Abstract
Australian native plants have adapted themselves to harsh climatic conditions enabling them to produce unique and high levels of secondary metabolites. Native fruits and vegetables have been an integral part of the Indigenous Australian diet and Bush medicine for centuries. They have recently gained popularity owing to their rich dietary fiber, minerals, polyphenolic and antioxidant contents. This review presents a comprehensive summary and critical assessment of the studies performed in the last few decades to understand the phytochemical and nutritional profiles and therapeutic properties of Australian native fruits and vegetables. Furthermore, the potential of these fruits and vegetables as functional food ingredients and in the prevention and treatment of different diseases is discussed. Research on the nutritional and phytochemical profiles and therapeutic activity of Australian vegetables is limited with most studies focused on native fruits. These fruits have demonstrated promising antioxidant, anticancer, anti-inflammatory and antimicrobial activities mostly in in vitro models. More research to a) identify novel bioactive compounds, b) define optimal post-harvest and extraction methods, and c) understand molecular mechanisms of pharmacological activity through preclinical and clinical studies is prudent for the prospective and wider use of Australian native fruits and vegetables by the food, pharmaceutical, and nutraceutical industries.
1. Introduction
Australian native plants have been playing an essential role in the Indigenous Australian diet and Bush medicine for thousands of years (Brand-Miller and Holt Citation1998; Clarke Citation2008). Because of the distinct climatic conditions of Australia, these plants have adapted to the biotic and abiotic stresses to produce fruits and vegetables that are rich in unique phytochemicals (secondary metabolites), dietary fiber, essential amino acids, minerals, and antioxidants with several potential health benefits (Singh, Raju, and Münch Citation2021). Due to their therapeutic activity as evident in the Australian Bush medicine, these fruits and vegetables have recently become attractive as functional food sources (nutraceuticals) and have been incorporated in different herbal formulations, supplements, and food products. The native edible plants, also known as Bush Food and Bush tucker, are becoming popular among modern Australians recently with a gross production value of $18–25 million (Richmond, Bowyer, and Vuong Citation2019). Many foods and nutraceutical companies are introducing products based on Australian native fruits and vegetables to meet the increasing consumer demands (Netzel et al. Citation2007). The national body- Australian Native Food and Botanicals (ANFAB Citation2021), which represents the rapidly-growing interests in the Australian native food and botanical sector, has listed a number of priority species including bush tomato (Solanum centrale), Davidson’s plum (Davidsonia pruriens), desert lime (Citrus glauca), finger lime (Citrus australasica), Kakadu plum (Terminalia ferdinandiana), lemon aspen (Acronychia acidula), lemon myrtle (Backhousia citriodora), muntries (Kunzea pomifera), quandong (Santalum acuminatum), Tasmanian pepperberry (Tasmannia lanceolata), riberry (Syzygium luehmannii), anise myrtle (Syzygium anisatum) and wattleseed (Acacia spp.) based on the industry demand and increasing research in the last decade. Of these species, 11 native fruits including bush tomato, Davidson’s plum, desert lime, finger lime, Kakadu plum, lemon aspen, muntries, quandong, Tasmanian pepperberry, and Illawarra plum (Podocarpus elatus) have been commercially produced in Australia (Richmond, Bowyer, and Vuong Citation2019).
As the current literature portrays several studies and reviews broadly on the bioactivity and industrial applications of Australian herbs and plants (Wilkinson and Cavanagh Citation2005; Sadgrove and Jones Citation2015; Sultanbawa and Sultanbawa Citation2017; Singh, Raju, and Münch Citation2021; Guo, Sakulnarmrat, and Konczak Citation2014; Bhuyan et al. Citation2018; Vuong et al. Citation2015; Vuong, Hirun, Phillips, et al. Citation2014), this review focuses specifically on the potential therapeutic activity of Australian native fruits and vegetables. The previous reviews by Vuong et al. (Citation2015) and Bhuyan et al. (Citation2018) were exclusively on Eucalypts which are generally inedible. Similarly, the review performed recently by Singh, Raju, and Münch (Citation2021) discussed the bioactive compounds found specifically in Australian rainforest plants including Endiandra anthropophagorum, Ochrosia moorei, Doryphora sassafras, Peripentadenia mearsii, Elaeocarpus grandis, Elaeocarpus eumundi, Ficus racemose, Tinospora smilacina, Melaleuca alternifolia, Pilidiostigma glabrum, Dodonaea polyandra, Lophostemon suaveolens, Geijera parviflora, Citrus garrawayi, Alphitonia petriei, Angophora costata, Ternstroemia cherry, Pleuranthodium racemigerum and Waterhousia mulgraveana with potential anti-neuroinflammatory activity. Another recent review by Richmond, Bowyer, and Vuong (Citation2019) highlighted the traditional, current and potential uses of Australian native fruits as functional food ingredients. In addition, the authors described the sensory properties of each native fruit. However, comprehensive reviews underlining chemical and nutritional compositions and broad-spectrum therapeutic activity of edible Australian native fruits and vegetables have not been conducted recently. Studies thus far have identified a number of phytochemicals in native fruits and vegetables and of the identified bioactive compounds, many have shown potential in improving health conditions or treating various diseases, including cancer (Netzel et al. Citation2007; Sakulnarmrat Citation2012; Sommano, Caffin, and Kerven Citation2013). This review draws a comprehensive summary and assessment of the studies available in the literature on the chemical composition, nutritional profile, and both in vitro and in vivo therapeutic activities of Australian native fruits and vegetables.
2. Nutritional composition of Australian native fruits and vegetables
Plant-derived natural compounds with multifunctional properties have been a global demand for application in the food, nutraceutical, and pharmaceutical industries (Chapman et al. Citation2021). Australian Indigenous communities have been using native fruits and vegetables as both food and medicine for centuries. Native plants have provided numerous nutritional benefits, such as a source of water-soluble vitamins, minerals, and dietary fiber for the Australian Indigenous peoples (Cherikoff and Isaacs Citation1989). These native fruits and vegetables are found to be nutritionally denser due to their low moisture content when compared to their non-native counterparts. For instance, higher fat, protein, and carbohydrate levels are reported in these fruits and vegetables (Brand and Cherikoff Citation1985; Williams and Chaliha Citation2016; Dyson Citation2006).
The most common native fruits and vegetables found in the market according to the ANFAB are bush tomatoes, Davidson’s plum, desert lime, Kakadu plum, lemon aspen, muntries, quandong, Tasmanian pepperberry, riberry, Murnong (Microseris lanceolata), and Warrigal greens (Tetragonia tetragonoides) (Richmond, Bowyer, and Vuong Citation2019; Gott Citation1983; Clarke Citation2012). Additionally, the Illawarra plum has been added to the list as an “evolving” commercially used Australian native species (Richmond, Bowyer, and Vuong Citation2019). Furthermore, an Australian food nutrition database has listed several native fruits and vegetables for their highest and lowest nutritional content. For example, riberry and bush tomato were reported to have 8 and 10 g/100 g protein, respectively, whereas blueberries and cherry tomato contain 0.5 g/100 g protein (NUTTAB Citation2011). Furthermore, the bush tomato was reported to have 47 g/100 g carbohydrate, and 6 g/100 g fat compared to the cherry tomato which contains 2.2 g/100 g carbohydrate and 0.1 g/100 g fat (NUTTAB Citation2011). There has been a significant interest in obtaining nutritional data of Australian native fruits and vegetables as evident in different studies published over the past three decades, however, many native fruits and vegetables still have not been explored for their nutritional composition and bioactivity. The existing nutritional composition of selected commercially grown native Australian fruits and vegetables are shown in .
Table 1. Nutritional composition of common commercially grown native Australian fruits and vegetables.
2.1. Proximate composition
The proximate composition analysis showed that Australian native fruits and vegetables are rich in carbohydrates and dietary fiber, followed by low amounts of protein and fat (). The total carbohydrate content of Australian native fruits is between 3 and 47 g/100 g fresh weight (FW) () inclusive of sugars, starches, and fiber. Bush tomato, Tasmanian pepperberry, Illawarra plum, and quandong contain the highest carbohydrate levels which are characteristically higher than the non-native fruits with 29–47, 34, 13–21, and 20.95 g/100 g FW, respectively (Brand-Miller and Holt Citation1998). In contrast, the unripe flesh of green plum (Buchanania obovate), which grows in the harsh environmental conditions of the Northern Territories of Queensland, was reported to contain only 4.5 g/100 g carbohydrates (Fyfe et al. Citation2020). The total content of sugars in finger lime and Illawarra plum varies from 1 − 1.2 g/100 g FW, whereas 24 − 29 g/100 g FW for pepperberry and bush tomato (). These native Australian fruits vary in taste and not all of them are sweet. Sugars in lemon aspen and Kakadu plum are primarily fructose. Bush tomato comprises of highest sugar levels of 29 g/100 g FW () with sucrose being the dominant sugar (Read Citation2012). Quandong, finger lime, and pepper berries contain a mix of fructose and glucose (Read Citation2012).
In comparison to Western fruits such as orange, apple, banana, plum, cherry tomato (Tom Thumb), blueberry, raspberry, lemon, lime, and strawberry, native fruits generally contain lower total sugar levels (NUTTAB Citation2011). For instance, Illawarra plum contains 17 g/100 g carbohydrate of which only 1 g/100 g is total sugars. Likewise, low levels of total sugar are also seen in Davidson’s plum, Kakadu plum, and lemon aspen when compared with their total carbohydrate content (< 50% of total carbohydrates are sugars) which could indicate the presence of other carbohydrates, i.e., starches or fibers. A high fiber diet is often linked with a healthy digestive system. Moreover, high fiber helps lower blood cholesterol levels and prevent constipation by improving bowel movement. The fiber content of Australian native fruits varies from 2.6 to 41.9 g/100 g () with Tasmanian pepperberry containing the highest reported content [41.9 g/100 g dried weight (DW)] and lemon aspen with the least recorded quantity of fiber at 2.6 g/100 g. Bush tomato, Kakadu plum, and finger lime comprise 7.8, 7.1, and 7.2 g/100 g of fiber, respectively. No fiber content has been reported for desert limes, muntries, and the riberry. According to the Food Standards Australia and New Zealand (Chapman et al. Citation2021), a serving of food with at least 4 to 7 g of fiber can be an excellent source of dietary fiber. Compared to Western fruits consisting between 1.7 g/100 g (cherry tomato) and 5.6 g/100 g (raspberry) with an average of 2.9 g/100 g fiber, native fruits with greater dietary fiber content can be the superior alternatives (NUTTAB Citation2011). For instance, the Australian native fruits green plum and Burdekin plum (Pleiogynium timorense) were found to contain 11.6 and 18.4 g/100 g of fiber, respectively (Fyfe et al. Citation2020). A recent study by Njume, McAinch, and Donkor (Citation2020) investigated the proximate compositions of edible portions of native Australian plants including pigface (Carpobrotus rossii), bulrush (Typha orientalis), native currant (Leucopogon parviflorus), chocolate lily (Arthropodium strictum), seaberry saltbush (Rhagodia candolleana), white correa (Correa alba), blueberry lily (Dianella revoluta) and long-leaved wattle (Acacia longifolia) and reported that the long-leaved wattle, bulrush, white Correa, and pigface had high fiber content. Other Australian Acacia species such as A. victoriae, A. coriacea and A. cowleana were also demonstrated to have high neutral detergent fiber in the range of 22.21 − 30.16 g/100 recently (Adiamo, Netzel, et al. Citation2021). Another recent study by Srivarathan et al. (2021a) found that Tecticornia sp. (Samphire), commonly used as a complementary vegetable, salads and salt substitute, albeit underutilized (Srivarathan et al. Citation2021b), can contain up to 46.8 g/100 g of fiber.
In addition to fibers, native fruits are also rich in proteins, antioxidants, and vitamins. The protein levels in native fruits range from 0.1 to 10.3 g/100 g (). Studies have demonstrated that native fruits have higher protein levels than Western fruits, stating their differences in moisture levels (Brand and Cherikoff Citation1985; NUTTAB Citation2011). The average protein contents of Western (0.73 g/100 g) and native fruits (3.25 g/100 g) indicate the potential nutritional benefits of the latter. For instance, green plum and Burdekin plum contain 2.69 and 1.3 g/100 g protein, respectively (Fyfe et al. Citation2020). Edible portions of the native Australian edible plants such as white Correa, blueberry lily, and long-leaved wattle were reported to be rich in protein (≥ 7.9 ± 0.02 g/100 g DW) and fatty acids (palmitic, oleic, and linoleic acids) (Njume, McAinch, and Donkor Citation2020). A recent study by Adiamo, Netzel, et al. (Citation2021) demonstrated that Australian Acacia species (wattleseed) have high protein contents (20.47–25.64 g/100 g DW) and are comparable to chickpea (21.9–26.8 g/100 g DW). The authors also reported that some wattleseed species such as A. cowleana are a good source of fat (average 14.61 g/100 g DW) (Adiamo, Netzel, et al. Citation2021). Fat contributes to most of the calories in native fruits. As a result, fat content in the native fruits varies from trace amounts (< 0.2 g/100 g) to 6.7 g/100 g () with an average of 2.29 g/100 g. Bush tomato and Tasmanian pepperberry have the highest fat content (6.0 and 6.7 g/100 g, respectively) whereas that of quandong, Illawarra plum, Kakadu plum, and riberry are negligible (< 0.2 g/100 g). Additionally, native fruits consist of a diverse composition of fatty acids which also varies among the fruit species. Finger lime and bush tomato have a distribution of approximately 1/3 saturated, monounsaturated and polyunsaturated fats in each featuring palmitic, oleic, and omega 6 as the three main contributing fatty acids. The study by Read (Citation2012) highlighted that finger lime also contains omega 3 in equivalent amounts to its omega 6 content. The Tasmanian pepperberry has a distribution of saturated: monounsaturated: polyunsaturated fats in a ratio of 10:10:80 that includes palmitic, oleic, and omega 6 as the major fatty acid components. Lemon aspen fats are close to a 25:25:50 distribution, with palmitic, palmitoleic, and omega 6 fatty acids being the dominant structural forms (Read Citation2012). Most Western fruits have shown low to negligible fat content (except for avocadoes with 21.6 g/100 g) and the lowest fat content has been recorded in blueberries, oranges, and apples (NUTTAB Citation2011). Kakadu plum kernels were also analyzed for their nutritional composition and found to contain high levels of fat (35% DW) and protein (32% DW) (Akter et al. Citation2018). Omega-6 fatty acid, linoleic acid, monounsaturated oleic acid and two saturated fatty acids- palmitic and stearic acids were found to be the major fatty acids of Kakadu plum kernels (Akter et al. Citation2018).
2.2. Vitamins
Vitamins such as A, B, C, and E (tocopherol) are also abundantly found in many native fruits which are of great importance to the overall health and well-being of the consumers (). Native fruits are known to have typically higher vitamin content than other fruits (Brand-Miller and Holt Citation1998). In particular, vitamin E, folate, and thiamine (vitamin B1) are higher on average when compared to Western fruits (NUTTAB Citation2011). For example, vitamin E levels on average are 10 times higher in native fruits (3.1 mg/100 g) than Western fruits (0.3 mg/100 g). The highest vitamin E level was recorded in Kakadu plum (6.1 mg/100 g), followed by quandong (4.8 mg/100 g) and bush tomato (4.6 mg/100 g) (). In comparison, the vitamin E content of Western plum is about 0.82 mg/100 g (highest values in western fruits), while cherry tomatoes have 0.2 mg/100 g vitamin E. Among the analyzed Australian native fruits, Tasmanian pepperberry has the lowest vitamin E content at 1.2 mg/100 g, which is still 10 times higher than the lowest value for vitamin E in Western fruits (banana 0.12 mg/100 g). Studies have recommended that men and women should consume 10 and 7 mg vitamin E/day, respectively to meet their daily vitamin E intake (National Health and Medical Research Council Citation2017). This equates to 164 g (for men) and 114 g (for women) of Kakadu plums, or 1.22 kg (for men) and 853.7 g (for women) of Western plums to meet the daily recommendations. Konczak and Roulle (Citation2011) reported that three forms of vitamin E: α-tocopherol, γ-tocopherol and δ-tocopherol were found in the lipophilic fractions of some native fruits. The highest proportion of α-tocopherol was identified in riberry (98.7%), followed by lemon aspen (96.6%) and quandong (90.4%). Davidson’s plum, on the other hand, exhibited lower vitamin E content (44.6% of α-tocopherol, 21.7% of γ-tocopherol, and 32.5% of δ-tocopherol (Konczak and Roulle Citation2011). α-tocopherol is the most active vitamin E component that protects cellular membranes from oxidative degradation of lipids (Miyazawa et al. Citation2019). Vitamin E is also widely used in cosmetic product to protect against the sun and provide assistance in age-related skin deterioration. Vitamin E is a lipid-soluble antioxidant that protects human cells against lipid peroxidation and provides protection against photoinhibition and photooxidative stress in plant cells (Konczak and Roulle Citation2011). Among the Western fruits, avocadoes are one of the richest sources of vitamin E with α-tocopherol as the main component (Bhuyan et al. Citation2019). In comparison to other countries, due to its location in the Southern Hemisphere, Australia has a higher level of solar UV radiation speculating that the high exposure to the sun could positively affect the levels of accumulation of phytochemicals, such as vitamin E in native plants. Quandong is a hemiparasitic plant originally found in the Western and Southern regions of Australia. Nowadays, quandong is among the leading commercially grown native Australian fruits used in various food products (Njume, Donkor, and McAinch Citation2019; Sakulnarmrat, Srzednicki, and Konczak Citation2014). Total folates in native fruits average at 47 µg/100 g, ranging from the lowest at 9 µg/100 g (riberry) to 191 µg/100 g in the (quandong), respectively. In comparison, the average of folates in Western fruits ranges from 5 − 29.7 µg/100 g (in plums) to 63 µg/100 g (in oranges). It is also recommended that adults consume 400 µg of folate to meet their daily intake (National Health and Medical Research Council Citation2017). In theory, 100 g each of quandong and riberry could provide 47.75% and 2.25% of daily folate requirements, respectively.
Vitamin C (ascorbic acid) levels in native fruits can reach up to 7000 mg/100 g. Moderate vitamin C levels are found in the bush tomato (17 mg/100 g), quandong (< 20 mg/100 g), and Davidson’s plum (< 30 mg/100 g), whereas finger lime, desert lime, and Kakadu plum have shown high levels of vitamin C, with up to 58.5, 188.6 and 7000 mg/100 g, respectively (). Riberry, lemon aspen, and Tasmanian pepperberry have not been reported to contain vitamin C. A specific variety of bush tomato (Solanum chippendalei) was considered to be a key source of vitamin C for the Indigenous Australians, recording levels of 12 − 49 mg/100 g (Brand-Miller and Holt Citation1998). Kakadu plum, also known as billy goat plum or salty plum, is one of the most popular Australian native fruits and is well-known to have the highest vitamin C concentration in the plant kingdom at up to 7000 mg/100 g DW () (Konczak, Maillot, and Dalar Citation2014). Another study by Netzel et al. (Citation2007) reported that the Kakadu plum (71.3 μmol/g FW) had a 938-fold higher vitamin C than that in blueberry (0.076 μmol/g FW). However, it should be noted that vitamin C content within the Kakadu plum is highly variable, and some studies report only 885 mg/100 g vitamin C (Konczak et al. Citation2010a). Despite this, the vitamin C content of Kakadu plums is still significantly higher than Western fruits, of which the orange is the richest source containing 52 mg/100 g (NUTTAB Citation2011). The vitamin C content of the other Western fruits averages to be 24.9 mg/100 g, with the blueberry having the lowest levels at 2 mg/100 g. The requirement for vitamin C intake in Australia is 45 mg/day. Lilly pilly (Syzygium paniculatum) is a native, medium-sized rainforest tree widely grown in eastern Australia. Vuong, Hirun, Chuen, et al. (Citation2014) reported that the vitamin C in lily pilly was higher than that of lemon aspen, ribberry, and quandong.
Vitamin A levels in Australian native fruits are found to be lower than other vitamins, averaging 2.8 mg/100 g retinol equivalents. In Australia, it is recommended that adult men and women should consume 900 and 700 µg/day of vitamin A, respectively. Only Davidson’s plum and quandong were found to contain vitamin A in 13 and 10 mg/100 g retinol equivalents, respectively (). In comparison, cherry tomatoes and plum have higher levels of vitamin A, with 82 and 25 µg/100 g, respectively. Vitamin A content in other Australian native fruits and vegetables was either not detected or there is no current data. Other Western fruits usually contain <10 µg/100 g of vitamin A. The reported vitamin A and C contents in native fruits present some issues, with most analyses typically being performed on dried fruits. Both these vitamins are affected by heat treatment and aerial oxidation, meaning losses may occur during the drying process (Rawson et al. Citation2011; Shahidi and Ambigaipalan Citation2015; Sommano, Caffin, and Kerven Citation2013). Alternatively, processing of the fruits may be required to prevent deterioration to accurately assess concentration. There are no known studies assessing fat-soluble vitamins D and K or water-soluble B vitamins: niacin, biotin, vitamin B5 or vitamin B6 in Australian native fruits and vegetables. Furthermore, information on the vitamin content of native fruits such as muntries is not available. Similarly, there are also no known studies on the vitamin content of Australian native vegetables ().
2.3. Minerals
Native fruits are notable for their calcium concentration and possess higher concentrations of the trace elements iron, copper, and zinc compared to Western fruits (Brand and Cherikoff Citation1985). Kakadu plum, for instance, is particularly high in minerals including magnesium, zinc, calcium, potassium, sodium, iron, phosphorus, manganese, copper, and molybdenum, and has shown high potassium:sodium ratio () (Cock and Mohanty Citation2011). Interestingly, the calcium content of the Kakadu plum was found to be positively correlated with the anti-nutritional factor phytic acid (tissues rich in phytic acid had higher levels of calcium), however, no correlation was observed with another anti-nutritional factor oxalic acid (Akter et al. 2020b). Kakadu plum kernels were also reported to contain high potassium (6693 mg/kg), calcium (5385 mg/kg DW), iron (61 mg/kg DW) and zinc (60 mg/kg DW) and had low levels of heavy metals (Akter et al. Citation2018). Moreover, riberry was found to have a high calcium content (RIRDC Citation2012). details the mineral compositions of some most common native fruits. Out of the selected native fruits, finger lime and Tasmania pepperberry were found to have the highest calcium content (34.34 and 13.6% Recommended Dietary Intake (RDI), respectively) as well as magnesium [30.56 and 31.9% RDI], phosphorus (15.43 and 11.59%RDI) and potassium (35.56 and 26.80%RDI) per 100 g (Richmond, Bowyer, and Vuong Citation2019). Comparatively, 100 g of Western limes has 2.2%RDI calcium, 2.68%RDI magnesium, 1.80%RDI phosphorus, and 3.95%RDI potassium (NUTTAB Citation2011) (Richmond, Bowyer, and Vuong Citation2019). Quandong also exhibited higher content of sodium (116 mg/100 g) and zinc (0.89 mg/100 g). Green plum and Burdekin plum displayed significantly higher levels of potassium (478 and 458 mg/100 g, respectively) compared to mango (168 mg/100 g) with Burdekin plum also showing higher calcium content (241 mg/100 g) than green plum (89 mg/100 g) and mango (11 mg/100 g) (Fyfe et al. Citation2020). The same study also demonstrated that green plum contains significantly higher levels of magnesium (120 mg/100 g) in comparison to mango (10 mg/100 g) and Burdekin plum (32 mg/100 g) (Fyfe et al. Citation2020).
Bush tomatoes were found to be rich in iron (6.1 mg/100 g) and copper (2.8 mg/100 g), and Tasmanian pepper berries are significantly higher in manganese (31.1 mg/100 g) compared to riberry which has the second-highest level of manganese (2.002 mg/100 g) (Richmond, Bowyer, and Vuong Citation2019). Samphires (belongs to the genus Tecticornia comprising of 44 species of Australian Indigenous edible halophytes) were found to be rich in iron (41.5 mg/100 g DW), magnesium (1.2 g/100 g DW) and sodium (16.7 g/100 g DW) (Srivarathan et al. 2021a). Acacia species (wattleseed) was recently reported to contain high potassium and iron (A. cowleana and A. coriacea) (Adiamo, Netzel, et al. Citation2021).
2.4. Amino acid profile
Studies analyzing the amino acid profiles of Australian native fruits and vegetables are limited. A study by Lim et al. (Citation2020) showed that Davidson’s plum, finger lime and Tasmanian pepperberry had high amounts of essential amino acids. Lysine was found to be the most abundant essential free amino acid in Davidson’s plum (74.11%), finger lime (53.74%) and Tasmanian pepperberry (67.88%). Other amino acids including isoleucine, cysteine and histidine were also reported in relatively high levels in the three fruits. Furthermore, Davidson’s plum had 11, finger lime had 19 and Tasmanian pepperberry had 14 free amino acids (Lim et al. Citation2020).
Konczak and Roulle (Citation2011) reported that the Kakadu plum is an important fruit for the Australian native food industry with the largest volumes of native fruit sold, reaching approximately 20000 kg per year. Over the past three decades, Kakadu plum is the most explored Australian native fruit in terms of its nutritional and chemical compositions for commercial aspects due to its higher content of vitamins, minerals and antioxidants when compared to other Australian native fruits and vegetables. For instance, a recent study by Bobasa, Netzel, Cozzolino, et al. (Citation2021) implemented a rapid hand-held near-infrared spectroscopy (NIR) method to measure the total soluble solids and moisture in dry powder and fruit puree of wild harvest Kakadu plum and recommended its use for other native fruits to develop a grading/sorting system for assessing fruit quality and safety. The same technology was also implemented to analyze and characterize kernel samples and tissues of wild harvest Kakadu plum (Bobasa, Netzel, Phan, et al. Citation2021). The feasibility of utilizing the handheld NIR and mid-infrared (MIR) tools to predict the chemical composition (bioactive compounds such as vitamin C and ellagic acid) of the whole, pureed and powdered Kakadu plum fruit as well as leaf samples was also evaluated (Bobasa, Phan, Netzel, Smyth, et al. Citation2021; Cozzolino et al. Citation2021b; Sultanbawa et al. Citation2021; Mulisa Bobasa et al. Citation2020). Furthermore, the same research group used MIR spectroscopy to quantify the chemical composition of 14 common edible wattleseed (Acacia) species before and after roasting (Adiamo, Sultanbawa, et al. Citation2021).
Nutritional data are currently limited for muntries, Warrigal greens, murnong, sarsaparilla, native currant, Molucca raspberry (Rubus moluccanus), cedar bay cherry (Eugenia carissoides), Burdekin plum (Pleiogynium timorense) and not comprehensive for other fruits such as Davidson’s plum, Illawarra plum, bush tomato, and Tasmanian pepperberry. Therefore, further studies should be undertaken to obtain a more comprehensive understanding of the nutritional compositions of Australian native fruits and vegetables.
3. Phytochemical composition and antioxidant properties of Australian native fruits and vegetables
Polyphenolic compounds are the secondary metabolites known to play a key role in the plant’s defensive mechanism (Manach et al. Citation2004). The polyphenolic content of fruits and vegetables is often linked with their antioxidant capacity. In recent years, the extraction, isolation, and identification of phenolic antioxidants from natural sources have become primary research focuses of the food, nutraceutical, and pharmaceutical industries (Bhuyan et al. Citation2019). However, detailed information related to the phytochemical extraction and composition of many native Australian fruits and vegetables is limited (Konczak and Roulle Citation2011). Some of the common phytochemicals and antioxidants found in Australian native fruits and vegetables are shown in and and and .
Figure 1. Some of the common phytochemicals and antioxidants found in Australian native fruits and vegetables.
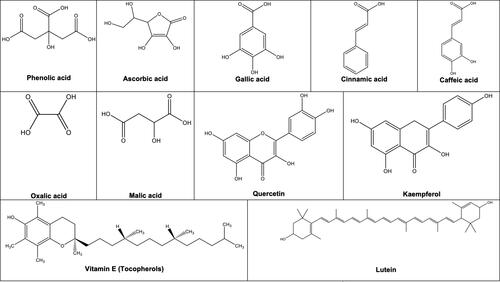
Table 2. Various phytochemicals identified in common Australian native fruits and vegetables.
Table 3. Antioxidants reported in Australian native fruits and vegetables.
Netzel et al. (Citation2006) investigated a wide range of Australian native fruits including riberry, brush cherry, muntries, Illawarra plum, Burdekin plum, cedar bay cherry, Davidson’s plum, Molucca raspberry and finger lime, Tasmanian pepperberry, and Kakadu plum for their chemical composition and antioxidant capacity. In that study, among the investigated fresh fruits, Burdekin plum exhibited the highest total phenolic content of 100.50 ± 7.40 μmol of gallic acid equivalent, whereas, Tasmanian pepperberry (21.13 μmol/g FW) and Illawarra plum (19.39 μmol/g FW) were abundant in anthocyanins, a subtype of the polyphenols, which was higher than that of any fruit in the study as well as most well-known berries. The anthocyanin mixtures of both native fruits were predominated by cyanidins, the most common anthocyanins found in food (Netzel et al. Citation2006). Illawarra plum was also reported to have a high content of anthocyanin-rich phenolics in other studies (Netzel et al. Citation2007; Symonds, Konczak, and Fenech Citation2013).
Both hydrophilic and lipophilic antioxidants are essential sources for preventing oxidative stress and can effectively scavenge free radicals (Prior et al. Citation2003). Konczak and Roulle (Citation2011) reported the presence of major phenolic compounds, organic acids, vitamin C, and antioxidants in lipophilic extracts obtained from commercially grown Australian native fruits. and summarize the phytochemicals and antioxidants currently identified in Australian native fruits and vegetables (Richmond, Bowyer, and Vuong Citation2019; Ahmed and Johnson Citation2000; Akter et al. Citation2016; Brand and Cherikoff Citation1985, Citation2019; Gott Citation1983; Netzel et al. Citation2006; Tan et al. Citation2011b; Konczak et al. Citation2010b; Netzel et al. Citation2007). Various native fruits and vegetables have been studied for their antioxidant properties using conventional spectroscopic assays such as 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC), cellular antioxidant activity (CAA), and ferric reducing ability of plasma (FRAP) as well as more sensitive analytical techniques including high-performance liquid chromatography (HPLC), HPLC-mass spectrometry (HPLC-MS) and HPLC-electrospray ionisation-MS (HPLC-ESI-MS). It is apparent from the studies performed so far that phenolic compounds (including phenolic, benzoic and cinnamic acids, flavonoids, and tannins), carotenoids, α, β, γ, and δ-tocopherols, monounsaturated and polyunsaturated fatty acids were found to be the key antioxidants in Australian native fruits and vegetables.
Kakadu plum is relatively more explored in terms of its phytochemical profile compared to other Australian native fruits. Interestingly, Kakadu plum displayed a 6-fold higher level of total phenolic compounds than blueberry in the Folin–Ciocalteu assay and was shown with superior oxygen radical-scavenging capacity (Konczak et al. Citation2010b). The phytochemical analysis of the Kakadu plum extract showed a complex mixture of over 100 compounds with the highest level of total phenolic compounds, among the tested fruits (Tan et al. Citation2011a). Vitamin C was found to be the main bioactive compound in Kakadu plum ranging from 0.2% − 5.9%, hence further marketing studies and optimization of vitamin C production were recommended for the fruit (McDonald et al. Citation2008). A recent study by Phan, Damyeh, et al. (Citation2021) showed that maturity and season can significantly affect the vitamin C and ellagic acid content of Kakadu plum. The authors observed a 20-fold increase in vitamin C and three times decrease in ellagic acid content in Kakadu plum from immature to mature stage (Phan, Damyeh, et al. Citation2021). The same research group recently recommended the use of 7.5–10% and 10–15% maltodextrin to preserve vitamin C and other quality parameters of the freeze‑dried and oven-dried Kakadu plum powders, respectively (Phan, Adiamo, Netzel, et al. Citation2021). Another study found that hydrophilic phenolic compounds (e.g., hydroxybenzoic acids, flavanols and flavanones) were predominant in Kakadu plum (Konczak et al. Citation2010a). The data obtained from the individual tree harvest analysis showed that Kakadu plum fruit is a rich source of free ellagic acids in fresh fruit and oxalate in frozen fruit. The analysis of the frozen harvest also revealed that the storage conditions increased free ellagic acid concentrations in the fruit (Williams, Edwards, Pun, et al. Citation2016). Another study by the same research group demonstrated that methanol and dimethylformamide (50:50 v/v) could be more suitable in extracting ellagic acid from Kakadu plum compared to methanol, acetone, acidified methanol or 80% acetone (Williams, Edwards, Chaliha, et al. Citation2016). The biochemical analysis of Kakadu plum supported the rationale behind its traditional use as food and medicine by the Indigenous Australians. Due to its high amount of ascorbic acid and a variety of other phenolic compounds, anthocyanins and flavonoids, Kakadu plum is a good source of antioxidants. The lipophilic fraction of Kakadu plum extract was also found to contain α-tocopherol (1.022 ± 0.1 mg/100 g, FW), lutein (0.26 ± 0.01 mg/100 g FW) and chlorophyll a and b (2.72 ± 0.1 and 0.54 ± 0.1 mg/100 g FW, respectively) (Konczak and Roulle Citation2011). Chlorophyll a and b can relieve oxidative stress (Konczak and Roulle Citation2011). Whilst only chlorophyll a, b and lutein have been characterized in Kakadu plum extracts, the total carotenoid content has not been reported. However, Konczak and Roulle (Citation2011) demonstrated that the lipophilic fraction of Kakadu plum rich in chlorophyll a and b, α-tocopherol and lutein exhibited 26.66% activity in the ORAC assay which was comparable to avocado with 28.6% activity (Konczak and Roulle Citation2011). That study utilized 80% methanol, 19.9% H2O and 0.1% HCl (v/v/v) or acetone as solvents for the extraction of the lipophilic fraction of Kakadu plum ().
Hesperitin, the glycosides kaempferol and luteolin, and the glucoside quercetin are other antioxidants present in Kakadu plum (Mohanty and Cock Citation2012). In another study by Netzel et al. (Citation2007), Kakadu plum and Tasmanian pepperberry were found to contain the highest antioxidant capacity and slightly lower phenolic content than other fruits indicating the nature of the polyphenols and not their quantity influences the antioxidant capacity. With a low correlation between the anthocyanidin glycosides and the antioxidant capacity, it can be inferred that anthocyanins may not necessarily contribute to the antioxidant activity and other unidentified phenolic compounds could also attribute to the bioactivity of Australian fruits (Netzel et al. Citation2006, Citation2007).
Lim et al. (Citation2020) recently assessed the phytochemical content of Davidson’s plum, finger lime, and Tasmanian pepperberry. The analyses of metabolomics profiles of these fruits revealed 604 aromatic compounds, an abundance of minerals, especially, potassium, and essential amino acids. The aromatic compounds: limonene, furfural, 1-R-α-pinene, and other compounds such as sugars, flavonoids, and terpenes were shown to be primarily responsible for their antioxidant activity (Lim et al. Citation2020). The antioxidant capacity of Australian native fruits, however, varied based on the biochemical assay used. For instance, Davidson’s plum showed the highest activity in the FRAP assay, finger lime in the ABTS assay, and Tasmanian pepperberry in the DPPH assay. Likewise, previous reports observed that despite its high phenolic content (890 mg GAE/100 g), Davidson’s plum exhibited low antioxidant activity (Sommano, Caffin, and Kerven Citation2013). Another study by Njume, Donkor, and McAinch (Citation2019) demonstrated that Davidson’s plum contained a considerable amount of anthocyanins, phenolic compounds (mainly flavonoids), and vitamin C. Davidson’s plum and Illawarra plum were also reported to have significantly higher (by several folds) antioxidant, total phenolic and anthocyanin contents compared to blueberry (Netzel et al. Citation2006, Citation2007; Symonds, Konczak, and Fenech Citation2013). A recent study by Nirmal et al. (Citation2021) showed that the infusions of Davidson’s plum species D. pruriens and D. jerseyana contained phenolics, minerals, organic acids and soluble sugars with high levels of gallic acid. The Davidson’s plum infusions displayed similar antioxidant capacity in the DPPH, reducing power, β-carotene-linoleate model and linoleic acid peroxidation assays to that of forest fruit infusion (containing hibiscus, poppy flowers, apple, rosehip, cherry stems, orange peel, blackberry, raspberry pieces and strawberry) (Nirmal et al. Citation2021). Tasmanian pepperberry is a shrub mostly found in the temperate rain forest of Tasmania and the Southeastern parts of Australia. The dried berries of the plant are used as flavorings in the traditional foods of Aboriginal Australians (Njume, Donkor, and McAinch Citation2019). The berries of Tasmanian pepper were found to contain flavonoids and phenolic compounds and have a high antioxidant capacity recently (Mani et al. Citation2021).
The phenolic content of lily pilly fruits is a mixture of flavonoids including gallic acid (0.39 mg/g), chlorogenic acid (2.35 mg/g), catechin (0.47 mg/g) and epicatechin (2.9 mg/g) as well as proanthocyanidins (Konda et al. Citation2019; Vuong, Hirun, Chuen, et al. Citation2014). The anthocyanins are responsible for the purple color of lily pilly fruit and are higher than that of Kakadu plum and lemon aspen (Vuong, Hirun, Chuen, et al. Citation2014). Although this plant has great potential, it is listed as vulnerable under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 (Ramírez and Kallarackal Citation2019; NSW Office of Environment and Heritage Citation2012).
Green plum showed a similar antioxidant capacity to that of Kakadu plum (Fyfe et al. Citation2018a). The flesh of green plum displayed high DPPH radical scavenging activity (106.3 ± 28.6 µM Trolox equivalent/g DW in methanol) and numerous polyphenols including gallic acid, ellagic acid, p-coumaric acid, kaempferol, quercetin, and trans-ferulic acid were found in the flesh and seed of green plum which may be responsible for their antioxidant activity (Fyfe et al. Citation2018a). Mazerand and Cock (Citation2019) also analyzed green plum for its phytochemicals and antioxidant potential. The authors reported high levels of phenolics, flavonoids, and tannins in line with the previous study (Fyfe et al. Citation2018a), as well as low to moderate levels of saponins in the green plum fruit extracts (Mazerand and Cock Citation2019). The methanolic, water and ethyl acetate extracts of green plum displayed high DPPH radical scavenging activity with 38, 55, and 48 µg ascorbic acid equivalents/g of dried fruit (Mazerand and Cock Citation2019).
Bush tomato, also known as desert raisin, is an Australian native fruit that has been found to contain a considerable amount of phenolic compounds such as hydroxybenzoic acid and ferulic acid (Konczak et al. Citation2010b) and a range of flavonoids including caffeic acid, ferulic acid, hesperetin and naringenin with radical scavenging activity (Sommano, Caffin, and Kerven Citation2013). Wright, Matthews, et al. (Citation2016) reported that methanolic and aqueous extracts of bush tomato had low to moderate levels of alkaloids content.
Brophy, Goldsack, and Forster (Citation2001) investigated the leaf oil of desert lime and detected α-pinene (59.5%), p-pinene (11.6%), the two furanoid forms of linalool oxide (total 5.3%), myrcene (2.2%), p-caryophyllene (1.4%), α-humulene (2.5%) and germacrene B (3.1%). In addition, desert lime was found to contain limonoids, coumarins, alkaloids, phenols, flavonoids, and flavanones. Wright, Matthews, et al. (Citation2016) demonstrated that methanolic extract of desert lime showed high antioxidant capacity (11.7 µg ascorbic acid equivalent) than that of aqueous extract (6.3 µg ascorbic acid equivalent). In addition, methanol extract of desert lime showed higher flavonoids, moderate triterpene and saponins and low levels of cardiac glycosides and total phenolics, whereas the aqueous extract had high flavonoids and total phenolics and moderate levels of alkaloids and triterpenes. The same study (Wright, Matthews, et al. Citation2016) also reported that muntries and Illawarra plum had moderate levels of tannins. The highest antioxidant capacity was exhibited by lemon aspen methanolic extract (15.9 mg ascorbic acid equivalents/g DW) and all methanolic extracts typically had higher antioxidant capacities than the corresponding aqueous extracts (Wright, Matthews, et al. Citation2016). Another study undertaken by Sirdaarta et al. (Citation2016), evaluated six Australian native fruits muntries, Illawarra plum, native tamarind (Diploglottis australis), lemon aspen, desert lime, and bush tomato and reported that the antioxidant activity of methanol extracts of all fruits and herbs were higher than the corresponding water, ethyl acetate, chloroform, or hexane extracts. Furthermore, lemon aspen fruit methanolic extract showed the highest antioxidant capacity at 15.9 mg ascorbic acid equivalent/g DW in that study (Sirdaarta et al. Citation2016). The HPLC-MS analysis putatively identified 20 compounds in the lemon aspen extracts (Sirdaarta et al. Citation2016).
Unlike many studies on native fruits, there are only limited studies on native vegetables. However, from the limited studies, it is interesting to note that many native vegetables have unique phytochemical compositions. For instance, a recent study by Njume, McAinch, and Donkor (Citation2020) demonstrated that seaberry saltbush lacked gallic acid but exhibited a very high betanin content (700 mg/L). Betanin replaces anthocyanins in vegetables and previously was linked to anti-inflammatory properties (Zhao et al. Citation2018). Interestingly, the seaberry saltbush and the pigface (244 mg/L) are the only two Australian species in that study that contained betanin () (Njume, McAinch, and Donkor Citation2020). The antioxidant capacity of bulrush that contained low phenolic content did not correlate with its polyphenol level (Njume, McAinch, and Donkor Citation2020). Another study by Agatonovic-Kustrin et al. (Citation2020) showed that sea celery (Apium prostratum) had a moderate DPPH radical scavenging activity (36 GAE g/10 L).
Konczak and Roulle (Citation2011) analyzed the lipophilic antioxidants present in native fruit extracts and noted that every lipophilic fraction had a different composition of bioactive compounds. Three components of vitamin E: α-tocopherol, γ-tocopherol and δ-tocopherol, and lutein were found to be the main constituents of the lipophilic fractions of some native fruits. The studies performed on the fruit extract fractions found a low correlation between the total level of identified lipophilic compounds and the antioxidant capacity. Furthermore, Western fruits are considered a poor source of lipophilic antioxidants (Prior et al. Citation2003). Several studies have reported that in addition to vitamin E, different Australian native plants consist of potent phenolic antioxidants such as chlorogenic acid, cyanidin 3-glucoside, cyanidin 3-arabinoside, delphinidin 3-glucoside, delphinidin 3-galactoside, ascorbic acid, cyanidin 3-galactoside, delphinidin 3-arabinoside, petunidin 3-galactoside, petunidin 3-glucoside, peonidin 3-galactoside, petunidin 3-arabinoside, peonidin 3-glucoside, malvidin 3-galactoside, malvidin 3-glucoside, malvidin 3-arabinoside, phenolic acids, gallic acid, catechin, epicatechin, quercetin rutinosides and quercetin hexoside () (Ahmed and Johnson Citation2000; Akter et al. Citation2016; Brand and Cherikoff Citation1985, Citation2019; Gott Citation1983; Tan et al. Citation2011b; Netzel et al. Citation2007; Richmond, Bowyer, and Vuong Citation2019). Lutein, a pigment known to improve visual function and symptoms in age-related macular degeneration (Richer et al. Citation2004), was detected in Kakadu plum, Davidson’s plum, and desert Lime (). Chlorophylls were also identified in desert lime and bush tomato (). Overall, studies so far have been able to identify the major lipophilic antioxidants in some of the commercially grown Australian native fruits with some degree of variation.
The discrepancies in the antioxidant and phytochemical profiles of Australian native fruits observed in different studies may be attributed to several factors including differences in biochemical assays used and the types of samples, solvents and techniques used for extraction in these studies. The findings thus far also support the argument that the phenolic compounds are not solely responsible for the antioxidant capacity of these fruits, but other bioactive compounds may also contribute to their overall antioxidant activity. The interactions among these compounds may also contribute to the variability antioxidant capacity of the Australian native fruit and vegetables. The antioxidant activity was previously linked to the number and position of the hydroxyl groups on the B-ring of flavonoids. For example, flavonols such as quercetin and myricetin in combination showed high antioxidant activity unlike pelargonidin-3-glucoside combined with either catechin, kaempferol or quercetin displaying antagonism and low DPPH scavenging activity (Hidalgo, Sánchez-Moreno, and de Pascual-Teresa Citation2010).
The drying technique employed during the processing of native fruits and vegetable samples can significantly impact their antioxidant capacity and phytochemical profiles. A report by McDonald et al. (Citation2008) highlighted the techniques implemented so far to dry commercially important bush tomato and Kakadu plum. Freeze drying, sun drying and oven drying were the most common techniques and solvents such as methanol, ethanol, acetone, hexane and distilled water were used for the extraction of bioactive compounds from bush tomato and Kakadu plum (McDonald et al. Citation2008). For bush tomatoes, the authors reported two post-harvest and handling methods- oven drying and sun drying (McDonald et al. Citation2008). The oven-dried bush tomato sample had higher bioactive content than its sun-dried counterpart. Furthermore, a higher rate of nutrient loss was observed in the sun-dried bush tomato sample when the moisture content was higher, indicating the importance of moisture content along with drying techniques influencing the stability and retention of bioactive compounds in the sample (McDonald et al. Citation2008). The authors recommended the implementation of freeze-drying prior to storage to preserve the vitamin content of bush tomato and Kakadu plum, however, underlined the need to optimize conditions for the post-harvest drying (McDonald et al. Citation2008). Similarly, a recent study demonstrated that freeze-drying was significantly better at retaining vitamin C, reducing the formation of non-enzymatic browning and oxidation products, as well as improving the powder color of Kakadu plum compared to oven drying (Phan, Adiamo, Netzel, et al. Citation2021). The advantage of freeze-drying over oven-drying was further validated by another study by utilizing a combination of two dimensional and NIR spectroscopies showing that the freeze-drying led to Kakadu plum samples with very similar and consistent levels of residual moisture (Cozzolino et al. Citation2021a). The same research group also established that MIR and NIR spectroscopies could measure and monitor the effect of the oven and freeze-drying and the addition of maltodextrin as a carrier to the Kakadu plum puree (Cozzolino et al. Citation2021c). In quandong, the commercial drying processes reduced the total oxygen radical scavenging capacity of fresh fruit by 11.1% (Konczak and Roulle Citation2011) as well as, reduced the anthocyanins content of Illawarra plum (0.996 μmol CEs/mg DW), and native currant (0.074 μmol CEs/mg DW) which are otherwise rich in anthocyanins (Tan et al. Citation2011a). Thus, factors such as drying techniques, temperature, and duration should be considered for post-harvest processing of native fruit and vegetable samples to maintain desired polyphenolic and antioxidant contents in the final products.
From the current literature, it is apparent that studies thus far have mostly focused on native fruits with very limited information on vegetables. Green techniques (which require less time and solvent and are more economical compared to traditional Soxhlet extraction) such as accelerated solvent extraction has recently been employed to prepare Kakadu plum, Davidson’s plum, quandong, Tasmanian pepperberry and green plum extracts with different solvents including methanol, ethanol, acetone, hexane, and distilled water (). Furthermore, recovery of bioactive compounds from Kakadu plum using enzyme assisted extraction has been optimized using a mathematical prediction model and solvent (propylene glycol) concentration of 1.5% (w/w), enzyme (pectolytic enzymes) concentration of 300 mg/L and extraction time of 15 h was recommended for optimal recovery (yield 51.3%) of free ellagic acid from freeze-dried Kakadu plum puree (Chaliha et al. 2017). However, this extraction technique has not been explored for other native fruit and vegetable samples with most studies implementing ultrasonic extraction or mechanical stirring (). Stability studies on most Australian native fruits and vegetables are also limited with only limited data available regarding their chemical compositions in the current literature. Further studies developing robust, green, and economical extraction techniques to isolate bioactive phytochemicals from Australian native fruits and vegetables and their chemical characterization are fundamental to cognize their potential health benefits. Moreover, in vivo and clinical studies to understand the bioavailability, pharmacokinetics and potential toxicity of these phytochemicals are also crucial.
4. Bioactivity of Australian native fruits and vegetables
4.1. Anticancer activity
Cancer is one of the biggest disease burdens which contributed to 9.6 million deaths worldwide in 2018, and the death rate is predicted to continue to rise (Bray et al. Citation2018). The International Agency for Research on Cancer, World Health Organization (WHO) estimated that by 2040 the incidence of cancer cases in Australia alone is going to increase by 42% (World Health Organization Citation2020). Today, lung cancer is the most diagnosed cancer type, followed by breast cancer in females and prostate cancer in males (Bray et al. Citation2018). Many cancer patients suffer from severe adverse effects from treatments including chemotherapy, radiotherapy, and resection (Pearce et al. Citation2017).
Many plant-derived bioactive compounds have demonstrated chemo-preventive activity against induced tumors in experimental models and clinical studies (Choudhari et al. Citation2019; Maru et al. Citation2016). Investigating natural compounds from various sources, including plants, animals, and microorganisms as potential anticancer strategies either alone or in conjunction with standard treatment regimens is an emerging area of biomedical research that may improve the undesired side-effects of various cancer therapies (Majolo et al. Citation2019). Phytochemicals can mediate redox changes and enzymatic activity in the cells, resulting in modification of gene expression. Studies have shown that including antioxidant-rich fruits and vegetables in the diet can reduce the risk of intracellular oxidative stress-related chronic diseases and cancer (Block, Patterson, and Subar Citation1992; Jideani et al. Citation2021). It has been demonstrated that the synergistic interactions among the bioactive compounds in plants can be utilized for the prevention and treatment of diseases via different molecular targets (Niedzwiecki et al. Citation2016). However, it is often difficult to reproduce preclinical in vitro and in vivo findings in the clinical settings primarily due to the complexity of the human body and cancer itself; and partially due to potential toxicity and poor bioavailability of phytochemicals leading to their lower efficacy in humans (Maru et al. Citation2016). Previous epidemiological studies have suggested that dietary phytochemicals from fruits and vegetables can significantly reduce the occurrence of many cancers (Block, Patterson, and Subar Citation1992). Carcinogenesis is a multi-step process during which cancer cells acquire multiple somatic mutations that allow cells to sustain proliferative signaling (Hanahan and Weinberg Citation2011).
4.1.1. In vitro studies directly on Australian native fruits and vegetables
The in vitro studies described below demonstrate that phytochemicals in Australian native fruits and vegetables naturally act as antioxidants to reduce cellular oxidative stress, disrupt proliferative signaling, and induce the cell cycle arrest leading to apoptosis in a wide range of cancer cell lines ( and ).
Figure 2. Structures of betanin in seaberry saltbush (Rhagodia candolleana) and the pigface (Carpobrotus rossii) (Njume, McAinch, and Donkor Citation2020); garcinol in yellow mangosteen (Garcinia dulcis) (Deachathai et al. Citation2005, John et al. 2020).
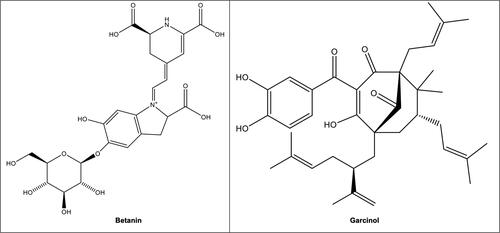
Table 4. Activity of Australian native fruits and vegetables against cancer cell lines.
Table 5. Australian native fruits and their interactions with gut microbial communities.
A study by Tan et al. (Citation2011a) examined the antiproliferative activity of polyphenols extracted from Illawarra plum, Kakadu plum, muntries, and native currant against a panel of cancer and normal cell lines containing AGS (gastric adenocarcinoma), HT-29 (colorectal adenocarcinoma), HL-60 (acute promyelocytic leukemia), CCD-18Co (colon normal), Hs 738.St/Int (mixed stomach and intestine normal) and peripheral blood mononuclear cells (PBMCs). The authors reported that each fruit selectively inhibited the growth of cancer cell lines in a dose-dependent manner with native currant exhibiting the lowest IC50 values against both the AGS (0.238 ± 0.027 mg/mL) and HT-29 (0.271 ± 0.076 mg/mL) cell lines. Furthermore, the Kakadu plum showed strong antiproliferative activity against the HT-29 with an IC50 value of 0.385 ± 0.036 mg/mL. All fruit extracts induced apoptosis (caspase-3 mediated) and DNA fragmentation in the HL-60 cells. In addition, Kakadu plum was found to activate the intrinsic apoptosis pathway via induction of caspase-7, −9, and poly (ADP-ribose) polymerase (PARP) in the HL-60 cells and cause direct DNA damage in the HT-29 cells. The IC50 values were in the range of 0.239 to > 2 mg/mL against the normal cell lines (CCD-18Co, Hs 738.St/Int and PBMCs). The observed antiproliferative activity of the fruit extracts against cancer cells was primarily ascribed to overall phenolic compounds and anthocyanins found in the extracts.
Another study by Joseph Sirdaarta et al. (Citation2016) evaluated the anti-proliferative activity of muntries, Illawarra plum, native tamarind, lemon aspen, desert lime, and bush tomato against the HeLa cervical adenocarcinoma and the CaCo2 colorectal adenocarcinoma cell lines. All the tested methanolic and chloroform extracts strongly inhibited the growth of the HeLa cells while only two of the aqueous extracts (muntries and Illawarra plum) significantly reduced the HeLa cell proliferation in a dose-dependent manner. Other seven extracts (aqueous: lemon aspen, desert lime, and bush tomato; ethyl acetate: Illawarra plum; hexane: muntries, Illawarra plum, and desert lime) displayed moderate HeLa cell growth inhibition in the study. Of the 30 fruit extracts tested, 22 displayed significant antiproliferative effects against the CaCo2 cells. All methanolic, aqueous (except for native tamarind), ethyl acetate (except for native tamarind and bush tomato), and chloroform extracts effectively inhibited the CaCo2 cell proliferation. Among the hexane extracts, only the lemon aspen inhibited the CaCo2 cell proliferation. Overall, the methanolic lemon aspen and desert lime extracts were most active against the HeLa and CaCo2 cells with the IC50 values in the range of 480–769 μg/mL. Furthermore, these fruit extracts displayed low to no toxicity in the Artemia franciscana nauplii lethality assays, indicating their therapeutic potential. The qualitative medium-pressure LC-MS quadrupole time-of-flight analysis of the methanolic and aqueous extracts of lemon aspen fruit putatively revealed 20 compounds with previously reported antioxidant and anticancer activity (Joseph Sirdaarta et al. Citation2016).
Mazerand and Cock (Citation2019) investigated green plum for its anti-proliferative activity against the Caco2 and HeLa human cancer cells. The methanol and aqueous extracts of green plum displayed potent inhibitory activity against the Caco2 cells with 80% and 72% inhibition, respectively compared to the untreated control. Similar values were also observed for the HeLa cells. The tested ethyl acetate, chloroform, and hexane were ineffective against the Caco2 cells, however, these extracts inhibited HeLa cell proliferation by 61%, 49%, and 51%, respectively. The antiproliferative activity of the fruit extracts might be attributed to the high levels of phenolics, flavonoids, and tannins as well as moderate levels of saponins reported in the study (Mazerand and Cock Citation2019).
A study by Symonds, Konczak, and Fenech (Citation2013) reported that anthocyanin-rich Illawarra plum extract inhibited HT-29 cell proliferation in a dose and time-dependent manner with the IC50 values of 4001, 1479, and 1318 μg/mL after 1, 24, and 48 h of treatment, respectively. However, the Illawarra plum extract did not show any activity against the normal mouse colonic epithelial YAMC cells but promoted their growth at the highest tested concentration of 1000 μg/mL (Symonds, Konczak, and Fenech Citation2013). The specificity of the drugs against abnormal cancer cells without affecting normal cells is a desirable trait in anticancer research, as many chemotherapy-related side effects are often caused by nonspecific drug-target interactions in the body. Additionally, Illawarra plum extract at 500 μg/mL after 24 h significantly decreased the cell population in the G0/G1 phase and increased the cell population in the S phase indicating a delay in the cell cycle progression, which is a desirable trait as some chemotherapeutic drugs are more effective when the cells reside in certain cell cycle phases (Mills, Kolb, and Sampson Citation2018). The Illawarra plum extract also altered the genomic expression characterized by decreased hTERT expression, increased SIRT1 expression, decreased TP53, down-regulation of telomerase activity, and decrease in telomeres length (Symonds, Konczak, and Fenech Citation2013). It also caused morphological alterations (formation of cytoplasmic vacuoles) of HT-29 cells suggesting the induction of starvation leading to autophagy, increased histone deacetylase (HDAC) activity, and class III HDAC sirtuin 1 expression. These findings indicated that the Illawarra plum extract may induce cell death of HT-29 via apoptotic pathways and possibly induced autophagy (Symonds, Konczak, and Fenech Citation2013). Overall, the anthocyanin-rich Illawarra plum inhibited cell proliferation by blocking signal transduction pathways, regulating gene expression, and inducing apoptosis. Previously, these events were shown to be triggered by the most common phenolic compounds including anthocyanins and flavanols (Jeong et al. Citation2009; Lin et al. Citation2017). However, the study by Symonds, Konczak, and Fenech (Citation2013) did not report the chemical profile of the Illawarra plum extract.
It should be noted that the choice of solvent for the extraction of phytochemicals from natural matrices can significantly influence the potency of the extracts against cancer cells. For instance, extracts of Kakadu plum fruit and leaf with methanol, sterile deionized water, ethyl acetate, chloroform, and hexane showed different activities against the Caco2, HeLa, Jeg-3 choriocarcinoma, JAR choriocarcinoma, MC3T3-E1 mouse preosteoblast, and MG63 human bone osteosarcoma cells (Shalom and Cock Citation2018). The leaf ethyl acetate extract was observed with the most potent activity against the MC3T3-E1 (IC50 = 6 µg/mL) and Caco2 cells (IC50 = 102 µg/mL), whereas the methanolic leaf extract inhibited the cell proliferation of Jeg-3 and MC3T3-E1 with the IC50 values of 147 and 40 µg/mL, respectively. The ethyl acetate, methanolic, and aqueous extracts of Kakadu plum fruit and leaf also induced apoptosis (as evident in morphological changes) in the Caco2 cells mediated via caspase 3 activity. In addition, the extracts displayed no or low toxicity in the Artemia franciscana bioassay and in a human dermal fibroblast (HDF) viability assay. The HPLC-MS QTOF analysis of the Kakadu plum leaf ethyl acetate extract putatively identified 18 compounds, dominated by tannins and flavonoids. The role of tannins and flavonoids in anticancer therapy has been emphasized previously (Teodor et al. Citation2020)
Jamieson, Sirdaartaa, and Cock (Citation2014) studied the correlation between antioxidant activity and anti-proliferative effects of riberry, brush cherry (Syzygium australe), Davidson’s plum, and blue quandong extracts (using different solvents and cold extraction at 4 °C) against the HeLa and CaCo2 cell lines. The authors reported that the antioxidant contents correlated with the anti-proliferative activity of the extracts against both the cancer cell lines. The aqueous brush cherry extracts exhibited the most potent activity with the IC50 values of 27 and 172 μg/mL against the CaCo2 and HeLa cells, respectively. The other tested fruit extracts displayed IC50 values in the ranges of 43 − 791 μg/mL against the CaCo2 and 58 − 884 μg/mL against the HeLa cells. The ethyl acetate extracts of the fruits displayed lower activity both in the antioxidant and cell growth inhibition assays in that study (Jamieson, Sirdaartaa, and Cock Citation2014). Qualitative analysis revealed that the methanol and water extracts of the fruits had moderate to high phenolics and flavonoids and low levels of saponins, triterpenoids, and tannins. Similarly, another study by Vuong, Hirun, Chuen, et al. (Citation2014) showed that the crude lily pilly fruit extract (200 μg/mL) significantly reduced the viability of the MiaPaCa-2 and ASPC-1 pancreatic cancer cells. These studies, however, did not report any molecular mechanisms of action of the fruit extracts against the cancer cell lines. Overall, these findings demonstrated that not only the type of phytochemicals but also extraction techniques and the choice of solvent play a key role in the potency and mechanisms of anticancer activity of Australian native fruit and vegetables.
4.1.2. In vivo studies performed on major phytochemicals also found in Australian native fruits and vegetables
The current literature does not report any direct in vivo studies exploring the anticancer activity of Australian native fruits and vegetables. However, the major phytochemicals present in these fruits and vegetables have been investigated in isolation against different in vivo models of cancer. This section underlines the key phytochemicals (that are also reported in Australian native fruits and vegetables) with potent in vivo anticancer activity.
The flavonol quercetin has been extensively studied against several cancer types in vivo and it is highly abundant in Illawarra plum (Symonds, Konczak, and Fenech Citation2013), Kakadu plum (Shalom and Cock Citation2018), and Davidson’s plum (Sakulnarmrat, Srzednicki, and Konczak Citation2014). Castillo et al. (Citation1989) investigated the antiproliferative effect of quercetin against HTB-43 (squamous cell carcinoma of pharyngeal origin) using immunocompetent rats and reported that it inhibited the growth of HTB-43 cells dose-dependently (Castillo et al. Citation1989). Caltagirone et al. (Citation2000) investigated the in vivo effect of quercetin on the growth and metastatic potential of the highly metastatic murine B16-BL6 melanoma in syngeneic mice and reported a nontoxic dose-dependent delay of tumor growth (Caltagirone et al. Citation2000). Furthermore, Zhou et al. (Citation2010) and Mouria et al. (Citation2002) demonstrated that quercetin exerted anti-proliferative and pro-apoptotic effects (Zhou et al. Citation2010), decreased primary tumor growth and prevented metastasis (Mouria et al. Citation2002) in a nude mouse model of pancreatic cancer.
The fruit and leaves of the Kakadu plum have shown very high levels of vitamin C (Gorman et al. Citation2020). Despite a few controversial opinions (Hoffer et al. Citation2008; Hoffer et al. Citation2015), there has been a growing scientific interest to investigate the therapeutic potential of vitamin C against cancer as demonstrated in several in vitro and in vivo studies (Reang et al. Citation2021). A study conducted by Park et al. (Citation2012) investigated the impact of combined treatment of vitamin C and paclitaxel using H1299 (a nonsmall cell lung cancer cell line) and BALB/c mice implanted with or without sarcoma 180 cancer cells. The findings of the study revealed that high doses of vitamin C could induce cell death in cancer cells, and when combined with paclitaxel it enhanced apoptosis of cancer cells. The combined treatment of vitamin C and paclitaxel could simultaneously ameliorate the side effects of the latter (Park et al. Citation2012). A similar type of combined approach was investigated by Kalita, Verma, and Prasad (Citation2014) that explored the modulatory effect of vitamin C on the efficacy of the anticancer drug chlorambucil by using Dalton’s ascites lymphoma in Swiss albino mice. Enhanced antitumor activity of the combined treatment was observed suggesting increased life span of mice as well as reduced histopathological changes in their kidneys, compared to the chlorambucil treatment alone (Kalita, Verma, and Prasad Citation2014).
Kakadu plum and Davidson’s plum are good sources of ellagic acid (Sakulnarmrat, Srzednicki, and Konczak Citation2015), with more than 70% of free ellagic acid in the Kakadu plum (Zeb Citation2018). Ellagic acid is another polyphenolic compound of the family ellagitannins and a dimeric derivative of gallic acid. Recently, hydrolyzable tannins that also include ellagitannins, epigallocatechin-3-gallate and gallotannins have gained significant interest due to their beneficial effects on human health, specifically their activity against cancer. An in vivo study conducted by Umesalma and Sudhandiran (Citation2010) revealed that administration of ellagic acid exerted a notable chemo-preventive activity in colon carcinoma induced male Wistar albino rats. Moreover, Aiyer and Gupta (Citation2010) showed that ellagic acid prevented estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism in vivo (Aiyer and Gupta Citation2010). Ceci et al. (Citation2016) confirmed the in vitro activity of ellagic acid by further in vivo studies depicting a significant growth reduction, and tumor-associated behavior of human bladder cancer xenografts.
4.2. Anti-inflammatory activity
The role of inflammation in the pathology of chronic diseases including rheumatoid arthritis, asthma, atherosclerosis, and neurodegenerative diseases is well-validated (Campbell Citation2004; Chen et al. Citation2018; Leyva-López et al. Citation2016). Additionally, inflammation also plays an integral part in cancer development and is defined as the seventh hallmark of carcinogenesis (Hanahan and Weinberg Citation2011). Hence, many anticancer agents often have anti-inflammatory activity. Australian native fruits and vegetables that have been reported in the literature with anti-inflammatory activity are shown in .
Figure 3. Anticancer activity of some Australian native fruits through induction of apoptosis. Muntries (Kunzea pomifera) and native currant (Antidesma erostre): ↑ caspase 3 mediated apoptosis in the HL-60 acute promyelocytic leukaemia cells (Tan, Konczak, Ramzan, et al. 2011a). Native tamarind (Diploglottis australis), desert lime (Citrus glauca), bush tomato (Solanum centrale) and lemon aspen (Acronychia acidula): ↑ caspase 3-mediated apoptosis by ↑ Bax in the HeLa cervical and the CaCo2 colorectal cancer cell lines (Joseph Sirdaarta et al. Citation2016). Illawarra plum (Podocarpus elatus): ↑ caspase 3-mediated apoptosis by ↑ SERT 1 [Sirtuin 1] expression which ↑ starvation-induced autophagy in the HeLa cervical and the CaCo2 colorectal cancer cells (Joseph Sirdaarta et al. 2016) and ↓ hTERT expression that affects cell telomere length and ↑ HDAC activity that silence the genes required for tumour. progression in the HT-29 human colorectal cancer cells (Symonds, Konczak, and Fenech 2013a). Kakadu plum (Terminalia ferdinandiana): ↑ caspase cascade (3, 7, 9) mediated apoptosis and activated of PARP [poly (ADP-ribose) polymerase] that induces intrinsic apoptotic pathways in the HL-60 acute promyelocytic leukaemia cells (Tan, Konczak, Ramzan, et al. 2011a) and the CaCo2 colorectal cancer cell lines (Shalom and Cock 2018a). Lemon aspen (Acronychia acidula): ↑ caspase cascade (3, 7, 8, 9) mediated apoptosis by ↑ DR4 and DR5 cell death receptors that facilitate the selective elimination of malignant cells through the ↑ apoptosis and ↓ the expression of PCNA (causing lethal DNA damage) and Cyclin D1 (inducing the cell-cycle arrest) in human lung cancer, pancreatic and HOS [Human osteo-sarcoma] carcinoma cell lines. ↑ of the p21 also inhibits the cell cycle progression (Joseph Sirdaarta et al. 2016)
.
![Figure 3. Anticancer activity of some Australian native fruits through induction of apoptosis. Muntries (Kunzea pomifera) and native currant (Antidesma erostre): ↑ caspase 3 mediated apoptosis in the HL-60 acute promyelocytic leukaemia cells (Tan, Konczak, Ramzan, et al. 2011a). Native tamarind (Diploglottis australis), desert lime (Citrus glauca), bush tomato (Solanum centrale) and lemon aspen (Acronychia acidula): ↑ caspase 3-mediated apoptosis by ↑ Bax in the HeLa cervical and the CaCo2 colorectal cancer cell lines (Joseph Sirdaarta et al. Citation2016). Illawarra plum (Podocarpus elatus): ↑ caspase 3-mediated apoptosis by ↑ SERT 1 [Sirtuin 1] expression which ↑ starvation-induced autophagy in the HeLa cervical and the CaCo2 colorectal cancer cells (Joseph Sirdaarta et al. 2016) and ↓ hTERT expression that affects cell telomere length and ↑ HDAC activity that silence the genes required for tumour. progression in the HT-29 human colorectal cancer cells (Symonds, Konczak, and Fenech 2013a). Kakadu plum (Terminalia ferdinandiana): ↑ caspase cascade (3, 7, 9) mediated apoptosis and activated of PARP [poly (ADP-ribose) polymerase] that induces intrinsic apoptotic pathways in the HL-60 acute promyelocytic leukaemia cells (Tan, Konczak, Ramzan, et al. 2011a) and the CaCo2 colorectal cancer cell lines (Shalom and Cock 2018a). Lemon aspen (Acronychia acidula): ↑ caspase cascade (3, 7, 8, 9) mediated apoptosis by ↑ DR4 and DR5 cell death receptors that facilitate the selective elimination of malignant cells through the ↑ apoptosis and ↓ the expression of PCNA (causing lethal DNA damage) and Cyclin D1 (inducing the cell-cycle arrest) in human lung cancer, pancreatic and HOS [Human osteo-sarcoma] carcinoma cell lines. ↑ of the p21 also inhibits the cell cycle progression (Joseph Sirdaarta et al. 2016).](/cms/asset/6a86a9a1-9cec-42a1-9482-6fdf6366ee67/bfsn_a_2057913_f0003_c.jpg)
4.2.1. In vitro studies directly on Australian native fruits and vegetables
A study by Tan et al. (Citation2011b) demonstrated that polyphenol-rich Kakadu Plum and muntries extracts protected the RAW 264.7 murine macrophages against H2O2-induced apoptosis in a dose-dependent manner. The same research group showed that the polyphenolic extracts of Illawarra plum and Kakadu plum suppressed two inflammatory enzymes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in a dose-dependent manner in the lipopolysaccharide (LPS)-activated murine RAW 264.7 macrophages (Tan, Hou, et al. Citation2011). Kakadu plum was also able to inhibit the activity of COX-1 at the highest tested concentration of 400 μg/mL whilst the native currant extract was only effective against iNOS (Tan, Hou, et al. Citation2011). Moreover, the products of these enzymes, nitric oxide (NO) and prostaglandin E2 (PGE2) were reduced primarily by Kakadu plum and muntries with native currant being the least effective. Similarly, Kakadu plum extract rich in ellagic acid showed significant anti-inflammatory and antioxidant activity in the Caco-2 and KERTr cell lines exposed to H2O2 by reducing ROS production, upregulating superoxide dismutase (SOD)-2 and downregulating iNOS, soluble cell adhesion molecule (sICAM), and COX-2 with no effects on COX-1 (Chaliha and Sultanbawa Citation2019).
Inhibition of COX-1 and COX-2 highlights the significance of those native fruit extracts in tumor progression and metastasis. Both forms of COX enzymes are expressed in various tissues and dysregulated in many cancer types (Morita Citation2002; Greenhough et al. Citation2009). A fraction of Kakadu plum extract showed upregulation of the nuclear erythroid-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) in the Hep G2 cells postulating an activation of p44/42 mitogen-activated protein kinase (MAPK) signaling pathway (Tan, Konczak, et al. Citation2011). The Nrf2/Keap1 signaling pathway regulates redox balance and protects the cell from oxidative stress. However, further studies by the same group clarified that the inhibition mechanism was mediated by nuclear factor-kappa B (NF-κB) upstream signaling pathway by inhibiting phosphorylation and degradation of inhibitor of kappa B-alpha (IкBα) (Tan, Hou, et al. Citation2011).
Queen Garnet plum (Prunus salicina) rich in anthocyanin- cyanidin 3-glucoside (C3G; up to 272 mg/100 g of fresh fruit; seven times higher than other anthocyanin-containing plums), has been found to attenuate inflammatory bowel disease (IBS) symptoms induced in rat models (Ghattamaneni, Panchal, and Brown Citation2019). Queen Garnet plum is a Japanese plum variety developed by the Department of Agriculture and Fisheries, Queensland Government, Australia using standard breeding techniques to contain a higher level of anthocyanins than other plum varieties (Fanning et al. Citation2014). Sivasinprasasn et al. (Citation2016) reported that the C3G attenuates angiotensin II-induced inflammation and oxidative stress in the human vascular endothelial EA.hy926cells in a dose-dependent manner. The authors demonstrated that C3G inhibited the NF-κB signaling pathway through the protection of IκB-α, inhibition of NF-κB expression and translocation into the nucleus, downregulation of NF-κB p65 and decreasing iNOS expression. In addition, pretreatment with C3G upregulated the endogenous antioxidant enzymes and promoted the activity of the Nrf2 signaling pathway (Sivasinprasasn et al. Citation2016).
4.2.2. In vivo studies performed on major phytochemicals also found in Australian native fruits and vegetables
Dietary flavonols (commonly found in Australian native fruits) such as catechin and quercetin in their pure forms alone or in combination were shown to reduce adipose inflammation in vivo through reducing phosphorylation of the MAPKs, c-Jun N-terminal kinase, and p38 (Vazquez Prieto et al. Citation2015). Kleemann et al. (Citation2011) investigated the anti-inflammatory activity of quercetin (also found abundantly in Illawarra plum, Kakadu plum and Davidson’s plum) both in vitro and in vivo using cultured human cells and human CRP transgenic mice, a humanized inflammation model in which CRP expression can be induced by cytokines such as IL-1β. In the in vivo model, quercetin quenched IL-1β-induced CRP expression, similar to sodium salicylate- the reference control (Kleemann et al. Citation2011).
The general role of ascorbic acid as a promising antioxidant implies that it could lead to the depletion of conditions associated with chronic inflammation and oxidative stress. For instance, Jeong et al. (Citation2014) evaluated the in vivo effect of ascorbic acid-treated mouse bone marrow-derived dendritic cells on CD8+ T cell differentiation using male C57BL/6 and BALB/mice between seven and nine weeks of age. The results of in vivo administration yielded a higher frequency of CD8+ effector and effector memory T cells, which showed an increased killing effect of tumor cells ex vivo (Jeong et al. Citation2014). Similarly, ellagic acid has also exhibited potent anti-inflammatory activity in both in vitro and in vivo models. Mansouri et al. (Citation2015) reported the molecular mechanism of action of ellagic acid in mediating anti-inflammatory activity in vivo. The authors hypothesized that ellagic acid might decrease the level of MDA, iNOS, and COX-2 in the edema paw via the suppression of pro-inflammatory cytokines (TNF-α and IL-1β), NO and PGE2 overproduction using a carrageenan-induced mouse paw edema model (Mansouri et al. Citation2015).
4.3. Antidiabetic activity and the effects on metabolic syndrome
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder. It is characterized by hyperglycemia and insulin resistance and is one of the major threats to global public health in the 21st century which has reached epidemic proportions with the worldwide prevalence approaching 400 million people (Nathan Citation2015). It is the fourth or fifth leading cause of death in developed countries and an increasing trajectory in newly industrialized economies (Hu Citation2011). Metabolic syndrome is a collection of risk factors such as dyslipidaemia, insulin resistance, central obesity, and hypertension that together may result in an increased risk of T2DM (Eckel, Grundy, and Zimmet Citation2005).
4.3.1. Studies directly on Australian native fruits and vegetables
Australian fruits and vegetables may offer promising prospects for the isolation and identification of novel agents for the treatment of metabolic syndrome and diabetes. The Australian native fruits and vegetables studied to date for their anti-diabetic activity and the effects on metabolic syndrome are- Davidson’s plum, quandong, lilly pilly, quinine bush (Petalostigma banksii), yellow mangosteen (Garcinia dulcis), anise myrtle and Tasmanian pepperberry.
An in vivo study on high-carbohydrate and high-fat diet rats demonstrated the potential of Davidson’s plum to ameliorate the symptoms of metabolic syndrome. The fruit decreased abdominal fat and adipose cells size, reduced the level of triglycerides, and improved liver function (John, Mouatt, Majzoub, et al. Citation2019). Davidson’s plum was found to alleviate metabolic syndrome symptoms across multiple body systems, including the heart, liver, and bone (John, Mouatt, Majzoub, et al. Citation2019). It was anticipated that anthocyanins were the primary constituents responsible for this activity, however, it was also emphasized that additional polyphenols, such as quercetin, may act synergistically with anthocyanins to produce the observed changes to the body systems (John, Mouatt, Majzoub, et al. Citation2019). That study utilized a rat model fed with a high-fat and high-carbohydrate diet demonstrating symptoms associated with metabolic syndrome, including disturbances to cardiovascular and liver functions established in a previous study (Panchal et al. Citation2011). In an in vitro study, the phenolic-rich extract of Davidson’s plum with high antioxidant capacity effectively inhibited the activities of angiotensin-converting enzyme and α-glucosidase (Sakulnarmrat, Srzednicki, and Konczak Citation2014). The results of these studies demonstrated the potential of Davidson’s plum to treat metabolic syndrome, however, more studies are required for a better understanding of its mechanisms of action and effectiveness.
An in vitro study showed that the polyphenolic-rich fraction of quandong is an effective inhibitor of the pancreatic lipase (Sakulnarmrat, Srzednicki, and Konczak Citation2014). Pancreatic lipase is one of the main digestive enzymes that break down dietary carbohydrates and lipids in the human digestive system. Therefore, inhibition of pancreatic lipase is an attractive approach for the treatment of high blood sugar and obesity (Buchholz and Melzig Citation2016). Further studies are required to investigate its antidiabetic activities.
Antihyperglycemic, antihyperlipidemic and antioxidant activities of the oral administration of the aqueous extract of lilly pilly fruit (100 mg/kg) have recently been evaluated in streptozotocin-induced diabetic rats (Konda et al. Citation2019). The results showed that the fruit extract has a potential effect on oxidative stress, glycemic control, and dyslipidaemia. It improved insulin secretion via β-cell restoration capacity (Konda et al. Citation2019). The leaves of the plant were also suggested to have potential anti-diabetic properties. In an in vitro study, different extracts of the leaves of lilly pilly showed inhibitory effects against α-amylase and α-glucosidase. The leaf extract also exhibited antiglycation activities (Kim et al. Citation2020). Protein glycation is a result of prolonged hyperglycemia and plays a significant role in the pathogenesis of diabetic complications. Reducing the formation of advanced glycation end-products (AGEs) can reduce the development of diabetic complications (Ahmed Citation2005).
The fruit of the quinine bush is rich in phenolic compounds and has shown a high antioxidant capacity in the FRAP and DPPH assays (Deo et al. Citation2016). In an in vitro study, the 80% v/v aqueous ethanol extract of the quinine bush fruit showed inhibitory activities against protein glycation, pancreatic α-amylase and α-glucosidase (Deo et al. Citation2016). The inhibition of these enzymes significantly prevented the postprandial increase of blood glucose levels and is a key approach for the management of hyperglycemia (Tundis, Loizzo, and Menichini Citation2010).
Yellow mangosteen usually grows in the rainforests of north Queensland, Australia, but is also found around South East Queensland (John, Mouatt, Majzoub, et al. Citation2019). The fruits contain some major bioactive compounds such as garcinol, camboginol, and morelloflavone (Deachathai et al. Citation2005; John, Mouatt, Majzoub, et al. Citation2019). Garcinol () has been shown with antioxidant, anti-inflammatory, and anti-diabetic properties (John, Mouatt, Majzoub, et al. Citation2019). Morelloflavone showed hypocholesterolaemic activity via inhibiting HMG-CoA reductase (Tuansulong et al. Citation2011) with potential anti-atherogenic properties (Pinkaew et al. Citation2009). Camboginol and morelloflavone exhibited strong antioxidant effects in both nonmetal induced human low-density lipoprotein (LDL) and Fe2+-mediated oxidation (Hutadilok-Towatana, Kongkachuay, and Mahabusarakam Citation2007). The fruit rind powder of yellow mangosteen was evaluated on a rat model of human metabolic syndrome (John, Mouatt, Majzoub, et al. Citation2019). The results demonstrated that the fruit rind can reduce obesity, hypertension, and fatty liver. It can also improve liver and cardiovascular structure and function (John, Mouatt, Majzoub, et al. Citation2019). Considering the above potential activities, yellow mangosteen and its bioactive compounds can further be investigated for attenuating metabolic syndrome through more rigorous studies.
Figure 4. Anti-inflammatory activity of Australian native fruits and their constituents mediated through different molecular pathways of oxidative stress. Kakadu plum (Terminalia ferdinandiana): ↓ inflammatory enzymes- cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), ↓ nitric oxide (NO) and Prostaglandin E2 (PGE2) (Tan, Hou, et al. Citation2011), ↑ the nuclear erythroidrelated factor 2 (Nrf2)/ Kelch-like ECH-associated protein 1 (Keap1) (Tan, Konczak, Ramzan, Zabaras, et al. 2011), inhibited phosphorylation and degradation of inhibitor of kappa B-alpha (IкBα) (Tan, Hou, et al. Citation2011). Illawarra plum (Podocarpus elatus): ↓ inflammatory enzymes- cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Tan, Hou, et al. Citation2011). Cyanidin 3-glucoside from Queen Garnet plum (Prunus salicina): Inhibited the NF-κB signalling pathway through the protection of IκBα, ↓ NF-κB and its translocation into the nucleus, ↓ NF-κB p65 and ↓ iNOS, ↑ endogenous antioxidant enzymes and ↑ Nrf2 signalling pathway activity.
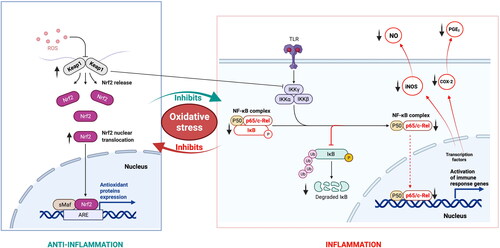
It has been observed that the anise myrtle plant contributed to the modulation of diabetic neuropathy through the regulation of digestive enzymes that are linked to the T2DM (Deo et al. Citation2016). Previous studies identified that anise myrtle increased α-glucosidase inhibition, however, had a minimal effect on the lipase (Sakulnarmrat and Konczak Citation2012). Comparatively, the Tasmanian pepperberry inhibited both α-glucosidase and pancreatic lipase to a significant extent implying an association between total phenolic content and enzyme-inhibitory activities of the assessed plant extracts (Sakulnarmrat and Konczak Citation2012). However, conflicting findings were reported by Deo et al. (Citation2016).
Geraghty et al. (Citation2011) demonstrated the in vitro antioxidant, antiplatelet, and anti-inflammatory activity of pigface extract in peripheral blood mononuclear cells. Furthermore, many Australian native fruits and vegetables including Illawarra plum, Kakadu plum, and bush tomato were reported to have high antioxidant and phenolic contents. Although there are no direct reports on these fruits and vegetables related to their effects on T2DM and metabolic syndrome, several studies have demonstrated that polyphenols can interfere with enzymatic activities of α-glucosidase and α-amylase in glucose metabolism. The inhibition of these key enzymes of glucose metabolism can reduce the absorbance of carbohydrates from the gut and ultimately reduce the blood glucose (McDougall et al. Citation2005). Similarly, as aforementioned in section 4.2, Illawarra plum and Kakadu plum have been linked with anti-inflammatory activity in murine macrophages through inhibition of nitric oxide, PGE2, and COX-2 (Njume, Donkor, and McAinch Citation2019; Tan, Hou, et al. Citation2011; Tan, Konczak, et al. Citation2011). Pre-clinical studies have shown that the antioxidant and anti-inflammatory effects of polyphenols can protect against T2DM (Njume, Donkor, and McAinch Citation2019). Therefore, more research is warranted to understand the effects of these fruits and vegetables on T2DM and metabolic syndrome.
4.3.2. Studies performed on major phytochemicals also found in Australian native fruits and vegetables
Quercetin has shown antidiabetic properties in vitro and in vivo and has gained popularity as a natural therapy for diabetes and its complications. Streptozotocin (STZ)-, alloxan-, and STZ- nicotinamide- induced rat models were assessed against oral administration of various doses of quercetin with doses ranging from 15–100 mg/kg for 14–70 days, which resulted in a significant reduction in blood glucose via pancreatic islet regeneration, serum insulin level elevation and enhanced insulin release (Shi et al. Citation2019). As aforementioned, ellagic acid is found in many Australian native fruits and has shown significant antidiabetic activity in an in vivo study carried out by Jadhav and Puchchakayala (Citation2012) using normoglycemic and STZ- nicotinamide induced diabetic rats. In normal rats administered with ellagic acid, the maximum reduction in the blood glucose level occurred at 2 h indicating that the mechanism of action may be by increasing the peripheral utilization of glucose and inhibiting the glucose transporter activity from the intestine (Jadhav and Puchchakayala Citation2012). Likewise, in STZ- nicotinamide induced diabetic rats, ellagic acid significantly (p < 0.001) reduced fasting blood glucose levels compared to the control group.
4.4. Antimicrobial activity
The development of multidrug resistance in pathogens against standard antimicrobial agents is one of the biggest concerns of medical science today. Antimicrobial compounds derived from natural sources have demonstrated great potential in the fight against multidrug-resistant bacterial and fungal strains (Hayashi, Bizerra, and Da Silva Citation2013). Combining natural products with standard antimicrobial agents to act on different pathways or molecular targets can be an effective strategy to combat the multidrug resistance (Fischbach Citation2011). Australian native fruit and vegetables with their diverse and unique chemical compositions can be potential sources to discover novel antimicrobial leads. In addition, these edible antimicrobial agents can also be used as preservatives to extend the shelf-life of food products.
In addition to the anti-inflammatory properties, certain species of Australian native fruits and vegetables have been investigated for their antimicrobial activity in multiple studies. Recently, Mani et al. (Citation2021) reviewed the therapeutic potential of Australian endemic plants and highlighted the antimicrobial activity of edible Kakadu plum () and Syzygium species. Sirdaarta et al. (Citation2015) reported that the methanol and ethyl acetate extracts of Kakadu plum displayed antibacterial activity against both reference and clinical strains of Acinetobacter baylyi and Pseudomonas aeruginosa with MIC values of >1000 µg/mL in the disk diffusion assay. The extracts exhibited no toxicity in the A. franciscana assay and several monoterpenoids and flavonoids were detected in the ethyl acetate extract using GC-MS (Sirdaarta et al. Citation2015). Furthermore, Mitchell Henry et al. (Citation2016) evaluated the antibacterial activity of different solvent extracts of Kakadu plum against Bacillus anthracis (the pathogen responsible for anthrax) using the disk diffusion method and determined the chemical composition of the most potent extracts using non-targeted GC-MS headspace analysis. The study reported that Kakadu plum fruit ethyl acetate and leaf methanolic extracts exhibited greater activity compared to other extracts with the MIC values of 451 and 377 μg/mL, respectively against B. anthracis. The MIC values of the extracts were in the range of 377 - >10,000 μg/mL. Additionally, the extracts did not exhibit any significant toxicity in the A. franciscana assay, with the LC50 values >1000 μg/mL. The fruit ethyl acetate and leaf methanolic extracts putatively identified several compounds which might be responsible for the observed antibacterial activity against B. anthracis. Recently, Cheesman et al. (Citation2019) also analyzed the ability of Kakadu plum fruit and leaf extracts in inhibiting the growth of β-lactam-sensitive and -resistant Escherichia coli and Methicillin-Resistant Staphylococcus aureus (MRSA) using disk diffusion liquid dilution techniques. The methanolic extract of the fruit and methanolic, aqueous, and ethyl acetate extracts of the leaf inhibited the growth of MRSA with the lowest MICs value of 223 µg/mL. However, the extracts were unable to inhibit the growth of β-lactam-resistant E. coli. The metabolomic fingerprint of the extracts revealed the abundance of tannins, flavonoids, and terpenoids, many of which have been shown to inhibit MRSA growth in previous studies. The extracts did not exhibit any toxicity in the A. franciscana and the HDF toxicity assays. Kakadu plum leaf extracts (aqueous and methanolic) inhibited the growth of S. aureus and the observed antibacterial activity was positively correlated with the total ascorbic acid and total phenolic content of the extracts (Chaliha et al. Citation2020). Another study by the same research group revealed that the extracts from Kakadu plum fruit and leaves showed greater activity compared to seedcoat and bark extracts against Listeria monocytogenes, Bacillus cereus, MRSA, P. aeruginosa clinical isolates (Akter, Netzel, et al. Citation2019). The authors attributed the observed antibacterial activity potentially to the presence of significant antioxidant and tannin contents in the Kakadu plum fruit and leaf extracts (Akter, Netzel, et al. Citation2019). Kakadu plum fruit and leaf extracts were also tested against malodour‐producing bacteria Brevibacterium linens (ATCC9172), Corynebacterium jeikeium (ATCC43734), and Propionibacterium acnes (ATCC6919), and Staphylococcus epidermidis using disk diffusion and microdilution methods (McManus et al. Citation2017). In that study, the Kakadu plum leaf methanolic extract showed greater activity compared to the tested fruit extracts with lower MIC values against C. jeikeium (233 μg/mL), S. epidermidis (220 μg/mL), P. acnes (625 μg/mL) and B. linens (523 μg/mL) with no toxicity in the A. franciscana assay. The observed activity might be attributed to the abundance of diverse tannins and the flavone luteolin in the most potent methanolic leaf extract as revealed by the untargeted HPLC-MS-TOF analysis (McManus et al. Citation2017). The same research group also previously assessed the antibacterial activity of other Australian Terminalia species- T. carpentariae (wild peach) and T. grandiflora (also known as yalu, plumwood or nutwood) against B. anthracis (Henry et al. Citation2016). The study utilized water, methanol, chloroform, hexane, and ethyl acetate to extract the plant materials and reported that the methanolic wild peach leaf and yalu nut extracts showed good antibacterial activity the MIC values of 74 and 155 µg/mL, respectively against B. anthracis. The potent wild peach leaf extracts were found to be nontoxic in the A. fransiscana assay (LC50 values >1000 µg/mL). Several compounds were putatively identified and highlighted in the potent extracts which might be responsible for the observed antibacterial activity (Henry et al. Citation2016). Another recent study on wild peach (T. carpentariae) showed that methanolic (80%) extract inhibited the growth of foodborne pathogens including S. aureus, MRSA, P. aeruginosa and Shewanella putrefaciens (Zhang et al. Citation2022). Furthermore, the methanolic extract was found to contain organic acids (ascorbic acid and gallic acid), gallotannins, ellagitannins, favone and favone derivatives and the observed antibacterial activity was mostly attributed to ellagitannins (Zhang et al. Citation2022).
Figure 5. Antimicrobial activity of two most investigated Australian native fruits – Kakadu plum (Terminalia ferdinandiana) and Tasmanian Pepperberry (Tasmannia lanceolata) (Sirdaarta et al. Citation2015; Mitchell Henry et al. Citation2016; Cheesman et al. Citation2019; McManus et al. Citation2017; Winnett et al. Citation2014). These two fruits showed no toxicity in the Artemia franciscana (brine shrimp) assay.
Syzygium species are commonly known as creek lilly pilly, brush cherry or satinash with 52 species found in Australia (Wrigley, Walter, and Fagg Citation2013). Despite their extensive medicinal use in other parts of the world, Australian Syzygium species have not been explored adequately (Mani et al. Citation2021). A few studies have reported the antibacterial and antifungal properties of Australian Syzygium species (Guleria et al. Citation2013; Murhekar et al. Citation2017; Mani et al. Citation2021). For instance, Murhekar et al. (Citation2017) evaluated the activity of S. australe (brush cherry) and S. luehmannii fruit and leaves extracts against the fish spoilage bacteria- S. putrefaciens strain 200, Shewanella baltica strain OS155, Shewanella frigidimarina strain NCIMB 400 and Shewanella loihica strain PV-4 using both disk diffusion and microdilution techniques. The methanolic S. australe fruit extract showed greater activity compared to other extracts against all Shewanella spp. with the MIC values in the range of 87–640 µg/mL and an LC50 value > 1000 µg/mL in the A. fransiscana assay. The aqueous and ethyl acetate S. australe fruit extracts were also potent against Shewanella spp. growth, although with higher MIC values. The antibacterial activity observed in this study might be attributed to the high antioxidant contents of the Australian Syzygium extracts, however, the specific phytochemicals responsible for the observed antibacterial activity were not reported. Wood et al. (Citation2017) reported that overall, the S. australe extracts were better inhibitors of B. linens and C. jeikeium growth compared to the S. luehmannii extracts in the disk diffusion assay. The ethyl acetate extracts of S. australe fruit and leaf displayed the MIC values of 300 and 857 μg/mL, respectively, against B. linens, and 1000 and 311 μg/mL against C. jeikeium. The ethyl acetate extracts of S. luehmannii fruit showed moderate activity against B. linens (MIC = 571 μg/mL) and C. jeikeium (MIC = 203 μg/mL). The most potent ethyl acetate extracts did not exhibit any toxicity in the A. franciscana assay (Wood et al. Citation2017). Similar to previous studies on Australian Syzygium extracts, this study did not quantify the phytochemicals present in the tested S. australe and S. luehmannii extracts. Previous in vitro studies have also observed similar antimicrobial activity of fruit and leaves extracts of Syzygium species including S. australe, S. leuhmannii, S. forte, S. francissi, S. moorei, S. puberulum and S. wilsonii against a number of bacterial and fungal pathogens by using disk diffusion technique (Sautron and Cock Citation2014; Chikowe, Mpala, and Cock Citation2013; Cock Citation2012).
Tasmanian pepperberry has been demonstrated to have no toxicity in the A. franciscana assay () (Winnett et al. Citation2014). An in vitro study by Winnett et al. (Citation2014) evaluated the antibacterial and antifungal activity of fruits, leaves and peppercorn extracts of Tasmanian pepperberry against Acinetobacter baylyi, Aeromonas hydrophila, Alcaligenes feacalis, B. cereus, Citrobacter freundii, Enterobacter aerogenes, Enterococcus faecalis, E. coli, Klebsiella pneumoniae, Proteus mirabilis, P. aeuroginosa, Pseudomonas fluorescens, Salmonella newport, Serratia marcescens, Shigella sonnei, S. aureus, S. epidermidis, Streptococcus pyogenes, Aspergillus niger, Candida albicans, Penicillium chrysogenum and Saccharomyces cerevisiae using disk diffusion method. The authors found that all extracts of Tasmanian pepperberry (methanolic, aqueous, and ethyl acetate) inhibited the growth of the tested Gram-negative and Gram-positive bacterial strains (Winnett et al. Citation2014) with the fruit methanolic extract exhibiting the highest antibacterial activity followed by fruit aqueous and fruit ethyl acetate extracts (). The MIC values were in the range of 4.8 − 7675 µg/mL against the tested bacterial strains. In contrast, only the Tasmanian pepperberry peppercorn extracts were moderately effective against C. albicans and S. cerevisiae with MIC values in the range of 180 − 316 µg/mL (Winnett et al. Citation2014). The leaf extracts of Tasmanian pepperberry also showed antibacterial and antifungal activity against Dekkera anomala, Schizosaccharomyces pombe, S. cerevisiae, C.albicans, Rhodotorula mucilaginosa, Candida krusei, S. aureus and E. coli in disk diffusion method (2 mg/6 mm disk) with hexane extract exhibiting a greater activity than the alcohol (ethanolic and methanolic) extracts (Alderees et al. Citation2018). The authors identified polygodial as the major compound in the Tasmanian pepperberry (Alderees et al. Citation2018). Furthermore, the same study demonstrated the antibacterial and antifungal activity of lemon myrtle and anise myrtle (Alderees et al. Citation2018).
Similar to Tasmanian pepperberry, Tasmannia stipitate commonly known as Dorrigo pepper, is a native Australian pepper that has also been used extensively by Indigenous Australians in cooking and Bush medicine (Hart et al. Citation2014) and becoming increasingly popular in modern Australian cuisine. A study by Hart et al. (Citation2014) evaluated the antibacterial, antifungal, and anti-Giardia activity as well as toxicity of different extracts (methanol, aqueous, and ethyl acetate) of Dorrigo pepper fruit and leaves. The study used microbial strains such as A. baylyi, A. hydrophila, A. feacalis, B. cereus, C. freundii, E. aerogenes, E. faecalis, E. coli, K. pneumoniae, P. mirabilis, Proteus vulgaris, P. aeruginosa, P. fluorescens, S. newport, S. marcescens, S. sonnei, S. aureus, S. epidermidis, S. pyogenes, A. niger, C. albicans, P. chrysogenum, S. cerevisiae, and Giardia duodenalis and implemented disk diffusion (for bacterial and fungal pathogens) and MTS cell proliferation (for G. duodenalis) assays. Similar to the study by Winnett et al. (Citation2014) on Tasmanian pepperberry, the fruit methanolic extract of Dorrigo pepper exhibited a greater antibacterial (22 of the 23 bacteria tested) and antifungal (against C. albicans and S. cerevisiae) activity compared to the other extracts, however, overall it displayed moderate to low antimicrobial activity. The extracts were able to inhibit Gram-negative bacteria more effectively than Gram-positive bacteria and fungi. Additionally, all fruit and leaf extracts of Dorrigo pepper showed significant anti-Giardia activity with no toxicity in the A. fransiscana assay (LC50 > 1000 µg/mL).
Mpala, Chikowe, and Cock (Citation2020) analyzed the antibacterial and antifungal activity of Davidson’s plum fruit and leaf methanolic and aqueous extracts against E. coli (ATCC157293), K. pneumoniae (ATCC31488), Proteus mirabilis (ATCC21721) and S. pyogenes (ATCC19615), Aeromonas hydrophilia, Alcaligenes feacalis, Aspergillus niger, B. cereus, C. albicans, Citrobacter freundii, P. fluorescens, S. cerviseae, Salmonella newport, Serratia marcescens, Shigella sonneii, S. aureus and S. epidermidis using disk diffusion method. Despite the high antioxidant activity, the extracts were inactive against the tested bacterial and fungal strains. Previous in vitro and in vivo studies have found that anthocyanins exhibit a diverse range of biological functions, including antimicrobial properties (Mazza Citation2005). As Davidson’s plum is rich in anthocyanin content, further investigation into other parts of the plant including flowers and bark with different extraction solvents and techniques can be performed to identify any potential antimicrobial activity. Other native fruit such as green plum flesh also exhibited antimicrobial properties against Gram-positive bacteria (Fyfe et al. Citation2018a).
These findings support the use of Australian native fruit and vegetables as the sources of natural food preservatives and antimicrobial compounds (Hart et al. Citation2014). However, further investigation is warranted to assess their antimicrobial activity against a wider range of microbial strains. The disk diffusion method used in most of these studies to evaluate antimicrobial activity has several drawbacks as this method cannot distinguish between bacteriostatic and bactericidal effects and it is impossible to quantify the amount of the antimicrobial agent diffused into the agar medium (Balouiri, Sadiki, and Ibnsouda Citation2016). Therefore, future studies should implement more sensitive methods such as microdilution, fluorescence-based assay, ATP bioluminescence, and flow cytometry to screen the antimicrobial activity of Australian native fruit and vegetable extracts. Similarly, studies to evaluate the molecular mechanisms of the antimicrobial activity of Australian fruits and vegetables are also necessary. Additionally, any potential synergistic interactions of extracts and/or bioactive compounds isolated from native fruit and vegetables with standard antimicrobial drugs should also be investigated using synergy models such as the fractional inhibitory concentration index and the combination index in future studies.
4.5. Interactions with gut microbiota and prebiotic activity
The close interactions between native Australian fruit and vegetable extracts and gut microbial communities have gained popularity in the scientific community in recent years (). Studies on animal models have observed that carbohydrate- and fat-rich diets disturb the gut microbial composition through a reduced concentration of the Bacteroidia species and an increase in the presence of Clostridia species (John, Mouatt, Majzoub, et al. Citation2019). Supplementation of yellow mangosteen was observed to attenuate these alterations to the gut microbiome in rats and effectively reduce the risk of metabolic syndrome and other inflammatory processes as a result of these changes (John, Mouatt, Majzoub, et al. Citation2019). Garcinol, a polyisoprenylated benzophenone metabolite, isolated from yellow mangosteen has also been observed as a potential gut microbiota modulator in the prevention of gut dysbiosis constituted by high-fat diets in mice (Lee et al. Citation2019). A study identified that the garcinol extract exhibits modulatory activity through an increase in the anti-obesity commensal Akkermansia bacterial population, which limits the dysbiosis caused by a high-fat diet through the administration of the native plant (Lee et al. Citation2019). A more recent study in obese rats acknowledged that decreased infiltration of inflammatory cells which decreased physiological, metabolic, liver and cardiovascular symptoms were conferred by the high concentrations of bioactive phenolic compounds found in the rind of purple mangosteen (Garcinia mangostana, a fruit native to Asia, Africa, Australia and Polynesia) (John et al. Citation2021). As such, the administration of garcinol may serve as a potential regulatory treatment in the maintenance of gut homeostasis.
The interplay between gut microbial communities and native plant species has also been investigated with the Australian fruit Davidson’s plum. This native fruit is rich in anthocyanin, which also exerted anti-obesity effects in rats via the regulation of the gut microbiome composition (John, Mouatt, Majzoub, et al. Citation2019). The findings elucidated that anthocyanin extracts led to increased growth of Akkermansia muciniphila, which has a positive influence on the gut microbiome through improved metabolic outcomes, such as a reduction in the gut and systemic inflammation, and an increase in gut mucin secretion in rats (John, Mouatt, Majzoub, et al. Citation2019). The therapeutic activity of anthocyanins was further supported by a study that examined the effects of anthocyanins extracted from Lycium ruthenicum plant (a native from the Central Asian region) on host health and identified that this polyphenolic compound exhibited modulatory action on gut microbial species, including an increase in short-chain fatty acids concentration (Peng et al. Citation2020). The observations from that study indicated that despite differences in the geographical location of Davidson’s plum and L. ruthenicum species, the anthocyanin extracts from each plant exhibited similar activity on gut microbiota. Additionally, Davidson’s plum, an edible source of ellagitannins, has been observed to possess antioxidant activity within the host organism in vivo (Williams et al. Citation2014). Ellagitannins are metabolized by the gut microbiota within the colon to produce bioactive urolithins to be absorbed by the body (Williams et al. Citation2014). As aforementioned, C3G can attenuate angiotensin II-induced inflammation and oxidative stress in human vascular endothelial cells (EA.hy926), which is a promising finding for the use of C3G as a prebiotic dietary supplement for the inhibition of inflammatory processes (Sivasinprasasn et al. Citation2016). However, studies have acknowledged that concentrations of anthocyanin extracted from Queen Garnet plum did not express significant effects on gut microbial communities, despite C3G attenuating inflammatory bowel disease symptoms in rat models (Ghattamaneni, Panchal, and Brown Citation2019; Igwe et al. Citation2020). In considering the available evidence, whilst certain anthocyanin variants express modulatory activity on gut-related processes, such as inflammation, other variants have a direct association with the gut microorganisms in the regulation of host health ().
5. Conclusion and future perspectives
As evident from the current literature, most Australian native fruits are rich in bioactive molecules and antioxidants and highly nutritious. For instance, native plum species such as Kakadu plum, Illawarra plum, and Davidson’s plum have shown significantly higher antioxidant content compared to most Western fruits in many studies. The chemical composition of these fruits has also been characterized with many reports on Davidson’s plum, finger lime, and native pepper exhibiting rich metabolomic profiles. As indicated in previous studies, these fruits can be a great source of functional food ingredients. Native fruits such as Kakadu plum and Illawarra plum have also exhibited potent anticancer, anti-inflammatory and antimicrobial properties in preclinical studies. However, studies on native vegetables are limited with only a few reports on the chemical composition and in vitro bioactivity of bush tomato. In addition, further research is needed to develop optimal, affordable, and green techniques to efficiently extract phytochemicals from Australian native fruits and vegetables. Overall, Kakadu plum is the most explored Australian native fruit thus far in terms of its post-harvest processing, extraction of bioactive compounds, chemical composition and bioactivity. More studies to define effective agricultural practices for large scale production of Australian native fruits and vegetables are necessary. Additionally, optimal post-harvest handling parameters including harvesting, handling, storage and stability, processing, packaging, transportation, and marketing of these native fruits and vegetables have not been defined clearly. Most studies performed to analyze the therapeutic activity of native fruits and vegetables are in vitro in nature with limited information on their molecular mechanisms of action. Therefore, future studies should prioritize understanding the molecular mechanisms of the therapeutic activity through in vivo and clinical trials. Although many Australian food, nutraceutical, and cosmetic companies have started utilizing native fruits in their formulations, more investigations are necessary for the wider use of both Australian fruits as well as vegetables by the food, pharmaceutical, and nutraceutical industries across the world.
Credit authorship contribution statement
Indeewarie Dissanayake: Design, Investigation, Writing- original draft, Figures. Valeria Zak: Design, Investigation, Writing- original draft. Kirandeep Kaur: Design, Investigation, Writing- original draft, Figures. Kayla Jaye: Design, Investigation, Writing- original draft. Zahra Ayati: Design, Investigation, Writing- original draft. Dennis Chang: Writing- review and editing. Chun Guang Li: Writing- review and editing. Deep Jyoti Bhuyan: Conceptualization, Design, Investigation, Writing- original draft, review and editing, Figures, Visualization, Supervision.
Acknowledgements
We acknowledge the support of Western Sydney University, Australia through the Master of Research International Scholarship Program (Indeewarie Dissanayake), the Summer Scholarship Program 2020 (Valeria Zak) and the Research Support Program Fellowship (Deep Jyoti Bhuyan) to conduct this research. were created with Biorender.com.
Disclosure statement
As a medical research institute, NICM Health Research Institute (NICM HRI) receives grants and donations from foundations, universities, government agencies, individuals, and industry. Sponsors and donors also provide untied funding to advance the vision and mission of NICM HRI. The authors declare no conflicts of interest.
References
- Adiamo, O., M. Netzel, L. Hoffman, M. Gidley, and Y. Sultanbawa. 2021. Nutritional, anti-nutritional, antioxidant, physicochemical and functional characterization of Australian acacia seed: Effect of species and regions. Journal of the Science of Food and Agriculture 101 (11):4681–90. doi: 10.1002/jsfa.11113. [pubmedMismatch]
- Adiamo, O., Y. Sultanbawa, and D. Cozzolino. 2021. Mid-infrared spectroscopy as a rapid tool to qualitatively predict the effects of species, regions and roasting on the nutritional composition of Australian acacia seed species. Molecules 26 (7):1879. doi: 10.3390/molecules26071879.
- Agatonovic-Kustrin, S., E. Doyle, V. Gegechkori, and D. Morton. 2020. High-performance thin-layer chromatography linked with (bio)assays and FTIR-ATR spectroscopy as a method for discovery and quantification of bioactive components in native Australian plants. Journal of Pharmaceutical and Biomedical Analysis 184:113208. doi: 10.1016/j.jpba.2020.113208.
- Ahmed, N. 2005. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Research and Clinical Practice 67 (1):3–21. doi: 10.1016/j.diabres.2004.09.004.
- Ahmed, A, and K. Johnson. 2000. Horticultural development of Australian native edible plants. Australian Journal of Botany 48 (4):417–26. doi: 10.1071/BT99042.
- Aiyer, H, and R. Gupta. 2010. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prevention Research 3 (6):727–37. doi: 10.1158/1940-6207.CAPR-09-0260.
- Akter, S., R. Addepalli, M. Netzel, M. Fletcher, Y. Sultanbawa, and S. Osborne. 2021. Impact of polyphenol-rich extracts of Terminalia ferdinandiana fruits and seeds on viability of human intestinal and liver cells in vitro. Food Chemistry: Molecular Sciences 2:100024. doi: 10.1016/j.fochms.2021.100024.
- Akter, S., R. Addepalli, M. Netzel, U. Tinggi, M. Fletcher, Y. Sultanbawa, and S. Osborne. 2019. Antioxidant-rich extracts of Terminalia ferdinandiana interfere with estimation of cell viability. Antioxidants 8 (6):191. doi: 10.3390/antiox8060191.
- Akter, S., R. Addepalli, M. Netzel, U. Tinggi, M. Fletcher, Y. Sultanbawa, and S. Osborne. 2022. In vitro bioaccessibility and intestinal absorption of selected bioactive compounds in Terminalia ferdinandiana. Frontiers in Nutrition 8: doi: 10.3389/fnut.2021.818195.
- Akter, K., E. Barnes, J. Brophy, D. Harrington, Y. Community Elders, S. Vemulpad, and J. Jamie. 2016. Phytochemical profile and antibacterial and antioxidant activities of medicinal plants used by aboriginal people of New South Wales, Australia. Evidence-Based Complementary and Alternative Medicine 2016:1–14. doi: 10.1155/2016/4683059.
- Akter, S., M. Netzel, M. Fletcher, U. Tinggi, and Y. Sultanbawa. 2018. Chemical and nutritional composition of Terminalia ferdinandiana (Kakadu Plum) kernels: a novel nutrition source. Foods 7 (4):60. doi: 10.3390/foods7040060.
- Akter, S., M. Netzel, U. Tinggi, M. Fletcher, S. Osborne, and Y. Sultanbawa. 2020. Interactions between phytochemicals and minerals in Terminalia ferdinandiana and implications for mineral bioavailability. Frontiers in Nutrition 7 doi: 10.3389/fnut.2020.598219.
- Akter, S., M. Netzel, U. Tinggi, S. Osborne, M. Fletcher, and Y. Sultanbawa. 2019. Antioxidant rich extracts of Terminalia ferdinandiana inhibit the growth of foodborne bacteria. Foods 8 (8):281. doi: 10.3390/foods8080281.
- Alderees, F., R. Mereddy, D. Webber, N. Nirmal, and Y. Sultanbawa. 2018. Mechanism of action against food spoilage yeasts and bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum plant solvent extracts. Foods 7 (11):179. doi: 10.3390/foods7110179.
- ANFAB. 2021. Australian native food and botanicals. Accessed 8 April. https://anfab.org.au/main.asp?_=SPECIES.
- Balouiri, M., M. Sadiki, and S. K. Ibnsouda. 2016. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis 6 (2):71–9. doi: 10.1016/j.jpha.2015.11.005.
- Bhuyan, D. J., M. Alsherbiny, S. Perera, M. Low, A. Basu, O. A. Devi, M. S. Barooah, C. G. Li, and K. Papoutsis. 2019. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants 8 (10):426. doi: 10.3390/antiox8100426.[Mismatch
- Bhuyan, D. J., Q. V. Vuong, A. C. Chalmers, M. C. Bowyer, and C. J. Scarlett. 2018. An array of bioactive compounds from australian eucalypts and their relevance in pancreatic cancer therapeutics. Pancreas 47 (6):690–707. doi: 10.1097/mpa.0000000000001074.
- Block, G., B. Patterson, and A. Subar. 1992. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutrition and Cancer 18 (1):1–29. doi: 10.1080/01635589209514201.
- Bobasa, E., M. Netzel, D. Cozzolino, A. D. T. Phan, and Y. Sultanbawa. 2021. Measurement of total soluble solids and moisture in puree and dry powder of Kakadu plum (Terminalia ferdinandiana) samples using hand-held near infrared spectroscopy. Journal of near Infrared Spectroscopy 29 (4):201–6. doi: 10.1177/0967033520982361.
- Bobasa, E., M. Netzel, A. Dao Thi Phan, H. Smyth, Y. Sultanbawa, and D. Cozzolino. 2021. Unlocking the secrets of terminalia kernels using near-infrared spectroscopy. Applied Spectroscopy 75 (7):834–8. doi: 10.1177/0003702821992136.
- Bobasa, E., A. D. T. Phan, M. Netzel, D. Cozzolino, and Y. Sultanbawa. 2021. Hydrolysable tannins in Terminalia ferdinandiana Exell fruit powder and comparison of their functional properties from different solvent extracts. Food Chemistry 358:129833. doi: 10.1016/j.foodchem.2021.129833.
- Bobasa, E., A. D. Phan, M. Netzel, H. Smyth, Y. Sultanbawa, and D. Cozzolino. 2021. The use of a micro near infrared portable instrument to predict bioactive compounds in a wild harvested fruit—Kakadu Plum (Terminalia ferdinandiana). Sensors 21 (4):1413. doi: 10.3390/s21041413.
- Brand, J, and V. Cherikoff. 1985. Australian aboriginal bushfoods: The nutritional composition of plants from arid and semi-arid areas. Australian Aboriginal Studies 38-46 (2)
- Brand, J, and V. Cherikoff. 2019. Australian aboriginal bushfoods: their nutritive value. Arid Lands: Today and Tomorrow:99–111.
- Brand-Miller, J, and S. Holt. 1998. Australian Aboriginal plant foods: A consideration of their nutritional composition and health implications. Nutrition Research Reviews 11 (1):5–23. doi: 10.1079/NRR19980003.
- Bray, F., J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 68 (6):394–424. doi: 10.3322/caac.21492.
- Brophy, J., R. Goldsack, and P. Forster. 2001. The leaf oils of the Australian species of citrus (Rutaceae). Journal of Essential Oil Research 13 (4):264–8. doi: 10.1080/10412905.2001.9699690.
- Buchholz, T, and M. Melzig. 2016. Medicinal plants traditionally used for treatment of obesity and diabetes Mellitus – screening for pancreatic lipase and α-amylase inhibition. Phytotherapy Research : PTR 30 (2):260–6. doi: 10.1002/ptr.5525.
- Caltagirone, S., C. Rossi, A. Poggi, F. Ranelletti, P. G. Natali, M. Brunetti, F. Aiello, and M. Piantelli. 2000. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. International Journal of Cancer 87 (4):595–600. doi: 10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5.
- Campbell, A. 2004. Inflammation, neurodegenerative diseases, and environmental exposures. Annals of the New York Academy of Sciences 1035 (1):117–32. doi: 10.1196/annals.1332.008.
- Castillo, M., E. Perkins, J. Campbell, R. Doerr, J. Hassett, C. Kandaswami, and E. Middleton. 1989. The effects of the bioflavonoid quercetin on squamous cell carcinoma of head and neck origin. The American Journal of Surgery 158 (4):351–5. doi: 10.1016/0002-9610(89)90132-3.
- Ceci, C., L. Tentori, M. Atzori, P. Lacal, E. Bonanno, M. Scimeca, R. Cicconi, M. Mattei, M. de Martino, G. Vespasiani, et al. 2016. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients 8 (11):744. doi: 10.3390/nu8110744.
- Chaliha, M., A. D. T. Phan, S. Cao, Q. Li, J. Gorman, Y. Sultanbawa, and D. Cozzolino. 2020. Antimicrobial activity, total phenolic and ascorbic acid content of Terminalia ferdinandiana leaves at various stages of maturity. Current Research in Nutrition and Food Science Journal 8 (3):744–56. doi: 10.12944/CRNFSJ.8.3.07.
- Chaliha, M., D. Williams, D. Edwards, S. Pun, H. Smyth, and Y. Sultanbawa. 2017. Bioactive rich extracts from Terminalia ferdinandiana by enzyme-assisted extraction: A simple food safe extraction method. Journal of Medicinal Plants Research 11 (5):96–106. doi: 10.5897/JMPR2016.6285.
- Chaliha, M, and Y. Sultanbawa. 2019. Terminalia ferdinandiana, a traditional medicinal plant of Australia, alleviates hydrogen peroxide induced oxidative stress and inflammation, in vitro. Journal of Complementary and Integrative Medicine 17 (1) doi: 10.1515/jcim-2019-0008.
- Chapman, J., A. Power, M. Netzel, Y. Sultanbawa, H. Smyth, V. K. Truong, and D. Cozzolino. 2021. Challenges and opportunities of the fourth revolution: A brief insight into the future of food. Critical Reviews in Food Science and Nutrition 1–9. doi: 10.1080/10408398.2020.1863328.
- Cheesman, M., A. White, B. Matthews, and I. Cock. 2019. Terminalia ferdinandiana fruit and leaf extracts inhibit methicillin-resistant Staphylococcus aureus growth. Planta Medica 85 (16):1253–62. doi: 10.1055/a-1013-0434.
- Chen, L., H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang, and L. Zhao. 2018. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9 (6):7204–18. doi: 10.18632/oncotarget.23208.
- Cherikoff, V, and J. Isaacs. 1989. Bush food handbook: Ti Tree Press.
- Chikowe, G., L. Mpala, and I. E. Cock. 2013. Antibacterial activity of selected australian syzygium species. Pharmacognosy Communications 3 (4):77–83.
- Choudhari, A. S., P. C. Mandave, M. Deshpande, P. Ranjekar, and O. Prakash. 2019. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Frontiers in Pharmacology 10:1614. doi: 10.3389/fphar.2019.01614.
- Clarke, M. 2012. Australian Natives Food Industry Stocktake. Australia: Australian Government.
- Clarke, P. A. 2008. Aboriginal healing practices and Australian bush medicine. Journal of the Anthropological Society of South Australia 33 (1):3–38.
- Cock, I. E. 2012. Antimicrobial activity of Syzygium australe and Syzygium leuhmannii leaf methanolic extracts. Pharmacognosy Communications 2 (2):71–7. doi: 10.5530/pc.2012.2.11.
- Cock, I. E, and S. Mohanty. 2011. Evaluation of the antibacterial activity and toxicity of Terminalia ferdinandia fruit extracts. Pharmacognosy Journal 3 (20):72–9. doi: 10.5530/pj.2011.20.14.
- Cozzolino, D., A. D. Phan, M. Netzel, H. Smyth, and Y. Sultanbawa. 2021a. Assessing the interaction between drying and addition of maltodextrin to Kakadu plum powder samples by two dimensional and near infrared spectroscopy. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy 247:119121. doi: 10.1016/j.saa.2020.119121.
- Cozzolino, D., A. D. T. Phan, M. Netzel, H. Smyth, and Y. Sultanbawa. 2021b. The use of vibrational spectroscopy to predict vitamin C in Kakadu plum powders (Terminalia ferdinandiana Exell, Combretaceae). Journal of the Science of Food and Agriculture 101 (8):3208–13. doi: 10.1002/jsfa.10950.
- Cozzolino, D., A. Phan, M. Netzel, H. Smyth, and Y. Sultanbawa. 2021c. Monitoring two different drying methods of Kakadu plum puree by combining infrared and chemometrics analysis. CyTA - Journal of Food 19 (1):183–9. doi: 10.1080/19476337.2021.1875052.
- Deachathai, S., W. Mahabusarakam, S. Phongpaichit, and W. C. Taylor. 2005. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry 66 (19):2368–75. doi: 10.1016/j.phytochem.2005.06.025.
- Deo, P., E. Hewawasam, A. Karakoulakis, D. Claudie, R. Nelson, B. Simpson, N. Smith, and S. Semple. 2016. In vitro inhibitory activities of selected Australian medicinal plant extracts against protein glycation, angiotensin converting enzyme (ACE) and digestive enzymes linked to type II diabetes. BMC Complementary and Alternative Medicine 16 (1):1–11. doi: 10.1186/s12906-016-1421-5.
- Dyson, L. E. 2006. Indigenous Australian cookery, past and present. Journal of Australian Studies 30 (87):5–18. doi: 10.1080/14443050609388047.
- Eckel, R., S. Grundy, and P. Zimmet. 2005. The metabolic syndrome. The Lancet 365 (9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7.
- Fanning, K., B. Topp, D. Russell, R. Stanley, and M. Netzel. 2014. Japanese plums (Prunus salicina Lindl.) and phytochemicals-breeding, horticultural practice, postharvest storage, processing and bioactivity. Journal of the Science of Food and Agriculture 94 (11):2137–47. doi: 10.1002/jsfa.6591.
- Fischbach, M. 2011. Combination therapies for combating antimicrobial resistance. Current Opinion in Microbiology 14 (5):519–23. doi: 10.1016/j.mib.2011.08.003.
- Fyfe, S., G. Netzel, M. Netzel, and Y. Sultanbawa. 2018a. Buchanania obovata: Functionality and phytochemical profiling of the Australian native green plum. Foods 7 (5):71. doi: 10.3390/foods7050071.
- Fyfe, S., M. Netzel, U. Tinggi, E. Biehl, and Y. Sultanbawa. 2018b. Buchanania obovata: An Australian indigenous food for diet diversification. Nutrition & Dietetics: The Journal of the Dietitians Association of Australia 75 (5):527–32. doi: 10.1111/1747-0080.12437.
- Fyfe, S., H. Smyth, H. J. Schirra, M. Rychlik, and Y. Sultanbawa. 2020. The nutritional potential of the native australian green plum (Buchanania obovata) compared to other anacardiaceae fruit and nuts. Frontiers in Nutrition 7 311. doi: 10.3389/fnut.2020.600215.
- Geraghty, D. P., K. D. Ahuja, J. Pittaway, C. Shing, G. A. Jacobson, N. Jager, S. Jurković, C. Narkowicz, C. I. Saunders, M. Ball, et al. 2011. In vitro antioxidant, antiplatelet and anti-inflammatory activity of Carpobrotus rossii (pigface) extract. Journal of Ethnopharmacology 134 (1):97–103. doi: 10.1016/j.jep.2010.11.060.
- Ghattamaneni, N., S. Panchal, and L. Brown. 2019. Cyanidin 3-glucoside from Queen Garnet plums and purple carrots attenuates DSS-induced inflammatory bowel disease in rats. Journal of Functional Foods 56:194–203. doi: 10.1016/j.jff.2019.01.028.
- Gorman, J., P. Wurm, S. Vemuri, C. Brady, and Y. Sultanbawa. 2020. Kakadu Plum (Terminalia ferdinandiana) as a sustainable indigenous agribusiness. Economic Botany 74 (1):74–91. doi: 10.1007/s12231-019-09479-8.
- Gott, B. 1983. Murnong-Microseris scapigera: A study of a staple food of Victorian aborigines. Australian Aboriginal Studies (2):2–18.
- Greenhough, A., H. Smartt, A. Moore, H. Roberts, A. Williams, C. Paraskeva, and A. Kaidi. 2009. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30 (3):377–86. doi: 10.1093/carcin/bgp014.
- Guleria, S., A. K. Tiku, G. Singh, A. Koul, S. Gupta, and S. Rana. 2013. In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. Journal of Plant Biochemistry and Biotechnology 22 (1):9–15. doi: 10.1007/s13562-012-0105-6.
- Guo, Y., K. Sakulnarmrat, and I. Konczak. 2014. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicology Reports 1:385–90. doi: 10.1016/j.toxrep.2014.06.011.
- Hanahan, D, and R. A. Weinberg. 2011. Hallmarks of cancer: The next generation. Cell 144 (5):646–74. doi: 10.1016/j.cell.2011.02.013.
- Hart, C., P. Ilanko, J. Sirdaarta, P. Rayan, P. A. McDonnella, and I. E. Cock. 2014. Tasmannia stipitata as a functional food/natural preservative: Antimicrobial activity and toxicity. Pharmacognosy Communications 4 (4):33–47.
- Hayashi, M., F. Bizerra, and P. I. Da Silva. 2013. Antimicrobial compounds from natural sources. Frontiers in Microbiology 4(:195. doi: 10.3389/fmicb.2013.00195.
- Henry, M., W. J. Sirdaarta, A. White, G. A. Carlson, and C. I. Edwin. 2016. Bacillus anthracis growth inhibitory properties of Australian Terminalia spp.: Putative identification of low polarity volatile components by GC-MS headspace analysis. Pharmacognosy Journal 8 (3):281–90. doi: 10.5530/pj.2016.3.18.
- Hidalgo, M., C. Sánchez-Moreno, and S. de Pascual-Teresa. 2010. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chemistry 121 (3):691–6. doi: 10.1016/j.foodchem.2009.12.097.
- Hoffer, L. J., M. Levine, S. Assouline, D. Melnychuk, S. J. Padayatty, K. Rosadiuk, C. Rousseau, L. Robitaille, and W. H. Miller. Jr. 2008. Phase I clinical trial of iv ascorbic acid in advanced malignancy. Annals of Oncology : official Journal of the European Society for Medical Oncology 19 (11):1969–74. doi: 10.1093/annonc/mdn377.
- Hoffer, J., L. Robitaille, R. Zakarian, D. Melnychuk, P. Kavan, J. Agulnik, V. Cohen, D. Small, and W. Miller. Jr. 2015. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PloS One 10 (4):e0120228. doi: 10.1371/journal.pone.0120228.
- Hu, F. 2011. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 34 (6):1249–57. doi: 10.2337/dc11-0442.
- Hutadilok-Towatana, N., S. Kongkachuay, and W. Mahabusarakam. 2007. Inhibition of human lipoprotein oxidation by morelloflavone and camboginol from Garcinia dulcis. Natural Product Research 21 (7):655–62. doi: 10.1080/14786410701371256.
- Igwe, E., S. Roodenrys, Y. Probst, V. do Rosario, M. Netzel, H. Hong, G. Netzel, A. Phan, and K. Charlton. 2020. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: A randomized crossover trial. Nutrition Research (New York, N.Y.) 82:74–87. doi: 10.1016/j.nutres.2020.08.003.
- Jadhav, R, and G. Puchchakayala. 2012. Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Group 1:100.
- Jamieson, N., J. Sirdaartaa, and I. E. Cock. 2014. The anti-proliferative properties of Australian plants with high antioxidant capacities against cancer cell lines. Pharmacognosy Communications 4 (4):71–82. doi: 10.5530/pc.2014.4.8.
- Jeong, J. H., J. Y. An, Y. T. Kwon, J. G. Rhee, and Y. J. Lee. 2009. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. Journal of Cellular Biochemistry 106 (1):73–82. doi: 10.1002/jcb.21977.
- Jeong, Y.-J., J.-H. Kim, J.-M. Hong, J. S. Kang, H.-R. Kim, W. J. Lee, and Y-i. Hwang. 2014. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology 219 (7):554–64. doi: 10.1016/j.imbio.2014.03.006.
- Jideani, A., H. Silungwe, T. Takalani, A. Omolola, H. Udeh, and T. Anyasi. 2021. Antioxidant-rich natural fruit and vegetable products and human health. International Journal of Food Properties 24 (1):41–67. doi: 10.1080/10942912.2020.1866597.
- John, O., P. Mouatt, M. Majzoub, T. Thomas, S. Panchal, and L. Brown. 2019. Physiological and metabolic effects of yellow mangosteen (Garcinia dulcis) rind in rats with diet-induced metabolic syndrome. International Journal of Molecular Sciences 21 (1):272. doi: 10.3390/ijms21010272.
- John, O., P. Mouatt, S. Panchal, and L. Brown. 2021. Rind from purple mangosteen (Garcinia mangostana) attenuates diet-induced physiological and metabolic changes in obese rats. Nutrients 13 (2):319. doi: 10.3390/nu13020319.
- John, O., P. Mouatt, I. Prasadam, Y. Xiao, S. Panchal, and L. Brown. 2019. The edible native Australian fruit, Davidson’s plum (Davidsonia pruriens), reduces symptoms in rats with diet-induced metabolic syndrome. Journal of Functional Foods 56:204–15. doi: 10.1016/j.jff.2019.03.018.
- Kalita, S.A. K. Verma, and S. B. Prasad. 2014. Chlorambucil and ascorbic acid-mediated anticancer activity and hematological toxicity in Dalton’s ascites lymphoma-bearing mice.
- Kim, S., S. Semple, B. Simpson, and P. Deo. 2020. Antioxidant and antiglycation activities of Syzygium paniculatum gaertn and inhibition of digestive enzymes relevant to type 2 diabetes mellitus. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 75 (4):621–7. doi: 10.1007/s11130-020-00858-4.
- Kleemann, R., L. Verschuren, M. Morrison, S. Zadelaar, M. van Erk, P. Wielinga, and T. Kooistra. 2011. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 218 (1):44–52. doi: 10.1016/j.atherosclerosis.2011.04.023.
- Konczak, I., F. Maillot, and A. Dalar. 2014. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chemistry 151:248–56. doi: 10.1016/j.foodchem.2013.11.049.
- Konczak, I, and P. Roulle. 2011. Nutritional properties of commercially grown native Australian fruits: Lipophilic antioxidants and minerals. Food Research International 44 (7):2339–44. doi: 10.1016/j.foodres.2011.02.023.
- Konczak, I., D. Zabaras, M. Dunstan, and P. Aguas. 2010a. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chemistry 123 (4):1048–54. doi: 10.1016/j.foodchem.2010.05.060.
- Konczak, I., D. Zabaras, M. Dunstan, and P. Aguas. 2010b. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chemistry 122 (1):260–6. doi: 10.1016/j.foodchem.2010.03.004.
- Konda, P. Y., S. Dasari, S. Konanki, and P. Nagarajan. 2019. In vivo antihyperglycemic, antihyperlipidemic, antioxidative stress and antioxidant potential activities of Syzygium paniculatum Gaertn. in Streptozotocin-induced diabetic rats . Heliyon 5 (3):e01373. doi: 10.1016/j.heliyon.2019.e01373.
- Lee, P. ‐S., Chia. ‐Y. Teng, N. Kalyanam, Chi. ‐T. Ho, and Min. ‐H. Pan. 2019. Garcinol reduces obesity in high‐fat‐diet‐fed mice by modulating gut microbiota composition. Molecular Nutrition & Food Research 63 (2):1800390. doi: 10.1002/mnfr.201800390.
- Leyva-López, N., E. Gutierrez-Grijalva, D. Ambriz-Perez, and J. Heredia. 2016. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. International Journal of Molecular Sciences 17 (6):921. doi: 10.3390/ijms17060921.
- Lim, V., S. G. Gorji, V. D. Daygon, and M. Fitzgerald. 2020. Untargeted and targeted metabolomic profiling of australian indigenous fruits. Metabolites 10 (3):114. doi: 10.3390/metabo10030114.
- Lin, B. W., C. C. Gong, H. F. Song, and Y. Y. Cui. 2017. Effects of anthocyanins on the prevention and treatment of cancer. British Journal of Pharmacology 174 (11):1226–43. doi: 10.1111/bph.13627.
- Majolo, F., L. Knabben de Oliveira Becker Delwing, D. J. Marmitt, I. Cunha Bustamante-Filho, and M. I. Goettert. 2019. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochemistry Letters 31:196–207. doi: 10.1016/j.phytol.2019.04.003.
- Manach, C., A. Scalbert, C. Morand, C. Rémésy, and L. Jiménez. 2004. Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition 79 (5):727–47. doi: 10.1093/ajcn/79.5.727.
- Mani, J., J. Johnson, H. Hosking, N. Ashwath, K. Walsh, P. Neilsen, D. Broszczak, and M. Naiker. 2021. Antioxidative and therapeutic potential of selected Australian plants: A review. Journal of Ethnopharmacology 268:113580. doi: 10.1016/j.jep.2020.113580.
- Mansouri, M. T., A. A. Hemmati, B. Naghizadeh, S. A. Mard, A. Rezaie, and B. Ghorbanzadeh. 2015. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian Journal of Pharmacology 47 (3):292–8. doi: 10.4103/0253-7613.157127.
- Maru, G., R. Hudlikar, G. Kumar, K. Gandhi, and M. Mahimkar. 2016. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World Journal of Biological Chemistry 7 (1):88–99. doi: 10.4331/wjbc.v7.i1.88.
- Mazerand, C, and I. E. Cock. 2019. An examination of the antibacterial, antifungal, anti-giardial and anticancer properties of Buchanania obovata Engl. fruit extracts. Pharmacognosy Communications 9 (1):07–14. doi: 10.5530/pc.2019.1.3.
- Mazza, G. 2005. Bioactivity, absorption and metabolism of anthocyanins. In International Symposium on Human Health Effects of Fruits and Vegetables 744.
- McDonald, J.N. A. Caffin, S. Sommano, and R. Cocksedge. 2008. The effect of post harvest handling on selected native food plants.
- McDougall, G., F. Shpiro, P. Dobson, P. Smith, A. Blake, and D. Stewart. 2005. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase . Journal of Agricultural and Food Chemistry 53 (7):2760–6. doi: 10.1021/jf0489926.
- McManus, K., A. Wood, M. H. Wright, B. Matthews, A. C. Greene, and I. E. Cock. 2017. Terminalia ferdinandiana Exell. Extracts inhibit the growth of body odour-forming bacteria. International Journal of Cosmetic Science 39 (5):500–10. doi: 10.1111/ics.12403.
- Mills, C. C., E. A. Kolb, and V. B. Sampson. 2018. Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Research 78 (2):320–5. doi: 10.1158/0008-5472.CAN-17-2782.
- Miyazawa, T., G. Burdeos, M. Itaya, K. Nakagawa, and T. Miyazawa. 2019. Vitamin E: regulatory redox interactions. IUBMB Life 71 (4):430–41. doi: 10.1002/iub.2008.
- Mohanty, S, and I. Cock. 2012. The chemotherapeutic potential of Terminalia ferdinandiana: Phytochemistry and bioactivity. Pharmacognosy Reviews 6 (11):29–36. doi: 10.4103/0973-7847.95855.
- Morita, I. 2002. Distinct functions of COX-1 and COX-2. Prostaglandins & Other Lipid Mediators 68-69:165–75. doi: 10.1016/S0090-6980(02)00029-1.
- Mouria, M., A. Gukovskaya, Y. Jung, P. Buechler, O. Hines, H. Reber, and S. Pandol. 2002. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. International Journal of Cancer 98 (5):761–9. doi: 10.1002/ijc.10202.
- Mpala, L., G. Chikowe, and I. E. Cock. 2020. Davidsonia pruriens F. Muell. Fruit and leaf extracts lack antibacterial and antifungal activity. Pharmacognosy Communications 10 (3):113–8. doi: 10.5530/pc.2020.3.23.
- Mulisa Bobasa, E., A. Dao Thi Phan, C. Manolis, M. Netzel, H. Smyth, D. Cozzolino, and Y. Sultanbawa. 2020. Effect of sample presentation on the near infrared spectra of wild harvest Kakadu plum fruits (Terminalia ferdinandiana). Infrared Physics & Technology 111:103560. doi: 10.1016/j.infrared.2020.103560.
- Murhekar, S., M. H. Wright, A. Carlson Greene, J. C. Brownlie, and I. E. Cock. 2017. Inhibition of Shewanella spp. growth by Syzygium australe and Syzygium luehmannii extracts: Natural methods for the prevention of fish spoilage. Journal of Food Science and Technology 54 (10):3314–26. doi: 10.1007/s13197-017-2782-6.
- Nathan, D. 2015. Diabetes: Advances in diagnosis and treatment. Jama 314 (10):1052–62. doi: 10.1001/jama.2015.9536.
- National Health and Medical Research Council. 2017. Nutrient Reference Values for Australia and New Zealand: Executive Summary. edited by Department of Health and Ageing. Australia: Australian Government.
- Netzel, M., G. Netzel, Q. Tian, S. Schwartz, and I. Konczak. 2006. Sources of antioxidant activity in Australian native fruits. Identification and quantification of anthocyanins. Journal of Agricultural and Food Chemistry 54 (26):9820–6. doi: 10.1021/jf0622735.
- Netzel, M., G. Netzel, Q. Tian, S. Schwartz, and I. Konczak. 2007. Native Australian fruits—a novel source of antioxidants for food. Innovative Food Science & Emerging Technologies 8 (3):339–46. doi: 10.1016/j.ifset.2007.03.007.
- Niedzwiecki, A., M. W. Roomi, T. Kalinovsky, and M. Rath. 2016. Anticancer efficacy of polyphenols and their combinations. Nutrients 8 (9):552. doi: 10.3390/nu8090:552.
- Nirmal, N., Prakash, R. Mereddy, D. Webber, and Y. Sultanbawa. 2021. Biochemical, antioxidant and sensory evaluation of Davidsonia pruriens and Davidsoina jerseyana fruit infusion. Food Chemistry 342:128349. doi: 10.1016/j.foodchem.2020.128349.
- Njume, C., O. Donkor, and A. McAinch. 2019. Predisposing factors of type 2 diabetes mellitus and the potential protective role of native plants with functional properties. Journal of Functional Foods 53:115–24. doi: 10.1016/j.jff.2018.12.001.
- Njume, C., A. McAinch, and O. Donkor. 2020. Proximate and phenolic composition of selected native Australian food plants. International Journal of Food Science & Technology 55 (5):2060–79. doi: 10.1111/ijfs.14400.
- NSW Office of Environment and Heritage. 2012. National Recovery Plan for Magenta Lilly Pilly (Syzygium paniculatum). Department of Agriculture, Water and the Environment, Australian Government. http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?showprofile=Y&taxon_id=20307.
- NUTTAB. 2011. Food Nutrient Database. https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/ausnutdatafiles/Pages/foodnutrient.aspx
- Panchal, S. K., H. Poudyal, A. Iyer, R. Nazer, M. A. Alam, V. Diwan, K. Kauter, C. Sernia, F. Campbell, L. Ward, et al. 2011. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. Journal of Cardiovascular Pharmacology 57 (5):611–24. doi: 10.1097/FJC.0b013e31821b1379.
- Park, J.-H., K. Davis, G. Lee, M. Jung, Y. Jung, J. Park, S.-Y. Yi, M.-A. Lee, S. Lee, C.-H. Yeom, et al. 2012. Ascorbic acid alleviates toxicity of paclitaxel without interfering with the anticancer efficacy in mice. Nutrition Research 32 (11):873–83. doi: 10.1016/j.nutres.2012.09.011.
- Pearce, A., M. Haas, R. Viney, S. A. Pearson, P. Haywood, C. Brown, and R. Ward. 2017. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PloS One 12 (10):e0184360. doi: 10.1371/journal.pone.0184360.
- Peng, Y., Y. Yan, P. Wan, W. Dong, K. Huang, L. Ran, J. Mi, L. Lu, X. Zeng, and Y. Cao. 2020. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Research International (Ottawa, Ont.) 130:108952. doi: 10.1016/j.foodres.2019.108952.
- Phan, A. D. T., O. Adiamo, S. Akter, M. Netzel, D. Cozzolino, and Y. Sultanbawa. 2021. Effects of drying methods and maltodextrin on vitamin C and quality of Terminalia ferdinandiana fruit powder, an emerging Australian functional food ingredient. Journal of the Science of Food and Agriculture 101 (12):5132–41. doi: 10.1002/jsfa.11159.
- Phan, A. D. T., M. S. Damyeh, M. Chaliha, S. Akter, S. Fyfe, M. Netzel, D. Cozzolino, and Y. Sultanbawa. 2021. The effect of maturity and season on health-related bioactive compounds in wild harvested fruit of Terminalia ferdinandiana (Exell). International Journal of Food Science & Technology 56 (12):6431–42. doi: 10.1111/ijfs.15350.
- Pinkaew, D., S. G. Cho, D. Hui, J. Wiktorowicz, N. Hutadilok-Towatana, W. Mahabusarakam, M. Tonganunt, L. Stafford, A. Phongdara, M. Liu, et al. 2009. Morelloflavone blocks injury-induced neointimal formation by inhibiting vascular smooth muscle cell migration. Biochimica et Biophysica Acta 1790 (1):31–9. doi: 10.1016/j.bbagen.2008.09.006.
- Prior, R., H. A. Hoang, L. Gu, X. Wu, M. Bacchiocca, L. Howard, M. Hampsch-Woodill, D. Huang, B. Ou, and R. Jacob. 2003. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. Journal of Agricultural and Food Chemistry 51 (11):3273–9. doi: 10.1021/jf0262256.
- Ramírez, F, and J. Kallarackal. 2019. The phenology and potential ecological associations of Magenta Lilly Pilly (Syzygium paniculatum Gaertn) a native vulnerable Australian tree growing in Bogotá, Colombia. Arboricultural Journal 41 (4):191–211. doi: 10.1080/03071375.2019.1642047.
- Rawson, A., A. Patras, B. K. Tiwari, F. Noci, T. Koutchma, and N. Brunton. 2011. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Research International 44 (7):1875–87. doi: 10.1016/j.foodres.2011.02.053.
- Read, C. 2012. Nutritional Data for Australian Native Foods. Rural Industries Research and Development Corporation, RIRDC Publication (12/099).
- Reang, J., P. C. Sharma, V. K. Thakur, and J. Majeed. 2021. Understanding the therapeutic potential of ascorbic acid in the battle to overcome cancer. Biomolecules 11 (8):1130. doi: 10.3390/biom11081130.
- Richer, S., W. Stiles, L. Statkute, J. Pulido, J. Frankowski, D. Rudy, K. Pei, M. Tsipursky, and J. Nyland. 2004. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry - Journal of the American Optometric Association 75 (4):216–29. doi: 10.1016/S1529-1839(04)70049-4.
- Richmond, R., M. Bowyer, and Q. Vuong. 2019. Australian native fruits: Potential uses as functional food ingredients. Journal of Functional Foods 62:103547. doi: 10.1016/j.jff.2019.103547.
- RIRDC. 2012. A Focus on Riberry. Rural Industries Research and Development Corporation.
- Sadgrove, N, and G. Jones. 2015. A contemporary introduction to essential oils: chemistry, bioactivity and prospects for australian agriculture. Agriculture 5 (1):48–102. doi: 10.3390/agriculture5010048.
- Sakulnarmrat, K. 2012. Potential health properties of selected commercially grown native Australian herbs and fruits. ed. G. Srzednicki, K. Izabela. Chemical Sciences & Engineering, Faculty of Engineering, UNSW and CSIRO Animal Food and Health Sciences.. https://www.unsworks.unsw.edu.au/primoexplore/fulldisplay/unsworks_10979/UNSWORKS
- Sakulnarmrat, K, and I. Konczak. 2012. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chemistry 134 (2):1011–9. doi: 10.1016/j.foodchem.2012.02.217.
- Sakulnarmrat, K., G. Srzednicki, and I. Konczak. 2014. Composition and inhibitory activities towards digestive enzymes of polyphenolic-rich fractions of Davidson’s plum and quandong. LWT - Food Science and Technology 57 (1):366–75. doi: 10.1016/j.lwt.2014.01.002.
- Sakulnarmrat, K., G. Srzednicki, and I. Konczak. 2015. Bioprospecting Davidson’s plum and quandong: Cytoprotective and proapoptotic activities. LWT - Food Science and Technology 61 (2):622–9. doi: 10.1016/j.lwt.2014.12.023.
- Sautron, C, and I. E. Cock. 2014. Antimicrobial activity and toxicity of Syzygium australe and Syzygium leuhmannii fruit extracts. Pharmacognosy Communication s 4 (1):53–60. doi: 10.5530/pc.2014.1.8.
- Shahidi, F, and P. Ambigaipalan. 2015. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. Journal of Functional Foods 18:820–97. doi: 10.1016/j.jff.2015.06.018.
- Shalom, J, and I. Cock. 2018. Terminalia ferdinandiana Exell. fruit and leaf extracts inhibit proliferation and induce apoptosis in selected human cancer cell lines. Nutrition and Cancer 70 (4):579–93. doi: 10.1080/01635581.2018.1460680.
- Shi, G.-J., Y. Li, Q.-H. Cao, H.-X. Wu, X.-Y. Tang, X.-H. Gao, J.-Q. Yu, Z. Chen, and Y. Yang. 2019. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 109:1085–99. doi: 10.1016/j.biopha.2018.10.130.
- Singh, A., R. Raju, and G. Münch. 2021. Potential anti-neuroinflammatory compounds from Australian plants - A review. Neurochemistry International 142:104897. doi: 10.1016/j.neuint.2020.104897.
- Sirdaarta, J., A. Maen, P. Rayan, B. Matthews, and I. E. Cock. 2016. High performance liquid chromatography-mass spectrometry analysis of high antioxidant Australian fruits with antiproliferative activity against cancer cells . Pharmacognosy Magazine 12 (Suppl 2):S181–S194. doi: 10.4103/0973‑1296.182178.
- Sirdaarta, J., B. Matthews, A. White, and I. E. Cock. 2015. GC-MS and LC-MS analysis of Kakadu plum fruit extracts displaying inhibitory activity against microbial triggers of multiple sclerosis. Pharmacognosy Communications 5 (2):100–15. doi: 10.5530/pc.2015.2.2.
- Sivasinprasasn, S., R. Pantan, S. Thummayot, J. Tocharus, A. Suksamrarn, and C. Tocharus. 2016. Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chemico-Biological Interactions 260:67–74. doi: 10.1016/j.cbi.2016.10.022.
- Sommano, S., N. Caffin, and G. Kerven. 2013. Screening for antioxidant activity, phenolic content, and flavonoids from Australian native food plants. International Journal of Food Properties 16 (6):1394–406. doi: 10.1080/10942912.2011.580485.
- Sommano, S., N. Caffin, J. McDonald, and R. Cocksedge. 2013. The impact of thermal processing on bioactive compounds in Australian native food products (bush tomato and Kakadu plum). Food Research International 50 (2):557–61. doi: 10.1016/j.foodres.2011.03.008.
- Srivarathan, S., A. Dao Thi Phan, H. T. Hong, E. Chua, O. Wright, Y. Sultanbawa, and M. Netzel. 2021a. Tecticornia sp. (Samphire)—A promising underutilized Australian indigenous edible halophyte. Frontiers in Nutrition 8 doi: 10.3389/fnut.2021.607799.
- Srivarathan, S., A. D. T. Phan, O. Wright, Y. Sultanbawa, M. Netzel, and D. Cozzolino. 2021b. The measurement of antioxidant capacity and colour attributes in wild harvest samphire (Tecticornia sp.) samples using mid-infrared spectroscopy. Food Analytical Methods 14 (11):2328–34. doi: 10.1007/s12161-021-02065-6.
- Sultanbawa, Y., M. Chaliha, A. D. Phan, S. Olarte Mantilla, G. Netzel, M. Netzel, H. Smyth, and D. Cozzolino. 2021. An Infrared analysis of Terminalia ferdinandiana Exell [Combretaceae] fruit and leaves—towards the development of biospectroscopy tools to characterise uniquely Australian foods. Food Analytical Methods 14 (3):423–9. doi: 10.1007/s12161-020-01915-z.
- Sultanbawa, Y, and F. Sultanbawa. 2017. Australian native plants: Cultivation and uses in the health and food industries. Boca Raton, FL: CRC Press.
- Sultanbawa, Y.D. Williams, M. Chaliha, I. Konczak, and H. Smyth. 2015. Changes in quality and bioactivity of native food during storage. Canberra, Australia: Rural Industries Research and Development Corporation (RIRDC), Australian Government. ISBN 978-1-74254-754-1, ISSN 1440-6845.
- Symonds, E. L., I. Konczak, and M. Fenech. 2013. The Australian fruit Illawarra plum (Podocarpus elatus Endl., Podocarpaceae) inhibits telomerase, increases histone deacetylase activity and decreases proliferation of colon cancer cells. British Journal of Nutrition 109 (12):2117–25. doi: 10.1017/S0007114512004333.
- Tan, A., D.-X. Hou, I. Konczak, S. Tanigawa, I. Ramzan, and D. Sze. 2011. Native Australian fruit polyphenols inhibit COX-2 and iNOS expression in LPS-activated murine macrophages. Food Research International 44 (7):2362–7. doi: 10.1016/j.foodres.2010.12.031.
- Tan, A. C., I. Konczak, I. Ramzan, and D. M. Sze. 2011a. Native Australian fruit polyphenols inhibit cell viability and induce apoptosis in human cancer cell lines. Nutrition and Cancer 63 (3):444–55. doi: 10.1080/01635581.2011.535953.
- Tan, A., I. Konczak, I. Ramzan, and D. M.-Y. Sze. 2011b. Antioxidant and cytoprotective activities of native Australian fruit polyphenols. Food Research International 44 (7):2034–40. doi: 10.1016/j.foodres.2010.10.023.
- Tan, A. C., I. Konczak, I. Ramzan, D. Zabaras, and D. M. Sze. 2011. Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of Kakadu plum and Illawarra plum polyphenolic fractions. Nutrition and Cancer 63 (7):1074–84. doi: 10.1080/01635581.2011.596646.
- Teodor, E. D., O. Ungureanu, F. Gatea, and G. L. Radu. 2020. The Potential of Flavonoids and Tannins from Medicinal Plants as Anticancer Agents. Anti-Cancer Agents in Medicinal Chemistry 20 (18):2216–27. doi: 10.2174/1871520620666200516150829.
- Tuansulong, K.-A., N. Hutadilok-Towatana, W. Mahabusarakam, D. Pinkaew, and K. Fujise. 2011. Morelloflavone from Garcinia dulcis as a novel biflavonoid inhibitor of HMG-CoA reductase. Phytotherapy Research : PTR 25 (3):424–8. doi: 10.1002/ptr.3286.
- Tundis, R., M. R. Loizzo, and F. Menichini. 2010. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Reviews in Medicinal Chemistry 10 (4):315–31. doi: 10.2174/138955710791331007.
- Umesalma, S, and G. Sudhandiran. 2010. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis . Basic & Clinical Pharmacology & Toxicology 107 (2):650–5. doi: 10.1111/j.1742-7843.2010.00565.x.
- Vazquez Prieto, M. A., A. Bettaieb, C. Rodriguez Lanzi, V. C. Soto, D. J. Perdicaro, C. R. Galmarini, F. G. Haj, R. M. Miatello, and P. I. Oteiza. 2015. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Molecular Nutrition & Food Research 59 (4):622–33. doi: 10.1002/mnfr.201400631.
- Vuong, Q., A. Chalmers, D. Jyoti Bhuyan, M. Bowyer, and C. Scarlett. 2015. Botanical, phytochemical, and anticancer properties of the Eucalyptus species. Chemistry & Biodiversity 12 (6):907–24. doi: 10.1002/cbdv.201400327.
- Vuong, Q., S. Hirun, T. Chuen, C. Goldsmith, M. Bowyer, A. Chalmers, P. Phillips, and C. Scarlett. 2014. Physicochemical composition, antioxidant and anti-proliferative capacity of a lilly pilly (Syzygium paniculatum) extract. Journal of Herbal Medicine 4 (3):134–40. doi: 10.1016/j.hermed.2014.04.003.
- Vuong, Q. V., S. Hirun, P. A. Phillips, T. L. K. Chuen, M. C. Bowyer, C. D. Goldsmith, and C. J. Scarlett. 2014. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from Australian native fruits. Journal of Ethnopharmacology 152 (2):227–42. doi: 10.1016/j.jep.2013.12.023.
- Wilkinson, J, and H. Cavanagh. 2005. Antibacterial activity of essential oils from Australian native plants. Phytotherapy Research : PTR 19 (7):643–6. doi: 10.1002/ptr.1716.
- Williams, D, and M. Chaliha. 2016. Nutritional characteristics and bioactive compounds in Australian native plants: a review. In Australian native plants: Cultivation and uses in the health and food industries, 223–36. Traditional Herbal Medicines for Modern Times. Boca Raton, FL: CRC Press. doi: 10.1201/b20635-20.
- Williams, D., D. Edwards, M. Chaliha, and Y. Sultanbawa. 2016. Measuring the three forms of ellagic acid: Suitability of extraction solvents. Chemical Papers 70 (2):144–52. doi: 10.1515/chempap-2015-0193.
- Williams, D. J., D. Edwards, S. Pun, M. Chaliha, B. Burren, U. Tinggi, and Y. Sultanbawa. 2016. Organic acids in Kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Research International (Ottawa, Ont.) 89 (Pt 1):237–44. doi: 10.1016/j.foodres.2016.08.004.
- Williams, D., D. Edwards, S. Pun, M. Chaliha, and Y. Sultanbawa. 2014. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Research International 66:100–6. doi: 10.1016/j.foodres.2014.09.003.
- Winnett, V., H. Boyer, J. Sirdaarta, and I. E. Cock. 2014. The potential of Tasmannia lanceolata as a natural preservative and medicinal agent: Antimicrobial activity and toxicity. Pharmacognosy Communication 4 (1):42–52. doi: 10.5530/pc.2014.1.7.
- Wood, A. J., K. McManus, M. H. Wright, A. C. Greene, and I. E. Cock. 2017. Growth inhibitory activity of selected Australian Syzygium species against malodour forming bacteria. Pharmacognosy Communications 7 (3):129–36. doi: 10.5530/pc.2017.3.19.
- World Health Organization. 2020. Cancer Tomorrow.
- Wright, M. H., B. Matthews, M. S. J. Arnold, A. Carlson Greene, and I. E. Cock. 2016. The prevention of fish spoilage by high antioxidant Australian culinary plants: Shewanella putrefaciens growth inhibition. International Journal of Food Science & Technology 51 (3):801–13. doi: 10.1111/ijfs.13026.
- Wright, M. H., J. Sirdaarta, A. White, A. C. Greene, and I. E. Cock. 2016. GC-MS headspace analysis of Terminalia ferdinandiana fruit and leaf extracts which inhibit Bacillus anthracis growth. Pharmacognosy Journal 9 (1):73–82. doi: 10.5530/pj.2017.1.14.
- Wrigley, J. Walter, and M. Fagg. 2013. Australian native plants: cultivation, use in landscaping and propagation. Wahroonga, NSW: Reed New Holland.
- Zeb, A. 2018. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Molecular and Cellular Biochemistry 448 (1–2):27–41. doi: 10.1007/s11010-018-3310-3.
- Zhang, J., A. Dao Thi Phan, S. Srivarathan, S. Akter, Y. Sultanbawa, and D. Cozzolino. 2022. Proximate composition, functional and antimicrobial properties of wild harvest Terminalia carpentariae fruit. Journal of Food Measurement and Characterization 16 (1):582–9. doi: 10.1007/s11694-021-01182-4.
- Zhao, G., F. He, C. Wu, P. Li, N. Li, J. Deng, G. Zhu, W. Ren, and Y. Peng. 2018. Betaine in inflammation: Mechanistic aspects and applications. Frontiers in Immunology 9(:1070. doi: 10.3389/fimmu.2018.01070.
- Zhou, W., G. Kallifatidis, B. Baumann, V. Rausch, J. Mattern, J. Gladkich, N. Giese, G. Moldenhauer, T. Wirth, M. Büchler, et al. 2010. Dietary polyphenol quercetin targets pancreatic cancer stem cells. International Journal of Oncology 37 (3):551–61. doi: 10.3892/ijo_00000704.
