Abstract
Abnormal cholesterol level is a major risk factor in the development of atherosclerosis, which is a fundamental derangement in cardiovascular diseases. Any efforts should be undertaken to lower blood cholesterol levels. Among dietary interventions, capsaicinoid supplementation is also considered as a novel cholesterol-lowering approach, but human studies concluded contradictory results about its effectiveness. The present meta-analysis aimed at determining the effects of capsaicinoids on serum lipid profile in humans. We searched the PubMed, EMBASE, and CENTRAL databases from inception to February 2021. We included 10 controlled studies, which involved 398 participants. We found that dietary capsaicinoid supplementation alone or in combination with other substances significantly (p = 0.004 and 0.001, respectively) reduced serum total cholesterol level compared to controls with an overall standardized mean difference of −0.52 (95% confidence interval: −0.83, −0.21). Capsaicinoids also decreased low-density lipoprotein level significantly (p = 0.035), whereas no effect was observed on serum levels of high-density lipoprotein and triglycerides. Our findings provide novel quantitative evidence for the efficacy of dietary capsaicin supplementation in lowering serum total cholesterol and low-density lipoprotein levels in humans. To validate our conclusion, further randomized controlled trials in a diverse population of adult humans receiving dietary capsaicinoid supplementation are warranted.
Introduction
Atherosclerosis represents a significant challenge for patients and healthcare worldwide, including developed and developing countries alike (Herrington et al. Citation2016), and it is a main underlying cause of ischemic heart disease and stroke, which, respectively, accounted for about 9 and 6 million deaths globally in 2015 (Roth et al. Citation2017). The development of atherosclerosis entails the formation of fatty deposits (plaques) in the vascular wall, which thickens the wall, but, in turn, narrows the lumen of the vessels, therefore reducing the blood flow and tissue blood supply (Lusis Citation2000).
Cholesterol is a key substance, which contributes to the buildup of plaques, hence high cholesterol level is a well-acknowledged risk factor for cardiovascular diseases, including atherosclerosis (Ference et al. Citation2017). Different pharmacological therapies, for example, statins, selective cholesterol absorption inhibitors, and resins, are used to decrease abnormally high serum cholesterol (Ray et al. Citation2019), but lifestyle changes are also fundamental in controlling cholesterol levels. Important advantages of changes in lifestyle (e.g., diet and physical activity) are the better compliance of the patients and the absence of possibly severe, adverse effects (e.g., liver failure, myopathy, and diabetes mellitus), both of which can hinder pharmacological therapies (Thompson, Clarkson, and Karas Citation2003; Sattar et al. Citation2010). Currently, there are different dietary recommendations that aim at reducing serum cholesterol levels (Arnett et al. Citation2019), but the hunt for novel and effective, cholesterol-lowering natural ingredients is still ongoing.
A candidate among such natural ingredients is capsaicin, which is the first known agonist of the transient receptor potential vanilloid-1 (TRPV1) channel, formerly also known as the capsaicin receptor [for a review, see Romanovsky et al. (Citation2009)]. TRPV1 contributes to the maintenance of energy metabolism in humans through different mechanisms, including the regulation of energy expenditure (Ludy, Moore, and Mattes Citation2012), body temperature (Garami et al. Citation2020), and body mass (Zsiboras et al. Citation2018). Data from animal experiments also suggest that TRPV1 activation increases fat oxidation in rodents (Leung Citation2014), and that capsaicin administration decreases serum levels of cholesterol and triglycerides in small mammals, such as mice, rats, hamsters, and rabbits, although it should be also noted that the beneficial effect was not shown in some studies [for a recent systematic review, see Sanati, Razavi, and Hosseinzadeh (Citation2018)]. At present, the results about the effect of TRPV1 agonists on serum cholesterol in human studies are inconclusive. In different trials, TRPV1 agonists caused a decrease (Yuan et al. Citation2016; Taghizadeh et al. Citation2017), no effect (Kim et al. Citation2010; Hochkogler et al. Citation2017) or an increase (Urbina et al. Citation2017) in serum cholesterol levels. In the present meta-analysis, we wanted to amalgamate the available data from human studies in order to determine the effects of the TRPV1 agonist capsaicinoids on serum cholesterol levels.
Methods
This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Moher et al. Citation2009) (Supplementary material Table S1). The question was raised in the PICO (Population, Intervention, Control, Outcome) format: in adult human subjects we wanted to compare the effect of TRPV1 agonists with placebo on serum total cholesterol level (as the primary outcome). Secondary outcome measures included triglyceride, lipoprotein component, fasting glucose and insulin levels. This meta-analysis has been registered with PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42020162735).
Table 1. Summary of study characteristics for publications included in the meta-analyses.
Search strategy
We searched the PubMed, EMBASE, and CENTRAL (Cochrane Central Register of Controlled Trials) databases from inception until February 20, 2021 to identify eligible papers for the meta-analysis. The used search term was “(capsa* OR capsi* OR TRPV1 OR vanilloid) AND (LDL OR HDL OR lipoprotein OR triglyceride OR cholester* OR "lipid").” The search was limited to human trials without language and publication date restrictions. Two authors (LK, AG) conducted the search separately; disagreements were resolved by consensus, if needed, with the help of a third party (ZR).
Selection of studies and data extraction
Two authors (LK, ZR) assessed study eligibility and extracted data from the selected studies independently. Studies were required to include the following: controlled human trial design; a group with TRPV1 agonist administration; a placebo group; serum cholesterol level among the outcome measures. The following data were extracted from the eligible studies: author names, publication year, age, body mass index (BMI), and number of the participants, as well as, different outcome measures, viz., serum levels of total cholesterol, high- and low-density lipoproteins (HDL and LDL, respectively), triglycerides, as well as, fasting glucose and insulin.
Evaluation of risk of bias and quality of evidence
The risk of bias was evaluated for all analyzed outcome measures, i.e., serum levels of total cholesterol, HDL, LDL, and triglycerides, as well as, for fasting plasma glucose and insulin levels, within the studies. According to the revised tool for assessing risk of bias in randomized trials (Rob 2) (Sterne et al. Citation2019), we assessed the bias in the following domains: randomization process, deviations from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result (Supplementary material Tables S2–S5). If the trial was not randomized, risk of bias was assessed with the ROBINS-I (Risk Of Bias In Non-randomized Studies of Interventions) tool (Supplementary material Table S6). The results of the assessments were visualized with the “robvis” tool (McGuinness and Higgins Citation2021).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the applicability of the evidence. According to the GRADE system (Atkins et al. Citation2004), each outcome was tested based on the following factors: study design, risk of bias, indirectness, inconsistency, imprecision, and publication bias. The overall quality of the evidence for each outcome could be “high,” “moderate,” “low,” or “very low.” The basal grade was high for randomized controlled studies and it was decreased by 1 grade for serious concerns or by 2 for very serious concerns.
Statistical analysis
The statistical analysis was performed according to the standard methods of meta-analysis by using the Stata/IC 16.0 software (StataCorp LLC, College Station, TX, USA). Subjects were grouped as either administered with a TRPV1 agonist (i.e., intervention group) or not (i.e., controls). We used standardized mean differences (SMDs) with 95% confidence intervals (CIs) between intervention and control groups as primary measure of the effect size on serum lipid, glucose, and insulin level response at the end of the substance administration. For standardization, the differences in means were divided by their corresponding pooled standard deviation (SD) values, which was required because the different measuring methods, reported units, and TRPV1 agonist dose ranges could result in different variances among the study groups, and, therefore, influence the results. SMD values were compared by using the random effect model by DerSimonian and Laird (Citation1986), and then presented as “forest plots”.
Statistical heterogeneity was determined by the I2 statistical test (p < 0.1 indicating significant heterogeneity), as previously (Olah et al. Citation2018). As an attempt to reduce heterogeneity, subgroups administered with capsaicin (and its derivates) only and with capsaicinoids in mixture of other supposedly active ingredients were analyzed separately. As in our recent study (Csenkey et al. Citation2019), publication bias was assessed by the visual inspection of funnel plots (Supplementary material Figures S3–S8). Quantitative evaluation by Egger’s test (Egger et al. Citation1997) could be performed only for total serum cholesterol and LDL levels, because in case of the other outcome parameters the number of eligible studies was lower than ten, which is the minimal number of studies recommended for the test by the Cochrane Handbook (Higgins et al. Citation2021).
Results
Search results
The search identified altogether 4,879 studies from the PubMed, EMBASE, and CENTRAL databases (). We used built-in filters available on the website of the databases to limit our search to humans and clinical trials, which resulted in exclusion of 4,443 records. After removing 35 duplicates, 401 papers remained, which were manually screened on title and abstract for inclusion criteria. Full texts of 39 articles were obtained and reviewed, which resulted in the selection of 11 studies that were found eligible for qualitative synthesis (Ahuja and Ball Citation2006; Inoue et al. Citation2007; Kim et al. Citation2010; Kang et al. Citation2016; Yuan et al. Citation2016; Hochkogler et al. Citation2017; Lee et al. Citation2017; Qin et al. Citation2017; Taghizadeh et al. Citation2017; Urbina et al. Citation2017; Arent et al. Citation2018). Manual search of the reference lists of the eligible articles lead to the identification of three additional studies (Snitker et al. Citation2009; Cha et al. Citation2013; Lim et al. Citation2015), thereby increasing the total number of eligible studies to 14. However, four of these studies could not be included in the quantitative analysis, because they did not report appropriate data for meta-analysis (Inoue et al. Citation2007; Snitker et al. Citation2009), or the authors (Lee et al. Citation2017) analyzed the same participants’ data as an earlier study by Cha et al. (Citation2013), which was already included in our meta-analysis, or because the subjects did not receive capsaicin, but instead they were administered with a low dose (0.15 mg/day) of nonivamide (Hochkogler et al. Citation2017), which has a potency of half of that of capsaicin (Skofitsch, Donnerer, and Lembeck Citation1984). As a result, 10 studies were included in the meta-analyses (Ahuja and Ball Citation2006; Kim et al. Citation2010; Cha et al. Citation2013; Lim et al. Citation2015; Kang et al. Citation2016; Yuan et al. Citation2016; Qin et al. Citation2017; Taghizadeh et al. Citation2017; Urbina et al. Citation2017; Arent et al. Citation2018). We confirmed that our search algorithm was sensitive enough by also adding “paprika species” and “herbal mixtures” to the search key, which extended search did not identify any additional studies eligible for quantitative synthesis.
Study characteristics and quality
The descriptive characteristics of the analyzed studies are presented in . All 10 studies were controlled trials: 8 with parallel placebo control (Kim et al. Citation2010; Yuan et al. Citation2016; Qin et al. Citation2017; Taghizadeh et al. Citation2017; Urbina et al. Citation2017; Arent et al. Citation2018), and 2 with crossover designs (Ahuja and Ball Citation2006; Kang et al. Citation2016). Except for one trial (Kang et al. Citation2016), all studies were randomized. The duration of the trials varied from 4 to 12 weeks, but all of them could be considered as short term, lasting for less than three months. Apart from a BMI of 26-32, the subjects had no diagnosed health issues, except for one study (Yuan et al. Citation2016), which recruited women with postprandial diabetes mellitus. With regards to substance administration, capsaicin was used in all studies, but in two trials (Taghizadeh et al. Citation2017; Arent et al. Citation2018), it was combined with other biologically active compounds (). The daily dose range of capsaicin (4-5 mg) was similar in four of the analyzed studies (Kang et al. Citation2016; Yuan et al. Citation2016; Qin et al. Citation2017; Urbina et al. Citation2017), while 100 mg was used in the study by Taghizadeh et al. (Citation2017) and 22 mg by Ahuja and Ball (Citation2006). The applied dosage was not reported in four papers (Kim et al. Citation2010; Cha et al. Citation2013; Lim et al. Citation2015; Arent et al. Citation2018).
According to the risk of bias analysis (Supplementary material Tables S2–S6), six studies were considered overall as high risk (Ahuja and Ball Citation2006; Kim et al. Citation2010; Cha et al. Citation2013; Lim et al. Citation2015; Urbina et al. Citation2017; Arent et al. Citation2018), two studies as moderate risk (Kang et al. Citation2016; Qin et al. Citation2017), and two studies as low risk (Yuan et al. Citation2016; Taghizadeh et al. Citation2017).
By using the GRADE approach, the overall quality of evidence was evaluated as low in case of lipid parameters, while as very low in case of glucose and insulin. The summary of findings and the detailed evaluation using the GRADE system are shown in Supplementary material Tables S7 and S8. It should be noted, however, that despite the suitability of GRADE for developing clinical guidelines, its applicability is questionable in food-based dietary guidelines, in which field there are still inconsistencies for rating evidence quality (Blake et al. Citation2018). Therefore, our GRADE results should be taken with care.
Effects on lipid parameters and other outcomes
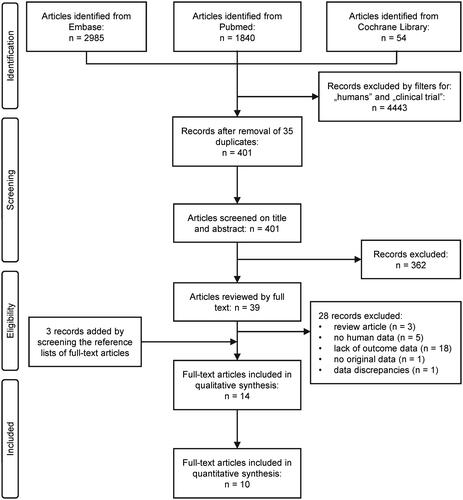
First, we analyzed the effect of dietary capsaicin supplementation on the serum total cholesterol level by comparing the cholesterol levels between the intervention and control groups at the end of the trials. Studies which used capsaicinoids only (Ahuja and Ball Citation2006; Kim et al. Citation2010; Cha et al. Citation2013; Lim et al. Citation2015; Kang et al. Citation2016; Yuan et al. Citation2016; Qin et al. Citation2017; Urbina et al. Citation2017) and capsaicinoids combined with other active ingredients (Taghizadeh et al. Citation2017; Arent et al. Citation2018) were included as separate subgroups in the forest plot (). Dietary capsaicinoid supplementation markedly (p = 0.004) reduced serum total cholesterol compared to the controls (SMD = −0.57; CI, −0.95, −0.18). With regards to capsaicinoid-containing mixtures, both of the included studies seemed to decrease serum total cholesterol, however, there was no significant effect in the individual studies or on average (SMD = −0.32; CI, −0.75, 0.11; p = 0.141). The overall effect of all capsaicionoid supplementations (i.e., alone and in combination; n = 195) was a significant (p = 0.001) decrease in serum total cholesterol, as compared to the control groups (n = 203) with an SMD of −0.52 (CI, −0.83, −0.21).
Figure 2. Forest plot of the effects of capsaicinoids (top) and capsaicinoid-containing mixtures (bottom) on serum total cholesterol level. Here, and in , for each study, we calculated the difference between serum cholesterol levels in the capsaicinoid-treated group and the control group at the end of the intervention period. For all studies, the differences in means were standardized (based on variances) to obtain standardized mean differences (SMDs). The SMDs and 95% confidence intervals (CI) are used as primary measures of effect size and are shown in the forest plot. Black circles represent the SMD for each study, while the left and right horizontal arms of the circles indicate the corresponding CI. The size of the gray box is proportional to the sample size and inversed variance. The rhombus represents the average SMD calculated from the SMDs of the individual studies in a subgroup (top and middle) and in all studies (bottom). The horizontal diagonal of the rhombus represents the CI, while the vertical diagonal of the rhombus points at the SMD value of the subgroup or of all studies. The dashed line is determined by the vertical diagonal of the bottom rhombus and indicates the SMD of all studies in the forest plot. There was no significant difference between the two subgroups (Q = 0.709; p = 0.400). SD, standard deviation.
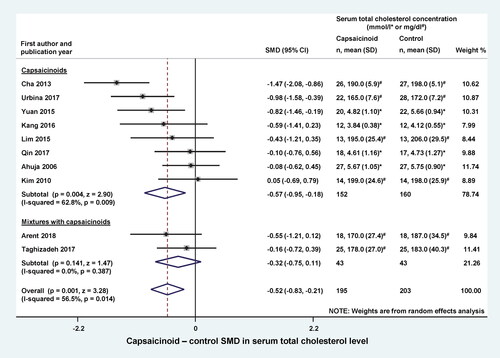
Next, we wanted to know which component of total cholesterol is affected the most by capsaicionoids. We found sufficient data to analyze the changes in LDL, triglyceride, and HDL levels in response to dietary capsaicinoid supplementation. Similarly as in , in the remaining forest plots we also analyzed the capsaicinoid alone and mixture subgroups separately, if applicable. At the end of the trials, the LDL level was lower (as indicated by a negative SMD) in the capsaicinoid-treated groups than in controls in the most of the studies (Ahuja and Ball Citation2006; Cha et al. Citation2013; Lim et al. Citation2015; Kang et al. Citation2016; Yuan et al. Citation2016; Urbina et al. Citation2017), whereas it was higher in two studies (Kim et al. Citation2010; Qin et al. Citation2017) (). The averaged SMD was not significantly different in either the capsaicinoid alone or mixture subgroups. However, the overall average SMD, calculated from all 10 studies, was −0.30 (CI −0.57, −0.02), indicating a significant (p = 0.035) decrease in the capsaicinoid-treated group.
Figure 3. Forest plot of the effects of capsaicinoids (top) and capsaicinoid-containing mixtures (bottom) on serum low-density lipoprotein (LDL) cholesterol level. There was no significant difference between the two subgroups (Q = 0.006; p = 0.940). CI, confidence interval; SD, standard deviation; SMD, standardized mean difference.
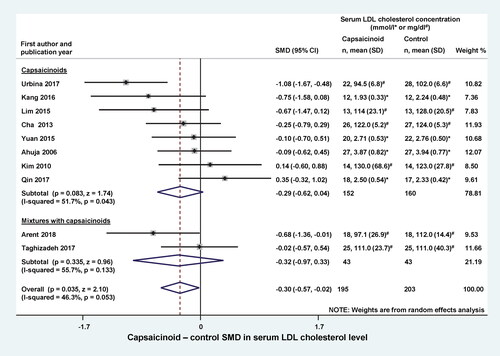
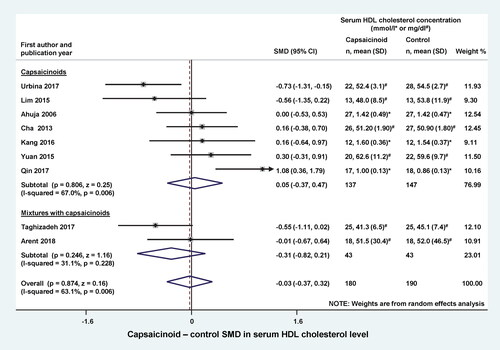
Capsaicinoids did not have a significant effect on serum triglyceride levels, when administered alone (SMD = −0.46; CI, −1.49, 0.57) or in a mixture (SMD = −0.19; CI, −0.62, 0.23) (). The overall effect (including both subgroups) was also not statistically significant (SMD = −0.39; CI, −1.15, 0.37). When we compared serum HDL levels between the capsaicinoid-treated and control groups, we found no significant change in SMD, regardless from whether the capsaicinoids were administered alone (SMD = 0.05; CI, −0.37, 0.47) or in combination with other substances (SMD = −0.31; CI, −0.82, 0.21) (). The overall effect was negligible (SMD = −0.03; CI, −0.37, 0.32).
Figure 4. Forest plot of the effects of capsaicinoids (top) and capsaicinoid-containing mixtures (bottom) on total serum triglyceride level. There was no significant difference between the two subgroups (Q = 0.216; p = 0.642). CI, confidence interval; SD, standard deviation; SMD, standardized mean difference.
Figure 5. Forest plot of the effects of capsaicinoids (top) and capsaicinoid-containing mixtures (bottom) on serum high-density lipoprotein (HDL) cholesterol level. There was no significant difference between the two subgroups (Q = 1.115; p = 0.291). CI, confidence interval; SD, standard deviation; SMD, standardized mean difference.
In addition to the lipid parameters, we also analyzed two indicators of carbohydrate metabolism: fasting blood glucose and insulin. We did not find a significant effect of dietary capsaicinoid supplementation on either of these parameters as compared to the placebo group (). The SMDs between capsaicinoid-treated and control groups were 0.41 (CI, −0.26, 1.07) for glucose and 0.28 (CI, −0.25, 0.82) for insulin.
Qualitative synthesis
Four studies were not included in the meta-analysis, but were retained for qualitative review. All four studies had randomized controlled design. In one of these articles, Lee et al. (Citation2017) analyzed the data from the same participants as a previous study by Cha et al. (Citation2013), which was included in the meta-analysis. Accordingly, the study design and characteristics of participants were the same in the two studies, and the effects of capsaicinoids on the outcome parameters were also similar.
In two studies, the authors stated that TRPV1 agonist supplementation did not have a significant effect on lipid parameters (total cholesterol, LDL, HDL, and triglyceride), but factual information about blood levels was not reported (Inoue et al. Citation2007; Snitker et al. Citation2009). Despite the lack of a significant effect on blood lipid parameters, both of these studies reported a beneficial effect of TRPV1 agonists on body fat mass. Inoue et al. (Citation2007) assigned 44 subjects into 3 groups: ingestion of non-pungent capsinoids at 3 or 10 mg/kg, or placebo, and showed that capsinoids enhance energy expenditure and fat oxidation. In the study by Snitker et al. (Citation2009), 80 subjects were assigned into a capsinoid-treated (6 mg) or placebo group, and the authors found that capsinoids caused an augmented abdominal fat loss and a near significant increase in fat oxidation.
Hochkogler et al. (Citation2017) studied the effects of nonivamide at a low dose of 0.15 mg/day (versus a control group) in 18 participants. Although the potency of nonivamide is only half of that of capsaicin (Skofitsch, Donnerer, and Lembeck Citation1984), it prevented dietary-induced fat accumulation even at the applied low dose. The authors hypothesized that nonivamide affects lipid assimilation by reducing adipogenesis, however, significant change in blood lipid parameters was not detected (Hochkogler et al. Citation2017).
Discussion
In the present study, we show that dietary intake of TRPV1 agonists reduced serum total cholesterol and LDL levels in humans, to our knowledge for the first time, with meta-analysis of the available controlled trials, including a total of 10 studies with 398 subjects.
High serum cholesterol is a main risk factor for atherosclerotic cardiovascular disease (Stone and Grundy Citation2019), which is the major component of cardiovascular diseases responsible for over 4 million deaths in Europe annually (Townsend et al. Citation2015). Lowering cholesterol levels reduces the risk for atherosclerosis, which explains why current guidelines recommend substantial reduction in serum cholesterol level, most of all in that of LDL (Mach et al. Citation2020). While different cholesterol-lowering pharmacological treatments are available, evidence on the influence of lifestyle changes and functional foods on lipoproteins is growing. Lifestyle changes, alone or in combination with drugs, are still among the recommended intervention strategies of reducing cardiovascular risk (Mach et al. Citation2020).
A beneficial effect of TRPV1 agonists, particularly capsaicin, on blood cholesterol has been long sought based on data obtained in animal experiments showing that capsaicin improved obesity-related metabolic disorders, also including dyslipidemia (Panchal, Bliss, and Brown Citation2018; Li et al. Citation2020). Human studies, however, lead to contradictory findings, thereby questioning whether the cholesterol-lowering effects of TRPV1 agonists observed in experimental models are applicable for humans. Indeed, the effect of dietary supplementation with capsaicinoids on serum cholesterol in humans ranged from beneficial to neutral to unfavorable. However, the number of the controlled human studies on the effect of capsaicinoids on cholesterol serum levels was too small until recently for a quantitative synthesis of their overall result, since six trials eligible for such synthesis were published in the last five years (Kang et al. Citation2016; Yuan et al. Citation2016; Qin et al. Citation2017; Taghizadeh et al. Citation2017; Urbina et al. Citation2017; Arent et al. Citation2018). In the present meta-analysis, we performed the long-sought quantitative synthesis of the available data and showed that dietary capsaicinoid supplementation lowers blood cholesterol levels in humans. When we looked at the lipid components, we found that capsaicinoids significantly decreased LDL levels, while the levels of triglycerides and HDL were not affected by the treatment. These findings indicate that the capsaicinoid-induced reduction of total cholesterol is associated with a beneficial alteration in lipid components, inasmuch as LDL, the harmful component (Ference et al. Citation2017), decreases, while HDL, the protective component (Rosenson et al. Citation2018), does not change. Unfortunately, there was not enough data available to study further lipid components (e.g., chylomicron, very-low- and intermediate-DL).
The exact mechanism by which TRPV1 agonists affect cholesterol levels could not be studied in the present work (due to its meta-analysis nature) and remains subject for further research. It is notable, however, that in healthy human subjects, capsaicin ingestion increased thermogenesis and activation of the sympathetic nervous system (Matsumoto et al. Citation2000) and augmented fat oxidation during exercise (Shin and Moritani Citation2007). Capsaicin and capsiate were also shown to increase energy expenditure in humans and proposed as novel therapeutic agents in obesity (Zsiboras et al. Citation2018). A site for thermogenesis in humans is the brown adipose tissue (Nedergaard and Cannon Citation2018), which can be stimulated via the sympathetic nervous system (e.g., in cold). It has been shown that adrenergic activation of brown fat is protective against hypercholesterolemia in mice (Berbee et al. Citation2015), and that its cold-induced activation by means of cold acclimation improved cholesterol metabolism in human patients with hypercholesterolemia (De Lorenzo et al. Citation1998). Adrenergic brown fat stimulation can trigger intracellular mechanisms capable of increasing the activation of lipases, which are responsible for the mobilization of fatty acids that can be used for thermogenesis (Xu and Lopez Citation2018). These findings suggest a link between capsaicin-induced thermogenesis, possibly via sympathetic activation in brown fat, and the reduction in blood cholesterol levels. It should be also noted that TRPV1 expression was described in brown and white adipocytes, therefore capsaicin can even directly (i.e., independently of adrenergic activation) regulate thermogenesis, adipocyte differentiation, and expression of uncoupling proteins [for a recent review, see Uchida et al. (Citation2018)].
Alternative interactions between the TRPV1 channel and cholesterol level may include the hunger hormone ghrelin. Studies in mice and rats suggested that ghrelin inhibits thermogenesis (Mano-Otagiri et al. Citation2009; Lin et al. Citation2014). Interestingly, one of the studies, which was included in our analysis, found that capsaicin administration markedly (p < 0.01) decreased serum ghrelin concentration in the participants (Kang et al. Citation2016). The presence of TRPV1 channels was shown in the human stomach (Faussone-Pellegrini et al. Citation2005), which is the main site of ghrelin production (Kojima and Kangawa Citation2005). Hence, a potential inhibitory action of capsaicin on ghrelin production can not be excluded. Moreover, a direct effect of cholesterol on TRPV1 was also suggested, as in cholesterol depletion the membrane trafficking of TRPV1 channels is negatively affected, while in cholesterol enrichment the activation of TRPV1 by capsaicin is inhibited (Morales-Lazaro and Rosenbaum Citation2019). However, the physiological significance of this interaction remains unknown.
The cholesterol-reducing effects of dietary TRPV1 agonists, as also shown here, are of high importance, especially considering that these compounds were found to evoke further advantageous effects on human bodily homeostasis. For instance, decreased body mass (Leung Citation2014), increased energy expenditure (Zsiboras et al. Citation2018), reduced blood pressure (Sanati, Razavi, and Hosseinzadeh Citation2018), as well as, lower blood sugar and increased insulin sensitivity (Panchal, Bliss, and Brown Citation2018) were all reported in response to the administration of different TRPV1 agonists. Moreover, potent and selective pharmacological agents for the modulation of TRPV1 activity were also developed and subjected to in-vivo testing. Depending on their pharmacological profiles against the TRPV1 channel, these compounds caused an increase, no change, or a decrease in the body temperature of laboratory animals (Garami et al. Citation2018) and humans (Garami et al. Citation2020). This raises the possibility that through targeted modulation of the TRPV1 channel, and thereby energy expenditure, some of the compounds might be also used in the control of lipid metabolism.
Some limitations of our study must be also mentioned. Although the number of studies was sufficient for meta-analysis, it was still relatively small. Only 10 controlled human studies (9 randomized and 1 non-randomized) could be included in the quantitative synthesis despite the extensive literature search. Consequently, we could not perform some of the planned analysis (e.g., subgroup analysis for sex and food intake) in our study because of data unavailability. Due to differences in study design and methodology, considerably high between-study heterogeneity (indicated by an I2 of 46-96%) was observed in our analysis (, ). To account for the presence of heterogeneity, we used the random-effects model in all forest plots of our meta-analyses and performed subgroup analysis. However, when we divided these studies into subgroups in some cases heterogeneity was still present and only two studies remained in a group, which does not allow one to draw firm conclusions about the overall results of such small subgroups. Based on visual inspection of the funnel plots (Supplementary material Figures S3–S8), some asymmetry may be present, indicating the possible existence of publication bias, although when Egger’s test could be performed, its results contradicted the presence of publication bias (p = 0.973 for total cholesterol and p = 0.523 for LDL). Nevertheless, two studies were identified by the literature search, but could not be included in the quantitative synthesis, because the authors opted not to report the results due to the lack of statistically significant difference between the treatment groups (Inoue et al. Citation2007; Snitker et al. Citation2009). Finally, it should be also noted that the overall risk of bias was judged as high in six of the ten analyzed studies (Supplementary material Tables S2–S5), and the GRADE certainty rating was low for the analyzed lipid parameters (Supplementary material Tables S7 and S8). It is possible that, despite all of our approaches to reduce methodological errors, the low number, different design and quality, and occasionally high heterogeneity of the analyzed studies may have negatively impacted our results.
In conclusion, the present study provides novel quantitative support to the beneficial effects of dietary capsaicin supplementation on serum total cholesterol and LDL levels in humans. Although the quantitative synthesis of the available data is, to our knowledge, the most extensive in its field, we also have to point out that the quality of evidence was evaluated as low in case of the analyzed lipid parameters and that a few studies which might argue against our results could not be included in the meta-analysis only in qualitative synthesis. Consequently, further randomized controlled trials are warranted to validate our findings and prove unequivocally that dietary intake of TRPV1 agonists is an effective intervention for lowering blood cholesterol levels.
Supplemental Material
Download PDF (877.2 KB)Additional information
Funding
References
- Ahuja, K. D., and M. J. Ball. 2006. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. British Journal of Nutrition 96 (2):239–42. doi: 10.1079/BJN20061788.
- Al Othman, Z. A., Y. B. Ahmed, M. A. Habila, and A. A. Ghafar. 2011. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 16 (10):8919–29. doi: 10.3390/molecules16108919.
- Arent, S. M., A. J. Walker, J. K. Pellegrino, D. J. Sanders, B. A. McFadden, T. N. Ziegenfuss, and H. L. Lopez. 2018. The combined effects of exercise, diet, and a multi-ingredient dietary supplement on body composition and adipokine changes in overweight adults. Journal of the American College of Nutrition 37 (2):111–20. doi: 10.1080/07315724.2017.1368039.
- Arnett, D. K., R. S. Blumenthal, M. A. Albert, A. B. Buroker, Z. D. Goldberger, E. J. Hahn, C. D. Himmelfarb, A. Khera, D. Lloyd-Jones, J. W. McEvoy, et al. 2019. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 140 (11):e596–e646. doi: 10.1161/cir.0000000000000678.
- Atkins, D., D. Best, P. A. Briss, M. Eccles, Y. Falck-Ytter, S. Flottorp, G. H. Guyatt, R. T. Harbour, M. C. Haugh, D. Henry, et al. 2004. Grading quality of evidence and strength of recommendations. BMJ 328 (7454):1490 doi: 10.1136/bmj.328.7454.1490.
- Berbee, J. F., M. R. Boon, P. P. Khedoe, A. Bartelt, C. Schlein, A. Worthmann, S. Kooijman, G. Hoeke, I. M. Mol, C. John, et al. 2015. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nature Communications 6:6356. doi: 10.1038/ncomms7356.
- Blake, P., S. Durao, C. E. Naude, and L. Bero. 2018. An analysis of methods used to synthesize evidence and grade recommendations in food-based dietary guidelines. Nutrition Reviews 76 (4):290–300. doi: 10.1093/nutrit/nux074.
- Cha, Y. S., S. R. Kim, J. A. Yang, H. I. Back, M. G. Kim, S. J. Jung, W. O. Song, and S. W. Chae. 2013. Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutrition & Metabolism 10 (1):24. doi: 10.1186/1743-7075-10-24.
- Csenkey, A., G. Jozsa, N. Gede, E. Pakai, B. Tinusz, Z. Rumbus, A. Lukacs, Z. Gyongyi, P. Hamar, R. Sepp, et al. 2019. Systemic antibiotic prophylaxis does not affect infectious complications in pediatric burn injury: A meta-analysis. PLoS One 14 (9):e0223063. doi: 10.1371/journal.pone.0223063.
- De Lorenzo, F., M. Mukherjee, Z. Kadziola, R. Sherwood, and V. V. Kakkar. 1998. Central cooling effects in patients with hypercholesterolaemia. Clinical Science 95 (2):213–7. doi: 10.1042/CS19980091.
- DerSimonian, R., and N. Laird. 1986. Meta-analysis in clinical trials. Controlled Clinical Trials 7 (3):177–88. doi: 10.1016/0197-2456(86)90046-2.
- Egger, M., G. D. Smith, M. Schneider, and C. Minder. 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109):629–34. doi: 10.1136/bmj.315.7109.629.
- Faussone-Pellegrini, M. S., A. Taddei, E. Bizzoco, M. Lazzeri, M. G. Vannucchi, and P. Bechi. 2005. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochemistry and Cell Biology 124 (1):61–8. doi: 10.1007/s00418-005-0025-9.
- Ference, B. A., H. N. Ginsberg, I. Graham, K. K. Ray, C. J. Packard, E. Bruckert, R. A. Hegele, R. M. Krauss, F. J. Raal, H. Schunkert, et al. 2017. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal 38 (32):2459–72. doi: 10.1093/eurheartj/ehx144.
- Garami, A., E. Pakai, H. A. McDonald, R. M. Reilly, A. Gomtsyan, J. J. Corrigan, E. Pinter, D. X. D. Zhu, S. G. Lehto, N. R. Gavva, et al. 2018. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds' pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiologica 223 (3):e13038. doi: 10.1111/apha.13038.
- Garami, A., Y. P. Shimansky, Z. Rumbus, R. C. L. Vizin, N. Farkas, J. Hegyi, Z. Szakacs, M. Solymar, A. Csenkey, D. A. Chiche, et al. 2020. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacology & Therapeutics 208:107474. doi: 10.1016/j.pharmthera.2020.107474.
- Herrington, W., B. Lacey, P. Sherliker, J. Armitage, and S. Lewington. 2016. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circulation Research 118 (4):535–46. doi: 10.1161/circresaha.115.307611.
- Higgins, J. P. T., J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Page, and V. A. Welch, eds. 2021. Cochrane handbook for systematic reviews of interventions (version 6.2), updated February Cochrane, 2021. http://www.training.cochrane.org/handbook
- Hochkogler, C. M., B. Lieder, P. Rust, D. Berry, S. M. Meier, M. Pignitter, A. Riva, A. Leitinger, A. Bruk, S. Wagner, et al. 2017. A 12-week intervention with nonivamide, a TRPV1 agonist, prevents a dietary-induced body fat gain and increases peripheral serotonin in moderately overweight subjects. Molecular Nutrition & Food Research 61 (5):1600731. doi: 10.1002/mnfr.201600731.
- Inoue, N., Y. Matsunaga, H. Satoh, and M. Takahashi. 2007. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Bioscience, Biotechnology, and Biochemistry 71 (2):380–9. doi: 10.1271/bbb.60341.
- Kang, C., Y. Zhang, X. Zhu, K. Liu, X. Wang, M. Chen, J. Wang, H. Chen, S. Hui, L. Huang, et al. 2016. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. The Journal of Clinical Endocrinology and Metabolism 101 (12):4681–9. doi: 10.1210/jc.2016-2786.
- Kim, Y., Y. J. Park, S. O. Yang, S. H. Kim, S. H. Hyun, S. Cho, Y. S. Kim, D. Y. Kwon, Y. S. Cha, S. Chae, et al. 2010. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutrition Research 30 (7):455–61. doi: 10.1016/j.nutres.2010.06.014.
- Kojima, M., and K. Kangawa. 2005. Ghrelin: Structure and function. Physiological Reviews 85 (2):495–522. doi: 10.1152/physrev.00012.2004.
- Lee, Y., Y. S. Cha, Y. Park, and M. Lee. 2017. PPARγ2 C1431T polymorphism interacts with the antiobesogenic effects of Kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: A 12-week, double-blind randomized clinical trial. Journal of Medicinal Food 20 (6):610–7. doi: 10.1089/jmf.2016.3911.
- Leung, F. W. 2014. Capsaicin as an anti-obesity drug. In Progress in drug research. Fortschritte der Arzneimittelforschung. Progres des recherches pharmaceutiques, eds O. Abdel-Salam, Vol. 68, 171–9. Basel: Springer doi: 10.1007/978-3-0348-0828-6_7.
- Li, D., T. Zhang, J. Lu, C. Peng, and L. Lin. 2020. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Critical Reviews in Food Science and Nutrition, 1–19. doi: 10.1080/10408398.2020.1768044.
- Lim, J. H., E. S. Jung, E. K. Choi, D. Y. Jeong, S. W. Jo, J. H. Jin, J. M. Lee, B. H. Park, and S. W. Chae. 2015. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clinical Nutrition 34 (3):383–7. doi: 10.1016/j.clnu.2014.05.013.
- Lin, L., J. H. Lee, O. Y. Bongmba, X. Ma, X. Zhu, D. Sheikh-Hamad, and Y. Sun. 2014. The suppression of ghrelin signaling mitigates age‐associated thermogenic impairment. Aging 6 (12):1019–32. doi: 10.18632/aging.100706.
- Ludy, M. J., G. E. Moore, and R. D. Mattes. 2012. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chemical Senses 37 (2):103–21. doi: 10.1093/chemse/bjr100.
- Lusis, A. J. 2000. Atherosclerosis. Nature 407 (6801):233–41. doi: 10.1038/35025203.
- Mach, F., C. Baigent, A. L. Catapano, K. C. Koskinas, M. Casula, L. Badimon, M. J. Chapman, G. G. De Backer, V. Delgado, B. A. Ference, et al. 2020. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. European Heart Journal 41 (1):111–88. doi: 10.1093/eurheartj/ehz455.
- Mano-Otagiri, A., H. Ohata, A. Iwasaki-Sekino, T. Nemoto, and T. Shibasaki. 2009. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. The Journal of Endocrinology 201 (3):341–9. doi: 10.1677/joe-08-0374.
- Matsumoto, T., C. Miyawaki, H. Ue, T. Yuasa, A. Miyatsuji, and T. Moritani. 2000. Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. Journal of Nutritional Science and Vitaminology 46 (6):309–15. doi: 10.3177/jnsv.46.309.
- McGuinness, L. A., and J. P. T. Higgins. 2021. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 12 (1):55–61. doi: 10.1002/jrsm.1411.
- Moher, D., A. Liberati, J. Tetzlaff, D. G. Altman, and PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6 (7):e1000097. doi: 10.1371/journal.pmed.1000097.
- Morales-Lazaro, S. L., and T. Rosenbaum. 2019. Cholesterol as a key molecule that regulates TRPV1 channel function. Advances in Experimental Medicine and Biology 1135:105–17. doi: 10.1007/978-3-030-14265-0_6.
- Nedergaard, J., and B. Cannon. 2018. Brown adipose tissue as a heat-producing thermoeffector. In Handbook of clinical neurology, eds Romanovsky A. A., Vol. 156, 137–52. Amsterdam: Elsevier. doi: 10.1016/b978-0-444-63912-7.00009-6.
- Olah, E., L. Poto, P. Hegyi, I. Szabo, P. Hartmann, M. Solymar, E. Petervari, M. Balasko, T. Habon, Z. Rumbus, et al. 2018. Therapeutic whole-body hypothermia reduces death in severe traumatic brain injury if the cooling index is sufficiently high: Meta-analyses of the effect of single cooling parameters and their integrated measure. Journal of Neurotrauma 35 (20):2407–17. doi: 10.1089/neu.2018.5649.
- Panchal, S. K., E. Bliss, and L. Brown. 2018. Capsaicin in metabolic syndrome. Nutrients 10 (5):630. doi: 10.3390/nu10050630.
- Qin, Y., L. Ran, J. Wang, L. Yu, H. D. Lang, X. L. Wang, M. T. Mi, and J. D. Zhu. 2017. Capsaicin supplementation improved risk factors of coronary heart disease in individuals with low HDL-C levels. Nutrients 9 (9):1037. doi: 10.3390/nu9091037.
- Ray, K. K., P. Corral, E. Morales, and S. J. Nicholls. 2019. Pharmacological lipid-modification therapies for prevention of ischaemic heart disease: Current and future options. The Lancet 394 (10199):697–708. doi: 10.1016/S0140-6736(19)31950-6.
- Romanovsky, A. A., M. C. Almeida, A. Garami, A. A. Steiner, M. H. Norman, S. F. Morrison, K. Nakamura, J. J. Burmeister, and T. B. Nucci. 2009. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. Pharmacological Reviews 61 (3):228–61. doi: 10.1124/pr.109.001263.
- Rosenson, R. S., H. B. Brewer, Jr., P. J. Barter, J. L. M. Bjorkegren, M. J. Chapman, D. Gaudet, D. S. Kim, E. Niesor, K. A. Rye, F. M. Sacks, et al. 2018. HDL and atherosclerotic cardiovascular disease: Genetic insights into complex biology. Nature Reviews. Cardiology 15 (1):9–19. doi: 10.1038/nrcardio.2017.115.
- Roth, G. A., C. Johnson, A. Abajobir, F. Abd-Allah, S. F. Abera, G. Abyu, M. Ahmed, B. Aksut, T. Alam, K. Alam, et al. 2017. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. Journal of the American College of Cardiology 70 (1):1–25. doi: 10.1016/j.jacc.2017.04.052.
- Sanati, S., B. M. Razavi, and H. Hosseinzadeh. 2018. A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iranian Journal of Basic Medical Sciences 21 (5):439–48. doi: 10.22038/ijbms.2018.25200.6238.
- Sattar, N., D. Preiss, H. M. Murray, P. Welsh, B. M. Buckley, A. J. de Craen, S. R. Seshasai, J. J. McMurray, D. J. Freeman, J. W. Jukema, et al. 2010. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. The Lancet 375 (9716):735–42. doi: 10.1016/S0140-6736(09)61965-6.
- Shin, K. O., and T. Moritani. 2007. Alterations of autonomic nervous activity and energy metabolism by capsaicin ingestion during aerobic exercise in healthy men. Journal of Nutritional Science and Vitaminology 53 (2):124–32. doi: 10.3177/jnsv.53.124.
- Skofitsch, G., J. Donnerer, and F. Lembeck. 1984. Comparison of nonivamide and capsaicin with regard to their pharmacokinetics and effects on sensory neurons. Arzneimittel-Forschung 34 (2):154–6.
- Snitker, S., Y. Fujishima, H. Shen, S. Ott, X. Pi-Sunyer, Y. Furuhata, H. Sato, and M. Takahashi. 2009. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. The American Journal of Clinical Nutrition 89 (1):45–50. doi: 10.3945/ajcn.2008.26561.
- Sterne, J. A. C., J. Savovic, M. J. Page, R. G. Elbers, N. S. Blencowe, I. Boutron, C. J. Cates, H. Y. Cheng, M. S. Corbett, S. M. Eldridge, et al. 2019. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898.
- Stone, N. J., and S. M. Grundy. 2019. The 2018 AHA/ACC/Multi-Society Cholesterol guidelines: Looking at past, present and future. Progress in Cardiovascular Diseases 62 (5):375–83. doi: 10.1016/j.pcad.2019.11.005.
- Taghizadeh, M., N. Farzin, S. Taheri, M. Mahlouji, H. Akbari, F. Karamali, and Z. Asemi. 2017. The effect of dietary supplements containing green tea, capsaicin and ginger extracts on weight loss and metabolic profiles in overweight women: A randomized double-blind placebo-controlled clinical trial. Annals of Nutrition and Metabolism 70 (4):277–85. doi: 10.1159/000471889.
- Thompson, P. D., P. Clarkson, and R. H. Karas. 2003. Statin-associated myopathy. JAMA 289 (13):1681–90. doi: 10.1001/jama.289.13.1681.
- Townsend, N., M. Nichols, P. Scarborough, and M. Rayner. 2015. Cardiovascular disease in Europe-epidemiological update 2015. European Heart Journal 36 (40):2696–705. doi: 10.1093/eurheartj/ehv428.
- Uchida, K., W. Sun, J. Yamazaki, and M. Tominaga. 2018. Role of thermo-sensitive transient receptor potential channels in brown adipose tissue. Biological & Pharmaceutical Bulletin 41 (8):1135–44. doi: 10.1248/bpb.b18-00063.
- Urbina, S. L., M. D. Roberts, W. C. Kephart, K. B. Villa, E. N. Santos, A. M. Olivencia, H. M. Bennett, M. D. Lara, C. A. Foster, M. Purpura, et al. 2017. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite 113:264–73. doi: 10.1016/j.appet.2017.02.025.
- Xu, Y., and M. Lopez. 2018. Central regulation of energy metabolism by estrogens. Molecular Metabolism 15:104–15. doi: 10.1016/j.molmet.2018.05.012.
- Yuan, L. J., Y. Qin, L. Wang, Y. Zeng, H. Chang, J. Wang, B. Wang, J. Wan, S. H. Chen, Q. Y. Zhang, et al. 2016. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clinical Nutrition 35 (2):388–93. doi: 10.1016/j.clnu.2015.02.011.
- Zsiboras, C., R. Matics, P. Hegyi, M. Balasko, E. Petervari, I. Szabo, P. Sarlos, A. Miko, J. Tenk, I. Rostas, et al. 2018. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Critical Reviews in Food Science and Nutrition 58 (9):1419–27. doi: 10.1080/10408398.2016.1262324.