , J. J. and Gudmand-Hoyer , E. 1986 . Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides . Gut , 27 : 1161 – 1168 .
- Stanhope , K. L. and Havel , P. J. 2010 . Fructose consumption: Recent results and their potential implications . Ann. NY Acad. Sci. , 1190 : 15 – 24 .
- Stanhope , K. L. , Schwarz , J. M. , Keim , N. L. , Griffen , S. C. , Bremer , A. A. , Graham , J. L. , Hatcher , B. , Cox , C. L. , Dyachenko , A. , Zhang , W. , McGahan , J. P. , Seibert , A. , Krauss , R. M. , Chiu , S. , Schaefer , E. J. , Ai , M. , Otokozawa , S. , Nakajima , K. , Nakano , T. , Beysen , C. , Hellerstein , M. K. , Berglund , L. and Havel , P. J. 2009 . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans . J. Cli. Invest. , 119 : 1322 – 1334 .
- Surwit , R. S. , Feinglos , M. N. , McCaskill , C. C. , Clay , S. L. , Babyak , M. A. , Brownlow , B. S. , Plaisted , C. S. and Lin , P. H. 1997 . Metabolic and behavioral effects of a high-sucrose diet during weight loss . Am. J. Clin. Nutr. , 65 : 908 – 915 .
- Tappy , L. , Le , K. A. , Tran , C. and Paquot , N. 2010 . Fructose and metabolic diseases: New findings, new questions . Nutrition , 26 : 1044 – 1049 .
- Truswell , A. S. 1994 . Food carbohydrates and plasma lipids–an update . Am. J. Clin. Nutr. , 59 : 710S – 718S .
- US Department of Health and Human Services and US Department of Agriculture . Dietary Guidelines for Americans 2005 , Washington , DC : U.S. Government Printing Office . Available at http://www.health.gov/dietaryguidelines
- Welsh , J. A. , Sharma , A. , Cunningham , S. A. and Vos , M. B. 2011 . Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents . Circulation , 123 : 249 – 257 .
- WHO/FAO . 2003 . Diet, nutrition and the prevention of chronic diseases . World Health Organ Tech. Rep. Ser. , 916 : 1 – 149 .
- Wu , C. H. and Shreeve , W. W. 1975 . Human plasma triglyceride labeling after high sucrose feeding II Study on triglyceride kinetics and postheparin lipolytic activity . Metabol.: Clin. Exp. , 24 : 755 – 766 .
- Yudkin , J. , Eisa , O. , Kang , S. S. , Meraji , S. and Bruckdorfer , K. R. 1986 . Dietary sucrose affects plasma HDL cholesterol concentration in young men . Ann. Nutr. Metabol. , 30 : 261 – 266 .