Abstract
Background
Endocrine-disrupting compounds (EDCs) are ubiquitous substances that are found in our everyday lives, including pesticides, plasticizers, pharmaceutical agents, personal care products, and also in food products and food packaging. Increasing epidemiological evidence suggest that EDCs may affect the development or progression of breast cancer and consequently lead to lifelong harmful health consequences, especially when exposure occurs during early life in humans. Yet so far no appraisal of the available evidence has been conducted on this topic.
Objective
To systematically review all the available epidemiological studies about the association of the levels of environmental exposures of EDCs with breast cancer risk.
Methods
The search was performed in accordance with the PRISMA guidelines. We retrieved articles from PubMed (MEDLINE) until 10 March 2021. The key words used in this research were: “Endocrine disruptor(s)” OR “Endocrine disrupting chemical(s)” OR any of the EDCs mentioned below AND “Breast cancer” to locate all relevant articles published. We included only cohort studies and case-control studies. All relevant articles were accessed in full text and were evaluated and summarized in tables.
Results
We identified 131 studies that met the search criteria and were included in this systematic review. EDCs reviewed herein included pesticides (e.g. p,p’-dichlorodiphenyltrichloroethane (DDT), p,p’-dichlorodiphenyldichloroethylene (DDE), atrazine, 2,3,7,8-tetrachloridibenzo-p-dioxin (TCDD or dioxin)), synthetic chemicals (e.g. bisphenol A (BPA), phthalates, per- and polyfluoroalkyl substances (PFAS), parabens, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), contraceptive pills), phytoestrogens (e.g. genistein, resveratrol), and certain mycotoxins (e.g. zearalenone). Most studies assessed environmental EDCs exposure via biomarker measurements.
Conclusion
We identified certain EDC exposures could potentially elevate the risk of breast cancer. As majority of EDCs are highly persistent in the environment and bio-accumulative, it is essential to assess the long-term impacts of EDC exposures, especially multi-generational and transgenerational. Also, since food is often a major route of exposure to EDCs, well-designed exposure assessments of potential EDCs in food and food packing are necessary and their potential link to breast cancer development need to be carefully evaluated for subsequent EDC policy making and regulations.
Introduction
Breast cancer accounts for the majority of the women cancer cases worldwide. According to GLOBOCAN 2020 (2021), there are approximately 2.3 million new breast cancer cases (11%) of all the new cancer cases diagnosed in 2020 and it contributes to the most women cancer death cases globally. The incidence of women breast cancer was the highest in developed countries such as Australia and New Zealand, Western and Northern Europe and Northern America; intermediate in South America, Eastern Europe, Latin America and Caribbean; and lowest in developing areas, such as Central America, Eastern and Middle Africa and South-Central Asia (Sung et al. Citation2021). This may be attributed to some of the established risk factors for breast cancer, such as early age at menarche, late age at first birth, menopause and other potential reproductive and family histories, but these may only explain breast cancer risk partially (Collaborative Group On Hormonal Factors In Breast Citation2012). Recently, there has been an increasing interest in whether certain environmental chemicals, especially those with evidence of being hormonally active, play a role in breast cancer risk.
Endocrine disrupting chemicals (EDCs) are ubiquitous in the environment and accumulating evidence show that estrogenic properties of EDCs are potentially linked to the increasing rates of breast cancer. Young women or girls reaching puberty will have higher susceptibility to cancer if they are constantly exposed to environmental toxicants such as EDCs, even at low concentrations since EDCs perturb the endocrine system in regulating growth and differentiation (Birnbaum and Fenton Citation2003).
Due to the wide presence of EDCs in the environment, they will end up in food chain and consumed by humans. Humans can be exposed to EDCs through different routes: (i) direct ingestion of contaminated food from packaging materials or additives that are deliberately added in food processing for maintaining the quality and safety of food products; (ii) drinking of contaminated water; and (iii) breathing of air and skin contacting contaminated soil, cosmetics and personal care products, etc. Since food is indispensable for life, it is considered as the major route of EDC exposure. It is worth noting that the choices of diet would affect the levels of exposure to EDCs in humans. For instance, even within the same geographical region, people who consume a large proportion of conventional food in their diet would have higher pesticide exposure than those consume more organic food (Lu et al. Citation2006). Likewise, people who eat a lot of oily fish, meat and dairy products would have a higher exposure to the more persistent EDCs such as DDT, than those who eat more vegetables, as the lipophilic persistent EDCs are bio-accumulated in the lipid fraction of these food (Darnerud et al. Citation2006).
Compounds with estrogenic activity (estrogen-mimic), are known as xenoestrogens, and they comprise a broad range of pesticides for agriculture uses (e.g. p,p’-dichlorodiphenyltrichloroethane (DDT), p,p’-dichlorodiphenyldichloroethylene (DDE), atrazine, 2,3,7,8-tetrachloridibenzo-p-dioxin (TCDD or dioxin)), synthetic chemicals (e.g. bisphenol A (BPA), phthalates, per- and polyfluoroalkyl substances (PFAS), parabens, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), contraceptive pills), phytoestrogens (e.g. genistein, resveratrol), and certain mycotoxins (e.g. zearalenone). To assess the potential relationship between EDCs exposure and breast cancer risks, we conducted a systematic review of epidemiologic studies that have evaluated the association between these EDCs exposure (using environmental measures, questionnaires or biomarker measures) and breast cancer incidences.
Methods
Data sources and search strategy: A comprehensive literature search in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) databases was conducted using the following search terms: (“Endocrine disruptor(s)” OR “Endocrine disrupting chemical(s)” OR any of the EDCs mentioned above) AND (“Breast Cancer”) to locate all relevant articles published. Titles and abstracts were first scanned; and full articles of potential eligible studies were reviewed independently by two investigators and were carefully examined for any duplicates. Any discrepancies were resolved by consensus. The evaluation of the included studies was conducted in accordance to the criteria defined by Longnecker et al. (Citation1988) and Appel et al. (Citation2002). We confirmed our criteria with the PRISMA checklist for completeness of systematic review findings (Moher et al. Citation2010).
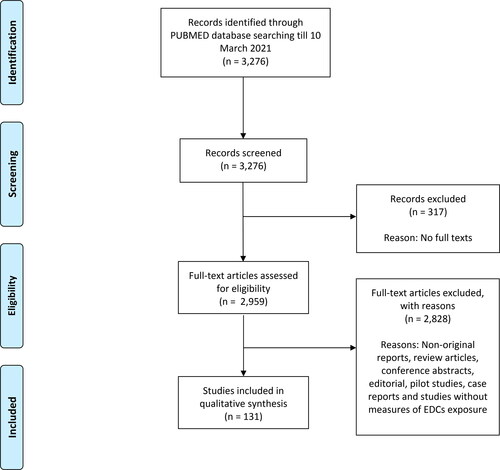
Data inclusion and exclusion: If all the above criteria were met, the studies were considered eligible for inclusion. Only cohort studies and case-control studies that evaluated the associations between EDCs and breast cancer risks were included, and we excluded non-original reports, review articles, conference abstracts, editorials, commentaries, experimental or pilot studies, case reports and case series, and studies without measures of EDCs exposure. The search was restricted to studies that were published in English. The data extracted from each study include the first author, country of origin, the duration of studies, sample size (number of breast cancer cases and control cases), biological specimen collected, biomarkers measured and breast cancer risk associations. The flowchart of our study selection is presented in .
Results
There was a total of 3,276 articles identified through PubMed database till 10 March 2021. 317 articles were first excluded due to the unavailability of full texts. The remaining articles were then assessed independently by two investigators, and 131 studies met our inclusion criteria () and were included in this review. The studies were conducted in the United States, France, Greenland, Sweden, China, Spain, Tunisia, Poland, Mexico, Denmark, Germany, Italy, Japan, Hong Kong, Belgium, Netherlands, Northern Ireland, Switzerland, Korea, Slovakia, South Africa, Canada, India, Indonesia, Jordan, Australia, Greece, United Kingdom, Taiwan, Singapore, Egypt, Norway, Brazil, Iran and Russia. We concluded that majority of the included studies were of limited quality and too diverse in outcome measures and exposure levels to allow for meaningful meta-analysis of all studies (Pittler 2002). Data were extracted and summarized in .
Table 1. Summary of papers for DDT or its metabolites exposure and breast cancer risk.
Table 2. Summary of papers for TCDD or dioxin exposure and breast cancer risk.
Table 3. Summary of papers for BPA exposure and breast cancer risk.
Table 4. Summary of papers for phthalates or its metabolites exposure and breast cancer risk.
Table 5. Summary of papers for PFOA exposure and breast cancer risk.
Table 6. Summary of papers for PCBs exposure and breast cancer risk.
Table 7. Summary of papers for PBDEs exposure and breast cancer risk.
Table 8. Summary of papers for oral contraceptive pills exposure and breast cancer risk.
Table 9. Summary of papers for genistein exposure and breast cancer risk.
Table 10. Summary of papers for mycoestrogen exposure and breast cancer risk.
Pesticides
Regarding links between breast cancer and environmental factors in humans (outside of diet), the most compelling data come from the exposure of pesticides through farming, which is associated with increased breast cancer incidences (Dorgan et al. Citation1999; Duell et al. Citation2000; Millikan et al. Citation2000; Brophy et al. Citation2002; Mathur et al. Citation2002; Engel et al. Citation2005; Xu et al. Citation2010; Brophy et al. Citation2012; Tayour et al. Citation2019). Certain organochlorine compounds, including agricultural pesticides and herbicides, such as p,p’-dichlorodiphenyltrichloroethane (DDT), p,p’-dichlorodiphenyldichloroethylene (DDE), atrazine, 2,3,7,8-tetrachloridibenzo-p-dioxin (TCDD or dioxin) may disrupt the endocrine system owing to their estrogenic properties and may cause breast cancer (Bustos et al. Citation1988; Ahlborg et al. Citation1995; Høyer et al. Citation1998; Zheng et al. Citation1999; Snedeker Citation2001).
p,p’-dichlorodiphenyltrichloroethane (DDT)/p,p’- dichlorodiphenyldichloroethylene (DDE)
DDT and/or its metabolite DDE are organochlorine pesticides that have always attracted a lot of attention worldwide because they are highly persistent EDCs in the environment. They are able to accumulate along the food chain, and therefore spontaneously be detected in breast milk and adipose tissues. Nowadays most countries impose bans on the use of DTT ever since it was discovered to have endocrine disrupting properties and cause birth defects in human and animals in 1970s (Eskenazi et al. Citation2009). Similar to most insecticides, DTT is neurotoxic, but DTT and its metabolites also cause reproductive and developmental effects (Faroon and Harris Citation2002). DDT promoted the growth of mammary tumors in some animal studies (Scribner and Mottet Citation1981; Robison, Sirbasku, and Stancel Citation1985; Brown and Lamartiniere Citation1995; Desaulniers et al. Citation2001; Johnson et al. Citation2012). Our search has found forty-three epidemiological studies regarding the DDT or its metabolites exposure on the breast cancer risk, which are summarized in . Among them, there are eleven epidemiological studies that reported positive associations between DDT or its metabolites in lipid, serum or plasma and breast cancer incidences (Wolff et al. Citation1993; Stellman et al. Citation1998; Høyer, Jørgensen, Brock, et al. Citation2000; Millikan et al. Citation2000; Mathur et al. Citation2002; Pavuk et al. Citation2003; Charlier, Foidart, et al. Citation2004; Cohn et al. Citation2007; Arrebola et al. Citation2015; Cohn, Cirillo, and Terry Citation2019; Parada et al. Citation2019). DDT is also shown to adversely affect survival following breast cancer diagnosis in a population-based study (Parada et al. Citation2016). Nine studies reported higher levels of DDT or DDE among women with breast cancer than among controls (Wassermann et al. Citation1976; Falck et al. Citation1992; Aronson et al. Citation2000; Ahmed, Loutfy, and Shiekh Citation2002; Charlier et al. Citation2003; Tang et al. Citation2014; He et al. Citation2017; Huang et al. Citation2019; Kaur et al. Citation2019). In two studies DDT was found to be linked to women with estrogen-receptor–positive breast cancer (Verreault et al. Citation1994; White et al. Citation2013), and in one study DDT increased risk of breast cancer among premenopausal women with CYP1A1 m2 variant genotype (Li et al. Citation2006). A case-control study by Chang et al. Citation2008 showed the interaction of genetic polymorphisms of glutathione S-transferase T1 with DDT residues which affected breast cancer risk (Chang et al. Citation2008). A case-control study showed that daughters whose mothers were exposed to DDT during pregnancy would have a higher risk for breast cancer later in life (Cohn et al. Citation2015). On the contrary, thirty-four studies reported no correlation between higher DDT or DDE levels in women and breast cancer risk (Hunter et al. Citation1997; López-Carrillo et al. Citation1997; van't Veer et al. Citation1997; Høyer et al. Citation1998; Moysich et al. Citation1998; Dorgan et al. Citation1999; Helzlsouer et al. Citation1999; Mendonça et al. Citation1999; Zheng et al. Citation1999; Bagga et al. Citation2000; Cocco, Kazerouni, and Zahm Citation2000; Demers et al. Citation2000; Stellman et al. Citation2000; Ward et al. Citation2000; Wolff, Berkowitz, et al. Citation2000; Wolff, Zeleniuch-Jacquotte, et al. Citation2000; Zheng, Holford, Tessari, et al. Citation2000; Laden, Collman, et al. Citation2001; Laden, Hankinson, et al. Citation2001; Snedeker Citation2001; Woolcott et al. Citation2001; Gammon et al. Citation2002; Muscat et al. Citation2003; López-Cervantes et al. Citation2004; McElroy et al. Citation2004; Raaschou-Nielsen et al. Citation2005; Gatto et al. Citation2007; Iwasaki et al. Citation2008; Itoh et al. Citation2009; Shakeel et al. Citation2010; Holmes et al. Citation2014; Louis et al. Citation2017; Rusiecki et al. Citation2020). It is suggested that time of first exposure and the levels of exposure at younger ages are critical for accurate assessment of the breast cancer risk (Johnson et al. Citation2012).
Atrazine
Atrazine was once one of the most commonly used agriculture herbicides worldwide with potent endocrine disruptor activity. Owing to the ubiquitous and unavoidable water and soil contamination, the use of atrazine was banned by European Union (EU) since October 2003 (Bethsass and Colangelo Citation2006).
In fact, atrazine is not only found in crops that are used as livestock feed but also found in milk and meat (Demarini and Zahm Citation1999). Atrazine is active and toxic at low environmentally relevant concentrations, but its mode of action is largely unknown. Atrazine is implicated to be an EDC as it has been demonstrated to cause reproductive dysfunction by inhibiting cAMP-specific phosphodiesterase-4 through the hypothalamic-pituitary-gonadal (HPG) axis (Wirbisky and Freeman Citation2015). According to IARC (Demarini and Zahm Citation1999), atrazine is classified as “unlikely to be carcinogenic to humans” but some animal studies have shown that atrazine exposure in adult rats delayed mammary gland growth and development, and long-term exposure of atrazine increased mammary tumor incidence (Laws et al. Citation2000; Stoker et al. Citation2000; Ueda et al. Citation2005; Enoch et al. Citation2007; Rosenberg et al. Citation2008; Davis et al. Citation2011; Hovey et al. Citation2011). However, epidemiological evidence for the association of atrazine exposure and breast cancer risk is still very limited. Two of them are ecological studies instead of case-control studies so they are not discussed here further (Hopenhayn-Rich, Stump, and Browning Citation2002; Freeman et al. Citation2011). There was only one case-control study evaluating the association of the exposure of atrazine and the incidences of breast cancer among women of rural Wisconsin, and the results did not demonstrate an increased breast cancer risk from atrazine exposure (Mcelroy et al. Citation2007).
2,3,7,8-Tetrachloridibenzo-p-dioxin (TCDD or dioxin)
2,3,7,8-tetrachloridibenzo-p-dioxin (TCDD or dioxin) is one of the most infamous organochlorines that is formed as a result of combustion and manufacturing chemicals. Human can be exposed to dioxins via ingestion of food that are high in fat content such as meat, poultry, milk, egg and their by-products. Dioxins are fat-soluble, very persistent and bio-accumulative in fatty tissues. They are considered as EDCs on the basis that they exert their estrogenic or anti-androgenic effects by binding to the aromatic hydrocarbon receptor (AhR) and inducing epigenetic alterations (Safe Citation1995).
According to IARC, dioxin is classified as a known human carcinogen, primarily based on occupational studies of increased mortality from all cancers combined (Dibenzofurans Citation1997). Overall, epidemiological studies did not provide a consistent link between dioxin exposure and risk of breast cancer in women. Our search has identified seven human studies about the dioxin exposure with breast cancer risk or mortality (). It was found that a higher incidence of breast cancer in women in Seveso, Italy which was associated with an elevated serum level of dioxin (Warner et al. Citation2002; Warner et al. Citation2011). The long-term effects of occupational exposure to dioxins, particularly with regards to cancer mortality, were also examined in a follow-up 23 years after closure of a German pesticide plant. An increase in breast cancer mortality was found among women workers exposed to herbicide and insecticide (Brody et al. Citation2007; Manuwald et al. Citation2012). Positive associations were found among Russian women living near a chemical plant, compared with women from a non-contaminated region (Revich et al. Citation2001). Similar results were observed in US women who resided close to incinerator plants for longer duration compared to those who lived farther (VoPham et al. Citation2020). However, in a case-control study nested within the French E3N prospective cohort, no increased risk of breast cancer was shown from higher airborne dioxin exposure, probably due to the small population size included in this study (Danjou et al. Citation2019).
Synthetic chemicals
Bisphenol A (BPA)
Bisphenol A (BPA) is a synthetic chemical used in manufacturing of polycarbonate plastics for food and beverage storage. Humans usually expose to BPA at low levels through eating food or drinking water stored in containers that contain BPA. BPA is a known endocrine disruptor, and it was considered as a relatively weak environmental estrogen because of their ability to bind either ERα and ERβ, or their capacity to activate ER-dependent transcription (Kuiper et al. Citation1998; Lemmen et al. Citation2004). Despite BPA has relatively lower binding affinity for ERs compared to estradiol (E2), some studies have demonstrated that BPA can trigger cellular responses even at very low concentrations. In some cases, BPA has similar potency as E2 (Quesada et al. Citation2002; Alonso-Magdalena et al. Citation2005; Hugo et al. Citation2008; Bouskine et al. Citation2009; Ge et al. Citation2014).
Due to its wide availability in the environment, adverse effects of BPA exposure on human health are possible. There are numerous studies examining BPA and human reproduction as well as other health outcomes (reviewed by Vandenberg et al. (Citation2013)). Furthermore, BPA is considered as a potential risk factor for breast cancer (Smith-Bindman Citation2012). Indeed there were some associations of BPA with breast cancer risks reported as summarized by Yang, Ryu, et al. (Citation2009) and in . It has been hypothesized that exposure of BPA during the early development may be the underlying cause of the increased incidence of infertility, genital tract abnormalities and breast cancer (Sharpe and Skakkebaek Citation1993; Yang, Ryu, et al. Citation2009). Also, BPA was shown to induce tumor aggressiveness in breast cancer patients, which may be associated with poor prognosis (Dairkee et al. Citation2008). There were, however, no significant associations between BPA and breast cancers found in two other studies (Trabert et al. Citation2014; Morgan et al. Citation2017). Considering potential linkages between BPA exposure and breast cancer risks, further biomonitoring studies of BPA are necessary for effective prevention against breast cancer.
Phthalates
Phthalates are a large class of chemicals that are constantly detected in food through food packaging materials and food contact substances used during processing and storage (Petersen and Jensen Citation2010). Their use is normally considered as harmless, but they are also known to induce cancer owing to its potential endocrine disrupting properties- the ability to interfere with the action or metabolism of androgens, thyroid hormones and glucocorticoids (Grindler et al. Citation2018). Majority of animal studies show that phthalates induce adverse effects on the male reproductive system (reviewed by Yost et al. (Yost et al. Citation2019)), however the precise underlying mechanisms remains largely unclear. In our search we identified six epidemiological studies that investigated the correlation of phthalates exposure and breast cancer risk by measuring different urinary phthalate metabolites as biomarkers (). Among them, four studies showed a positive association of phthalates with breast cancer risk (López-Carrillo et al. Citation2010; Holmes et al. Citation2014; Mérida-Ortega et al. Citation2016; Parada et al. Citation2018). In the study by Mérida-Ortega et al. (Citation2016), it reported the synergistic intereactions of some flavonoid from vegetables (anthocyanidins, flavan-3-ols and flavones) with phthalate exposure on the risk of breast cancer, but it was inconclusive due to the small population size.
Per- and polyfluoroalkyl substances (PFAS)
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals that can contaminate drinking water. They can enter the food chain and be consumed by human through food packaging materials and contaminated soil. It has been reported that PFAS can mimic fatty acid and bio-accumulate in adipose tissues, therefore exposure to PFAS chemicals can cause adverse health effects. Furthermore, PFAS bind to plasma proteins, which can then be passed on to the offspring via the placenta and breast milk (Inoue et al. Citation2004).
The most-studied PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). They are considered as potential EDCs, since they have been shown to cause endocrine disruption by binding and activating the peroxisome proliferator activated receptor (PPAR)-α/γ signaling pathways, which then increases hepatic aromatase concentrations and subsequent estrogen levels (DeWitt et al. Citation2009). Reproductive and developmental toxicities of PFAS have been documented in animals and humans (Lau et al. Citation2006; Yang, Tan, et al. Citation2009; Zhao et al. Citation2010; Macon et al. Citation2011; Dixon et al. Citation2012; Tsai et al. Citation2015; Anderko and Pennea Citation2020; Blake and Fenton Citation2020). After gestational exposure of rodents, PFOA was found in the placenta, amniotic fluid, breast milk and fetus (Fenton et al. Citation2009). The maternal level of PFOA was found to be associated with various reproductive and child health outcomes (Fei et al. Citation2007).
Currently, there are only five epidemiological studies about the association of PFOA with breast cancer risk (). A study of Inuit women showed a positive association of serum perfluorinated compounds along with other persistent organic chemicals and breast cancer risk (Bonefeld-Jørgensen et al. Citation2011). It was found that the genetic polymorphisms in CYP 1A1(Val) and CYP 17(A1) may increase the breast cancer risk among Inuit women, and that the risk increases with higher serum levels of PFOA and another persistent environmental contaminant, PFOS (perfluorooctane sulfonate). Three studies found positive associations between breast cancer risk and perfluoroalkylated substances, including PFOA, in Danish premenopausal women, Greenlandic Inuit and French women, respectively (Bonefeld-Jørgensen et al. Citation2014; Wielsøe, Kern, and Bonefeld-Jørgensen Citation2017; Mancini et al. Citation2020). However, a recent nested case-control study within the California Teachers showed no correlation of serum PFAS levels measured after diagnosis with breast cancer risk (Hurley et al. Citation2018).
Parabens
Parabens are synthetic chemicals that are commonly used as preservatives in many foods, for examples, beer, sauces, jams, soft drinks, as well as personal care products such as cosmetics (Golden, Gandy, and Vollmer Citation2005). In spite of their relatively low toxicity profiles and a long history of safe uses, these chemicals are considered as potential carcinogens and endocrine disruptors, because they can mimic estrogen in the body and disrupt the hormone balances (Golden, Gandy, and Vollmer Citation2005). In animal studies, parabens have been shown to affect both male and female reproductive developments (Oishi Citation2001; Kang et al. Citation2002; Kawaguchi et al. Citation2009; Vo et al. Citation2010; Ahn et al. Citation2012; Guerra, Sanabria, Cagliarani, et al. Citation2017; Guerra, Sanabria, Leite, et al. Citation2017; Fisher et al. Citation2020). In humans, parabens have been detected in normal breast cells and maternal urine and breast milk (Barr et al. Citation2012; Fisher et al. Citation2017; Amin et al. Citation2019). These studies have revealed that the estrogenic properties of parabens may perturb the mechanism of normal breast cells and potentially contribute to their abnormal growth and thus increase the risk of the breast cancer (Barr et al. Citation2012; Fisher et al. Citation2017; Amin et al. Citation2019). By far there are only one epidemiological study that reported the association of paraben exposures and breast cancer risk and mortality following breast cancer (Parada et al. Citation2019).
Polychlorinated biphenyls (PCBs)
Polychlorinated biphenyls (PCBs) are a halogenated aromatic group of ubiquitous, persistent environmental pollutants, and they can be present in high concentrations in fatty foods such as such as meat, fish and dairy products (Guo et al. Citation2019). Similar to DTT and dioxins, these chemicals are lipophilic, bio-accumulate in adipose tissue, persistent in animal tissues and excreted in breast milk (Jackson et al. Citation2017).
PCBs are a class of 209 possible congeners, and they are different from each other in the numbers and positions of chlorine atoms substituted on the aromatic rings. However, not all PCBs act alike. Some congeners seem to be estrogenic, especially those that contain less than 48% chlorine, in particular after hydroxylation (Ahlborg et al. Citation1995; Negri et al. Citation2003), with the ability to mimic and induce metabolism of different hormones such as estradiol E2, that can lead to infertility, cancers and other hormone-related disorders (Bonefeld-Jørgensen et al. Citation2001; Negri et al. Citation2003). These congeners, such as Aroclor 1221, were shown to increase uterine weight and cause precocious puberty (Gellert Citation1978). Some can also bind to the estrogen receptor (Korach et al. Citation1988) and enhance the proliferation of MCF-7 cells, as well as induction of gene expression (Nesaretnam et al. Citation1996; Andersson et al. Citation1999; Arcaro et al. Citation1999; Ptak, Mazur, and Gregoraszczuk Citation2011). Other congeners, however, bear structural similarities to dioxins, which exhibit anti-estrogenic activity via AhR which induces down-regulation of ER, interfere with ligand activated ER binding to DNA response element and/or induction of cytochrome P450 monooxygenase 1A1, 1A2 or 1B1 activities that are involved in the metabolism of E2 (Bonefeld-Jørgensen et al. Citation2001).
One of the most studied PCBs, 3,3,4,4,5-pentachlorobiphenyl (PCB 126), is classified as a “dioxin-like compound”. A study by Muto et al. (Citation2002) showed that prenatal exposure to a relatively low dose of PCB126 increased the rate of DMBA-induced rat mammary carcinoma, while a high dose decreased it. In addition exposures at these levels also reduced serum E2. In vitro studies have demonstrated that PCB126, and other congeners, including PCB77, PCB88 and PCB169 inhibited E2-stimulated cell proliferation dose-dependently in estrogen-responsive breast tumor T47D and ZR-75-1 cells (Oenga, Spink, and Carpenter Citation2004). Clearly, this complex array of potential PCB effects could be missed by evaluating solely the total level of PCBs instead of individual congeners.
In a review of epidemiology studies, the association between total PCBs and breast cancer risk was found to be inconsistent, regardless of the exposure measure (). While there are 29 studies showing no correlation of PCBs with breast cancer risk (Hunter et al. Citation1997; Høyer et al. Citation1998; Moysich et al. Citation1998; Stellman et al. Citation1998; Dorgan et al. Citation1999; Helzlsouer et al. Citation1999; Aronson et al. Citation2000; Bagga et al. Citation2000; Demers et al. Citation2000; Ward et al. Citation2000; Wolff, Berkowitz, et al. Citation2000; Zheng, Holford, Mayne, et al. Citation2000; Zheng, Holford, Tessari, et al. Citation2000; Høyer et al. Citation2001; Laden, Hankinson, et al. Citation2001; Woolcott et al. Citation2001; Gammon et al. Citation2002; López-Carrillo et al. Citation2002; Muscat et al. Citation2003; Pavuk et al. Citation2003; Raaschou-Nielsen et al. Citation2005; Prince et al. Citation2006; Gatto et al. Citation2007; Itoh et al. Citation2009; Bonefeld-Jørgensen et al. Citation2011; Holmes et al. Citation2014; Donat-Vargas et al. Citation2016; Li et al. Citation2019; Rusiecki et al. Citation2020), 19 studies found a positive association between certain PCB exposures and elevated incidence for breast cancer (Moysich et al. Citation1999; Høyer, Jørgensen, Grandjean, et al. Citation2000; Millikan et al. Citation2000; Stellman et al. Citation2000; Ahmed, Loutfy, and Shiekh Citation2002; Laden et al. Citation2002; Charlier, Albert, et al. Citation2004; Li et al. 2004; McElroy et al. Citation2004; Zhang et al. Citation2004; Silver et al. Citation2009; Villeneuve et al. Citation2010; Recio‐Vega et al. Citation2011; Cohn et al. Citation2012; Zimeri et al. Citation2015; He et al. Citation2017; Morgan et al. Citation2017; Wielsøe, Kern, and Bonefeld-Jørgensen Citation2017; Huang et al. Citation2019). It is suggested that certain polymorphisms, such as cytochrome P450 1A1 (CYP1A1) M2-containing genotypes modify the association between PCB exposure and risk of breast cancer, and genotyping of CYP1A1 could help to resolve inconsistencies in past epidemiologic studies (Moysich et al. Citation1999; Laden et al. Citation2002; Li et al. Citation2004; Zhang et al. Citation2004).
Polybrominated diphenyl ethers (PBDEs)
Polybrominated diphenyl ethers (PBDEs) are a group of synthetic halogenated compounds that have been widely distributed as environmental contaminants due to their extensive uses as additive flame retardants in many household products, such as furniture, hard plastic coating in appliances, etc (Pohl et al. Citation2017). They are highly persistent, and they can enter the food chain and be accumulated in wildlife such as fish, and other higher predators that are eventually consumed by humans (Ross et al. Citation2009). Due to their structural similarities to PCBs, they are also known to be suspected human chemical carcinogens. According to IARC (Citation2012), PCBs are not classified as carcinogenic to humans.
However, there is a raising concern over the use of PBDEs in humans as several animal studies have shown that PBDEs are able to cause endocrine disruptions and affect sexual, reproductive functions and developments (Branchi, Alleva, and Costa Citation2002; Kuriyama et al. Citation2005; Lilienthal et al. Citation2006; Tseng et al. Citation2008). This is largely due to their interference with estrogen and androgen signaling pathways. High levels of PBDEs are consistently detected in human breast tissues (She et al. Citation2002; Petreas et al. Citation2011).
Moreover, owing to their endocrine-disrupting effects, PBDEs attract a lot of interest in their connection with breast cancer. Certain PBDE congeners were shown to stimulate the proliferation of human breast cancer cells (Li, Liu, et al. Citation2012; Kanaya et al. Citation2019), In mouse models, PBDE congeners were able to promote breast tumor growth as indicated by influencing cell cycle regulation as well as increasing expression of Ki-67, a cancer proliferation marker (Kanaya et al. Citation2019). A recent study by Wei et al. (Citation2020) has revealed a positive correlation of breast cancer growth with exposure to BDE-47, one of the PBDE congeners with intense focus, owing to its extensive existence in the environment and adverse health effects. Human data are still limited but our search has identified seven case-control studies (). Among them, five studies showed no significant associations with PBDEs (McElroy et al. Citation2004; Hurley et al. Citation2011; Holmes et al. Citation2014; Hurley et al. Citation2019; Mancini et al. Citation2020). A case-control study, in contrast, showed that the BDE-47 levels in adipose tissue were positively correlated with breast cancer risk in Chinese women (He et al. Citation2018). A similar possible association of PBDE levels and breast cancer risk was observed in young women from the Ontario Environment and Health Study (Kachuri et al. Citation2018). However, significant difference in PBDE levels were not observed when examining malignant and benign tumor tissues (Li et al. Citation2019).
Oral contraceptive pills
Oral contraceptives, which contain both levonorgestrel (a progestin) and ethinyl estradiol (an estrogen), are considered as toxic EDCs because they alter normal reproductive function by mimicking the action of estrogen and thus preventing normal hormone production. A large body of evidence showed that the ethinyl estradiol in oral contraceptive pills not only affect the fertility and sexual behavior of mice but also affects fetal growth and survival, as well as causes disruption of the fetal development of the prostate, urethra, especially when the mice were exposed to ethinyl estradiol during early pregnancy (Yasuda, Kihara, and Nishimura Citation1981; Vom Saal et al. Citation1997; Timms et al. Citation2005; Della Seta et al. Citation2008; Derouiche et al. Citation2015; Kolla et al. Citation2018; Meyer et al. Citation2019).
Due to wide availability and prevalent usage of oral contraceptives among women nowadays, there are considerable interest in investigating the linkage between contraceptive pills consumption and breast cancer risk. Seven prospective and retrospective studies identified an increased breast cancer risk associated with contraceptive pills use, regardless of contraceptive pills type () (Rosenberg et al. Citation1996, Citation2009; Hunter et al. Citation2010; Bethea et al. Citation2015; Soroush et al. Citation2016; Wahidin, Djuwita, and Adisasmita Citation2018; Bardaweel et al. Citation2019), but only one study reported no such association (Vessey and Yeates Citation2013). However, there were controversies on whether duration of oral contraceptive use affect cancer risk. Bethea et al. (Citation2015) demonstrated that recency and duration of oral contraceptive use increased breast cancer risk. While discontinued use would lower the risk slightly, the risk was still higher compared to non-users. In addition, the increase in risk was more pronounced in overweight or obese women, though the authors did not suggest an explanation for this. The studies by Mørch et al. (Citation2017) and Van Hoften et al. (Citation2000) also reported that long term use of oral contraceptive increased breast cancer risk, but their studies also suggested that the increased risk would gradually reduce after discontinuation. On the other hand, three studies showed that the duration of oral contraceptive use was not associated with increased breast cancer risk (Hankinson et al. Citation1997; Urban et al. Citation2012; Bardaweel et al. Citation2019). Moreover, Rosenberg et al. (Citation1996) and Meirik et al. (Citation1986) reported that use of oral contraceptive pills at young ages increased breast cancer risk, but opposing results were observed in other studies (Hennekens et al. Citation1984).
Phytoestrogens
The chemicals that have been discussed by far are synthetic compounds that disrupt the endocrine system by mimicking or inhibiting endogenous hormones. However, there are also some naturally occurring chemicals that can mimic or inhibit the effects of endogenous hormones. Phytoestrogens are naturally occurring estrogens that can be found abundantly in plants. They are considered to be EDCs. In contrast to industrial or communal EDCs, some believe that in some cases, phytoestrogens can be beneficial, such as to treat and/or prevent breast cancer. Yet, negative effects associated with phytoestrogens were also reported. Life-long exposure to estrogenic substance, especially during critical periods of development, can lead to the severe long-term consequences, for instance, leading to the formation of malignancies and several abnormalities of the reproduction systems (Pryor et al. Citation2000).
Genistein
Genistein is a naturally occurring phytoestrogen found in most soy products. In spite of the fact that it is a polyphenol, genistein shares structural similarity with E2 and has been shown to act in a similar way as an estrogen. In vivo and in vitro data indicate that genistein can signal through both ERα and ERβ (Mueller et al. Citation2004). It has also been suggested that whether or not genistein exerts its beneficial or adverse effects depends on the timing of exposure, dose levels and endpoints examined. For example, prepubertal exposure to genistein prevented carcinogen-induced mammary gland cancer in rats (Murrill et al. Citation1996; Lamartiniere, Zhang, and Cotroneo Citation1998; Hilakivi-Clarke, Onojafe, et al. Citation1999), whereas another study showed that maternal exposure to genistein during pregnancy increased carcinogen-induced mammary tumorigenesis (Hilakivi-Clarke, Cho, et al. Citation1999). Neonatal exposure to genistein resulted in a higher incidence of uterine adenocarcinoma in mice, similar to that following diethylstilbestrol exposure (Newbold et al. Citation2001). In two multigenerational studies conducted by the National Toxicology Program, developmental genistein exposures in rats increased incidence of adenomas, adenocarcinomas and mammary hyperplasia in male offspring compared to controls (National Toxicology Program Citation2008; Latendresse et al. Citation2009). In ovariectomized athymic mice implanted with MCF-7 breast cancer cells, dietary genistein stimulated mammary gland growth and enhanced the growth of MCF-7 cell tumors, which was similar to that seen in vitro (Hsieh et al. Citation1998; Ju et al. Citation2001).
There are only sparse human data regarding the relationship between dietary exposure of soy products containing phytoestrogens such as genistein and breast cancer risk (). Although ten studies have shown that a high soy diet/supplementation reduced breast cancer risk in women (Lee et al. Citation1991; Wu et al. Citation1996, Citation2002, Citation2008; Yamamoto et al. Citation2003; Trock, Hilakivi-Clarke, and Clarke Citation2006; Do et al. Citation2007; Korde et al. Citation2009; Dong and Qin Citation2011; He and Chen Citation2013), the causative role of soy food intake in reducing breast cancer risk is still controversial. Several of these studies reported that Asian women who consumed a traditional diet (high in soy products) had lower incidence of breast cancer compared to Western women (Lee et al. Citation1991; Wu et al. Citation1996; Yamamoto et al. Citation2003; He and Chen Citation2013). Interestingly, the incidence of breast cancer in Asian-American populations was higher than native Asians, suggesting that breast cancer occurrence is not solely due to genetics but also diet or environmental factors, for example, due to reduced intakes of soy products (Wu et al. Citation1996). A case-control study by Veheus et al. (Citation2007) also showed that increased levels of isoflavones in blood samples are associated with lower risk of breast cancer among Dutch women. Yuan et al. (Yuan et al. Citation1995), however, reported no protection against breast cancer from soy consumption. A retrospective cohort study found that young women fed soy-based infant formula reported slightly longer duration of menstrual bleeding and menstrual discomfort than those who were fed a non-soy based formula (Strom et al. Citation2001). On the other hand, in an epidemiological study, pregnant women consuming vegetarian diets (with high soy isoflavones levels) during pregnancy gave birth to a male offspring with a higher incidence of hypospadias (North, Golding, and Team Citation2000).
Resveratrol (RES)
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) (RES) is one of the polyphenols abundant in red wine and grape skin. In recent years, RES has been proven to have numerous health-promoting benefits, for examples, inhibiting inflammation, viral infection, oxidative stress, and platelet aggregation and the growth of a variety of cancer cells (Athar et al. Citation2009), as well as breast cancer (Levi et al. Citation2005).
RES is structurally similar to E2 and has endocrine disrupting properties. Unlike other phytoestrogens, RES binds ERβ with higher affinity than ERα (Bowers et al. Citation2000). In the absence of E2, resveratrol exerts mixed estrogen agonist/antagonist activities in some mammary cancer cell lines, but in the presence of E2, RES functioned as an anti-estrogen (Bhat et al. Citation2001). Whether the RES-bound ER has agonist or antagonist activity in breast cancer is not clear. RES was shown to increase the expression of native estrogen-regulated genes and stimulated the proliferation of estrogen-dependent T47D breast cancer cells, and that the stimulation was blocked by the estrogen antagonist ICI 182, 780 (Gehm et al. Citation1997). Other studies have shown that RES decreased proliferation of both ER-positive (MCF-7, T47D and KPL-1) and ER-negative (MDA-MB-231 and MKL-F) breast cancer cells (Mgbonyebi, Russo, and Russo Citation1998; Lu and Serrero Citation1999; Damianaki et al. Citation2000; Nakagawa et al. Citation2001), implying that the interaction of RES with ER may not fully explain its inhibitory effect on proliferation.
Several animal studies have shown that RES is protective against mammary tumorigenesis (Bhat et al. Citation2001; Banerjee, Bueso-Ramos, and Aggarwal Citation2002; Nikaido et al. Citation2004; Provinciali et al. Citation2005; Whitsett and Lamartiniere Citation2006). Anti-breast cancer effects of RES were also documented in animals. A significant decrease in tumor growth and angiogenesis, as well as an increase in apoptotic index was demonstrated in mice treated with RES (Garvin, Öllinger, and Dabrosin Citation2006). However, RES had no effect on tumor growth or metastasis in mice administered intraperitoneally daily with RES (Bove, Lincoln, and Tsan Citation2002). Castillo-Pichardo et al. reported that RES accelerated mammary tumor growth and metastasis in nude mice, which was accompanied by a significant induction of tumoral Rac activity and a trend of increased expression of the Rac downstream effector PAK1 and other tumor promoting molecules (Castillo-Pichardo, Cubano, and Dharmawardhane Citation2013). However, there was only one case-control study in the Swiss Canton of Vaud, which reported an inverse correlation between resveratrol and breast cancer risk (La Vecchia and Bosetti Citation2006). Taken together, although RES has shown remarkable promise as a potent chemopreventive and anti-cancer drug in breast cancer, however low concentrations of RES may potentially promote breast cancer as demonstrated in animal studies. Further studies are needed to establish its usefulness as a chemopreventive agent.
Mycotoxins
Mycotoxins are highly toxic secondary metabolites of fungi. Human are exposed to these mycotoxins through ingestion of contaminated foods and feeds. Among the most important mycotoxin producing fungi are Fusarium, Aspergillus and Penicillium spp. Out of the many classes of mycotoxins produced by Fusarium fungi, resorcyclic acid lactones (RALs) are of particular concern with respect to endocrine disruption. The most prominent representative of the RALs is zearalenone (ZEA).
Zearalenone (ZEA) and its metabolites
Zearalenone (ZEA) is a macrocyclic β-RAL produced by Fusarium spp. It is a common plant and crop contaminants around the world. The oestrogenicity of ZEA and its metabolites, particularly α-zearalenol (ZOL), are attributed to their ability to bind to human estrogen receptors, although an action through a disturbance of the steroid metabolism cannot be excluded. In vitro and in vivo studies have demonstrated that ZEA and its metabolites are capable of binding to ERs which initiate estrogenic effects (Celius et al. Citation1999; Takemura et al. Citation2007; Frizzell et al. Citation2011; Li, Burns, et al. Citation2012). The binding affinity and potency are in the order of α-ZOL ∼ E2 ≫ ZEA > β-ZOL (Celius et al. Citation1999; Shier et al. Citation2001; Cozzini and Dellafiora Citation2012). Due to the structural similarity with E2, its direct estrogenic effects are expected to be similar to that of E2, and its oestrogenicity is orders of magnitudes higher than those of synthetic endocrine disruptors such as BPA, DTT, etc (Coldham et al. Citation1997). In fact, ZEA has been found to activate a variety of estrogenic genes (Parveen, Zhu, and Kiyama Citation2009) via the nuclear ERs (Frizzell et al. Citation2011) and secondary signaling (Ahamed et al. Citation2001). As a result, ZEA would increase the estrogen burden in the animals, disrupt the endocrine systems and possibly have a role in the cancer development. In vitro exposure to relatively low dose in the nano-molar range of ZEA caused proliferation of breast cancer cells (Dees et al. Citation1997; Yu et al. Citation2005; Yip et al. Citation2017); and exposure to ZEA related compound α-zeralanol (zeranol; ZAL) increased proliferation of rat breast epithelial and stroma cells (Zhong et al. Citation2011), and pre-adipocytes in young cow in vivo (Ye et al. Citation2009). However, a study has revealed that α-ZAL could exert a biphasic behavior by stimulating breast cancer at low doses but increasing apoptosis and cell cycle arrest at high doses (Yuri et al. Citation2006).
Among the few epidemiological studies on this topic (), the findings on ZEA exposure and breast cancer risk were also controversial. A case-control study in Tunisia suggested a positive association between urinary α-zeralanol concentration and breast cancer risk (Belhassen et al. Citation2015). Another study also found that ZEA promoted the growth of mammary gland tumors, and the authors advised ZEA as a potential risk factor for breast cancer (Kuciel-Lisieska et al. Citation2008). On the other hand, Pillay et al. (Citation2002) found no significant difference between plasma concentrations of ZEA, α-ZOL and β-ZOL in breast cancer patients and healthy volunteers. One possible explanation for the different conclusions could be inconsistencies in measuring the in vivo levels of ZEA and its metabolites, where some studies measured only the free forms of the mycotoxin while others accounted for the total levels in the biological samples (Bandera et al. Citation2011; Belhassen et al. Citation2015).
Discussion
This paper provides a comprehensive review of all the epidemiological studies about the association of common EDCs found in pesticides, herbicides, plant crops and food additives/packaging and breast cancer risks.
From these studies, it is first worth mentioning that for EDCs to cause impairment of endocrine functions, time of exposure is as important as dose, duration and age at exposure. Indeed, an EDC can have different effects in different cell/tissue types, species, and at different time during the life cycle (Zoeller et al. Citation2012). Moreover, the relationship between EDC exposure and breast cancer risk is not always clear-cut. Several studies suggest that genetics could play a vital role, and genotypes could determine whether EDC exposure affects host susceptibility and risks. One example is the polymorphism of the CYP1A1 gene, which is responsible for estrogen metabolism. Two case-control studies reported the association of CYP1A1 genotype to one’s susceptibility to increased breast cancer risk following PCB exposure (Moysich et al. Citation1998; Laden et al. Citation2002). Women with at least one valine CYP1A1 allele are more likely to be affected by high serum PCBs levels compared to other CYP1A1 genotypes (Moysich et al. Citation1998). However, there are only a few studies considering the effects of genetic variations on EDC and breast cancer risk. Further research on this topic is needed to gain a more comprehensive understanding of the relationship between EDC exposure and breast cancer.
Moreover, there are not many of the population-based case-control studies reporting an increased risk of breast cancer with exposure to chemicals such as DDT and PCBs. The inconsistency and heterogeneity of the studies could be attributed to potential confounders or modifiers that might affect the relationship between DDT or PCBs and breast cancer incidences such as the collection time of biological samples, instruments of measurements and misclassification of the exposure. For example, DDT can stay in the body for an extended period of time, thus it is difficult to accurately identify the latent period of exposures during diagnosis (Park et al. Citation2014). For PCBs, some of the PCB congeners have short life and are quickly metabolized. Therefore, the results of existing studies may not be able to assess the exposure of short-lived organochlorine compounds accurately (Calle et al. Citation2002).
Furthermore, most of studies regarding the association of EDCs and breast cancer risk were case-control studies. Although case-control studies are an efficient method for the study of rare outcomes, they incur several limitations, for example, bias can occur during recollection of samples for exposure estimations (Melamed and Robinson Citation2019). Also, case-control studies are always retrospective which are often started with an outcome then traced back to investigate exposures. In other words, case-control studies may only prove an association but they do not reveal any causation (Lewallen and Courtright Citation1998).
The other limitation of the existing research studies is the negligence of interactive effect of different chemicals in the natural environment. For example, many persistent organic pollutants, including DDT can have either an agonistic role in estrogenic effects, or an antagonistic role. Current estimations may rule out the possibility that there is a more pronounced adverse effect resulting from interactions of several chemicals. To make things worse, there are quick emergence of thousands of new and untested synthetic chemicals in addition to the numerous naturally existing ones, and the adverse effects that could be caused by these EDCs, especially the mixture, are still not fully known. As a result, the WHO/UNEP (2013) urged the development of validated test methods for identification of EDCs, which target larger range of endocrine disrupting effects, and also the investigation of the mixture effects of EDCs.
Traditionally biomarkers of exposure are usually based on the costly and time-consuming measures of certain chemicals or chemical metabolites in the body or after excretion from the body. In this review we find that the assessment of breast cancer risk is mainly based on the measurement of EDCs and their metabolites in urine, serum, plasma or adipose tissues. However, these methods do not account for the delayed effects or multigenerational effects of EDCs on disease development. During the developmental stages, humans are usually more sensitive to stressors, and exposure to stressors in this time could lead to more devastating or permanent changes that will increase the susceptibility to some other diseases. Therefore, timing of collection of the biomarker, together with patient conditions will need to be delineated more clearly for more precise prediction of cancer risks. Also, in certain occasions, for example, when estimating the levels of exposure to oral contraceptive pills, the only source of information was based on self-reported questionnaires, which may lead to biased and imprecise estimation of breast cancer risks.
Finally, it is important to note that epidemiological studies on the association between EDCs exposure and breast cancer risk are usually diverse. Thus, one could not easily apply meta-analysis as well as graphical or statistical methods to accurately assess any publication-related biases. Large-scale well-designed studies with long-term follow-up are necessary to document the clinical importance of exposure to environmental chemicals, by taking into considerations all the relevant confounders and characterization of the exposure.
Conclusion and future perspectives
In conclusion, exposure of these EDCs via food appears to be inevitable and this can indeed significantly result in long-term adverse consequences to human health and well-being, especially in pregnant women, developing fetuses and young children, who are often considered as highly susceptible populations to EDC exposures. Accumulating body of evidence from epidemiology studies has already shown that many EDCs may influence the development or progression of breast cancer. However, owing to the co-existence of multiple EDCs in the environment, more research studies are necessary in order to adequately evaluate the human risk for breast cancer upon the exposure to mixtures of EDCs in the environment.
To date, with the extraordinary advance in next-generation technologies, such as genome sequencing, proteomics or epigenomics, these can help identify the key molecular changes associated with EDC exposure which can then be developed as potential exposure biomarkers with better sensitivity and specificity. These technologies will also pave way to better assessment of past exposure and prediction of future risks, by taking into account an individual’s genetic profile. However, for a biomarker to be used clinically, it needs to be validated in multiple and large population cohorts to ensure its reliability and demonstrate the ability to provide incremental prognostic information over conventional marker.
Competing interests
The authors declare they have no actual or potential competing financial interests.
Acknowledgements
M.W. and V.C. performed the systematic review and wrote the paper. H.E. checked the extracted data and provided input and suggestions for revision.
References
- Ahamed, S., J. S. Foster, A. Bukovsky, and J. Wimalasena. 2001. Signal transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Molecular Carcinogenesis 30 (2):88–98. doi: 10.1002/1098-2744(200102)30:2<88::AID-MC1017>3.0.CO;2-E.
- Ahlborg, U. G., L. Lipworth, L. Titus-Ernstoff, C.C. Hsieh, A. Hanberg, J. Baron, D. Trichopoulos, and H.O. Adami. 1995. Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: An assessment of the biological and epidemiological evidence. Critical Reviews in Toxicology 25 (6):463–531. doi: 10.3109/10408449509017924.
- Ahmed, M. T., N. Loutfy, and E. E. Shiekh. 2002. Residue levels of DDE and PCBs in the blood serum of women in the Port Said region of Egypt. Journal of Hazardous Materials 89 (1):41–8. doi: 10.1016/S0304-3894(01)00283-7.
- Ahn, H. J., B. S. An, E. M. Jung, H. Yang, K. C. Choi, and E. B. Jeung. 2012. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Molecular Reproduction and Development 79 (9):626–36. doi: 10.1002/mrd.22070.
- Alonso-Magdalena, P., O. Laribi, A. B. Ropero, E. Fuentes, C. Ripoll, B. Soria, and A. Nadal. 2005. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans . Environmental Health Perspectives 113 (8):969–77. doi: 10.1289/ehp.8002.
- Amin, M. M., M. Tabatabaeian, A. Chavoshani, E. Amjadi, M. Hashemi, K. Ebrahimpour, R. Klishadi, S. Khazaei, and M. Mansourian. 2019. Paraben content in adjacent normal-malignant breast tissues from women with breast cancer. Biomedical and Environmental Sciences 32 (12):893–904.
- Anderko, L., and E. Pennea. 2020. Exposures to per-and polyfluoroalkyl substances (PFAS): Potential risks to reproductive and children's health. Current Problems in Pediatric and Adolescent Health Care 50 (2):100760. doi: 10.1016/j.cppeds.2020.100760.
- Andersson, P., A. Blom, A. Johannisson, M. Pesonen, M. Tysklind, A. Berg, P.-E. Olsson, and L. Norrgren. 1999. Assessment of PCBs and hydroxylated PCBs as potential xenoestrogens: In vitro studies based on MCF-7 cell proliferation and induction of vitellogenin in primary culture of rainbow trout hepatocytes. Archives of Environmental Contamination and Toxicology 37 (2):145–50. doi: 10.1007/s002449900499.
- Appel, L. J., K. A. Robinson, E. Guallar, T. Erlinger, S. Masood, M. Jehn, L. Fleisher, N. Powe, and E. B. Bass. 2002. Utility of blood pressure monitoring outside of the clinic setting. Evidence Report/Technology Assessment (Summary) 63:1–5.
- Arcaro, K. F., L. Yi, R. F. Seegal, D. D. Vakharia, Y. Yang, D. C. Spink, K. Brosch, and J. F. Gierthy. 1999. 2, 2', 6, 6'‐tetrachlorobiphenyl is estrogenic in vitro and in vivo. Journal of Cellular Biochemistry 72 (1):94–102. doi: 10.1002/(SICI)1097-4644(19990101)72:1<94::AID-JCB10>3.0.CO;2-Y.
- Aronson, K. J., A. B. Miller, C. G. Woolcott, E. E. Sterns, D. R. McCready, L. A. Lickley, E. B. Fish, G. Y. Hiraki, C. Holloway, T. Ross, et al. 2000. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention 9 (1):55.
- Arrebola, J. P., H. Belhassen, F. Artacho-Cordón, R. Ghali, H. Ghorbel, H. Boussen, F. M. Perez-Carrascosa, J. Expósito, A. Hedhili, and N. Olea. 2015. Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: A case–control study in Tunisia. Science of the Total Environment 520:106–13. doi: 10.1016/j.scitotenv.2015.03.045.
- Arrebola, J. P., M. Cuellar, J. P. Bonde, B. González-Alzaga, and L. A. Mercado. 2016. Associations of maternal o,p′-DDT and p,p′-DDE levels with birth outcomes in a Bolivian cohort. Environmental Research 151:469–477. doi: 10.1016/j.envres.2016.08.008.
- Athar, M., J. H. Back, L. Kopelovich, D. R. Bickers, and A. L. Kim. 2009. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Archives of Biochemistry and Biophysics 486 (2):95–102. doi: 10.1016/j.abb.2009.01.018.
- Bagga, D., E. Roberts, H.-J. Wang, J. A. Glaspy, and K. H. Anders. 2000. Organochlorine pesticide content of breast adipose tissue from women with breast cancer and control subjects. Journal of the National Cancer Institute 92 (9):750–3. doi: 10.1093/jnci/92.9.750.
- Bandera, E. V., U. Chandran, B. Buckley, Y. Lin, S. Isukapalli, I. Marshall, M. King, and H. Zarbl. 2011. Urinary mycoestrogens, body size and breast development in New Jersey girls. The Science of the Total Environment 409 (24):5221–7. doi: 10.1016/j.scitotenv.2011.09.029.
- Banerjee, S., C. Bueso-Ramos, and B. B. Aggarwal. 2002. Suppression of 7, 12-dimethylbenz (a) anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Research 62 (17):4945–54.
- Bardaweel, S. K., A. A. Akour, S. Al-Muhaissen, H. A. AlSalamat, and K. Ammar. 2019. Oral contraceptive and breast cancer: Do benefits outweigh the risks? A case – control study from Jordan. BMC Womens Health 19 (1):72.
- Barr, L., G. Metaxas, C. Harbach, L. Savoy, and P. Darbre. 2012. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. Journal of Applied Toxicology 32 (3):219–32. doi: 10.1002/jat.1786.
- Belhassen, H., I. Jiménez-Díaz, J. Arrebola, R. Ghali, H. Ghorbel, N. Olea, and A. Hedili. 2015. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere 128:1–6. doi: 10.1016/j.chemosphere.2014.12.055.
- Bethea, T. N., L. Rosenberg, C.-C. Hong, M. A. Troester, K. L. Lunetta, E. V. Bandera, P. Schedin, L. N. Kolonel, A. F. Olshan, C. B. Ambrosone, et al. 2015. A case-control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast Cancer Research: BCR 17 (1):22. doi: 10.1186/s13058-015-0535-x.
- Bethsass, J., and A. Colangelo. 2006. European Union bans atrazine, while the United States negotiates continued use. International Journal of Occupational and Environmental Health 12 (3):260–267. doi: 10.1179/oeh.2006.12.3.260.
- Bhat, K. P., D. Lantvit, K. Christov, R. G. Mehta, R. C. Moon, and J. M. Pezzuto. 2001. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Research 61 (20):7456–7463.
- Birnbaum, L. S., and S. E. Fenton. 2003. Cancer and developmental exposure to endocrine disruptors. Environmental Health Perspectives 111 (4):389–394. doi: 10.1289/ehp.5686.
- Blake, B. E., and S. E. Fenton. 2020. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri-and postnatal effects. Toxicology 443:152565. doi: 10.1016/j.tox.2020.152565.
- Bonefeld-Jørgensen, E. C., H. R. Andersen, T. H. Rasmussen, and A. M. Vinggaard. 2001. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158 (3):141–153. doi: 10.1016/S0300-483X(00)00368-1.
- Ahlborg, U. G., L. Lipworth, L. Titus-Ernstoff, C.C. Hsieh, A. Hanberg, J. Baron, D. Trichopoulos, and H.O. Adami. 1995. Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: An assessment of the biological and epidemiological evidence. Critical Reviews in Toxicology 25 (6):463–531. doi: 10.3109/10408449509017924.
- Bonefeld-Jørgensen, E. C., M. Long, S. O. Fredslund, R. Bossi, and J. Olsen. 2014. Breast cancer risk after exposure to perfluorinated compounds in Danish women: A case-control study nested in the Danish National Birth Cohort. Cancer Causes & Control: CCC 25 (11):1439–1448. doi: 10.1007/s10552-014-0446-7.
- Bosetti, C., L. Spertini, M. Parpinel, P. Gnagnarella, P. Lagiou, E. Negri, S. Franceschi, M. Montella, J. Peterson, J. Dwyer, A. Giacosa, and C. La Vecchia. 2005. Flavonoids and breast cancer risk in Italy. Cancer Epidemiology, Biomarkers & Prevention 14 (4):805–808. doi: 10.1158/1055-9965.EPI-04-0838.
- Bouskine, A., M. Nebout, F. Brucker-Davis, M. Benahmed, and P. Fenichel. 2009. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environmental Health Perspectives 117 (7):1053–1058. doi: 10.1289/ehp.0800367.
- Bove, K., D. W. Lincoln, and M.-F. Tsan. 2002. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochemical and Biophysical Research Communications 291 (4):1001–1005. doi: 10.1006/bbrc.2002.6554.
- Bowers, J. L., V. V. Tyulmenkov, S. C. Jernigan, and C. M. Klinge. 2000. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 141 (10):3657–3667. doi: 10.1210/endo.141.10.7721.
- Branchi, I., E. Alleva, and L. G. Costa. 2002. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioural development. Neurotoxicology 23 (3):375–384. doi: 10.1016/S0161-813X(02)00078-5.
- Brody, J. G., K. B. Moysich, O. Humblet, K. R. Attfield, G. P. Beehler, and R. A. Rudel. 2007. Environmental pollutants and breast cancer: Epidemiologic studies. Cancer 109 (12 Suppl):2667–2711. doi: 10.1002/cncr.22655.
- Brophy, J. T., M. M. Keith, A. Watterson, R. Park, M. Gilbertson, E. Maticka-Tyndale, M. Beck, H. Abu-Zahra, K. Schneider, A. Reinhartz, et al. 2012. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: A Canadian case-control study. Environmental Health : a Global Access Science Source 11 (1):87. doi: 10.1186/1476-069X-11-87.
- Brophy, J. T., M. M. Keith, K. M. Gorey, E. Laukkanen, D. Hellyer, A. Watterson, A. Reinhartz, and M. Gilberston. 2002. Occupational histories of cancer patients in a Canadian cancer treatment center and the generated hypothesis regarding breast cancer and farming. International Journal of Occupational and Environmental Health 8 (4):346–353. doi: 10.1179/oeh.2002.8.4.346.
- Brown, N. M., and C. A. Lamartiniere. 1995. Xenoestrogens alter mammary gland differentiation and cell proliferation in the rat. Environmental Health Perspectives 103 (7–8):708–713. doi: 10.2307/3432863.
- Bustos, S., J. C. Denegri, F. Diaz, and A. N. Tchernitchin. 1988. p,p'-DDT is an estrogenic compound. Bulletin of Environmental Contamination and Toxicology 41 (4):496–501. doi: 10.1007/BF02020992.
- Calle, E. E., H. Frumkin, S. J. Henley, D. A. Savitz, and M. J. Thun. 2002. Organochlorines and breast cancer risk. CA: A Cancer Journal for Clinicians 52 (5):301–309. doi: 10.3322/canjclin.52.5.301.
- Castillo-Pichardo, L., L. A. Cubano, and S. Dharmawardhane. 2013. Dietary grape polyphenol resveratrol increases mammary tumor growth and metastasis in immunocompromised mice. BMC Complementary and Alternative Medicine 13 (1):6. doi: 10.1186/1472-6882-13-6.
- Celius, T., T. B. Haugen, T. Grotmol, and B. T. Walther. 1999. A sensitive zonagenetic assay for rapid in vitro assessment of estrogenic potency of xenobiotics and mycotoxins. Environmental Health Perspectives 107 (1):63–68. doi: 10.1289/ehp.9910763.
- Chang, Y., J. Li, S. Yao, W. Hu, S. Jiang, Z. Guo, L. Yang, D. Li, Y. Li, and Y. Liu. 2008. A case-control study on serum organochlorines residues, genetic polymorphisms of glutathione S-transferase T1 and the risks of breast cancer. Zhonghua Liu Xing Bing Xue za Zhi = Zhonghua Liuxingbingxue Zazhi 29 (8):763–766.
- Charlier, C. J., A. I. Albert, L. Zhang, N. G. Dubois, and G. J. Plomteux. 2004. Polychlorinated biphenyls contamination in women with breast cancer. Clinica Chimica Acta 347 (1–2):177–181. doi: 10.1016/j.cccn.2004.04.025.
- Charlier, C., A. Albert, P. Herman, E. Hamoir, U. Gaspard, M. Meurisse, and G. Plomteux. 2003. Breast cancer and serum organochlorine residues. Occupational and Environmental Medicine 60 (5):348–351. doi: 10.1136/oem.60.5.348.
- Charlier, C., J.-M. Foidart, F. Pitance, P. Herman, U. Gaspard, M. Meurisse, and G. Plomteux. 2004. Environmental dichlorodiphenyltrichlorethane or hexachlorobenzene exposure and breast cancer: Is there a risk? Clinical Chemistry and Laboratory Medicine 42 (2):222–227.
- Cocco, P., N. Kazerouni, and S. H. Zahm. 2000. Cancer mortality and environmental exposure to DDE in the United States. Environmental Health Perspectives 108 (1):1–4. doi: 10.2307/3454288.
- Cohn, B. A., M. B. Terry, M. Plumb, and P. M. Cirillo. 2012. Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50. Breast Cancer Research and Treatment 136 (1):267–275. doi: 10.1007/s10549-012-2257-4.
- Cohn, B. A., M. La Merrill, N. Y. Krigbaum, G. Yeh, J.-S. Park, L. Zimmermann, and P. M. Cirillo. 2015. DDT exposure in utero and breast cancer. The Journal of Clinical Endocrinology and Metabolism 100 (8):2865–2872. doi: 10.1210/jc.2015-1841.
- Cohn, B. A., M. S. Wolff, P. M. Cirillo, and R. I. Sholtz. 2007. DDT and breast cancer in young women: New data on the significance of age at exposure. Environmental Health Perspectives 115 (10):1406–1414. doi: 10.1289/ehp.10260.
- Cohn, B. A., P. M. Cirillo, and M. B. Terry. 2019. DDT and breast cancer: Prospective study of induction time and susceptibility windows. JNCI: Journal of the National Cancer Institute 111 (8):803–810. doi: 10.1093/jnci/djy198.
- Coldham, N. G., M. Dave, S. Sivapathasundaram, D. P. McDonnell, C. Connor, and M. J. Sauer. 1997. Evaluation of a recombinant yeast cell estrogen screening assay. Environmental Health Perspectives 105 (7):734–742. doi: 10.1289/ehp.97105734.
- Collaborative Group On Hormonal Factors In Breast, C.. 2012. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncology 13 (11):1141–1151.
- Cozzini, P., and L. Dellafiora. 2012. In silico approach to evaluate molecular interaction between mycotoxins and the estrogen receptors ligand binding domain: A case study on zearalenone and its metabolites. Toxicology Letters 214 (1):81–85. doi: 10.1016/j.toxlet.2012.07.023.
- Dai, Q., X. O. Shu, F. Jin, J. D. Potter, L. H. Kushi, J. Teas, Y. T. Gao, and W. Zheng. 2001. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. British Journal of Cancer 85 (3):372–378. doi: 10.1054/bjoc.2001.1873.
- Dairkee, S. H., J. Seok, S. Champion, A. Sayeed, M. Mindrinos, W. Xiao, R. W. Davis, and W. H. Goodson. 2008. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Research 68 (7):2076–2080. doi: 10.1158/0008-5472.CAN-07-6526.
- Damianaki, A., E. Bakogeorgou, M. Kampa, G. Notas, A. Hatzoglou, S. Panagiotou, C. Gemetzi, E. Kouroumalis, P. M. Martin, and E. Castanas. 2000. Potent inhibitory action of red wine polyphenols on human breast cancer cells. Journal of Cellular Biochemistry 78 (3):429–441. doi: 10.1002/1097-4644(20000901)78:3<429::AID-JCB8>3.0.CO;2-M.
- Danjou, A. M. N., T. Coudon, D. Praud, E. Lévêque, E. Faure, P. Salizzoni, M. L. Romancer, G. Severi, F. R. Mancini, K. Leffondré, et al. 2019. Long-term airborne dioxin exposure and breast cancer risk in a case-control study nested within the French E3N prospective cohort. Environment International 124:236–248. doi: 10.1016/j.envint.2019.01.001.
- Darnerud, P. O., S. Atuma, M. Aune, R. Bjerselius, A. Glynn, K. P. Grawe, and W. Becker. 2006. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 44 (9):1597–1606. doi: 10.1016/j.fct.2006.03.011.
- Davis, L. K., A. S. Murr, D. S. Best, M. J. Fraites, L. M. Zorrilla, M. G. Narotsky, T. E. Stoker, J. M. Goldman, and R. L. Cooper. 2011. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reproductive Toxicology (Elmsford, N. Y.) 32 (1):43–51. doi: 10.1016/j.reprotox.2011.04.004.
- Dees, C., J. S. Foster, S. Ahamed, and J. Wimalasena. 1997. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environmental Health Perspectives 105 (Suppl 3):633–636. doi: 10.1289/ehp.97105s3633.
- Della Seta, D., F. Farabollini, F. Dessì-Fulgheri, and L. Fusani. 2008. Environmental-like exposure to low levels of estrogen affects sexual behavior and physiology of female rats. Endocrinology 149 (11):5592–5598. doi: 10.1210/en.2008-0113.
- Demarini, D. M., and S. H. Zahm. 1999. Atrazine. IARC Monographs 73: 196–205.
- Demers, A., P. Ayotte, J. Brisson, S. Dodin, J. Robert, and E. Dewailly. 2000. Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 9 (2):161–166.
- Derouiche, L., M. Keller, A. H. Duittoz, and D. Pillon. 2015. Developmental exposure to Ethinylestradiol affects transgenerationally sexual behavior and neuroendocrine networks in male mice. Scientific Reports 5 (1):1–12. doi: 10.1038/srep17457.
- Desaulniers, D., K. Leingartner, J. Russo, G. Perkins, B. G. Chittim, M. C. Archer, M. Wade, and J. Yang. 2001. Modulatory effects of neonatal exposure to TCDD, or a mixture of PCBs, p, p'-DDT, and p-p'-DDE, on methylnitrosourea-induced mammary tumor development in the rat. Environmental Health Perspectives 109 (7):739. doi: 10.2307/3454792.
- DeWitt, J. C., A. Shnyra, M. Z. Badr, S. E. Loveless, D. Hoban, S. R. Frame, R. Cunard, S. E. Anderson, B. J. Meade, M. M. Peden-Adams, et al. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Critical Reviews in Toxicology 39 (1):76–94. doi: 10.1080/10408440802209804.
- Dibenzofurans, P. 1997. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. In IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 69.
- Dixon, D., C. E. Reed, A. B. Moore, E. A. Gibbs-Flournoy, E. P. Hines, E. A. Wallace, J. P. Stanko, Y. Lu, W. N. Jefferson, R. R. Newbold, et al. 2012. Histopathologic changes in the uterus, cervix and vagina of immature CD-1 mice exposed to low doses of perfluorooctanoic acid (PFOA) in a uterotrophic assay. Reproductive Toxicology 33 (4):506–512. doi: 10.1016/j.reprotox.2011.10.011.
- Do, M. H., S. S. Lee, P. J. Jung, and M. H. Lee. 2007. Intake of fruits, vegetables, and soy foods in relation to breast cancer risk in Korean women: A case-control study. Nutrition and Cancer 57 (1):20–27. doi: 10.1080/01635580701268063.
- Donat-Vargas, C., A. Åkesson, M. Berglund, A. Glynn, A. Wolk, and M. Kippler. 2016. Dietary exposure to polychlorinated biphenyls and risk of breast, endometrial and ovarian cancer in a prospective cohort. British Journal of Cancer 115 (9):1113–1121. doi: 10.1038/bjc.2016.282.
- Dong, J.-Y., and L.-Q. Qin. 2011. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Research and Treatment 125 (2):315–323. doi: 10.1007/s10549-010-1270-8.
- Dorgan, J. F., J. W. Brock, N. Rothman, L. L. Needham, R. Miller, H. E. Stephenson, N. Schussler, and P. R. Taylor. 1999. Serum organochlorine pesticides and PCBs and breast cancer risk: Results from a prospective analysis (USA). Cancer Causes & Control: CCC 10 (1):1–11. doi: 10.1023/A:1008824131727.
- Duell, E. J., R. C. Millikan, D. A. Savitz, B. Newman, J. C. Smith, M. J. Schell, and D. P. Sandler. 2000. A population-based case-control study of farming and breast cancer in North Carolina. Epidemiology (Cambridge, Mass.) 11 (5):523–531. doi: 10.1097/00001648-200009000-00007.
- Engel, L. S., D. A. Hill, J. A. Hoppin, J. H. Lubin, C. F. Lynch, J. Pierce, C. Samanic, D. P. Sandler, A. Blair, and M. C. Alavanja. 2005. Pesticide use and breast cancer risk among farmers' wives in the agricultural health study. American Journal of Epidemiology 161 (2):121–135. doi: 10.1093/aje/kwi022.
- Enoch, R. R., J. P. Stanko, S. N. Greiner, G. L. Youngblood, J. L. Rayner, and S. E. Fenton. 2007. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environmental Health Perspectives 115 (4):541–547. doi: 10.1289/ehp.9612.
- Eskenazi, B., J. Chevrier, L. G. Rosas, H. A. Anderson, M. S. Bornman, H. Bouwman, A. Chen, B. A. Cohn, C. de Jager, D. S. Henshel, et al. 2009. The Pine River statement: Human health consequences of DDT use. Environmental Health Perspectives 117 (9):1359–1367. doi: 10.1289/ehp.11748.
- Falck, J. F., J. A. Ricci, M. S. Wolff, J. Godbold, and P. Deckers. 1992. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Archives of Environmental Health 47 (2):143–146.
- Faroon, O., and M. O. Harris. 2002. Toxicological profile for DDT, DDE, and DDD. Georgia, US: Agency for Toxic Substances and Disease Registry, Division of Toxicology/Toxicology Information Branch.
- Fei, C., J. K. McLaughlin, R. E. Tarone, and J. Olsen. 2007. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environmental Health Perspectives 115 (11):1677–1682. doi: 10.1289/ehp.10506.
- Fenton, S. E., J. L. Reiner, S. F. Nakayama, A. D. Delinsky, J. P. Stanko, E. P. Hines, S. S. White, A. B. Lindstrom, M. J. Strynar, and S.S E. Petropoulou. 2009. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reproductive Toxicology (Elmsford, N.Y.) 27 (3–4):365–372. doi: 10.1016/j.reprotox.2009.02.012.
- Fink, B. N., S. E. Steck, M. S. Wolff, J. A. Britton, G. C. Kabat, J. C. Schroeder, S. L. Teitelbaum, A. I. Neugut, and M. D. Gammon. 2007. Dietary flavonoid intake and breast cancer risk among women on Long Island. American Journal of Epidemiology 165 (5):514–523. doi: 10.1093/aje/kwk033.
- Fisher, B. G., A. Thankamony, J. Mendiola, C. J. Petry, H. Frederiksen, A. M. Andersson, A. Juul, K. K. Ong, D. B. Dunger, I. A. Hughes, et al. 2020. Maternal serum concentrations of bisphenol A and propyl paraben in early pregnancy are associated with male infant genital development. Human Reproduction (Oxford, England) 35 (4):913–928. doi: 10.1093/humrep/deaa045.
- Fisher, M., S. MacPherson, J. M. Braun, R. Hauser, M. Walker, M. Feeley, R. Mallick, R. Bérubé, and T. E. Arbuckle. 2017. Paraben concentrations in maternal urine and breast milk and its association with personal care product use. Environmental Science & Technology 51 (7):4009–4017. doi: 10.1021/acs.est.6b04302.
- Freeman, L. E. B., J. A. Rusiecki, J. A. Hoppin, J. H. Lubin, S. Koutros, G. Andreotti, S. H. Zahm, C. J. Hines, J. B. Coble, F. Barone-Adesi, et al. 2011. Atrazine and cancer incidence among pesticide applicators in the agricultural health study (1994–2007). Environmental Health Perspectives 119 (9):1253–1259. doi: 10.1289/ehp.1103561.
- Frizzell, C., D. Ndossi, S. Verhaegen, E. Dahl, G. Eriksen, M. Sorlie, E. Ropstad, M. Muller, C. T. Elliott, and L. Connolly. 2011. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicology Letters 206 (2):210–217. doi: 10.1016/j.toxlet.2011.07.015.
- Gammon, M. D., M. S. Wolff, A. I. Neugut, S. M. Eng, S. L. Teitelbaum, J. A. Britton, M. B. Terry, B. Levin, S. D. Stellman, and G. C. Kabat. 2002. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 11 (8):686–697.
- Garvin, S., K. Öllinger, and C. Dabrosin. 2006. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Letters 231 (1):113–122. doi: 10.1016/j.canlet.2005.01.031.
- Gatto, N. M., M. P. Longnecker, M. F. Press, J. Sullivan-Halley, R. McKean-Cowdin, and L. Bernstein. 2007. Serum organochlorines and breast cancer: A case-control study among African-American women. Cancer Causes & Control : CCC 18 (1):29–39. doi: 10.1007/s10552-006-0070-2.
- Ge, L.-C., Z.-J. Chen, H.-Y. Liu, K.-S. Zhang, H. Liu, H.-B. Huang, G. Zhang, C. K. C. Wong, J. P. Giesy, J. Du, et al. 2014. Involvement of activating ERK1/2 through G protein coupled receptor 30 and estrogen receptor α/β in low doses of bisphenol A promoting growth of Sertoli TM4 cells. Toxicology Letters 226 (1):81–89. doi: 10.1016/j.toxlet.2014.01.035.
- Gehm, B. D., J. M. McAndrews, P.-Y. Chien, and J. L. Jameson. 1997. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America 94 (25):14138–14143. doi: 10.1073/pnas.94.25.14138.
- Gellert, R. J. 1978. Uterotrophic activity of polychlorinated biphenyls (PCB) and induction of precocious reproductive aging in neonatally treated female rats. Environmental Research 16 (1–3):123–130. doi: 10.1016/0013-9351(78)90149-4.
- Golden, R., J. Gandy, and G. Vollmer. 2005. A review of the endocrine activity of parabens and implications for potential risks to human health. Critical Reviews in Toxicology 35 (5):435–458. doi: 10.1080/10408440490920104.
- Grindler, N. M., L. Vanderlinden, R. Karthikraj, K. Kannan, S. Teal, A. J. Polotsky, T. L. Powell, I. V. Yang, and T. Jansson. 2018. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Scientific Reports 8 (1):6086. doi: 10.1038/s41598-018-24505-w.
- Guerra, M. T., M. Sanabria, G. A. Leite, C. S. Borges, M. S. Cucielo, J. A. Anselmo‐Franci, W. Foster, and W. Kempinas. 2017. Maternal exposure to butyl paraben impairs testicular structure and sperm quality on male rats. Environmental Toxicology 32 (4):1273–1289. doi: 10.1002/tox.22323.
- Guerra, M. T., M. Sanabria, S. V. Cagliarani, G. A. A. Leite, C. d S. Borges, and W. De Grava Kempinas. 2017. Long-term effects of in utero and lactational exposure to butyl paraben in female rats. Environmental Toxicology 32 (3):776–788. doi: 10.1002/tox.22277.
- Guha, N., M. L. Kwan, C. P. Quesenberry, E. K. Weltzien, A. L. Castillo, and B. J. Caan. 2009. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The Life After Cancer Epidemiology study. Breast Cancer Research and Treatment 118 (2):395–405. doi: 10.1007/s10549-009-0321-5.
- Guo, W., B. Pan, S. Sakkiah, G. Yavas, W. Ge, W. Zou, W. Tong, and H. Hong. 2019. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. International Journal of Environmental Research and Public Health 16 (22):4361. doi: 10.3390/ijerph16224361.
- Hankinson, S. E., G. A. Colditz, J. E. Manson, W. C. Willett, D. J. Hunter, M. J. Stampfer, and F. E. Speizer. 1997. A prospective study of oral contraceptive use and risk of breast cancer (Nurses' Health Study, United States). Cancer Causes & Control : CCC 8 (1):65–72.
- He, F. J., and J. Q. Chen. 2013. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Science and Human Wellness 2 (3–4):146–161. doi: 10.1016/j.fshw.2013.08.002.
- He, T. T., A. J. Zuo, J. G. Wang, and P. Zhao. 2017. Organochlorine pesticides accumulation and breast cancer: A hospital-based case-control study. Tumour Biology : The Journal of the International Society for Oncodevelopmental Biology and Medicine 39 (5):1010428317699114. doi: 10.1177/1010428317699114.
- He, Y., L. Peng, W. Zhang, C. Liu, Q. Yang, S. Zheng, M. Bao, Y. Huang, and K. Wu. 2018. Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: A case–control study. Environmental Research 167:160–168. doi: 10.1016/j.envres.2018.07.009.
- Helzlsouer, K. J., A. J. Alberg, H.-Y. Huang, S. C. Hoffman, P. T. Strickland, J. W. Brock, V. W. Burse, L. L. Needham, D. A. Bell, J. A. Lavigne, et al. 1999. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 8 (6):525.
- Hennekens, C. H., F. E. Speizer, R. J. Lipnick, B. Rosner, C. Bain, C. Belanger, M. J. Stampfer, W. Willett, and R. Peto. 1984. A case-control study of oral contraceptive use and breast cancer. Journal of the National Cancer Institute 72 (1):39–42. doi: 10.1093/jnci/72.1.39.
- Hilakivi-Clarke, L., E. Cho, I. Onojafe, M. Raygada, and R. Clarke. 1999. Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat offspring. Oncology Reports 6 (5):1089–1184. doi: 10.3892/or.6.5.1089.
- Hilakivi-Clarke, L., I. Onojafe, M. Raygada, E. Cho, T. Skaar, I. Russo, and R. Clarke. 1999. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. British Journal of Cancer 80 (11):1682–1688. doi: 10.1038/sj.bjc.6690584.
- Hirose, K., N. Imaeda, Y. Tokudome, C. Goto, K. Wakai, K. Matsuo, H. Ito, T. Toyama, H. Iwata, S. Tokudome, and K. Tajima. 2005. Soybean products and reduction of breast cancer risk: A case–control study in Japan. British Journal of Cancer 93 (1):15–22. doi: 10.1038/sj.bjc.6602659.
- Ho, S., M. Schooling, L. Hui, S. M. McGhee, K. Mak, and T. Lam. 2006. Soy consumption and mortality in Hong Kong: Proxy-reported case–control study of all older adult deaths in 1998. Preventive Medicine 43 (1):20–26.
- Holmes, A. K., K. R. Koller, S. M. Kieszak, A. Sjodin, A. M. Calafat, F. D. Sacco, D. W. Varner, A. P. Lanier, and C. H. Rubin. 2014. Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska Native women. International Journal of Circumpolar Health 73 (1):25760 doi: 10.3402/ijch.v73.25760.
- Hopenhayn-Rich, C., M. Stump, and S. Browning. 2002. Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Archives of Environmental Contamination and Toxicology 42 (1):127–136.
- Horn-Ross, P. L., E. M. John, M. Lee, S. L. Stewart, J. Koo, L. C. Sakoda, A. C. Shiau, J. Goldstein, P. Davis, and E. J. Perez-Stable. 2001. Phytoestrogen consumption and breast cancer risk in a multiethnic population: The Bay Area Breast Cancer Study. American Journal of Epidemiology 154 (5):434–441. doi: 10.1093/aje/154.5.434.
- Hovey, R. C., P. S. Coder, J. C. Wolf, R. L. Sielken, Jr, M. O. Tisdel, and C. B. Breckenridge. 2011. Quantitative assessment of mammary gland development in female Long Evans rats following in utero exposure to atrazine. Toxicological Sciences: An Official Journal of the Society of Toxicology 119 (2):380–390. doi: 10.1093/toxsci/kfq337.
- Høyer, A. P., P. Grandjean, T. Jørgensen, J. W. Brock, and H. B. Hartvig. 1998. Organochlorine exposure and risk of breast cancer. Lancet (London, England) 352 (9143):1816–1820. doi: 10.1016/S0140-6736(98)04504-8.
- Høyer, A. P., T. Jørgensen, F. Rank, and P. Grandjean. 2001. Organochlorine exposures influence on breast cancer risk and survival according to estrogen receptor status: A Danish cohort-nested case-control study. BMC Cancer 1 (1):8. doi: 10.1186/1471-2407-1-8.
- Høyer, A. P., T. Jørgensen, J. W. Brock, and P. Grandjean. 2000. Organochlorine exposure and breast cancer survival. Journal of Clinical Epidemiology 53 (3):323–330. doi: 10.1016/S0895-4356(99)00165-1.
- Høyer, A. P., T. Jørgensen, P. Grandjean, and H. B. Hartvig. 2000. Repeated measurements of organochlorine exposure and breast cancer risk (Denmark). Cancer Causes & Control : CCC 11 (2):177–184. doi: 10.1023/A:1008926219539.
- Hsieh, C.-Y., R. C. Santell, S. Z. Haslam, and W. G. Helferich. 1998. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Research 58 (17):3833–3838.
- Huang, W., Y. He, J. Xiao, Y. Huang, A. Li, M. He, and K. Wu. 2019. Risk of breast cancer and adipose tissue concentrations of polychlorinated biphenyls and organochlorine pesticides: A hospital-based case-control study in Chinese women. Environmental Science and Pollution Research 26 (31):32128–32136. doi: 10.1007/s11356-019-06404-3.
- Hugo, E. R., T. D. Brandebourg, J. G. Woo, J. Loftus, J. W. Alexander, and N. Ben-Jonathan. 2008. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environmental Health Perspectives 116 (12):1642–1647. doi: 10.1289/ehp.11537.
- Hunter, D. J., G. A. Colditz, S. E. Hankinson, S. Malspeis, D. Spiegelman, W. Chen, M. J. Stampfer, and W. C. Willett. 2010. Oral contraceptive use and breast cancer: A prospective study of young women. Cancer Epidemiology Biomarkers & Prevention 19 (10):2496–2502. doi: 10.1158/1055-9965.EPI-10-0747.
- Hunter, D. J., S. E. Hankinson, F. Laden, G. A. Colditz, J. E. Manson, W. C. Willett, F. E. Speizer, and M. S. Wolff. 1997. Plasma organochlorine levels and the risk of breast cancer. The New England Journal of Medicine 337 (18):1253–1258. doi: 10.1056/NEJM199710303371801.
- Hurley, S., D. Goldberg, J.-S. Park, M. Petreas, L. Bernstein, H. Anton-Culver, S. L. Neuhausen, D. O. Nelson, and P. Reynolds. 2019. A breast cancer case-control study of polybrominated diphenyl ether (PBDE) serum levels among California women. Environment International 127:412–419. doi: 10.1016/j.envint.2019.03.043.
- Hurley, S., D. Goldberg, M. Wang, J.-S. Park, M. Petreas, L. Bernstein, H. Anton-Culver, D. O. Nelson, and P. Reynolds. 2018. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: A case-control study nested in the California Teachers Study. Environment Health : a Global Access Science Source 17 (1):83.
- Hurley, S., P. Reynolds, D. Goldberg, D. O. Nelson, S. S. Jeffrey, and M. Petreas. 2011. Adipose levels of polybrominated diphenyl ethers and risk of breast cancer. Breast Cancer Research and Treatment 129 (2):505–511. doi: 10.1007/s10549-011-1481-7.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2012. Chemical agents and related occupations. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 100:9.
- Ingram, D., K. Sanders, M. Kolybaba, and D. Lopez. 1997. Case-control study of phyto-oestrogens and breast cancer. Lancet 350 (9083):990–994. doi: 10.1016/S0140-6736(97)01339-1.
- Inoue, K., F. Okada, R. Ito, S. Kato, S. Sasaki, S. Nakajima, A. Uno, Y. Saijo, F. Sata, Y. Yoshimura, et al. 2004. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: Assessment of PFOS exposure in a susceptible population during pregnancy. Environmental Health Perspectives 112 (11):1204–1207. doi: 10.1289/ehp.6864.
- Itoh, H., M. Iwasaki, T. Hanaoka, Y. Kasuga, S. Yokoyama, H. Onuma, H. Nishimura, R. Kusama, and S. Tsugane. 2009. Serum organochlorines and breast cancer risk in Japanese women: A case-control study. Cancer Causes & Control : CCC 20 (5):567–580. doi: 10.1007/s10552-008-9265-z.
- Iwasaki, M., M. Inoue, S. Sasazuki, N. Kurahashi, H. Itoh, M. Usuda, and S. Tsugane. 2008. Plasma organochlorine levels and subsequent risk of breast cancer among Japanese women: A nested case-control study. The Science of the Total Environment 402 (2–3):176–183. doi: 10.1016/j.scitotenv.2008.05.009.
- Iwasaki, M., G. S. Hamada, I. N. Nishimoto, M. M. Netto, J. Motola, F. M. Laginha, Y. Kasuga, S. Yokoyama, H. Onuma, H. Nishimura, R. Kusama, M. Kobayashi, J. Ishihara, S. Yamamoto, T. Hanaoka, and S. Tsugane. 2009. Dietary isoflavone intake and breast cancer risk in case–control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Breast Cancer Research and Treatment 116 (2):401–411. doi: 10.1007/s10549-008-0168-1.
- Jackson, E., R. Shoemaker, N. Larian, and L. Cassis. 2017. Adipose tissue as a site of toxin accumulation. Comprehensive Physiology 7 (4):1085–1135.
- Johnson, N. A., A. Ho, J. M. Cline, C. L. Hughes, W. G. Foster, and V. L. Davis. 2012. Accelerated mammary tumor onset in a HER2/Neu mouse model exposed to DDT metabolites locally delivered to the mammary gland. Environmental Health Perspectives 120 (8):1170–1176. doi: 10.1289/ehp.1104327.
- Ju, Y. H., C. D. Allred, K. F. Allred, K. L. Karko, D. R. Doerge, and W. G. Helferich. 2001. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. The Journal of Nutrition 131 (11):2957–2962. doi: 10.1093/jn/131.11.2957.
- Kachuri, L., P. Villeneuve, S. Siddique, C. Kubwabo, L. Latifovic, B. Boucher, L. Ritter, M. Cotterchio, J. Knight, and S. Harris. 2018. Serum concentrations of Polybrominated Diphenyl Ethers (PBDEs) and breast cancer risk in young women: Results from the Ontario Environment and Health Study. ISEE Conference Abstracts 2018 (1). doi: 10.1289/isesisee.2018.P03.2940.
- Kanaya, N., L. Bernal, G. Chang, T. Yamamoto, D. Nguyen, Y.-Z. Wang, J.-S. Park, C. Warden, J. Wang, X. Wu, et al. 2019. Molecular mechanisms of polybrominated diphenyl ethers (BDE-47, BDE-100, and BDE-153) in human breast cancer cells and patient-derived xenografts. Toxicological Sciences : An Official Journal of the Society of Toxicology 169 (2):380–398. doi: 10.1093/toxsci/kfz054.
- Kang, K.-S., J.-H. Che, D.-Y. Ryu, T.-W. Kim, G.-X. Li, and Y.-S. Lee. 2002. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben). The Journal of Veterinary Medical Science 64 (3):227–235. doi: 10.1292/jvms.64.227.
- Kaur, N., S. K. Swain, B. D. Banerjee, T. Sharma, and T. Krishnalata. 2019. Organochlorine pesticide exposure as a risk factor for breast cancer in young Indian women: A case-control study. South Asian Journal of Cancer 8 (4):212–214. doi: 10.4103/sajc.sajc_427_18.
- Kawaguchi, M., K. Morohoshi, J. Masuda, G. Watanabe, M. Morita, H. Imai, K. Taya, and T. Himi. 2009. Maternal isobutyl-paraben exposure decreases the plasma corticosterone level in dams and sensitivity to estrogen in female offspring rats. The Journal of Veterinary Medical Science 71 (8):1027–1033. doi: 10.1292/jvms.71.1027.
- Keinan-Boker, L., Y. T. van Der Schouw, D. E. Grobbee, and P. H. M. Peeters. 2004. Dietary phytoestrogens and breast cancer risk. American Journal of Clinical Nutrition 79 (2):282–288. doi: 10.1093/ajcn/79.2.282.
- Kolla, S., M. Morcos, B. Martin, and L. N. Vandenberg. 2018. Low dose bisphenol S or ethinyl estradiol exposures during the perinatal period alter female mouse mammary gland development. Reproductive Toxicology (Elmsford, N.Y.) 78:50–59. doi: 10.1016/j.reprotox.2018.03.003.
- Korach, K. S., P. Sarver, K. Chae, J. A. McLachlan, and J. D. McKinney. 1988. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: Conformationally restricted structural probes. Molecular Pharmacology 33 (1):120–126.
- Korde, L. A., A. H. Wu, T. Fears, A. M. Nomura, D. W. West, L. N. Kolonel, M. C. Pike, R. N. Hoover, and R. G. Ziegler. 2009. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 18 (4):1050–1059. doi: 10.1158/1055-9965.EPI-08-0405.
- Kuciel-Lisieska, G., K. Obremski, J. Stelmachów, M. Gajecka, Ł. Zielonka, E. Jakimiuk, and M. Gajecki. 2008. Presence of zearalenone in blood plasma in women with neoplastic lesions in the mammary gland. Bulletin of the Veterinary Institute in Pulawy 52:671–674.
- Kuiper, G. G., J. G. Lemmen, B. Carlsson, J. C. Corton, S. H. Safe, P. T. van der Saag, B. van der Burg, and J. A. Gustafsson. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139 (10):4252–4263. doi: 10.1210/endo.139.10.6216.
- Kumle, M., E. Weiderpass, T. Braaten, I. Persson, H. Adami, and E. Lund. 2002. Use of oral contraceptives and breast cancer risk. The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiology, Biomarkers & Prevention 11 (11):1375–1381.
- Kuriyama, S. N., C. E. Talsness, K. Grote, and I. Chahoud. 2005. Developmental exposure to low dose PBDE 99: Effects on male fertility and neurobehavior in rat offspring . Environmental Health Perspectives 113 (2):149–154. doi: 10.1289/ehp.7421.
- La Vecchia, C., and C. Bosetti. 2006. Diet and cancer risk in Mediterranean countries: Open issues. Public Health Nutrition 9 (8A):1077–1082. doi: 10.1017/S1368980007668475.
- Laden, F., G. Collman, K. Iwamoto, A. J. Alberg, G. S. Berkowitz, J. L. Freudenheim, S. E. Hankinson, K. J. Helzlsouer, T. R. Holford, H. Y. Huang, et al. 2001. 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls and breast cancer: Combined analysis of five U.S. studies . Journal of the National Cancer Institute 93 (10):768–775. doi: 10.1093/jnci/93.10.768.
- Laden, F., N. Ishibe, S. E. Hankinson, M. S. Wolff, D. M. Gertig, D. J. Hunter, and K. T. Kelsey. 2002. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the Nurses’ Health Study. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 11 (12):1560.
- Laden, F., S. E. Hankinson, M. S. Wolff, G. A. Colditz, W. C. Willett, F. E. Speizer, and D. J. Hunter. 2001. Plasma organochlorine levels and the risk of breast cancer: An extended follow-up in the Nurses' Health Study. International Journal of Cancer 91 (4):568–574. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1081>3.0.CO;2-W.
- Lamartiniere, C. A., J.-X. Zhang, and M. S. Cotroneo. 1998. Genistein studies in rats: Potential for breast cancer prevention and reproductive and developmental toxicity. The American Journal of Clinical Nutrition 68 (6 Suppl):1400S–1405S. doi: 10.1093/ajcn/68.6.1400S.
- Latendresse, J. R., T. J. Bucci, G. Olson, P. Mellick, C. C. Weis, B. Thorn, R. R. Newbold, and K. B. Delclos. 2009. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reproductive Toxicology (Elmsford, N.Y.) 28 (3):342–353. doi: 10.1016/j.reprotox.2009.04.006.
- Lau, C., J. R. Thibodeaux, R. G. Hanson, M. G. Narotsky, J. M. Rogers, A. B. Lindstrom, and M. J. Strynar. 2006. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicological Sciences : An Official Journal of the Society of Toxicology 90 (2):510–518. doi: 10.1093/toxsci/kfj105.
- Laws, S. C., J. M. Ferrell, T. E. Stoker, J. Schmid, and R. L. Cooper. 2000. The effects of atrazine on female Wistar rats: An evaluation of the protocol for assessing pubertal development and thyroid function. Toxicological Sciences : An Official Journal of the Society of Toxicology 58 (2):366–376. doi: 10.1093/toxsci/58.2.366.
- Lee, H., J. Lee, L. Gourley, S. Duffy, N. Day, and J. Estève. 1991. Dietary effects on breast-cancer risk in Singapore. Lancet (London, England) 337 (8751):1197–1200. doi: 10.1016/0140-6736(91)92867-2.
- Lee, M. M., I. Y. H. Chang, C. F. Horng, J. S. Chang, S. H. Cheng, and A. Huang. 2005. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control 16 (8):929–937. doi: 10.1007/s10552-005-4932-9.
- Lemmen, J. G., R. J. Arends, P. T. van der Saag, and B. van der Burg. 2004. In vivo imaging of activated estrogen receptors in utero by estrogens and bisphenol A. Environmental Health Perspectives 112 (15):1544–1549. doi: 10.1289/ehp.7155.
- Levi, F., C. Pasche, F. Lucchini, R. Ghidoni, M. Ferraroni, and C. L. Vecchia. 2005. Resveratrol and breast cancer risk. European Journal of Cancer Prevention : The Official Journal of the European Cancer Prevention Organisation (ECP) 14 (2):139–142. doi: 10.1097/00008469-200504000-00009.
- Lewallen, S., and P. Courtright. 1998. Epidemiology in practice: Case-control studies. Community Eye Health Journal 11 (28):57–58.
- Li, A. J., S. M. Feldman, R. K. McNally, and K. Kannan. 2019. Distribution of organohalogen and synthetic musk compounds in breast adipose tissue of breast cancer patients in Ulster County, New York, USA. Archives of Environmental Contamination and Toxicology 77 (1):68–78. doi: 10.1007/s00244-019-00621-0.
- Li, J. Y., D. S. Wu, F. Yang, H. Y. Zeng, F. M. Lei, W. D. Zhou, H. Li, and P. Tao. 2006. Study on serum organochlorines pesticides (DDTs) level, CYP1A1 genetic polymorphism and risk of breast cancer: A case control study. Zhonghua Liu Xing Bing Xue za Zhi = Zhonghua Liuxingbingxue Zazhi 27 (3):217–22.
- Li, Y., K. A. Burns, Y. Arao, C. J. Luh, and K. S. Korach. 2012. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environmental Health Perspectives 120 (7):1029–35. doi: 10.1289/ehp.1104689.
- Li, Y., R. C. Millikan, D. A. Bell, L. Cui, C. J. Tse, B. Newman, and K. Conway. 2004. Polychlorinated biphenyls, cytochrome P450 1A1 (CYP1A1) polymorphisms, and breast cancer risk among African American women and white women in North Carolina: A population-based case–control study. Breast Cancer Research 7 (1):R12. doi: 10.1186/bcr941.
- Li, Z. H., X. Y. Liu, N. Wang, J. S. Chen, Y. H. Chen, J. T. Huang, C. H. Su, F. Xie, B. Yu, and D. J. Chen. 2012. Effects of decabrominated diphenyl ether (PBDE-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environmental Health Perspectives 120 (4):541–6. doi: 10.1289/ehp.1104051.
- Lilienthal, H., A. Hack, A. Roth-Härer, S. W. Grande, and C. E. Talsness. 2006. Effects of developmental exposure to 2, 2′, 4, 4′, 5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environmental Health Perspectives 114 (2):194–201. doi: 10.1289/ehp.8391.
- Longnecker, M. P., J. A. Berlin, M. J. Orza, and T. C. Chalmers. 1988. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA 260 (5):652–6. doi: 10.1001/jama.1988.03410050072032.
- López-Carrillo, L., A. Blair, M. López-Cervantes, M. Cebrián, C. Rueda, R. Reyes, A. Mohar, and J. Bravo. 1997. Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: A case-control study from Mexico. Cancer Research 57 (17):3728–32.
- López-Carrillo, L., M. López-Cervantes, L. Torres-Sánchez, A. Blair, M. E. Cebrián, and R. M. García. 2002. Serum levels of beta-hexachlorocyclohexane, hexachlorobenzene and polychlorinated biphenyls and breast cancer in Mexican women. European Journal of Cancer Prevention : The Official Journal of the European Cancer Prevention Organisation (Ecp) 11 (2):129–35.
- López-Carrillo, L., R. U. Hernández-Ramírez, A. M. Calafat, L. Torres-Sánchez, M. Galván-Portillo, L. L. Needham, R. Ruiz-Ramos, and M. E. Cebrián. 2010. Exposure to phthalates and breast cancer risk in Northern Mexico. Environmental Health Perspectives 118 (4):539–44. doi: 10.1289/ehp.0901091.
- López-Cervantes, M., L. Torres-Sánchez, A. Tobías, and L. López-Carrillo. 2004. Dichlorodiphenyldichloroethane burden and breast cancer risk: A meta-analysis of the epidemiologic evidence. Environmental Health Perspectives 112 (2):207–14. doi: 10.1289/ehp.112-1241830.
- Louis, L. M., C. C. Lerro, M. C. Friesen, G. Andreotti, S. Koutros, D. P. Sandler, A. Blair, M. G. Robson, and L. E. B. Freeman. 2017. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environmental Health : A Global Access Science Source 16 (1):95. doi: 10.1186/s12940-017-0298-1.
- Lu, C., K. Toepel, R. Irish, R. A. Fenske, D. B. Barr, and R. Bravo. 2006. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environmental Health Perspectives 114 (2):260–3. doi: 10.1289/ehp.8418.
- Lu, R., and G. Serrero. 1999. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. Journal of Cellular Physiology 179 (3):297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P.
- Macon, M. B., L. R. Villanueva, K. Tatum-Gibbs, R. D. Zehr, M. J. Strynar, J. P. Stanko, S. S. White, L. Helfant, and S. E. Fenton. 2011. Prenatal perfluorooctanoic acid exposure in CD-1 mice: Low-dose developmental effects and internal dosimetry. Toxicological Sciences : An Official Journal of the Society of Toxicology 122 (1):134–45. doi: 10.1093/toxsci/kfr076.
- Mancini, F. R., G. Cano-Sancho, J. Gambaretti, P. Marchand, M. Boutron-Ruault, G. Severi, P. Arveux, J. Antignac, and M. Kvaskoff. 2020. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. International Journal of Cancer 146 (4):917–28. doi: 10.1002/ijc.32357.
- Manuwald, U., M. V. Garrido, J. Berger, A. Manz, and X. Baur. 2012. Mortality study of chemical workers exposed to dioxins: Follow-up 23 years after chemical plant closure. Occupational and Environmental Medicine 69 (9):636–42. doi: 10.1136/oemed-2012-100682.
- Mathur, V., P. Bhatnagar, R. G. Sharma, V. Acharya, and R. Sexana. 2002. Breast cancer incidence and exposure to pesticides among women originating from Jaipur. Environment International 28 (5):331–6. doi: 10.1016/S0160-4120(02)00031-4.
- McElroy, J. A., M. S. Kanarek, A. Trentham-Dietz, S. A. Robert, J. M. Hampton, P. A. Newcomb, H. A. Anderson, and P. L. Remington. 2004. Potential exposure to PCBs, DDT, and PBDEs from sport-caught fish consumption in relation to breast cancer risk in Wisconsin. Environmental Health Perspectives 112 (2):156–62. doi: 10.1289/ehp.6506.
- Mcelroy, J. A., R. E. Gangnon, P. A. Newcomb, M. S. Kanarek, H. A. Anderson, J. V. Brook, A. Trentham-Dietz, and P. L. Remington. 2007. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. Journal of Exposure Science & Environmental Epidemiology 17 (2):207–14. doi: 10.1038/sj.jes.7500511.
- Hurley, S., D. Goldberg, M. Wang, J.S. Park, M. Petreas, L. Bernstein, H. Anton-Culver, D. O. Nelson, and P. Reynolds. 2018. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: A case-control study nested in the California Teachers Study. Environment Health : a Global Access Science Source 17 (1):83.
- Hurley, S., D. Goldberg, M. Wang, J.S. Park, M. Petreas, L. Bernstein, H. Anton-Culver, D. O. Nelson, and P. Reynolds. 2018. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: A case-control study nested in the California Teachers Study. Environment Health : a Global Access Science Source 17 (1):83.
- Melamed, A., and J. N. Robinson. 2019. Case-control studies can be useful but have many limitations: Study design: Case-control studies. BJOG : An International Journal of Obstetrics and Gynaecology 126 (1):23. doi: 10.1111/1471-0528.15200.
- Mendonça, G. A. S., J. Eluf‐Neto, M. J. Andrada-Serpa, P. A. O. Carmo, H. H. C. Barreto, O. N. K. Inomata, and T. A. Kussumi. 1999. Organochlorines and breast cancer: A case-control study in Brazil. International Journal of Cancer 83 (5):596–600. doi: 10.1002/(SICI)1097-0215(19991126)83:5<596::AID-IJC4>3.0.CO;2-P.
- Mérida-Ortega, Á., C. Hernández-Alcaraz, R. U. Hernández-Ramírez, A. García-Martínez, B. Trejo-Valdivia, A. Salinas-Rodríguez, K. Svensson, M. E. Cebrián, F. Franco-Marina, and L. López-Carrillo. 2016. Phthalate exposure, flavonoid consumption and breast cancer risk among Mexican women. Environment International 96:167–172. doi: 10.1016/j.envint.2016.08.023.
- Meyer, N., C. G. Santamaria, J. E. Müller, A. Schumacher, H. A. Rodriguez, and A. C. Zenclussen. 2019. Exposure to 17α-ethinyl estradiol during early pregnancy affects fetal growth and survival in mice. Environmental Pollution (Barking, Essex : 1987) 251:493–501. doi: 10.1016/j.envpol.2019.04.144.
- Mgbonyebi, O. P., J. Russo, and I. H. Russo. 1998. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. International Journal of Oncology 12 (4):865–874. doi: 10.3892/ijo.12.4.865.
- Millikan, R., E. DeVoto, E. J. Duell, C.-K. Tse, D. A. Savitz, J. Beach, S. Edmiston, S. Jackson, and B. Newman. 2000. Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Cancer Epidemiology, Biomarkers & Prevention 9 (11):1233–1240.
- Moher, D., A. Liberati, J. Tetzlaff, and D. G. Altman. 2010. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery (London, England) 8 (5):336–341. doi: 10.1016/j.ijsu.2010.02.007.
- Mørch, L. S., C. W. Skovlund, P. C. Hannaford, L. Iversen, S. Fielding, and Ø. Lidegaard. 2017. Contemporary hormonal contraception and the risk of breast cancer. New England Journal of Medicine 377 (23):2228–2239. doi: 10.1056/NEJMoa1700732.
- Morgan, M., A. Deoraj, Q. Felty, and D. Roy. 2017. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Molecular and Cellular Endocrinology 457:89–102. doi: 10.1016/j.mce.2016.10.003.
- Moysich, K. B., C. B. Ambrosone, J. E. Vena, P. G. Shields, P. Mendola, P. Kostyniak, H. Greizerstein, S. Graham, J. R. Marshall, and E. F. Schisterman. 1998. Environmental organochlorine exposure and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 7 (3):181–188.
- Moysich, K. B., P. G. Shields, J. L. Freudenheim, E. F. Schisterman, J. E. Vena, P. Kostyniak, H. Greizerstein, J. R. Marshall, S. Graham, and C. B. Ambrosone. 1999. Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 8 (1):41.
- Mueller, S. O., S. Simon, K. Chae, M. Metzler, and K. S. Korach. 2004. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicological Sciences : An Official Journal of the Society of Toxicology 80 (1):14–25. doi: 10.1093/toxsci/kfh147.
- Murkies, A., F. S. Dalais, E. M. Briganti, H. G. Burger, D. L. Healy, M. L. Wahlqvist, and S. R. Davis. 2000. Phytoestrogens and breast cancer in postmenopausal women: A case control study. Menopause 7 (5):289–296.
- Murrill, W. B., N. M. Brown, J.-X. Zhang, P. A. Manzolillo, S. Barnes, and C. A. Lamartiniere. 1996. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis 17 (7):1451–1458. doi: 10.1093/carcin/17.7.1451.
- Muscat, J. E., J. A. Britton, M. V. Djordjevic, M. L. Citron, M. Kemeny, E. Busch-Devereaux, B. Pittman, and S. D. Stellman. 2003. Adipose concentrations of organochlorine compounds and breast cancer recurrence in Long Island. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 12 (12):1474–1478.
- Muto, T., S. Wakui, N. Imano, K. Nakaaki, H. Takahashi, H. Hano, M. Furusato, and T. Masaoka. 2002. Mammary gland differentiation in female rats after prenatal exposure to 3, 3′, 4, 4′, 5-pentachlorobiphenyl. Toxicology 177 (2–3):197–205. doi: 10.1016/S0300-483X(02)00224-X.
- Nakagawa, H., Y. Kiyozuka, Y. Uemura, H. Senzaki, N. Shikata, K. Hioki, and A. Tsubura. 2001. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. Journal of Cancer Research and Clinical Oncology 127 (4):258–264. doi: 10.1007/s004320000190.
- National Toxicology Program. 2008. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). National Toxicology Program technical report series 539:1–266.
- Negri, E., C. Bosetti, E. Fattore, and C. L. Vecchia. 2003. Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: A systematic review of the epidemiological evidence. European Journal of Cancer Prevention : The Official Journal of the European Cancer Prevention Organisation (Ecp) 12 (6):509–516.
- Nesaretnam, K., D. Corcoran, R. Dils, and P. Darbre. 1996. 3,4,3',4'-Tetrachlorobiphenyl acts as an estrogen in vitro and in vivo . Molecular Endocrinology (Baltimore, Md.) 10 (8):923–936. doi: 10.1210/mend.10.8.8843409.
- Newbold, R. R., E. P. Banks, B. Bullock, and W. N. Jefferson. 2001. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Research 61 (11):4325–4328.
- Nikaido, Y., K. Yoshizawa, N. Danbara, M. Tsujita-Kyutoku, T. Yuri, N. Uehara, and A. Tsubura. 2004. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reproductive Toxicology (Elmsford, N.Y.) 18 (6):803–811. doi: 10.1016/j.reprotox.2004.05.002.
- Nishio, K., Y. Niwa, H. Toyoshima, K. Tamakoshi, T. Kondo, H. Yatsuya, A. Yamamoto, S. Suzuki, S. Tokudome, Y. Lin, K. Wakai, N. Hamajima, and A. Tamakoshi. 2007. Consumption of soy foods and the risk of breast cancer: findings from the Japan Collaborative Cohort (JACC) Study. Cancer Causes Control 18 (8):801–808. doi: 10.1007/s10552-007-9023-7.
- North, K., J. Golding, and A. S. Team. 2000. A maternal vegetarian diet in pregnancy is associated with hypospadias. BJU International 85 (1):107–113. doi: 10.1046/j.1464-410x.2000.00436.x.
- Nur, U., D. El Reda, D. Hashim, and E. Weiderpass. 2019. A prospective investigation of oral contraceptive use and breast cancer mortality: Findings from the Swedish women's lifestyle and health cohort. BMC Cancer 19 (1):807–807. doi: 10.1186/s12885-019-5985-6.
- Oenga, G. N., D. C. Spink, and D. O. Carpenter. 2004. TCDD and PCBs inhibit breast cancer cell proliferation in vitro. Toxicology in Vitro : An International Journal Published in Association with BIBRA 18 (6):811–819. doi: 10.1016/j.tiv.2004.04.004.
- Oishi, S. 2001. Effects of butylparaben on the male reproductive system in rats. Toxicology and Industrial Health 17 (1):31–39. doi: 10.1191/0748233701th093oa.
- Parada, H., M. D. Gammon, J. Chen, A. M. Calafat, A. I. Neugut, R. M. Santella, M. S. Wolff, and S. L. Teitelbaum. 2018. Urinary phthalate metabolite concentrations and breast cancer incidence and survival following breast cancer: The Long Island Breast Cancer Study Project. Environmental Health Perspectives 126 (4):047013. doi: 10.1289/EHP2083.
- Parada, H., M. S. Jr., Wolff, L. S. Engel, A. J. White, S. M. Eng, R. J. Cleveland, N. K. Khankari, S. L. Teitelbaum, A. I. Neugut, and M. D. Gammon. 2016. Organochlorine insecticides DDT and chlordane in relation to survival following breast cancer. International Journal of Cancer 138 (3):565–575. doi: 10.1002/ijc.29806.
- Parada, H., X. Sun, C.-K. Tse, L. S. Engel, A. F. Olshan, and M. A. Troester. 2019. Plasma levels of dichlorodiphenyldichloroethene (DDE) and dichlorodiphenyltrichloroethane (DDT) and survival following breast cancer in the Carolina Breast Cancer Study. Environment International 125:161–171. doi: 10.1016/j.envint.2019.01.032.
- Park, J.-H., E. S. Cha, Y. Ko, M.-S. Hwang, J.-H. Hong, and W. J. Lee. 2014. Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: A systematic review and meta-analysis. Osong Public Health and Research Perspectives 5 (2):77–84. doi: 10.1016/j.phrp.2014.02.001.
- Parveen, M., Y. Zhu, and R. Kiyama. 2009. Expression profiling of the genes responding to zearalenone and its analogues using estrogen-responsive genes. FEBS Letters 583 (14):2377–2384. doi: 10.1016/j.febslet.2009.06.035.
- Paydar, P., G. Asadikaram, H. Fallah, H. Zeynali Nejad, H. Akbari, M. Abolhassani, V. Moazed, P. Khazaeli, and M. R. Heidari. 2019. Serum levels of organochlorine pesticides and breast cancer risk in Iranian women. Archives of Environmental Contamination and Toxicology 77 (4):480–489. doi: 10.1007/s00244-019-00648-3.
- Pavuk, M., J. R. Cerhan, C. F. Lynch, A. Kocan, J. Petrik, and J. Chovancova. 2003. Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia. Journal of Exposure Analysis and Environmental Epidemiology 13 (4):267–275. doi: 10.1038/sj.jea.7500277.
- Petersen, J. H., and L. K. Jensen. 2010. Phthalates and food-contact materials: Enforcing the 2008 European Union plastics legislation. Food Additives & Contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 27 (11):1608–1616. doi: 10.1080/19440049.2010.501825.
- Petreas, M., D. Nelson, F. R. Brown, D. Goldberg, S. Hurley, and P. Reynolds. 2011. High concentrations of polybrominated diphenylethers (PBDEs) in breast adipose tissue of California women. Environment International 37 (1):190–197. doi: 10.1016/j.envint.2010.09.001.
- Peterson, J., P. Lagiou, E. Samoli, A. Lagiou, K. Katsouyanni, C. La Vecchia, J. Dwyer, and D. Trichopoulos. 2003. Flavonoid intake and breast cancer risk: A case–control study in Greece. British Journal of Cancer 89 (7):1255–1259. doi: 10.1038/sj.bjc.6601271.
- Pillay, D., A. A. Chuturgoon, E. Nevines, T. Manickum, W. Deppe, and M. F. Dutton. 2002. The quantitative analysis of zearalenone and its derivatives in plasma of patients with breast and cervical cancer. Clinical Chemistry and Laboratory Medicine 40 (9):946–951.
- Pittler, M. 2002. Systematic reviews in health care: Meta-analysis in context. Focus on Alternative and Complementary Therapies 7 (2):206–207. doi: 10.1111/j.2042-7166.2002.tb05479.x.
- Pohl, H. R., M. Odin, P. R. McClure, K. Zaccaria, F. Llados, M. Kawa, and M. Citra. 2017. "Toxicological profile for polybrominated diphenyl ethers (PBDEs)."
- Prince, M. M., A. M. Ruder, M. J. Hein, M. A. Waters, E. A. Whelan, N. Nilsen, E. M. Ward, T. M. Schnorr, P. A. Laber, and K. E. Davis-King. 2006. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). Environmental Health Perspectives 114 (10):1508–1514. doi: 10.1289/ehp.9175.
- Provinciali, M., F. Re, A. Donnini, F. Orlando, B. Bartozzi, G. D. Stasio, and A. Smorlesi. 2005. Effect of resveratrol on the development of spontaneous mammary tumors in HER‐2/neu transgenic mice. International Journal of Cancer 115 (1):36–45. doi: 10.1002/ijc.20874.
- Pryor, J. L., C. Hughes, W. Foster, B. F. Hales, and B. Robaire. 2000. Critical windows of exposure for children's health: The reproductive system in animals and humans. Environmental Health Perspectives 108 (suppl 3):491–503. doi: 10.1289/ehp.00108s3491.
- Ptak, A., K. Mazur, and E. L. Gregoraszczuk. 2011. Comparison of combinatory effects of PCBs (118, 138, 153 and 180) with 17 beta-estradiol on proliferation and apoptosis in MCF-7 breast cancer cells . Toxicology and Industrial Health 27 (4):315–321. doi: 10.1177/0748233710387003.
- Quesada, I., E. Fuentes, M. C. Viso-León, B. Soria, C. Ripoll, and A. Nadal. 2002. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB . FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 16 (12):1671–1673. doi: 10.1096/fj.02-0313fje.
- Raaschou-Nielsen, O., M. Pavuk, A. LeBlanc, P. Dumas, J. Philippe Weber, A. Olsen, A. Tjønneland, K. Overvad, and J. H. Olsen. 2005. Adipose organochlorine concentrations and risk of breast cancer among postmenopausal Danish women. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 14 (1):67.
- Recio‐Vega, R., V. Velazco‐Rodriguez, G. Ocampo‐Gómez, S. Hernandez‐Gonzalez, P. Ruiz‐Flores, and F. Lopez‐Marquez. 2011. Serum levels of polychlorinated biphenyls in Mexican women and breast cancer risk. Journal of Applied Toxicology 31 (3):270–278.
- Reeves, K. W., M. Díaz Santana, J. E. Manson, S. E. Hankinson, R. T. Zoeller, C. Bigelow, S. R. Sturgeon, D. Spiegelman, L. Tinker, J. Luo, B. Chen, J. Meliker, M. R. Bonner, M. L. Cote, T.-Y. D. Cheng, and A. M. Calafat. 2019. Urinary Phthalate Biomarker Concentrations and Postmenopausal Breast Cancer Risk. JNCI: Journal of the National Cancer Institute 111 (10):1059–1067. doi: 10.1093/jnci/djz002.
- Revich, B., E. Aksel, T. Ushakova, I. Ivanova, N. Zhuchenko, N. Klyuev, B. Brodsky, and Y. Sotskov. 2001. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 43 (4–7):951–966. doi: 10.1016/S0045-6535(00)00456-2.
- Robison, A. K., D. A. Sirbasku, and G. M. Stancel. 1985. DDT supports the growth of an estrogen-responsive tumor: Mammary tumor growth; pesticides. Toxicology Letters 27 (1–3):109–113. doi: 10.1016/0378-4274(85)90127-4.
- Rosenberg, B. G., H. Chen, J. Folmer, J. Liu, V. Papadopoulos, and B. R. Zirkin. 2008. Gestational exposure to atrazine: Effects on the postnatal development of male offspring. Journal of Andrology 29 (3):304–311. doi: 10.2164/jandrol.107.003020.
- Rosenberg, L., J. R. Palmer, R. S. Rao, A. G. Zauber, B. L. Strom, M. E. Warshauer, S. Harlap, and S. Shapiro. 1996. Case-control study of oral contraceptive use and risk of breast cancer. American Journal of Epidemiology 143 (1):25–37. doi: 10.1093/oxfordjournals.aje.a008654.
- Rosenberg, L., Y. Zhang, P. F. Coogan, B. L. Strom, and J. R. Palmer. 2009. A case-control study of oral contraceptive use and incident breast cancer. American Journal of Epidemiology 169 (4):473–479. doi: 10.1093/aje/kwn360.
- Ross, P. S., C. M. Couillard, M. G. Ikonomou, S. C. Johannessen, M. Lebeuf, R. W. Macdonald, and G. T. Tomy. 2009. Large and growing environmental reservoirs of Deca-BDE present an emerging health risk for fish and marine mammals. Marine Pollution Bulletin 58 (1):7–10. doi: 10.1016/j.marpolbul.2008.09.002.
- Ruder, A. M., M. J. Hein, N. B. Hopf, and M. A. Waters. 2014. Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: A ten-year update. The International Journal of Hygiene and Environmental Health 217 (2–3):176–187. doi: 10.1016/j.ijheh.2013.04.006.
- Ruder, A. M., M. J. Hein, N. B. Hopf, and M. A. Waters. 2017. Cancer incidence among capacitor manufacturing workers exposed to polychlorinated biphenyls. American Journal of Industrial Medicine 60 (2):198–207. doi: 10.1002/ajim.22657.
- Rusiecki, J. A., H. Denic-Roberts, C. Byrne, J. Cash, C. F. Raines, L. A. Brinton, S. H. Zahm, T. Mason, M. R. Bonner, A. Blair, et al. 2020. Serum concentrations of DDE, PCBs, and other persistent organic pollutants and mammographic breast density in Triana, Alabama, a highly exposed population. Environmental Research 182:109068. doi: 10.1016/j.envres.2019.109068.
- Safe, S. H. 1995. Modulation of gene expression and endocrine response pathways by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacology & Therapeutics 67 (2):247–281. doi: 10.1016/0163-7258(95)00017-B.
- Sartippour, M. R., J. Y. Rao, S. Apple, D. Wu, S. Henning, H. Wang, R. Elashoff, R. Rubio, D. Heber, and M. N. Brooks. 2004. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutrition and Cancer 49 (1):59–65. doi: 10.1207/s15327914nc4901_8.
- Scribner, J. D., and N. K. Mottet. 1981. DDT acceleration of mammary gland tumors induced in the male Sprague-Dawley rat by 2-acetamidophenanthrene. Carcinogenesis 2 (12):1235–1239. doi: 10.1093/carcin/2.12.1235.
- Shakeel, M. K., P. S. George, J. Jose, J. Jose, and A. Mathew. 2010. Pesticides and breast cancer risk: A comparison between developed and developing countries. Asian Pacific Journal of Cancer Prevention : APJCP 11 (1):173–180.
- Shannon, J., R. Ray, C. Wu, Z. Nelson, D. L. Gao, W. Li, W. Hu, J. Lampe, N. Horner, J. Satia, R. Patterson, D. Fitzgibbons, P. Porter, and D. Thomas. 2005. Food and botanical groupings and risk of breast cancer: A case–control study in Shanghai, China. Cancer Epidemiology, Biomarkers & Prevention 14 (1):81–90.
- Sharpe, R. M., and N. E. Skakkebaek. 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet (London, England) 341 (8857):1392–1396. doi: 10.1016/0140-6736(93)90953-E.
- She, J., M. Petreas, J. Winkler, P. Visita, M. McKinney, and D. Kopec. 2002. PBDEs in the San Francisco Bay area: Measurements in harbor seal blubber and human breast adipose tissue. Chemosphere 46 (5):697–707. doi: 10.1016/S0045-6535(01)00234-X.
- Shier, W. T., A. C. Shier, W. Xie, and C. J. Mirocha. 2001. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon : Official Journal of the International Society on Toxinology 39 (9):1435–1438. doi: 10.1016/S0041-0101(00)00259-2.
- Silva, I., P. Mangtani, V. McCormack, D. Bhakta, A. J. McMichael, and L. Sevak. 2004. Phyto-oestrogen intake and breast cancer risk in South Asian women in England: Findings from a population-based case-control study. Cancer Causes Control 15 (8):805–818. doi: 10.1023/B:CACO.0000043431.85706.d8.
- Silver, S. R., E. A. Whelan, J. A. Deddens, N. K. Steenland, N. B. Hopf, M. A. Waters, A. M. Ruder, M. M. Prince, L. C. Yong, M. J. Hein, et al. 2009. Occupational exposure to polychlorinated biphenyls and risk of breast cancer. Environmental Health Perspectives 117 (2):276–282. doi: 10.1289/ehp.11774.
- Smith-Bindman, R. 2012. Environmental causes of breast cancer and radiation from medical imaging: Findings from the Institute of Medicine report. Archives of Internal Medicine 172 (13):1023–1027. doi: 10.1001/archinternmed.2012.2329.
- Snedeker, S. M. 2001. Pesticides and breast cancer risk: A review of DDT, DDE, and dieldrin. Environmental Health Perspectives 109 (Suppl 1):35–47.
- Soroush, A., N. Farshchian, S. Komasi, N. Izadi, N. Amirifard, and A. Shahmohammadi. 2016. The role of oral contraceptive pills on increased risk of breast cancer in Iranian populations: A meta-analysis. Journal of Cancer Prevention 21 (4):294–301. doi: 10.15430/JCP.2016.21.4.294.
- Stellman, S. D., M. V. Djordjevic, J. A. Britton, J. E. Muscat, M. L. Citron, M. Kemeny, E. Busch, and L. Gong. 2000. Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 9 (11):1241–1249.
- Stellman, S. D., M. V. Djordjevic, J. E. Muscat, L. Gong, D. Bernstein, M. L. Citron, A. White, M. Kemeny, E. Busch, and A. N. Nafziger. 1998. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiology, Biomarkers & Prevention 7 (6):489.
- Stoker, T. E., S. Laws, D. Guidici, and R. Cooper. 2000. The effect of atrazine on puberty in male Wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicological Sciences : An Official Journal of the Society of Toxicology 58 (1):50–59. doi: 10.1093/toxsci/58.1.50.
- Strom, B. L., R. Schinnar, E. E. Ziegler, K. T. Barnhart, M. D. Sammel, G. A. Macones, V. A. Stallings, J. M. Drulis, S. E. Nelson, and S. A. Hanson. 2001. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 286 (7):807–814. doi: 10.1001/jama.286.7.807.
- Sung, H., J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, and F. Bray. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 1–41.
- Takemura, H., J. Y. Shim, K. Sayama, A. Tsubura, B. T. Zhu, and K. Shimoi. 2007. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. The Journal of Steroid Biochemistry and Molecular Biology 103 (2):170–177. doi: 10.1016/j.jsbmb.2006.08.008.
- Tang, M., M. Zhao, Z. Shanshan, K. Chen, C. Zhang, and W. Liu. 2014. Assessing the underlying breast cancer risk of Chinese females contributed by dietary intake of residual DDT from agricultural soils. Environment International 73:208–215. doi: 10.1016/j.envint.2014.08.001.
- Tayour, C., B. Ritz, B. Langholz, P. K. Mills, A. Wu, J. P. Wilson, K. Shahabi, and M. Cockburn. 2019. A case–control study of breast cancer risk and ambient exposure to pesticides. Environmental Epidemiology (Philadelphia, Pa.) 3 (5):e070. doi: 10.1097/EE9.0000000000000070.
- Thanos, J., M. Cotterchio, B. A. Boucher, N. Kreiger, and L. U. Thompson. 2006. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control 17 (10):1253–1261. doi: 10.1007/s10552-006-0062-2.
- Timms, B. G., K. L. Howdeshell, L. Barton, S. Bradley, C. A. Richter, and F. S. Vom Saal. 2005. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proceedings of the National Academy of Sciences of the United States of America 102 (19):7014–7019. doi: 10.1073/pnas.0502544102.
- Toi, M., S. Hirota, A. Tomotaki, N. Sato, Y. Hozumi, K. Anan, T. Nagashima, Y. Tokuda, N. Masuda, S. Ohsumi, S. Ohno, M. Takahashi, H. Hayashi, S. Yamamoto, and Y. Ohashi. 2013. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case–control study. Current Nutrition & Food Science 9 (3):194–200. doi: 10.2174/15734013113099990001.
- Tonkelaar, I., L. Keinan-Boker, P. Van’t Veer, C. J. M. Arts, H. Adlercreutz, J. H. H. Thijssen, and P. H. M. Peeters. 2001. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention 10 (3):223–228.
- Touillaud, M. S., A. C. M. Thiébaut, M. Niravong, M. Boutron-Ruault, and F. Clavel-Chapelon. 2006. No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiology, Biomarkers & Prevention 15 (12):2574–2576. doi: 10.1158/1055-9965.EPI-06-0543.
- Trabert, B., R. T. Falk, J. D. Figueroa, B. I. Graubard, M. Garcia-Closas, J. Lissowska, B. Peplonska, S. D. Fox, and L. A. Brinton. 2014. Urinary bisphenol A-glucuronide and postmenopausal breast cancer in Poland. Cancer Causes & Control : CCC 25 (12):1587–1593. doi: 10.1007/s10552-014-0461-8.
- Trock, B. J., L. Hilakivi-Clarke, and R. Clarke. 2006. Meta-analysis of soy intake and breast cancer risk. Journal of the National Cancer Institute 98 (7):459–471. doi: 10.1093/jnci/djj102.
- Tsai, M.S., C.Y. Lin, C.C. Lin, M.H. Chen, S. H. Hsu, K.L. Chien, F.C. Sung, P.C. Chen, and T.C. Su. 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. International Journal of Hygiene and Environmental Health 218 (5):437–443. doi: 10.1016/j.ijheh.2015.03.008.
- Tseng, L.H., M.H. Li, S.S. Tsai, C.W. Lee, M.H. Pan, W.J. Yao, and P.C. Hsu. 2008. Developmental exposure to decabromodiphenyl ether (PBDE 209): Effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere 70 (4):640–647. doi: 10.1016/j.chemosphere.2007.06.078.
- Ueda, M., T. Imai, T. Takizawa, H. Onodera, K. Mitsumori, T. Matsui, and M. Hirose. 2005. Possible enhancing effects of atrazine on growth of 7, 12‐dimethylbenz (a) anthracene‐induced mammary tumors in ovariectomized Sprague–Dawley rats. Cancer Science 96 (1):19–25. doi: 10.1111/j.1349-7006.2005.00008.x.
- Urban, M., E. Banks, S. Egger, K. Canfell, D. O'Connell, V. Beral, and F. Sitas. 2012. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women: Case-control study. PLoS Medicine 9 (3):e1001182. e1001182. doi: 10.1371/journal.pmed.1001182.
- Van Hoften, C., H. Burger, P. H. M. Peeters, D. E. Grobbee, P. A. H. Van Noord, and H. G. M. Leufkens. 2000. Long-term oral contraceptive use increases breast cancer risk in women over 55 years of age: The DOM cohort. International Journal of Cancer 87 (4):591–594. doi: 10.1002/1097-0215(20000815)87:4<591::AID-IJC20>3.0.CO;2-C.
- Vandenberg, L. N., S. Ehrlich, S. M. Belcher, N. Ben-Jonathan, D. C. Dolinoy, E. R. Hugo, P. A. Hunt, R. R. Newbold, B. S. Rubin, K. S. Saili, et al. 2013. Low dose effects of bisphenol A. Endocrine Disruptors 1 (1):e26490. doi: 10.4161/endo.26490.
- van't Veer, P., I. E. Lobbezoo, J. M. Martín-Moreno, E. Guallar, J. Gómez-Aracena, A. F. Kardinaal, L. Kohlmeier, B. C. Martin, J. J. Strain, M. Thamm, et al. 1997. DDT (dicophane) and postmenopausal breast cancer in Europe: Case-control study. British Medical Journal (Clinical Research ed.) 315 (7100):81–85. doi: 10.1136/bmj.315.7100.81.
- Verheus, M., C. H. van Gils, L. Keinan-Boker, P. B. Grace, S. A. Bingham, and P. H. Peeters. 2007. Plasma phytoestrogens and subsequent breast cancer risk. Journal of Clinical Oncology 25 (6):648–655. doi: 10.1200/JCO.2006.06.0244.
- Verreault, R., P. Ayotte, L. Sauvi, J. Morin, and J. Brisson. 1994. High organochlorine body burden in women with estrogen receptor-positive breast cancer. Journal of the National Cancer Institute 86 (3):232–234.
- Vessey, M., and D. Yeates. 2013. Oral contraceptive use and cancer: Final report from the Oxford-Family Planning Association contraceptive study. Contraception 88 (6):678–683. doi: 10.1016/j.contraception.2013.08.008.
- Villeneuve, S., D. Cyr, E. Lynge, L. Orsi, S. Sabroe, F. Merletti, G. Gorini, M. Morales-Suarez-Varela, W. Ahrens, C. Baumgardt-Elms, et al. 2010. Occupation and occupational exposure to endocrine disrupting chemicals in male breast cancer: A case-control study in Europe–. Occupational and Environmental Medicine 67 (12):837–844. doi: 10.1136/oem.2009.052175.
- Vo, T. T., Y.M. Yoo, K.-C. Choi, and E.B. Jeung. 2010. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reproductive Toxicology (Elmsford, N.Y.) 29 (3):306–316. doi: 10.1016/j.reprotox.2010.01.013.
- Vom Saal, F. S., B. G. Timms, M. M. Montano, P. Palanza, K. A. Thayer, S. C. Nagel, M. D. Dhar, V. Ganjam, S. Parmigiani, and W. V. Welshons. 1997. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proceedings of the National Academy of Sciences of the United States of America 94 (5):2056–2061. doi: 10.1073/pnas.94.5.2056.
- VoPham, T., K. A. Bertrand, R. R. Jones, N. C. Deziel, N. C. DuPré, P. James, Y. Liu, V. M. Vieira, R. M. Tamimi, J. E. Hart, et al. 2020. Dioxin exposure and breast cancer risk in a prospective cohort study. Environmental Research 186:109516. doi: 10.1016/j.envres.2020.109516.
- Wada, K., K. Nakamura, Y. Tamai, M. Tsuji, T. Kawachi, A. Hori, N. Takeyama, S. Tanabashi, S. Matsushita, N. Tokimitsu, and C. Nagata. 2013. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. International Journal of Cancer 133 (4):952–960. doi: 10.1002/ijc.28088.
- Wahidin, M., R. Djuwita, and A. Adisasmita. 2018. Oral contraceptive and breast cancer risks: A case control study in six referral hospitals in Indonesia. Asian Pacific Journal of Cancer Prevention 19 (8):2199–2203.
- Ward, E. M., P. Schulte, B. Grajewski, A. Andersen, D. G. Patterson, W. Turner, E. Jellum, J. A. Deddens, J. Friedland, and N. Roeleveld. 2000. Serum organochlorine levels and breast cancer: A nested case-control study of Norwegian women. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 9 (12):1357–1367.
- Ward, H., G. Chapelais, G. G. C. Kuhnle, R. Luben, K. Khaw, and S. A. Bingham. 2008. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Research 10 (2):R32. doi: 10.1186/bcr1995.
- Warner, M., B. Eskenazi, P. Mocarelli, P. M. Gerthoux, S. Samuels, L. Needham, D. Patterson, and P. Brambilla. 2002. Serum dioxin concentrations and breast cancer risk in the Seveso Women's Health Study. Environmental Health Perspectives 110 (7):625–628. doi: 10.1289/ehp.02110625.
- Warner, M., P. Mocarelli, S. Samuels, L. Needham, P. Brambilla, and B. Eskenazi. 2011. Dioxin exposure and cancer risk in the Seveso Women's Health Study. Environmental Health Perspectives 119 (12):1700–1705. doi: 10.1289/ehp.1103720.
- Wassermann, M., D. Nogueira, L. Tomatis, A. Mirra, H. Shibata, G. Arie, S. Cucos, and D. Wassermann. 1976. Organochlorine compounds in neoplastic and adjacent apparently normal breast tissue. Bulletin of Environmental Contamination and Toxicology 15 (4):478–484. doi: 10.1007/BF01685076.
- Wei, Y., J. Lv, Y. Guo, Z. Bian, M. Gao, H. Du, L. Yang, Y. Chen, X. Zhang, T. Wang, J. Chen, Z. Chen, C. Yu, D. Huo, and L. Li. 2019. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response meta-analysis. European Journal of Epidemiology 35 (6):567–578.
- Wei, J., X. Li, L. Xiang, Y. Song, Y. Liu, Y. Jiang, and Z. Cai. 2020. Metabolomics and lipidomics study unveils the impact of polybrominated diphenyl ether-47 on breast cancer mice. Journal of Hazardous Materials 390:121451. doi: 10.1016/j.jhazmat.2019.121451.
- White, A. J., S. L. Teitelbaum, M. S. Wolff, S. D. Stellman, A. I. Neugut, and M. D. Gammon. 2013. Exposure to fogger trucks and breast cancer incidence in the Long Island Breast Cancer Study Project: A case-control study. Environmental Health : A Global Access Science Source 12:24–24.
- Whitsett Jr, T. G., and C. A., Lamartiniere. 2006. Genistein and resveratrol: Mammary cancer chemoprevention and mechanisms of action in the rat. Expert Review of Anticancer Therapy 6 (12):1699–1706. doi: 10.1586/14737140.6.12.1699.
- Wielsøe, M., P. Kern, and E. C. Bonefeld-Jørgensen. 2017. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: A case control study. Environmental Health : A Global Access Science Source 16 (1):56–56.
- Wirbisky, S. E., and J. L. Freeman. 2015. Atrazine exposure and reproductive dysfunction through the hypothalamus-pituitary-gonadal (HPG) axis. Toxics 3 (4):414–450. doi: 10.3390/toxics3040414.
- Wolff, M. S., A. Zeleniuch-Jacquotte, N. Dubin, and P. Toniolo. 2000. Risk of breast cancer and organochlorine exposure. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 9 (3):271–277.
- Wolff, M. S., G. S. Berkowitz, S. Brower, R. Senie, I. J. Bleiweiss, P. Tartter, B. Pace, N. Roy, S. Wallenstein, and A. Weston. 2000. Organochlorine exposures and breast cancer risk in New York city women. Environmental Research 84 (2):151–161. doi: 10.1006/enrs.2000.4075.
- Wolff, M. S., P. G. Toniolo, E. W. Lee, M. Rivera, and N. Dubin. 1993. Blood levels of organochlorine residues and risk of breast cancer. Journal of the National Cancer Institute 85 (8):648–652. doi: 10.1093/jnci/85.8.648.
- Woolcott, C. G., K. J. Aronson, W. M. Hanna, S. K. SenGupta, D. R. McCready, E. E. Sterns, and A. B. Miller. 2001. Organochlorines and breast cancer risk by receptor status, tumor size, and grade (Canada). Cancer Causes and Control 12 (5):395–404. doi: 10.1023/A:1011289905751.
- World Health Organization and United Nations Environment Programme (WHO/UNEP). 2013. State of the science of endocrine disrupting chemicals – 2012. WHO Press.
- Wu, A. H., P. Wan, J. Hankin, C.C. Tseng, M. C. Yu, and M. C. Pike. 2002. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 23 (9):1491–1496. doi: 10.1093/carcin/23.9.1491.
- Wu, A. H., M. C. Yu, C. Tseng, N. C. Twaddle, and D. R. Doerge. 2004. Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County. Carcinogenesis 25 (1):77–81. doi: 10.1093/carcin/bgg189.
- Wu, A. H., R. G. Ziegler, P. L. Horn-Ross, A. Nomura, D. W. West, L. N. Kolonel, J. F. Rosenthal, R. N. Hoover, and M. C. Pike. 1996. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 5 (11):901–906.
- Wu, A. H., W. P. Koh, R. Wang, H. P. Lee, and M. C. Yu. 2008. Soy intake and breast cancer risk in Singapore Chinese Health Study. British Journal of Cancer 99 (1):196–200. doi: 10.1038/sj.bjc.6604448.
- Xu, X., A. B. Dailey, E. O. Talbott, V. A. Ilacqua, G. Kearney, and N. R. Asal. 2010. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in US adults. Environmental Health Perspectives 118 (1):60–66. doi: 10.1289/ehp.0900919.
- Yamamoto, S., T. Sobue, M. Kobayashi, S. Sasaki, and S. Tsugane. 2003. Soy, isoflavones, and breast cancer risk in Japan. Journal of the National Cancer Institute 95 (12):906–913. doi: 10.1093/jnci/95.12.906.
- Yang, C., Y. S. Tan, J. R. Harkema, and S. Z. Haslam. 2009. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reproductive Toxicology (Elmsford, N.Y.) 27 (3–4):299–306. doi: 10.1016/j.reprotox.2008.10.003.
- Zheng, T., T. R. Holford, S. T. Mayne, B. Ward, D. Carter, P. H. Owens, R. Dubrow, S. H. Zahm, P. Boyle, S. Archibeque, et al. 1999. DDE and DDT in breast adipose tissue and risk of female breast cancer. American Journal of Epidemiology 150 (5):453–458. doi: 10.1093/oxfordjournals.aje.a010033.
- Zheng, T., T. R. Holford, S. T. Mayne, B. Ward, D. Carter, P. H. Owens, R. Dubrow, S. H. Zahm, P. Boyle, S. Archibeque, and J. Tessari, et al. 1999. DDE and DDT in breast adipose tissue and risk of female breast cancer. American Journal of Epidemiology 150 (5):453–458. doi: 10.1093/oxfordjournals.aje.a010033.
- Yasuda, Y., T. Kihara, and H. Nishimura. 1981. Effect of ethinyl estradiol on development of mouse fetuses. Teratology 23 (2):233–239. doi: 10.1002/tera.1420230208.
- Ye, W., P. Xu, W. R. Threlfall, R. Jen, H. Li, S. H. Lin, C. T. Kuo, and Y. C. Lin. 2009. Zeranol enhances the proliferation of pre-adipocytes in beef heifers. Anticancer Research 29 (12):5045–5052.
- Yip, K. Y., M. L. Y. Wan, A. S. T. Wong, K. S. Korach, and H. El-Nezami. 2017. Combined low-dose zearalenone and aflatoxin B1 on cell growth and cell-cycle progression in breast cancer MCF-7 cells. Toxicology Letters 281:139–151. doi: 10.1016/j.toxlet.2017.09.022.
- Yost, E. E., S. Y. Euling, J. A. Weaver, B. E. J. Beverly, N. Keshava, A. Mudipalli, X. Arzuaga, T. Blessinger, L. Dishaw, A. Hotchkiss, et al. 2019. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environment International : a Global Access Science Source 125:579–594. doi: 10.1016/j.envint.2018.09.038.
- Yu, Z., L. Zhang, D. Wu, and F. Liu. 2005. Anti-apoptotic action of zearalenone in MCF-7 cells. Ecotoxicology and Environmental Safety 62 (3):441–446. doi: 10.1016/j.ecoenv.2004.10.003.
- Yuan, J., Q. Wang, R. Ross, B. Henderson, and M. Yu. 1995. Diet and breast cancer in Shanghai and Tianjin, China. British Journal of Cancer 71 (6):1353–1358. doi: 10.1038/bjc.1995.263.
- Yuri, T., R. Tsukamoto, K. Miki, N. Uehara, Y. Matsuoka, and A. Tsubura. 2006. Biphasic effects of zeranol on the growth of estrogen receptor-positive human breast carcinoma cells. Oncology Reports 16 (6):1307–1312. doi: 10.3892/or.16.6.1307.
- Zhang, B., H. Xie, J. Rusiecki, J. P. Wise, K. Zou, P. H. Owens, P. Boyle, S. H. Zahm, T. R. Holford, T. Zheng, et al. 2004. Serum polychlorinated biphenyls, cytochrome P-450 1A1 polymorphisms, and risk of breast cancer in Connecticut women. American Journal of Epidemiology 160 (12):1177–1183. doi: 10.1093/aje/kwh346.
- Zhao, Y., Y. S. Tan, S. Z. Haslam, and C. Yang. 2010. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicological Sciences : An Official Journal of the Society of Toxicology 115 (1):214–224. doi: 10.1093/toxsci/kfq030.
- Zheng, T., T. R. Holford, J. Tessari, S. T. Mayne, P. H. Owens, B. Ward, D. Carter, P. Boyle, R. Dubrow, S. Archibeque-Engle, et al. 2000. Breast cancer risk associated with congeners of polychlorinated biphenyls. American Journal of Epidemiology 152 (1):50–58. doi: 10.1093/aje/152.1.50.
- Zheng, T., T. R. Holford, S. T. Mayne, B. Ward, D. Carter, P. H. Owens, R. Dubrow, S. H. Zahm, P. Boyle, S. Archibeque, and J. Tessari. 1999. DDE and DDT in breast adipose tissue and risk of female breast cancer. American Journal of Epidemiology 150 (5):453–458. doi: 10.1093/oxfordjournals.aje.a010033.
- Zheng, T., T. R. Holford, S. T. Mayne, J. Tessari, B. Ward, D. Carter, P. H. Owens, P. Boyle, R. Dubrow, and S. Archibeque-Engle. 2000. Risk of female breast cancer associated with serum polychlorinated biphenyls and 1, 1-dichloro-2, 2′-bis (p-chlorophenyl) ethylene. Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 9 (2):167–174.
- Zhong, S., W. Ye, S. H. Lin, J. Y. Liu, J. Leong, C. Ma, and Y. C. Lin. 2011. Zeranol induces cell proliferation and protein disulfide isomerase expression in mammary gland of ACI rat. Anticancer Research 31 (5):1659–1665.
- Zimeri, A. M., S. W. Robb, S. M. Hassan, R. R. Hire, and M. B. Davis. 2015. Assessing heavy metal and PCB exposure from tap water by measuring levels in plasma from sporadic breast cancer patients, a pilot study. International Journal of Environmental Research and Public Health 12 (12):15683–15691. doi: 10.3390/ijerph121215013.
- Zoeller, R. T., T. R. Brown, L. L. Doan, A. C. Gore, N. E. Skakkebaek, A. M. Soto, T. J. Woodruff, and F. S. Vom Saal. 2012. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 153 (9):4097–4110. doi: 10.1210/en.2012-1422.