Abstract
During the COVID-19 pandemic, the botanical product market saw a consumer interest increase in immune health supplements. While data are currently insufficient to support public health guidance for using foods and dietary supplements to prevent or treat COVID-19 and other immune disorders, consumer surveys indicate that immune support is the second-most cited reason for supplement use in the United States. Meanwhile, consumers showed increased attention to dietary supplement ingredient labels, especially concerning authenticity and ingredient claims. Top-selling botanical ingredients such as elderberry, turmeric, and functional mushrooms have been increasingly marketed toward consumers to promote immune health, but these popular products succumb to adulteration with inaccurate labeling due to the intentional or unintentional addition of lower grade ingredients, non-target plants, and synthetic compounds, partially due to pandemic-related supply chain issues. This review highlights the regulatory requirements and recommendations for analytical approaches, including chromatography, spectroscopy, and DNA approaches for ingredient claim verification. Demonstrating elderberry, turmeric, and functional mushrooms as examples, this review aims to provide industrial professionals and scientists an overview of current United States regulations, testing approaches, and trends for label compliance verification to ensure the safety of botanical products marketed for “immune health.”
Introduction
Labeling policy and laws in the United States
Labeling regulation for dietary supplements and foods
In the United States, the Food and Drug Administration (FDA) oversees the safety and proper labeling of foods and dietary supplements. Dietary supplements are classified under the same umbrella as foods and are subject to labeling laws and regulations that apply to foods as well as supplement specific requirements, such as adverse event reporting. While FDA does not pre-approve food and dietary supplement labels, manufacturers and distributors are responsible for ensuring products offered for sale in the United States are labeled in accordance with applicable laws and regulations, including the Federal Food, Drug and Cosmetic Act (FDCA) and Fair Packaging and Labeling Act.
Product labeling must be truthful and not misleading to the consumer, otherwise the product could be deemed misbranded and/or adulterated, allowing the FDA to take actions to remove the products from the market. In general, five label statements are required for foods and dietary supplements: (1) the statement of identity, (2) the net quantity of contents statement (amount of the food or dietary supplement), (3) the nutrition labeling (the nutrition or supplement facts panel), (4) the ingredient list, and (5) the name and place of business of the manufacturer, packer, or distributor.
While the FDA’s nutrition labeling regulations cover both foods and dietary supplements, there are requirements unique to dietary supplements. Nutrition labeling for dietary supplements is contained in the “Supplement Facts” panel while nutrition labeling for food products is contained in the “Nutrition Facts” panel; both contain information on the calories, serving size, the name and amount per serving (quantity) of the macronutrients and micronutrients in the product. By definition, dietary supplements contain “dietary ingredients,” which could be vitamins, minerals, amino acids, herbs and botanicals. Further, dietary ingredients can be a dietary substance used to supplement the diet, or an extract, metabolite, or concentrate of the previously mentioned categories, as defined in the Dietary Supplement Health and Education Act (DSHEA) of 1994 (FDA Citation1994). The common or usual name of the dietary ingredients must be declared in the Supplement Facts. Herbal or botanical dietary ingredients may include the Latin binomial, and must identify the part of the plant that is used as the dietary ingredient. In addition to mandatory information, the labeling of dietary supplements and foods may contain optional statements or claims, provided they are truthful and not misleading and comply with regulations. The FDA has defined six types of claims that may exist on dietary supplement labels (1) nutrient content claims; (2) antioxidant claims; (3) high potency claims; (4) percentage claims; (5) health claims; and (6) structure/function claims (FDA Citation2005).
Nutrient content claims characterize the level of a nutrient contained in a dietary supplement or food product. There are conditions for using terms such as high and low when characterizing a level of a nutrient (e.g., high in antioxidants). Similarly, there are criteria for comparing the level of a nutrient in different foods and supplements using terms such as more or reduced (e.g., more fiber). For dietary supplements, percentage claims can be used to describe the percentage level of a dietary ingredient in a product.
Health claims describe a well-established relationship between a dietary ingredient or a food and reduction of risk of a disease or health-related condition. Product labeling may contain one (or more) of twelve health claims that are currently FDA-approved and affirmed to meet a high evidentiary standard of significant scientific agreement (SSA) among qualified experts (FDA Citation2006). When the scientific evidence falls below the SSA standard, FDA may use its discretion to allow the use of a Qualified Health Claim on product labeling. Qualified Health Claims describe emerging and limited scientific evidence demonstrating a relationship between a dietary ingredient or food and a reduced risk of disease or health condition (FDA Citation2006). Qualifying language is included to reflect the limited evidence supporting the claim. Currently, no health claims pertaining to immunity are permitted by FDA.
Structure/function claims are statements of nutritional support often made in the labeling of dietary supplements and functional foods. This category includes claims of general well-being from consumption of a nutrient or dietary ingredient, benefits related to a nutrient deficiency disease, roles of a nutrient or dietary ingredient in affecting the structure or function of the body, or that characterizes the mechanism of action to maintain a structure or function. FDA considers claims to “support the immune system” to be acceptable structure/function claims. In contrast, claims that a dietary supplement fights disease or enhances disease-fighting functions of the body (e.g., “supports the body’s ability to resist infection” and “supports the body’s antiviral capabilities”) are viewed as disease claims by FDA (FDA 2002). From early in the pandemic, FDA, the Federal Trade Commission, and other government bodies have targeted companies making claims that products can prevent, treat, or cure coronavirus disease 2019 (COVID-19) with warning letters and legal action (Anon Citation2020).
Structure/function claims are not pre-approved by FDA, but dietary supplement marketers must have substantiation for the claims and notify FDA of the claims within 30 days of first marketing. Dietary supplement labeling containing statements of nutritional support must include the disclaimer: “This statement has not been evaluated by FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” The disclaimer makes clear that structure/function claims are not health claims, which are evaluated by FDA, nor are they drug claims, which refer to preventing or treating disease. FDA does not require food manufacturers to notify the agency prior to marketing products containing structure/function claims or display a disclaimer on food product labeling (FDA Citation2022a).
Definition of adulteration by the US FDA
The US 21 CFR 111.70 cGMP regulation requires dietary supplement manufacturers to establish specifications for each component, including dietary ingredients that are necessary to ensure that specifications for identity, purity, strength, composition, and limits on contamination for the dietary supplement are met (FDA Citation2022b). Per 21 CFR 111.75, manufacturers are required to conduct at least one test to verify the identity of each dietary ingredient and to use tests and examinations to verify that dietary supplement finished products meet the established specifications. Under section 402(g)(1) of the Act [21 U.S.C. § 342(g)(1)], a dietary supplement shall be deemed to be adulterated if it has been prepared, packed, or held under conditions that do not meet cGMP regulations for dietary supplements (United States Code 2006). In sum, products are adulterated if they do not meet established finished product, ingredient, or label specifications. This US federal definition of adulteration has a wider scope than the common-sense “economically-motivated adulteration” definition that is usually understood as someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food or dietary supplement.
Analytical testing regulatory requirement
Analytical testing is an important and usually inevitable way to ensure dietary supplement products meet their established specifications, and to support the label statements and claims. 21 CFR 111.320 requires manufacturers to (a) verify that the laboratory examination and testing methodologies are appropriate for their intended use and (b) identify and use an appropriate scientifically valid method for each established specification for which testing or examination is required to determine whether the specification is met. Per the Federal Register of June 25, 2007 (72 FR 34853), FDA defines a scientifically valid method to be one that is accurate, precise, and specific for its intended purpose. An analytical method will meet these criteria if they are carefully designed and implemented according to guidelines published by ICH, AOAC, FDA, USP, or other governmental or scientific organizations (FDA Citation2007).
Various technologies are used to assess whether botanical products meet the specifications established for identity, purity, strength, composition, and limits on contamination that may adulterate the finished product, as required by regulation. Identity testing means testing to make sure the botanical material is what it’s claimed to be. Adulteration and purity tests look for adulterants and contaminants to ensure the product is safe, pure to use, and relatively free of extraneous matter whether harmful or not to the recipient of the product. Strength testing is used to determine if the quality of an ingredient is meeting its label and specification, often by testing the concentration of certain phytochemical marker compounds that are unique to the plants. Dietary supplement composition testing ensures the label claims and all components of the dietary supplement are accurately listed to reflect dietary ingredients and non-dietary ingredients that are defined by the FDA (Bath 2018).
In addition to the mandatory quality control regulations mentioned above, US FDA has convened the Botanical Safety Consortium (BSC) to enhance the toolkit (including analytical methods) for conducting the safety evaluation of botanicals. The BSC serves as a global forum for various stakeholders to work together on adapting and integrating new approach methodologies into routine botanical safety assessments. The chemical analysis technical work group of BSC has its focus on developing and setting up analytical approaches for the phytochemical characterization and quality control of botanical ingredients (Mitchell et al. Citation2022).
Dietary supplement use for “immune health”
In recent times, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which can cause the infection commonly referred to as COVID-19, has led people to seek additional protection through various dietary supplements, functional foods, and nutraceuticals that are marketed to have beneficial effects for the immune system (Kamarli Altun, Karacil Ermumcu, and Seremet Kurklu Citation2021; Lordan, Rando, and Greene Citation2021). Although dietary supplementation is not a novel idea for improving immune health, there has been a large increase in sales and available market products since 2020 (Council for Responsible Nutrition Citation2022). While research is inconclusive and far from making reliable claims of dietary supplements preventing and reducing the symptoms of COVID-19 and other immune ailments, consumers are still purchasing the products, so their safety, purity, and identify must be analytically evaluated.
Immune health and support
The immune system is comprised of innate and adaptive responses, and it defends against attack from disease causing pathogens (Mrityunjaya et al. Citation2020; Hoebe, Janssen, and Beutler Citation2004). Many factors are involved in maintaining a healthy immune system; different authorities and organizations have provided various guidelines or recommendations for dietary considerations to remain healthy during the pandemic (World Health Organization Citation2022; UNICEF Citation2022; Food and Agriculture Organization of the United Nations Citation2020; Dietitians Australia Citation2022; de Faria Coelho-Ravagnani et al. Citation2021). In general, the normal functions of the immune system need vitamins and minerals, and their deficiencies can lead to susceptibility to viral infections, obesity, metabolic syndrome, and other diseases. To prevent deficiencies, nutritious functional foods such as fruits, vegetables, fish, whole grains, and nuts are often recommended. Consumers may also turn to botanical products, such as elderberry, turmeric, and functional mushrooms, to improve the overall function of the immune system (Council for Responsible Nutrition Citation2020). However, because the immune system is a complex network of organs, tissues, and cells, it is difficult to set proper endpoints and analyze the immune function of a certain nutrient or botanical ingredient, especially for its effectiveness against COVID-19 infection and treatment (National Institutes of Health Citation2022). Therefore, as of August 2022, the U.S. National Institutes of Health (NIH) maintains that data are insufficient to support recommendations for the use of dietary supplements to prevent or treat COVID-19 (National Institutes of Health Citation2022).
Current consumer demand for immune support products
Nevertheless, sales of dietary supplements marketed for immune health support have increased significantly during the pandemic. COVID-19 prompted many consumers to reevaluate and alter their health and lifestyle habits. In 2020, 91% of those who changed their supplement routine as a result of the COVID-19 pandemic increased their supplement intake, with 57% of the increase due to overall immune support (Council for Responsible Nutrition Citation2022). While overall health/wellness benefits remain the top reason for taking supplements, immune health has gained significant traction since the onset of COVID-19, overtaking energy as the second-most cited reason for supplement use in 2020 (Council for Responsible Nutrition Citation2020). Since 2019, supplement users citing immune health support as a reason for their use has increased nine percentage points (36% in 2021) (Council for Responsible Nutrition Citation2021).
Dietary supplement ingredients marketed for immune health: three case studies for ingredient verification testing
Elderberry, turmeric, and mushroom supplements ranked No. 2, 3, and 5 of top-selling herbal supplements in 2020 in US Natural Channel while elderberry sales grew 68.2% and mushroom sales grew 41.8% compared to the 2019 record (HerbalGram Citation2022). According to the 2021 Council for Responsible Nutrition consumer survey data, turmeric is used by 16% of supplement users, and elderberry has grown from 4 to 8% since 2019 and mushroom supplement use has remained steady at 3% (Council for Responsible Nutrition Citation2020; Council for Responsible Nutrition Citation2021; Council for Responsible Nutrition Citation2022). SPINS, a market research firm, and Nutrition Business Journal, a natural products industry publication, published the US retail sales data for these botanical products. To demonstrate how to ensure label compliance and to give an overview about the advancement of quality control testing technologies for botanicals marketed for immune health, we reviewed the analytical testing methodologies for elderberry, turmeric, and functional mushrooms as three case studies. This summary focuses on ingredient verification (what is in the product) and strength (how much of an ingredient is in the product), and does not cover conformation of structure/function claims (does the product do what it says it does).
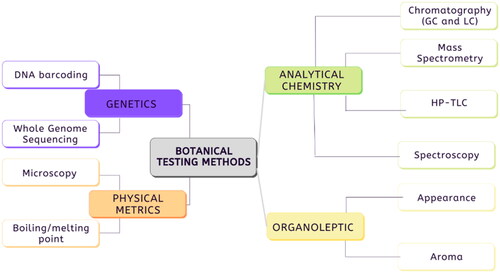
An overview of the methods described in the following case studies is provided in . Testing methods include genetic testing, including barcoding methods like ITS2 and RAPD fingerprinting, as well as physical characteristics, like boiling and melting points and morphological characteristics. Most botanical product testing can be broadly classified as analytical chemistry: chromatography (LC and GC), TLC, spectroscopy (FT-NIR, UV-Vis), and mass spectrometry.
Elderberry
Elderberry and its potential immune functions
Elderberry (Sambucus nigra L.), also known as European elderberry, is a flowering plant that belongs to the Adoxaceae family native to Europe, northern Africa, and western Asia (Gafner et al. Citation2021). It has a diverse set of chemicals credited for its immune properties, as demonstrated in .
Figure 2. Immune compounds in elderberry supplements. These compounds have various functional effects, and are often used for analytical testing.
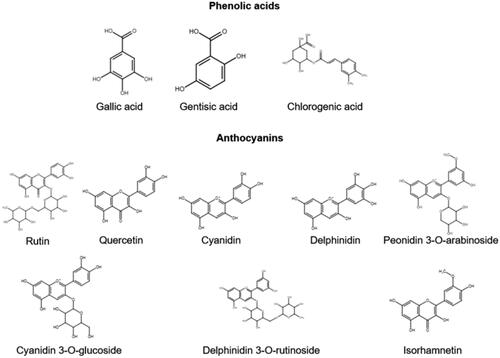
Elderberry has a long history of traditional medicinal use; in the literature it is most often suggested for influenza and immune stimulation. As such, interest in using elderberry for stimulating immune responses against viral infections is growing (Brendler et al. Citation2021). While many studies exist investigating elderberry’s immune potential, there are few that justify its use as an immune supplement in humans at this time. Clinical trials demonstrate that a elderberry extracts can reduce the duration of influenza and cold symptoms in children and adults with limited over-stimulatory immune effects (Zakay-Rones et al. Citation2004; Zakay-Rones et al. Citation1995; Tiralongo, Wee, and Lea Citation2016; Wieland et al. Citation2021). The range of health effects are associated with the high levels of polyphenolic compounds, including gallic acid, gentisic acid, rutin, and quercetin (Liu et al. Citation2022). Some studies show that supplements can reduce the duration and possibility of adverse events of influenza and similar viral diseases compared to placebos (Zakay-Rones et al. Citation2004), while others show that it is only as effective as or less effective than alternative treatments (Rauš et al. Citation2015). Currently, the evidence to support elderberry supplements to prevent or reduce the symptoms of COVID-19 is inconclusive (Wieland et al. Citation2021). Thus, more research is needed before recommending elderberry-based products for the treatment of COVID-19 and other viral infections or as an immunomodulatory supplement.
Elderberry quality control testing methodologies
Elderberry was historically used to adulterate wine and bilberry extracts as it was a relatively inexpensive anthocyanin colorant source (Bridle and García-Viguera Citation1996; Foster and Blumenthal Citation2012). However, due to consumers’ increased interest in elderberry dietary supplements during COVID-19, elderberry extracts and finished products have become valuable dietary ingredients, raising concerns of low quality and adulteration (Gafner et al. Citation2021). According to the Botanical Adulterants Prevention Program (BAPP), there was a high amount of adulteration in elderberry in 2021. Since elderberry adulteration is an emerging issue, limited studies are available on this topic to date. Elderberry polyphenols, particularly their anthocyanins, can be beneficial in oxidative stress-related diseases (Zielińska-Wasielica et al. Citation2019). Thus, elderberry polyphenols are often used as markers for quality control. Due to the high abundance of anthocyanins, strength testing in finished products is usually carried out via anthocyanin quantification. In some cases, other marker compounds such as chlorogenic acid, rutin and isoquercitrin are used to determine the strength of elderberry in marketed supplements (Avula et al. Citation2022).
Analytical technologies for elderberry testing include high-performance thin-layer chromatography (HPTLC), high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LCMS), nuclear magnetic resonance (NMR), DNA-based methodologies, morphological verification, UV spectroscopy, etc. with the first two being the most prevalent.
For identity and adulteration testing, HPTLC has been historically used to identify botanical materials in crude, extracted or finished products (Gunjal Sanket and Dighe Citation2022). HPTLC identification of elderberry is attained by a visual comparison between test samples and authentic materials for the number, sequence, position and color of separated zones (Nicoletti Citation2011). Depending on the interpretation strategy, the HPTLC identification can be either component-based (marker) or pattern-based (Sudberg et al. Citation2010). In highly complex matrices, marker compounds present in limited amount or are accompanied by similar compounds, so HPTLC is not always ideal for marker-based identification and authentication.
HPLC performs great separation efficiency, selectivity and sensitivity, thus is popular among various analytical techniques (Ganzera and Sturm Citation2018). HPLC is probably the most frequently used technique for berry authentication (Salo et al. Citation2021). A significant amount of elderberry authentication is based on the analysis of the anthocyanin profile. For example, European elderberry can be differentiated by its relatively large amount of cyanidin-3-sambubioside. Potential adulterants such as black chokeberry (Aronia melanocarpa), black soybean (Glycine max), black rice (Oryza sativa), mulberry species (Morus australis) and wild cherry (Prunus avium) lack this mono-saccharide anthocyanin (Gardana et al. Citation2014). American elderberry (Sambucus canadensis) is predominant in acylated anthocyanins, thus can be easily distinguished from the European ones (Zhou, Gao, and Giusti Citation2020).
Lately, there are efforts to combine LC or LCMS fingerprinting results with chemometric analysis for adulteration detection and quality control. In study by Viapiana and Wesolowski, a reference chromatographic fingerprint of representative elderberry extract was generated by HPLC as a base for pattern recognition instead of concentrating on one or a few analytes. Correlation coefficients between reference chromatographic fingerprints and the study samples were calculated and the outlying (low quality/adulterated) samples were determined by hierarchical cluster analysis (HCA) and principal component analysis (PCA) (Viapiana and Wesolowski Citation2016).
DNA-based molecular analysis includes highly sensitive and reliable methodologies to identify the composition of complex mixture of food and herbal products (Raime, Krjutškov, and Remm Citation2020). DNA-based adulteration detection can be divided into PCR-based and sequencing-based techniques (Salo et al. Citation2021). PCR-RFLP profiling can differentiate elderberry juice from other fruit juice such as apple, blueberry, grape, pear and pomegranate juice by their different PCR amplicon sizes (Clarke et al. Citation2020). By sequencing-based techniques, Karapatzak et al authenticated elderberry by internal transcribed spacer (ITS) barcoding sequence (Karapatzak et al. Citation2022).
A variety of other micro- and macroscopic tools exist for elderberry evaluation. Microscopic diagnostics is a traditional pharmacopeia method used for commercial herbal product authentication, and the morphological characteristics of the plants, such as xylem cells, stone cells and stomatal complexes form the base of authentication (Ichim, Häser, and Nick Citation2020). NMR (nuclear magnetic resonance) spectroscopy can simultaneously detect a diverse range of abundant primary and secondary metabolites thus being a suitable tool for macroscopic view of the plant metabolome (Wei et al. Citation2022).
Strength testing usually relies on UV spectroscopy, HPTLC, HPLC, and LCMS. The spectrophotometric method is a rapid and relative low-cost approach to quantify anthocyanin content in raw material and finished products. Anthocyanins are predominate in the red-colored flavylium cation form at pH 1.0 and turn to colorless at pH 4.5 (Alappat and Alappat Citation2020). The difference of absorbance at 520 nm under two pH values is proportional to anthocyanin concentration, thus can be employed for quantification purposes (Lee, Durst, and Wrolstad Citation2005). Due to multiple anthocyanins present in elderberry, anthocyanin content is typically calculated as cyanidin-3-glucoside equivalent. Bioactive compounds in elderberry can be quantified by HPTLC, and the content of marker compounds can be simply estimated by applying standards of known concentration along with the sample and visually examined in visible and UV light to determine the relative content. Chromatographic quantification has higher specificity than spectrophotometric and HPTLC methods as it also affords excellent fingerprint profiles (Chandra Singh et al. Citation2020). It can quantify not only anthocyanin content but also a series of other bioactive compounds such as phenolic acid and flavonoids. LCMS is also used frequently for strength testing. More accurate quantification can be obtained under Multiple Reaction Monitoring (MRM) mode when tandem MS is applied, and a low limit of quantification (LOQ) can be reached consequently (Johnson et al. Citation2017).
A summary of literature search results is presented in .
Table 1. Summary of methodologies for elderberry quality control testing.
Turmeric
Turmeric and its immune functions
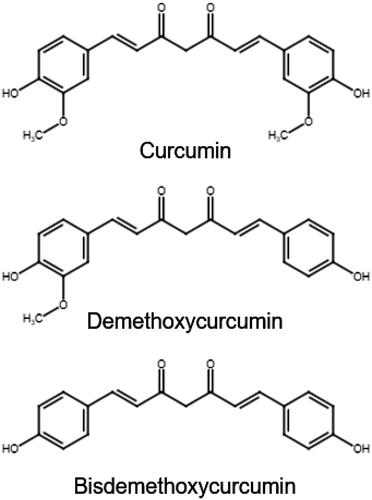
Turmeric (Curcuma longa) is a plant prized for its uses in food and traditional Ayurvedic medicine. Its rhizome has emerged in recent years as a botanical ingredient of interest in addressing a variety of ailments. Many of its actions are attributed to the polyphenol curcumin and related compounds found in the turmeric rhizome that have potential involvement in the modulation of immune pathways, mainly in in vitro studies (Gupta, Patchva, and Aggarwal Citation2013). These compounds are shown in .
While clinical trials exist to evaluate the use of turmeric’s most notable compound curcumin in altering immune function, there is not substantial evidence to support turmeric supplement use for prevention or treatment of immune disorders. Clinical trials have shown reduction of inflammation using curcumin extracts in patients with irritable bowel syndrome, ulcerative colitis, chronic kidney disease, and rheumatoid arthritis (Portincasa et al. Citation2016; Sadeghi et al. Citation2020; Amalraj et al. Citation2017). In osteoarthritis patients, oral curcumin was shown to reduce the numbers of immune response cells, but increased the levels of immunosuppressive Treg cells, potentially modulating an appropriate immune response (Atabaki et al. Citation2020). Additionally, the oral administration of nano-curcumin capsules to adult patients with COVID-19 caused significant decreases in IL-1β and IL-6, both pro-inflammatory cytokines with elevated levels in COVID-19 cases (Valizadeh et al. Citation2020). However, this does not support the use of turmeric of curcumin as a COVID-19 treatment at this time.
Turmeric quality control testing methodologies
Given the increased popularity of blanket turmeric usage in the modern era, it is more critical than ever to ensure the authenticity and strength of turmeric products. A “Turmeric Raw Material and Products Laboratory Guidance Document” was published by the American Botanical Council to overview some of the practices that were used for turmeric testing (Cardellina Citation2020). For identity and adulteration testing, spectroscopy was often employed for testing turmeric-containing powders and supplements. Thangavel et al. applied Fourier Transform-Near Infrared (FT-IR) spectroscopy to estimate curcumin, starch, and moisture content in different turmeric samples (Thangavel and Dhivya Citation2019). Spectroscopy methods coupled with PCA can be utilized for detecting the percentage of tartrazine and colored rice flour adulterants in turmeric samples (Chaminda Bandara et al. Citation2020). Adulterants may be added to products for their color to make the product more appealing or look more pure. That is why lead chromate or metanil yellow, which are vibrant yellow in color, have also been used to adulterate turmeric products. Spectroscopy-based methods are also employed for the rapid detection of lead chromate or metanil yellow in turmeric (Erasmus et al. Citation2021; Kar et al. Citation2018). A common method for identifying Curcuma species is based on PCR to amplify the DNA of the material. Sasikumar et al. (Citation2004) used PCR to amplify DNA of three market samples of turmeric powder. When compared to two species, Curcuma zedoaria (wild species) and Curcuma longa L. (common in turmeric), the PCR amplification banding pattern of the market samples showed the presence of more C. zedoaria powder than C. longa powder (Sasikumar et al. Citation2004). An orthogonal approach was also proposed to test the adulteration of synthetic curcuminoids in “natural” turmeric dietary supplements using Accelerater Mass Spectrometry (AMS) and HPLC (You et al. Citation2022).
Often, the same markers that poses bioactive properties are used for identity testing. Thus, the ability to find and quantify a bioactive marker allows simultaneous identification and strength testing. The most abundant bioactive marker components of turmeric are curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin). The most widely used methods for curcuminoid determination are chromatography techniques. One of the early curcuminoid test methods developed for quantification was performed by capillary zone electrophoresis (CZE) with photodiode array detection (PDA). Several HPLC methods are available for identifying and quantifying curcuminoids in commercial food products, finished products (dietary supplements), and turmeric rhizomes (raw materials), including an AOAC First Action Method that has been suggested for Final Action Status (Mudge et al. Citation2016; Mudge et al. Citation2020). Many of these methods are tested for optimal extraction efficiency/quantification of curcuminoids by studying different conditions for extraction solvent, type of column, column temperature, and gradient program. Some labs have optimized a previous HPLC method for UPLC to decrease the run time, lower mobile phase consumption and decrease sample volume (Cheng et al. Citation2010; Poudel, Pandey, and Lee Citation2019). For rapid analysis, an UV spectroscopy method was also used for estimating curcumin in cream products (Kadam, Palamthodi, and Lele Citation2019) and turmeric rhizome samples (Pawar, Gavasane, and Choudhary Citation2018).
A summary of literature search results is presented in .
Table 2. Summary of methodologies for turmeric quality control testing.
Functional mushrooms
Functional mushrooms and their immune functions
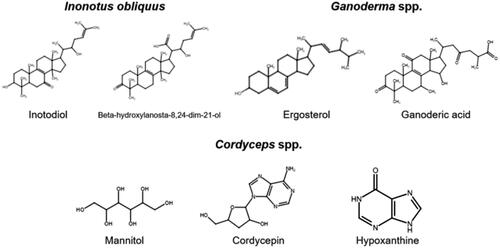
Mushrooms have a long history of traditional use worldwide, with medicinal origins in oriental medicine, European folk remedies, and North American indigenous culture. Out of over 14,000 species of mushrooms, only about 700 have suggested biofunctional properties (Sullivan, Smith, and Rowan Citation2006). We have outlined a small selection of functional mushrooms below. Within the diversity of functional mushrooms, there exists a diversity of functional compounds, as shown in .
Different mushrooms have different potential immune effects, and most studies have not included valid human clinical trials. For example, chaga (Inonotus obliquus) has high levels of polysaccharides that are suggested to have a variety of immunomodulatory, anti-inflammatory, and anti-tumor effects in mice models (Mishra et al. Citation2012; Choi et al. Citation2010; Li et al. Citation2021), whole cell assays (Van et al. Citation2009; Song et al. Citation2013), and in vitro assays (Hu et al. Citation2009). Oyster mushroom (Pleurotus ostreatus) extracts also contain a myriad of polysaccharides and proteins that contribute to an immunostimulative effect (Mariga et al. Citation2014; Ullah et al. Citation2015; Mishra et al. Citation2021), like β-glucans, which exert an antiallergic immunomodulatory effect and provide respiratory tract infection relief in adults and children (Devi et al. Citation2013; Dobšíková et al. Citation2012; Dewi and Mukti Citation2022; Majtan Citation2012; Jesenak et al. Citation2014). Similarly, the shiitake (Lentinus edodes) polysaccharide lentinan, isolated from the mushroom fruiting bodies, stimulates various kinds of immune cell reactivates in vitro (Yap and Ng Citation2003). Another popular mushroom, reishi (Ganoderma lucidum) has a documented effect on immune cell and protein function in rats (Kubota et al. Citation2018; Altai, Altobje, and Dawood Citation2022), and is a rich source of vitamins and minerals, triterpenoids, polysaccharides, and proteins, which may collectively contribute to health (Sheikha and Farag Citation2022).
Interestingly, turkey tail (Trametes versicolor) has been used as a commercial drug; in 1965 Krestin, was developed in Japan from T. versicolor with a marked effect against various types of tumors in experimental animals when administered intraperitoneally or orally (Mizuno Citation1999). Currently there is at least one clinical trial evaluating turkey tails’ potential as a COVID-19 antagonist (Slomski Citation2021) and a 2021 in silico study demonstrated that chaga compounds have a strong binding interaction with the S1-carboxy-terminal domain of the SARS-CoV-2 receptor-binding domain in a molecular docking study (Eid et al. Citation2021).
While studies suggest functional mushrooms are beneficial for immune health, there is not yet substantial evidence to support their use as a therapeutic agent and more studies in humans are necessary before a clinical transition. There are a few reviews summarizing the potential use of functional mushrooms as COVID-19 prevention, symptom reduction, and overall respiratory health (El-Ramady et al. Citation2022; Booi et al. Citation2022; Hapuarachchi and Wen Citation2022). However, these mainly cover studies in non-COVID respiratory illnesses, and lack clinical backing. As more research is conducted, better recommendations for use of functional mushrooms will become available.
Functional mushroom control testing methodologies
The standardization of mushrooms’ quality control testing methodologies has been a glaring problem in the industry for decades. So far, limited methodologies have been published and most of them are difficult to be adopted as consensus industrial standards, partially because of the lack of commercially available reference standards or the expense of special instrumentation requirements. Identity and adulteration testing to differentiate between species of mushrooms present in raw materials or supplements starts with identifying marker compounds that are present in a particular species of mushroom. Once marker compounds are identified, they can be tested for within unknown samples to determine if they are from a certain species of mushroom. Frommenwiler et al. (Citation2020) applied HPTLC fingerprinting by detecting triterpene acids in Ganoderma lucidum fruiting body (Frommenwiler et al. Citation2020). Using HPLC, Inotodiol and 3ß-hydroxylanosta-8,24-dien-21-al were shown to be two markers that can identify a mushroom as Chaga (Inonotus obliquus) (Kim, Choi, et al. Citation2020). Hericerin A and isohericenone J were identified as markers for Lion’s Mane (Hericium erinaceus) and could also be used to distinguish between unknown mushroom samples using 2D NMR spectroscopy and LCMS (Li et al. Citation2015). A study in 2015 (Wang et al.) examined the chemical composition of natural and cultured Cordyceps sinesis with an amino acid analyzer (Wang et al. Citation2015). This could be applied to other species of mushroom and allow for identification of species by amino acid composition. Other than fingerprinting based on chemical composition, DNA can also be extracted from mushrooms and amplified by PCR or other DNA-based methods and compared to reference materials using banding patterns on an agarose gel. Several studies on Reishi mushroom (G. lucidum) have applied DNA-based methods. In one method by Gunnels et al. (Citation2020) DNA barcoding is used to distinguish between G. lucidum and Ganoderma lingzhi. Using best-hit BLAST and molecular phylogenic analyses they identified the presence of G. lingzhi in the seven supplements they tested (Gunnels et al. Citation2020). Using orthogonal testing approach, a research group evaluated the quality of dietary supplements of G. lucidum from the United States using HPTLC, carbohydrate gel electrophoresis (PACE), and GCMS (Wu et al. Citation2017). Of the samples tested, only 26.3% (5 out of 19) of them contained G. lucidum as indicated by their product labels, which results exemplified the need for adulteration testing in functional mushroom products.
For strength testing in mushrooms, HPLC and LCMS methods are conventional techniques. As discussed, specific markers (active ingredients) are the focus of quantification techniques. For mushrooms, the markers for individual mushrooms can be single compounds, such as inotodiol in Chaga (Kim et al. Citation2021), or an array of compounds, such as triterpenes in Ganoderma species (Liu et al. Citation2017). For Cordyceps, spectroscopy could be used to estimate levels of adenosine and cordycepin in Cordyceps militaris fruiting bodies, followed by quantification by HPLC (Singpoonga et al. Citation2020). Additionally, HPLC-MS-MS could be utilized for the separation of Cordyceps sinesis and C. ciadae, based on 13 compounds such as cytidine, uracil, and 2′-deoxyuridine (Meng et al. Citation2019). Dong et al. (Citation2018) used stable isotope labeling-LCMS to quantify free amino acids found in shiitake mushrooms (Dong et al. Citation2018). A few laboratories focus on techniques for extracting compounds from mushrooms. One lab studied the effects of pH and water temperature for the extraction of Chaga mushroom (Abu-Reidah et al. Citation2021). Another research group optimized Soxhlet extraction time, ethanol content, and temperature (for heat-assisted extraction) or ultrasonic power (for ultrasound assisted extraction) for the extraction of triterpenoids and phenolic compounds from Ganoderma lucidum using response surface methodology (Oludemi et al. Citation2018). Microwave-assisted extraction was studied for the recovery of polyphenols in shiitake mushrooms (Xiaokang, Lyng, et al. Citation2020). These improved extractions allowed for the best conditions in extracting compounds of interest in different mushrooms. Following optimized extraction conditions, HPLC or LCMS techniques were employed for detection and quantification of recovered compound markers.
A summary of literature search results is presented in .
Table 3. Summary of methodologies for functional mushroom quality control testing.
Advantages and disadvantages of different analytical approaches
With so many analytical options, it can seem overwhelming to determine the best approach for specific products, botanicals, formulations, and needs. A detailed comparison of the different techniques is outside the scope of this article, but has been extensively reviewed elsewhere (Balekundri and Mannur Citation2020; Fibigr, Šatínský, and Solich Citation2018; Upton et al. Citation2020). We have briefly outlined the advantages and disadvantages of different approaches below which may serve as a starting point for understanding differences in methodology.
Modern analytical approaches for the authenticity and strength testing of botanical supplement label compliance have largely abandoned traditional nonspecific wet chemistry approaches such as gravimetric and titrimetric analysis. The industry is pursuing specific, rapid, and accurate methodologies that provide concurrent compound separation, identification, and quantification. Broad advantages and disadvantages for each of the 3 major methodology categories are listed below.
Chromatography: The most common chromatography techniques for botanical strength and identity analysis are HPLC, HPTLC, and GC, all of which have been discussed throughout this paper. It is the most popular analytical approach for a reason: chromatography requires a very small amount of sample, can separate a broad range of compounds, can detect and identify trace amounts of analytes, and remains consistent between runs. To measure the abundance and identity of the target compounds, chromatography methods usually couple with one or multiple detectors, such as UV-vis spectrophotometer and Mass Spectrometry for HPLC, densitometry for HPTLC, and flame ionization detector for GC. Among those, Mass Spectrometry instrumentation is constantly improving and can now provide high resolution, accurate mass information for hundreds of compounds, meaning detailed supplement profiles are available. There are disadvantages though, including the need for trained specialists to operate instruments and for data analysis, expensive parts and maintenance, and high organic solvent requirements.
Spectroscopy: Spectroscopy is a popular approach for botanical authentication analysis. In general, spectroscopy methodologies measure absorption and emission of light and other radiation by matter. Ultraviolet (UV), visible (Vis), fluorescence (FL), infrared (IR), raman, and nuclear magnetic resonance (NMR) are the common types of spectroscopy for botanical analysis. In some cases, like colorful anthocyanins, light in the visual spectrum is appropriate. This allows a simple, fast, and cost effective measurement when coupled with specialized extractions for the compounds of interest. However, these assay based measurements lack absolute quantification and identification for multiple phytochemicals because they do not provide compound separation. Stand-alone UV-vis spectroscopy is typically more cost effective with easier analysis than spectrometry methods that are coupled with chromatography techniques, but it cannot identify specific compounds within an absorption range and analysis is limited to compounds that can absorb UV light. Additionally, IR and NMR methodologies are fast and require minimal sample preparation (can be used on mixtures) and provide a large amount of organic compound information. When used correctly, this large amount of information can be instrumental for multivariate analysis for botanical authentication determination. However, these methodologies rely heavily on well-established database that is composed with information from authentic botanical reference materials and are also difficult to be applied to complex mixtures and dietary supplement finished products.
Genetic methods: DNA analysis for botanical identity determination appears frequently in literature and academic studies, but rarely in analytical testing labs in the industry. While PCR based approaches, which amplify and compare segments of DNA between samples to determine genetic relationships, can distinguish samples to a subspecies and chemotype level, they are not specific enough to use on a single sample in isolation. There is also the limitation of needing high-quality tissue for successful DNA extractions – often botanical products are oils, tinctures, powders, etc that cannot be used for DNA extractions. Next-generation sequencing is a trending technique that can provide parallelization of sequencing reaction to generate multiple reads to identify low-level fragmented DNA in botanical products. It complements the shortcomings of PCR based approaches and has potential to be used for botanical identification testing for label compliance verification. However, limited data is available to determine the feasibility and cost-effectiveness of this technique. Thus, more studies and industrial applications for next-generation sequencing are needed before it can be widely used.
Future research needs and key questions
Due to high market demand during the pandemic, “immune health” dietary supplement formulators are seeking new approaches to better deliver health benefits to consumers. Multi-ingredient products are becoming popular due to potential synergistic effects among different bioactive ingredients. For example, studies have revealed that administration of vitamin C in combination with quercetin provides synergistic antiviral, antioxidant and immunomodulatory effects (Mrityunjaya et al. Citation2020). On the other hand, multi-ingredient products are more likely to contain incorrect amounts of one or more ingredients, and they are more likely to be contaminated or adulterated (Anon Citation2016). Multi-ingredient products can sometimes contain as many as 30–40 ingredients (Binns, Lee, and Lee Citation2018), which complicates label compliance and quality control testing.
For single ingredient products, pharmacopia monographs (USP, ChP, Ph.Eur., JP., etc.) and international standards (AOAC, ISO, AOCS, etc.) are official resources to find analytical methodologies and acceptance criteria to test the identity, strength, purity, composition, and adulteration of dietary supplements for their label claim compliance. However, when “immune health” dietary supplements contain multiple botanical ingredients, they are hard to be assessed by existing monographs or standard methodologies, because it is impossible to develop methodologies for each dietary ingredient combination. Since 2020, USP has started the process to generate a general methodology development guideline (Anon Citation2022a). However, it had not been published until May 2022 due to the complexity of the issue. Future research on the quality control methodologies of multi-ingredient immune health botanicals is encouraged to focus on the development of scientifically valid approaches focused on the specificity, accuracy, and precision of the methodologies. Non-target ingredients from the multi-ingredient formulations introduce unexpected analytical interferences and are easily to generate fake-negative results for identity testing, overestimated results for strength testing, and inaccurate results for adulterant detection. Therefore, methodology developers need to pay extra attention to the specificity of the analytical procedure to make sure it can determine the target analyte in the presence of interferences. When HPLC-DAD or LCMS MRM methods are employed to test multi-ingredient products, it is important to examine the UV-Vis spectra or quantifier/qualifier ion ratios of each target analyte and compare them against purified reference standard to confirm peak purity. When using HPTLC for identity or adulteration testing, it would be preferred to prepare a reference mixture sample from the available reference materials. Although time-consuming, it is also valuable to test reference mixtures lacking one ingredient at a time.
Common dosage forms that can be found in the “immune health” dietary supplement market are tablets, chewable tablets, capsules, soft gels, caplets, tincture, or liquid, which are similar to the ones used in the pharmaceutical industry (You et al. Citation2019; Liu et al. Citation2020). However, recent trends showed that novel forms such as chewing gums, gummies, chocolates, beadlets, shots, and nutrition bars are getting more and more popular as the line blurs between dietary supplement and functional foods from the product development standpoints (Anon Citation2022b; Bussy, You, and Kwik-Uribe Citation2022). Current trends for pharmaceutical dosage forms include patient-centered care, inhalants, compartmentalized dosage forms, robotic pills, and custom 3D printed forms (Anon Citation2022c). Limited dietary supplements are in these forms currently, and not all of these forms are legal for dietary supplements. But they are worthy of method developer’s attentions.
For multi-ingredient and new form “immune health” dietary supplements, sample preparation is particularly important as solubility of different ingredients could vary significantly. The combination between botanical ingredients and excipients/coloring agents further complicate sample preparation and analysis. The food-like dietary supplements such as nutrition bar or chocolate also contain significant amount of fat, protein, and emulsifier, which cannot be easily dissolved and homogenized for instrument analysis. Micro-encapsulated or nano-encapsulated ingredients coated with gelatin and starch also need additional sample preparation steps to free target analytes from the encapsulation matrix. One key element to testing is to ensure that the method of sample preparation provides a reproducible and homogenous solution suitable for analysis (Etse Citation2011). Additionally, a solution of the sample must be free of interferences, stable in the solvent of choice, and compatible with any downstream analysis strategies. Sonication in an organic solvent is a common technique for extraction of the analyte from its dosage form. More difficult matrices like softgels may require special care such as extracting first in water to dissolve the shell. Heating may also be used if the analyte does not degrade in the presence of heat. The extraction solvent, length of extraction time, extraction temperature, and extraction equipment need to be optimized to fully release and separate target marker compounds prior to testing. Sample transfer is preferred to be minimized to diminish analyte loss and improve method precision. Design of Experiments (DoE) is an approach or a set of tools to discover which parameters make the biggest impact to the method output and how to find their optimized conditions. Using DoE tools such as Box-Behnken Response Surface Methodology, Plackett-Burman design, and Youden’s Ruggedness Trial for sample preparation optimization experimental design can make the method development procedures more standardized and systematic (Qiu et al. Citation2019; Doldolova et al. Citation2021; You et al. Citation2020). Limited studies have been performed to evaluate testing strategies for recent novel dosage forms like beadlet formulations or multi-compartment pills. 3D printing dosage form development studies should also be considered to analyze the recovery of target analytes from the new design.
Orthogonal and chemometric approaches
It should be emphasized that adulteration testing is quite onerous, and no individual technique can address all potential adulterants; thus, an orthogonal approach (apply multiple methods for testing) can sometimes achieve a more accurate analytical outcome. Similar to adulteration testing, two or more identification approaches are sometimes combined to overcome spoofs that a single methodology cannot confidently detect. For example, combining DNA barcoding and LCMS fingerprinting have successfully been combined to distinguish between species of multiple taxa.
Non-targeted and semi-targeted metabolomic workflows are also gaining popularity in the botanical testing space. While most of the methods described in this review look at the presence or quantity of a specific chemical markers to access authenticity and strength determination, non-targeted approaches look at the overall metabolite profile (sometimes up to thousands of compounds) to find patterns specific to a species or treatment (Abraham and Kellogg Citation2021). When evaluating a new sample, like to perform identity testing, its overall chemical profile is compared to that of authentic samples to determine its similarity to the expected compound distribution (Kellogg, Kvalheim, and Cech Citation2020). In addition to species-level identification, these approaches can distinguish other factors, like geographic origin (Peng et al. Citation2020), environmental conditions (Costa et al. Citation2022), and storage or processing differences (Khin et al. Citation2020). As technology advances, high-resolution, accurate-mass mass spectrometry instruments allow compound identification and quantification within non-targeted workflows, meaning researchers can investigate both the overall chemical complexity of a sample and single-compound quantification for improved quality control (Garvey et al. Citation2020).
Orthogonal and chemical fingerprinting approaches are increasingly supplemented with advanced statistical visualization tools, including supervised and unsupervised models. Generally, the term chemometrics is used to describe the use of statistical modeling to visualize large chemical datasets. Chemometric approaches are necessary to understand the underlying chemical patterns that differentiate between species or other categories. Unsupervised methods, including principal component analysis (PCA), use non-targeted metabolomic data to determine the innate variations between samples, and can be used to determine if the chemical composition of a new samples is similar to that of a target (reference) dataset (Kellogg, Kvalheim, and Cech Citation2020). Importantly, PCA provides a loadings plot that identifies the compounds most responsible for the distinction between groupings. This is the most common chemometric approach, however other unsupervised methods, like hierarchical cluster analysis (HCA) provide more detailed overviews of sample relationships (Kammoun, Altyar, and Gad Citation2021). Supervised analysis, including partial least squares (PLS), random forest (RF), and support vector machine (SVM) are guided by pre-determined classifications (like adulterated or authentic samples) and allow prediction of a new samples class based on similarity to the chemical profile of each category in the model (Lu et al. Citation2021; You et al. Citation2022; Abraham and Kellogg Citation2021). The benefit of supervised methods over unsupervised methods is the possibility of prediction of a new samples classification based only on its chemical data, with no prior knowledge of species or grouping necessary; this provides an impartial classification based on reference materials and decreases computational time (Pan et al. Citation2020). The downside of supervised methods is the high likelihood of overfitting, where differences in categories are forced when there may not be any true differences (Ghosh et al. Citation2020). As more methods are produced and familiarity with chemometrics increases, the reliability and community acceptance of modeling based approaches to botanical quality control continues to improve.
While these approaches are increasing in popularity, the transition from academic research to an industry setting is slow. This is largely due to needing a diversity of reference standards to build reliable models. To fully encompass the chemical variation present in market available botanicals, models need be constructed with large variation in the experimental design. The future of botanical testing will require reference standard companies, monograph developers (USP, AOAC, etc), and botanists to work together to build robust libraries of reference materials to integrate chemometric approaches into the natural product industry.
Conclusions
The sales of “immune health” botanical ingredients and dietary supplements has increased significantly during the COVID-19 pandemic. With more ingredients and new practitioners flowing into this industry, these products are subjected to quality control testing and label compliance issues. Using elderberry, turmeric, and functional mushrooms as examples, we overviewed analytical approaches and future technology trends for testing the identity, strength, and adulteration of botanical products marketed for “immune health.”
Declaration of interest statement
The authors declare no direct conflicts of interests.
Acknowledgements
We would like to thank Dr. Ryan Snodgrass from USDA for his editorial suggestions. We would also like to acknowledge Institute of Food Technologists - Nutraceuticals and Functional Foods Division and Eurofins Marketing team for their support on this manuscript.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Abraham, E. J., and J. J. Kellogg. 2021. Chemometric-guided approaches for profiling and authenticating botanical materials. Frontiers in Nutrition 8:780228. doi: 10.3389/fnut.2021.780228.
- Abu-Reidah, I. M., A. L. Critch, C. F. Manful, A. Rajakaruna, N. P. Vidal, T. H. Pham, M. Cheema, and R. Thomas. 2021. Effects of pH and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (Inonotus obliquus) mushroom. Antioxidants (Basel, Switzerland) 10 (8):1322. doi: 10.3390/antiox10081322.
- Alappat, B., and J. Alappat. 2020. Anthocyanin pigments: Beyond aesthetics. Molecules 25 (23):5500. doi: 10.3390/molecules25235500.
- Altai, H., M. Altobje, and A. Dawood. 2022. The effect of Ganoderma lucidum powder on some immune parameters in rat. Rafidain Journal of Science 31 (1):1–10. doi: 10.33899/rjs.2022.172921.
- Amalraj, A., K. Varma, J. Jacob, C. Divya, A. B. Kunnumakkara, S. J. Stohs, and S. Gopi. 2017. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. Journal of Medicinal Food 20 (10):1022–30. doi: 10.1089/jmf.2017.3930.
- Anon. 2016. Is it better to buy supplements which are combination formulas or single-ingredient? https://www.consumerlab.com/answers/is-it-better-to-buy-supplements-that-are-combination-formulas-or-single-ingredient/single-ingredient-vs-combination/.
- Anon. 2020. Coronavirus response: Enforcement actions. Federal Trade Commission. https://www.ftc.gov/news-events/features/coronavirus/enforcement.
- Anon. 2022a. 5 Trends spotted at Expo West 2022. WholeFoods Magazine. https://wholefoodsmagazine.com/columns/editorial/5-trends-spotted-at-expo-west-2022/.
- Anon. 2022b. Inside the bottle: Top 10 supplement trends as seen at Expo West. New Hope Network. https://www.newhope.com/vitamins-and-supplements/inside-bottle-top-10-supplement-trends-seen-expo-west?utm_campaign=HLN00INS-CB-ITB-Newsletter-Mar22-033122&utm_emailname=HLN00INS-CB-ITB-Newsletter-Mar22-033122&utm_medium=email&utm_source=Eloqua&utm_MDMContactID=687ba813-45d5-4204-81d3-609e993f8395&utm_campaigntype=Others&utm_sub=Supplement%20industry%20news%20and%20updates%21%20-%20March%202022&eM=a6159ccefc35ebc9c5eea326ad7fca78589676e01a1b265405a80f6994c301f3&eventSeriesCode=ES_NATPRINSDRDGTL&eventEditionCode=HLN00INS&sessionCode=NULL.
- Anon. 2022c. Multi-ingredient dietary supplement products—Development of quality tests. Accessed April 22, 2022. https://www.uspnf.com/notices/gc-prospectus-2800-multi-ingredient-ds-product-dev-quality-tests.
- Atabaki, M., Z. Shariati-Sarabi, J. Tavakkol-Afshari, and M. Mohammadi. 2020. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. International Immunopharmacology 85:106607. doi: 10.1016/j.intimp.2020.106607.
- Avula, B., K. Katragunta, Y.-H. Wang, Z. Ali, R. Srivedavyasasri, S. Gafner, R. Slimestad, and I. A. Khan. 2022. Chemical profiling and UHPLC-QToF analysis for the simultaneous determination of anthocyanins and flavonoids in Sambucus berries and authentication and detection of adulteration in elderberry dietary supplements using UHPLC. International Journal of Food, Agriculture and Environment 110:104584.
- Balekundri, A., and V. Mannur. 2020. Quality control of the traditional herbs and herbal products: A review. Future Journal of Pharmaceutical Sciences 6 (1):67. doi: 10.1186/s43094-020-00091-5.
- Binns, C. W., M. K. Lee, and A. H. Lee. 2018. Problems and prospects: Public health regulation of dietary supplements. Annual Review of Public Health 39:403–20. doi: 10.1146/annurev-publhealth-040617-013638.
- Booi, H.-N., M.-K. Lee, S.-Y. Fung, S.-T. Ng, C.-S. Tan, K.-H. Lim, R. Roberts, and K.-N. Ting. 2022. Medicinal mushrooms and their use to strengthen respiratory health during and post-COVID-19 pandemic. International Journal of Medicinal Mushrooms 24:1–14.
- Brendler, T., A. Al-Harrasi, R. Bauer, S. Gafner, M. L. Hardy, M. Heinrich, H. Hosseinzadeh, A. A. Izzo, M. Michaelis, M. Nassiri-Asl, et al. 2021. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytotherapy Research: PTR 35 (6):3013–31. doi: 10.1002/ptr.7008.
- Bridle, P., and C. García-Viguera. 1996. A simple technique for the detection of red wine adulteration with elderberry pigments. Food Chemistry 55 (2):111–3. doi: 10.1016/0308-8146(95)00179-4.
- Bussy, U., H. You, and C. Kwik-Uribe. 2022. Determination of cocoa flavanols and procyanidins (by degree of polymerization DP1-7) in cocoa-based products by hydrophilic interaction chromatography coupled with fluorescence detection, collaborative study. Journal of AOAC International 105 (4):1060–8. doi: 10.1093/jaoacint/qsac007.
- Cardellina, J. H.II. 2020. Turmeric raw material and products laboratory guidance document. Austin, TX: ABC-AHP-NCNPR Botanical Adulterants Prevention Program.
- Chaminda Bandara, W. G., G. W. Kasun Prabhath, D. W. Sahan Chinthana Bandara Dissanayake, V. R. Herath, G. M. Roshan Indika Godaliyadda, M. P. Bandara Ekanayake, D. Demini, and T. Madhujith. 2020. Validation of multispectral imaging for the detection of selected adulterants in turmeric samples. Journal of Food Engineering 266:109700. doi: 10.1016/j.jfoodeng.2019.109700.
- Chandra Singh, M., C. Kelso, W. E. Price, and Y. Probst. 2020. Validated liquid chromatography separation methods for identification and quantification of anthocyanins in fruit and vegetables: A systematic review. Food Research International (Ottawa, Ont.) 138 (Pt A):109754. doi: 10.1016/j.foodres.2020.109754.
- Cheng, J., K. Weijun, L. Yun, W. Jiabo, W. Haitao, L. Qingmiao, and X. Xiaohe. 2010. Development and validation of UPLC method for quality control of Curcuma longa Linn.: Fast simultaneous quantitation of three curcuminoids. Journal of Pharmaceutical and Biomedical Analysis 53 (1):43–9. doi: 10.1016/j.jpba.2010.03.021.
- Choi, S. Y., S. J. Hur, C. S. An, Y. H. Jeon, Y. J. Jeoung, J. P. Bak, and B. O. Lim. 2010. Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. Journal of Biomedicine & Biotechnology 2010:943516. doi: 10.1155/2010/943516.
- Clarke, M.-A. L., J. J. Dooley, S. D. Garrett, and H. M. Brown. 2020. An investigation into the use of PCR–RFLP profiling for the identification of fruit species in fruit juices. Project Code: Q01111. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.631.8663&rep=rep1&type=pdf.
- Costa, A. R., J. R. de Lima Silva, P. S. Pereira, J. W. Almeida-Bezerra, M. A. S. de Oliveira, P. R. V. Ribeiro, E. S. de Brito, M. A. Drumond, J. T. C. Júnior, J. P. Kamdem, et al. 2022. Influence of abiotic factors on phytochemical diversity of Anacardium occidentale L. Food Bioscience 49:101911. doi: 10.1016/j.fbio.2022.101911.
- Council for Responsible Nutrition. 2020. 2020 CRN consumer survey on dietary supplements. https://www.crnusa.org/resources/2020-crn-consumer-survey-dietary-supplements.
- Council for Responsible Nutrition. 2021. Consumer intelligence to enhance business outcomes. https://www.crnusa.org/resources/consumer-intelligence-enhance-business-outcomes.
- Council for Responsible Nutrition. 2022. 2019 CRN consumer survey on dietary supplements. Accessed February 22, 2022. https://www.crnusa.org/resources/2019-crn-consumer-survey-dietary-supplements.
- Da, J., C.-R. Cheng, S. Yao, H.-L. Long, Y.-H. Wang, I. A. Khan, Y.-F. Li, Q.-R. Wang, L.-Y. Cai, B.-H. Jiang, et al. 2015. A reproducible analytical system based on the multi-component analysis of triterpene acids in Ganoderma lucidum. Phytochemistry 114:146–54. doi: 10.1016/j.phytochem.2014.08.007.
- Devi, K. S. P., B. Roy, P. Patra, B. Sahoo, S. S. Islam, and T. K. Maiti. 2013. Characterization and lectin microarray of an immunomodulatory heteroglucan from Pleurotus ostreatus mycelia. Carbohydrate Polymers 94 (2):857–65. doi: 10.1016/j.carbpol.2013.02.017.
- Dewi, A. D. R., and Y. P. Mukti. 2022. Immunostimulant potential of oyster mushroom (Pleourotus ostreatus) nugget. Jurnal Pangan Dan Agroindustri 10 (2):78–82.
- Dietitians Australia. 2022. COVID-19 tips and resources: Planning your pantry for COVID-19. https://dietitiansaustralia.org.au/smart-eating-for-you/smart-eating-fast-facts/planning-your-pantry-during-the-covid-19-pandemic/.
- Dobšíková, R., J. Blahová, A. Franc, J. Jakubík, I. Mikulíková, H. Modrá, K. Novotná, Z. Svobodová. 2012. Effect of β-1.3/1.6-D-glucan derived from oyster mushroom Pleurotus ostreatus on biometrical, haematological, biochemical, and immunological indices in rainbow trout (Oncorhynchus mykiss). Neuroendocrinology Letters 33 (Suppl 3):96–106.
- Doldolova, K., M. Bener, M. Lalikoğlu, Y. S. Aşçı, R. Arat, and R. Apak. 2021. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chemistry 353:129337. doi: 10.1016/j.foodchem.2021.129337.
- Dong, M., L. Qin, J. Xue, M. Du, S.-Y. Lin, X.-B. Xu, and B.-W. Zhu. 2018. Simultaneous quantification of free amino acids and 5’-nucleotides in shiitake mushrooms by stable isotope labeling-LC-MS/MS analysis. Food Chemistry 268:57–65. doi: 10.1016/j.foodchem.2018.06.054.
- Eid, J. I., B. Das, M. M. Al-Tuwaijri, and W. T. Basal. 2021. Targeting SARS-CoV-2 with chaga mushroom: An in silico study toward developing a natural antiviral compound. Food Science & Nutrition 9 (12):6513–23. doi: 10.1002/fsn3.2576.
- El-Ramady, H., N. Abdalla, K. Badgar, X. Llanaj, G. Törős, P. Hajdú, Y. Eid, and J. Prokisch. 2022. Edible mushrooms for sustainable and healthy human food: Nutritional and medicinal attributes. Sustainability 14 (9):4941. doi: 10.3390/su14094941.
- Erasmus, S. W., L. van Hasselt, L. M. Ebbinge, and S. M. van Ruth. 2021. Real or fake yellow in the vibrant colour craze: Rapid detection of lead chromate in turmeric. Food Control 121:107714. doi: 10.1016/j.foodcont.2020.107714.
- Etse, J. 2011. 6. Novel dosage form analysis. In Separation science and technology, eds. S. Ahuja and S. Scypinski, vol. 10, 225–49. Cambridge, MA: Academic Press.
- de Faria Coelho-Ravagnani, C., F. Campos Corgosinho, F. L. F. Ziegler Sanches, C. Marques Maia Prado, A. Laviano, and J. F. Mota. 2021. Dietary recommendations during the COVID-19 pandemic: An extract. Kompass Nutrition & Dietetics 1 (1):3–7. doi: 10.1159/000513449.
- FDA. 1994. Dietary Supplement Health and Education Act (DSHEA).
- FDA. 2005. Dietary Supplement Labeling Guide. Accessed September 14, 2022. https://www.fda.gov/food/dietary-supplements-guidance-documentsregulatory-information/dietary-supplement-labeling-guide.
- FDA. 2006. Guidance for Industry: FDA’s Implementation of Qualified Health Claims. Accessed September 14, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fdas-implementation-qualified-healthclaims.
- FDA. 2007. Current good manufacturing practice in manufacturing, packaging, labeling, or holding operations for dietary supplements, 21 CFR part 111 [Docket No. 1996N-0417] (Formerly Docket No. 96N-0417) RIN 0910-AB88) (In: 72 Federal Register 34752-34958, June 25, 2007). The Office of the Federal Register, Washington, DC.
- FDA. 2021. Dietary Supplement Labeling Guide. Accessed September 14, 2022. https://www.fda.gov/food/dietary-supplements-guidance-documentsregulatory-information/dietary-supplement-labeling-guide.
- FDA. 2022a. Label Claims for Conventional Foods and Dietary Supplements. Accessed September 14, 2022. https://www.fda.gov/food/food-labelingnutrition/label-claims-conventional-foods-and-dietary-supplements.
- FDA. 2022b. CFR - Code of Federal Regulations Title 21. Accessed September 14, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=111.70.
- Fejer, S., I. Salamon, D. Grulova, S. Michalek, and M. Zvalova. 2013. Elderberry (Sambucus nigra) cultivation in Slovak Republic and identification and quantification of anthocyanins. On Elderberry 1061. https://www.actahort.org/books/1061/1061_28.htm.
- Fibigr, J., D. Šatínský, and P. Solich. 2018. Current trends in the analysis and quality control of food supplements based on plant extracts. Analytica Chimica Acta 1036:1–15. doi: 10.1016/j.aca.2018.08.017.
- Food and Agriculture Organization of the United Nations. 2020. Maintaining a healthy diet during the COVID-19 pandemic. https://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1269769/.
- Foster, S., and M. Blumenthal. 2012. The adulteration of commercial bilberry extracts. HerbalGram. https://www.academia.edu/download/41820469/Bilberry-HerbalGram-96-2012.pdf.
- Frommenwiler, D. A., D. Trefzer, M. Schmid, S. Cañigueral, and E. Reich. 2020. Comprehensive HPTLC fingerprinting: A novel economic approach to evaluating the quality of Ganoderma lucidum fruiting body. Journal of Liquid Chromatography & Related Technologies 43 (11–12):414–23. doi: 10.1080/10826076.2020.1725560.
- Gafner, S., T. Borchard, M. Bush, S. Sudberg, N. G. Feuillère, M. Y. Tenone, J. H. Jolibois, et al. 2021. Tales from the elder: Adulteration issues of elder berry. HerbalEGramno 130:24–32.
- Galetti, J. A. 2016. A competitive assessment commercial elderberry (Sambucus sp.) products and the evaluation of Copigmentation within elderberry tinctures. https://search.proquest.com/openview/aa27b54fa99659962f282a902b1da472/1?pq-origsite=gscholar&cbl=18750.
- Ganzera, M., and S. Sturm. 2018. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. Journal of Pharmaceutical and Biomedical Analysis 147:211–33. doi: 10.1016/j.jpba.2017.07.038.
- Gardana, C., S. Ciappellano, L. Marinoni, C. Fachechi, and P. Simonetti. 2014. Bilberry adulteration: Identification and chemical profiling of anthocyanins by different analytical methods. Journal of Agricultural and Food Chemistry 62 (45):10998–1004. doi: 10.1021/jf504078v.
- Garvey, J., T. Walsh, E. Devaney, T. King, and R. Kilduff. 2020. Multi-residue analysis of pesticide residues and polychlorinated biphenyls in fruit and vegetables using orbital ion trap high-resolution accurate mass spectrometry. Analytical and Bioanalytical Chemistry 412 (26):7113–21. doi: 10.1007/s00216-020-02844-w.
- Ghosh, T., W. Zhang, D. Ghosh, and K. Kechris. 2020. Predictive modeling for metabolomics data. Methods in Molecular Biology (Clifton, N.J.) 2104:313–36. doi: 10.1007/978-1-0716-0239-3_16.
- Govindaraghavan, S. 2014. Pharmacopeial HPLC identification methods are not sufficient to detect adulterations in commercial bilberry (Vaccinium myrtillus) extracts. Anthocyanin profile provides additional clues. Fitoterapia 99:124–38. doi: 10.1016/j.fitote.2014.09.007.
- Gunjal Sanket, B., and P. R. Dighe. 2022. Analysis of herbal drugs by HPTLC: A review. Asian Journal of Pharmaceutical Research 10 (2):125–8.
- Gunnels, T., M. Creswell, J. McFerrin, and J. B. Whittall. 2020. The ITS region provides a reliable DNA barcode for identifying Reishi/Lingzhi (Ganoderma) from herbal supplements. PLoS One 15 (11):e0236774. doi: 10.1371/journal.pone.0236774.
- Gupta, S. C., S. Patchva, and B. B. Aggarwal. 2013. Therapeutic roles of curcumin: Lessons learned from clinical trials. The AAPS Journal 15 (1):195–218. doi: 10.1208/s12248-012-9432-8.
- Güzelmeriç, E., C. Çelik, N. B. Şen, M. A. Oçkun, and E. Yeşilada. 2021. Quali/quantitative research on herbal supplements containing black elder (Sambucus nigra L.) fruits. JCT Research 25 (3):238–48.
- Hapuarachchi, K. K, and T. C. Wen. 2022. Therapeutic potential of medicinal mushrooms: Insights into its use against Covid-19. In Biology, cultivation and applications of mushrooms, eds. A. Arya and K. Rusevska, 27–46. Singapore: Springer. doi: 10.1007/978-981-16-6257-7_2.
- HerbalGram. 2022. Herbal supplement sales in US increase by record-breaking 17.3% in 2020—American Botanical Council. Accessed February 17, 2022. https://www.herbalgram.org/resources/herbalgram/issues/131/table-of-contents/hg131-mkrpt/.
- Ho, G. T. T., H. Wangensteen, and H. Barsett. 2017. Elderberry and elderflower extracts, phenolic compounds, and metabolites and their effect on complement, RAW 264.7 macrophages and dendritic cells. International Journal of Molecular Sciences 18 (3):584. doi: 10.3390/ijms18030584.
- Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nature Immunology 5 (10):971–4. doi: 10.1038/ni1004-971.
- Hu, H., Z. Zhang, Z. Lei, Y. Yang, and N. Sugiura. 2009. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. Journal of Bioscience and Bioengineering 107 (1):42–8. doi: 10.1016/j.jbiosc.2008.09.004.
- Huang, L.-F., M.-J. Wu, X.-J. Sun, F.-Q. Guo, Y.-Z. Liang, and X.-R. Li. 2004. Simultaneous determination of adenine, uridine and adenosine in cordyceps sinensis and its substitutes by LC/ESI-MS. Journal of Central South University of Technology 11 (3):295–9. doi: 10.1007/s11771-004-0060-z.
- Ichim, M. C., A. Häser, and P. Nick. 2020. Microscopic authentication of commercial herbal products in the globalized market: Potential and limitations. Frontiers in Pharmacology 11:876. doi: 10.3389/fphar.2020.00876.
- Jadhav, B.-K., K.-R. Mahadik, and A.-R. Paradkar. 2007. Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatographia 65 (7–8):483–8. doi: 10.1365/s10337-006-0164-8.
- Jesenak, M., M. Hrubisko, J. Majtan, Z. Rennerova, and P. Banovcin. 2014. Anti-allergic effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Phytotherapy Research: PTR 28 (3):471–4. doi: 10.1002/ptr.5020.
- Johnson, M., C. M. Dela Libera Tres, A. L. Thomas, G. E. Rottinghaus, and C. M. Greenlief. 2017. Discriminant analyses of the polyphenol content of American elderberry juice from multiple environments provide genotype fingerprint. Journal of Agricultural and Food Chemistry 65 (20):4044–50. doi: 10.1021/acs.jafc.6b05675.
- Kadam, D., S. Palamthodi, and S. S. Lele. 2019. Complexation of curcumin with Lepidium sativum protein hydrolysate as a novel curcumin delivery system. Food Chemistry 298:125091. doi: 10.1016/j.foodchem.2019.125091.
- Kamarli Altun, H., M. S. Karacil Ermumcu, and N. Seremet Kurklu. 2021. Evaluation of dietary supplement, functional food and herbal medicine use by dietitians during the COVID-19 pandemic. Public Health Nutrition 24 (5):861–9. doi: 10.1017/S1368980020005297.
- Kammoun, A. K., A. E. Altyar, and H. A. Gad. 2021. Comparative metabolic study of citrus sinensis leaves cultivars based on GC-MS and their cytotoxic activity. Journal of Pharmaceutical and Biomedical Analysis 198:113991. doi: 10.1016/j.jpba.2021.113991.
- Kar, S., B. Tudu, A. K. Bag, and R. Bandyopadhyay. 2018. Application of near-infrared spectroscopy for the detection of metanil yellow in turmeric powder. Food Analytical Methods 11 (5):1291–302. doi: 10.1007/s12161-017-1106-9.
- Karapatzak, E., O. Dichala, I. Ganopoulos, A. Karydas, K. Papanastasi, D. Kyrkas, P. Yfanti, N. Nikisianis, D. Fotakis, G. Patakioutas, et al. 2022. Molecular authentication, propagation trials and field establishment of Greek native genotypes of Sambucus nigra L. (Caprifoliaceae): Setting the basis for domestication and sustainable utilization. Agronomy 12 (1):114. doi: 10.3390/agronomy12010114.
- Kellogg, J. J., O. M. Kvalheim, and N. B. Cech. 2020. Composite score analysis for unsupervised comparison and network visualization of metabolomics data. Analytica Chimica Acta 1095:38–47. doi: 10.1016/j.aca.2019.10.029.
- Khin, M., N. B. Cech, J. J. Kellogg, and L. K. Caesar. 2020. Chemical evaluation of the effects of storage conditions on the botanical goldenseal using marker-based and metabolomics approaches. The Yale Journal of Biology and Medicine 93 (2):265–75.
- Kim, J., S. C. Yang, A. Y. Hwang, H. Cho, and K. T. Hwang. 2020. Composition of triterpenoids in Inonotus obliquus and their anti-proliferative activity on cancer cell lines. Molecules 25 (18):4066. doi: 10.3390/molecules25184066.
- Kim, J. H., D. Gao, C. W. Cho, I. Hwang, H. M. Kim, and J. S. Kang. 2021. A novel bioanalytical method for determination of inotodiol isolated from Inonotus obliquus and its application to pharmacokinetic study. Plants 10 (8):1631. doi: 10.3390/plants10081631.
- Kim, T. I., J.-G. Choi, J. H. Kim, W. Li, and H.-S. Chung. 2020. Blocking effect of chaga mushroom (Inonotus oliquus) extract for immune checkpoint CTLA-4/CD80 interaction. NATO Advanced Science Institutes Series E: Applied Sciences 10 (17):5774.
- Krüger, S., M. Mirgos, and G. E. Morlock. 2015. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. Journal of Chromatography. A 1426:209–19. doi: 10.1016/j.chroma.2015.11.021.
- Kubota, A., M. Kobayashi, S. Sarashina, R. Takeno, K. Okamoto, K. Narumi, A. Furugen, Y. Suzuki, N. Takahashi, and K. Iseki. 2018. Reishi mushroom Ganoderma lucidum modulates IgA production and alpha-defensin expression in the rat small intestine. Journal of Ethnopharmacology 214:240–3. doi: 10.1016/j.jep.2017.12.010.
- Lechtenberg, M., B. Quandt, and A. Nahrstedt. 2004. Quantitative determination of curcuminoids in curcuma rhizomes and rapid differentiation of Curcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochemical Analysis: PCA 15 (3):152–8. doi: 10.1002/pca.759.
- Lee, J., R. W. Durst, and R. E. Wrolstad. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International 88 (5):1269–78.
- Lee, J., and C. E. Finn. 2007. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. Journal of the Science of Food and Agriculture 87 (14):2665–75. doi: 10.1002/jsfa.3029.
- Lee, J. H., and M.-G. Choung. 2011. Determination of curcuminoid colouring principles in commercial foods by HPLC. Food Chemistry 124 (3):1217–22. doi: 10.1016/j.foodchem.2010.07.049.
- Li, J., C. Qu, F. Li, Y. Chen, J. Zheng, Y. Xiao, Q. Jin, G. Jin, X. Huang, and D. Jin. 2021. Inonotus obliquus polysaccharide ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer in mice via activation of the NLRP3 inflammasome. Frontiers in Pharmacology 11:621835. doi: 10.3389/fphar.2020.621835.
- Li, W., W. Zhou, E.-J. Kim, S. H. Shim, H. K. Kang, and Y. H. Kim. 2015. Isolation and identification of aromatic compounds in lion’s mane mushroom and their anticancer activities. Food Chemistry 170:336–42. doi: 10.1016/j.foodchem.2014.08.078.
- Liu, D., X.-Q. He, D.-T. Wu, H.-B. Li, Y.-B. Feng, L. Zou, and R.-Y. Gan. 2022. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. Journal of Agricultural and Food Chemistry 70 (14):4202–20. doi: 10.1021/acs.jafc.2c00010.
- Liu, T., Q. Tang, Y. Yang, S. Zhou, D. Wu, C. Tang, Z. Zhang, M. Yan, J. Feng, and J.-S. Zhang. 2017. Characterization of polysaccharides from the fruiting bodies of two species of genus Ganoderma (agaricomycetes) and determination of water-soluble β-D-glucan using high-performance liquid chromatography. Journal of Medicinal and Aromatic Plant Sciences 19 (1):75–85.
- Liu, Z., K. Ren, Y. Feng, T. Uong, S. Krepich, and H. You. 2020. Rapid and economic determination of 13 steviol glycosides in market-available food, dietary supplements, and ingredients: Single-laboratory validation of an HPLC method. Journal of Agricultural and Food Chemistry 68 (37):10142–8. doi: 10.1021/acs.jafc.0c03453.
- Lordan, R., H. M. Rando, and C. S. Greene. 2021. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. mSystems 6 (3):e00122-21. doi: 10.1128/mSystems.00122-21.
- Lu, Z., S. M. Handy, N. Zhang, Z. Quan, Q. Xu, M. Ambrose, G. Giancaspro, and N. D. Sarma. 2021. Development and validation of a species-specific PCR method for the identification of ginseng species using orthogonal approaches. Planta Medica. doi: 10.1055/a-1478-9143.
- Majtan, J. 2012. Pleuran (β-glucan from Pleurotus ostreatus): An effective nutritional supplement against upper respiratory tract infections? Acute Topics in Sport Nutrition 59:57–61.
- Mariga, A. M., W.-J. Yang, D. K. Mugambi, F. Pei, L.-Y. Zhao, Y.-N. Shao, and Q. Hu. 2014. Antiproliferative and immunostimulatory activity of a protein from Pleurotus eryngii. Journal of the Science of Food and Agriculture 94 (15):3152–62. doi: 10.1002/jsfa.6665.
- Meng, C., Q. Han, X. Wang, X. Liu, X. Fan, R. Liu, Q. Wang, and C. Wang. 2019. Determination and quantitative comparison of nucleosides in two cordyceps by HPLC-ESI-MS-MS. Journal of Chromatographic Science 57 (5):426–33. doi: 10.1093/chromsci/bmz012.
- Mishra, S. K., J.-H. Kang, D.-K. Kim, S. H. Oh, and M. K. Kim. 2012. Orally administered aqueous extract of inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. Journal of Ethnopharmacology 143 (2):524–32. doi: 10.1016/j.jep.2012.07.008.
- Mishra, V., S. Tomar, P. Yadav, and M. P. Singh. 2021. Promising anticancer activity of polysaccharides and other macromolecules derived from oyster mushroom (Pleurotus sp.): An updated review. International Journal of Biological Macromolecules 182:1628–37. doi: 10.1016/j.ijbiomac.2021.05.102.
- Mitchell, C. A., J. T. Dever, S. Gafner, J. C. Griffiths, D. S. Marsman, C. Rider, C. Welch, and M. R. Embry. 2022. The Botanical Safety Consortium: A public-private partnership to enhance the botanical safety toolkit. Regulatory Toxicology and Pharmacology: RTP 128:105090. doi: 10.1016/j.yrtph.2021.105090.
- Mizuno, T. 1999. The extraction and development of antitumor-active polysaccharides from medicinal mushrooms in Japan (Review). International Journal of Medicinal Mushrooms 1 (1):9–29. doi: 10.1615/IntJMedMushrooms.v1.i1.20.
- Mrityunjaya, M., V. Pavithra, R. Neelam, P. Janhavi, P. M. Halami, and P. V. Ravindra. 2020. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Frontiers in Immunology 11:570122. doi: 10.3389/fimmu.2020.570122.
- Mudge, E., M. Chan, S. Venkataraman, and P. N. Brown. 2016. Curcuminoids in turmeric roots and supplements: Method optimization and validation. Food Analytical Methods 9 (5):1428–35. doi: 10.1007/s12161-015-0326-0.
- Mudge, E. M., P. N. Brown, C. A. Rimmer, and M. M. Phillips. 2020. Determination of curcuminoids in turmeric dietary supplements by HPLC-DAD: Multi-laboratory study through the NIH-ODS/NIST quality assurance program. Journal of AOAC International 103 (6):1625–32. doi: 10.1093/jaoacint/qsaa069.
- National Institutes of Health. 2022. Dietary supplements in the time of COVID-19. https://ods.od.nih.gov/factsheets/COVID19-HealthProfessional/.
- Nicoletti, M. 2011. HPTLC fingerprint: A modern approach for the analytical determination of botanicals. Revista Brasileira de Farmacognosia 21 (5):818–23. doi: 10.1590/S0102-695X2011005000131.
- Oludemi, T., L. Barros, M. A. Prieto, S. A. Heleno, M. F. Barreiro, and I. Ferreira. 2018. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food & Function 9 (1):209–26. doi: 10.1039/c7fo01601h.
- Oniszczuk, A., M. Olech, T. Oniszczuk, K. Wojtunik-Kulesza, and A. Wójtowicz. 2019. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arabian Journal of Chemistry 12 (8):4719–30. doi: 10.1016/j.arabjc.2016.09.003.
- Pan, H., C. Yao, S. Yao, W. Yang, W. Wu, and D. Guo. 2020. A metabolomics strategy for authentication of plant medicines with multiple botanical origins, a case study of Uncariae rammulus cum uncis. Journal of Separation Science 43 (6):1043–50. doi: 10.1002/jssc.201901064.
- Pawar, H. A., A. J. Gavasane, and P. D. Choudhary. 2018. A novel and simple approach for extraction and isolation of curcuminoids from turmeric rhizomes. Natural Products Chemistry & Research 6 (1):300.
- Peng, Q., Q. Xu, B. G. Dula, J. Wang, J. Fu, L. Wang, B. Qian, J. Zhou, J. Wu, J. Wang, et al. 2020. Discrimination of geographical origin of camellia seed oils using electronic nose characteristics and chemometrics. Journal of Consumer Protection and Food Safety 15 (3):263–70. doi: 10.1007/s00003-020-01278-x.
- Portincasa, P., L. Bonfrate, M. Scribano, A. Kohn, N. Caporaso, D. Festi, M. C. Campanale, T. Di Rienzo, M. Guarino, M. Taddia, et al. 2016. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. Journal of Gastrointestinal and Liver Diseases 25 (2):151–7. doi: 10.15403/jgld.2014.1121.252.ccm.
- Poudel, A., J. Pandey, and H.-K. Lee. 2019. Geographical discrimination in curcuminoids content of turmeric assessed by rapid UPLC-DAD validated analytical method. Molecules 24 (9):1805. doi: 10.3390/molecules24091805.
- Qi, Y., L. Zhao, and H. H. Sun. 2012. Development of a rapid and confirmatory method to identify ganoderic acids in Ganoderma mushrooms. Frontiers in Pharmacology 3:85. doi: 10.3389/fphar.2012.00085.
- Qiu, X., H. Zhang, Y. Yin, H. Brandes, T. Marsala, K. Stenerson, H. Cramer, and H. You. 2019. Determination of active vitamin B12 (cobalamin) in dietary supplements and ingredients by reversed-phase liquid chromatography: Single-laboratory validation. Food Chemistry 298:125010. doi: 10.1016/j.foodchem.2019.125010.
- Raime, K., K. Krjutškov, and M. Remm. 2020. Method for the identification of plant DNA in food using alignment-free analysis of sequencing reads: A case study on lupin. Frontiers in Plant Science 11:646. doi: 10.3389/fpls.2020.00646.
- Rauš, K., S. Pleschka, P. Klein, R. Schoop, and P. Fisher. 2015. Effect of an echinacea-based hot drink versus oseltamivir in influenza treatment: A randomized, double-blind, double-dummy, multicenter, noninferiority clinical trial. Current Therapeutic Research, Clinical and Experimental 77:66–72. doi: 10.1016/j.curtheres.2015.04.001.
- Sadeghi, N., A. Mansoori, A. Shayesteh, and S. J. Hashemi. 2020. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytotherapy Research: PTR 34 (5):1123–33. doi: 10.1002/ptr.6581.
- Sahu, P. K., J. Panda, Y. V. V. Jogendra Kumar, and S. K. Ranjitha. 2020. A robust RP-HPLC method for determination of turmeric adulteration. Journal of Liquid Chromatography & Related Technologies 43 (7–8):247–54. doi: 10.1080/10826076.2020.1722162.
- Salo, H. M., N. Nguyen, E. Alakärppä, L. Klavins, A. L. Hykkerud, K. Karppinen, L. Jaakola, M. Klavins, and H. Häggman. 2021. Authentication of berries and berry-based food products. Comprehensive Reviews in Food Science and Food Safety 20 (5):5197–225. doi: 10.1111/1541-4337.12811.
- Sasikumar, B., S. Syamkumar, R. Remya, and T. J. Zachariah. 2004. PCR based detection of adulteration in the market samples of turmeric powder. Food Biotechnology 18 (3):299–306. doi: 10.1081/FBT-200035022.
- Sheikha, E., and A. Farag. 2022. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods 11 (7):1030.
- Singpoonga, N., R. Rittiron, B. Seang-On, P. Chaiprasart, and Y. Bantadjan. 2020. Determination of adenosine and cordycepin concentrations in cordyceps militaris fruiting bodies using near-infrared spectroscopy. ACS Omega 5 (42):27235–44. doi: 10.1021/acsomega.0c03403.
- Slomski, A. 2021. Trials test mushrooms and herbs as anti–COVID-19 agents. JAMA 326 (20):1997–9. doi: 10.1001/jama.2021.19388.
- Song, F.-Q., Y. Liu, X.-S. Kong, W. Chang, and G. Song. 2013. Progress on understanding the anticancer mechanisms of medicinal mushroom: Inonotus obliquus. Asian Pacific Journal of Cancer Prevention: APJCP 14 (3):1571–8. doi: 10.7314/apjcp.2013.14.3.1571.
- Sudberg, S., E. M. Sudberg, J. Terrazas, S. Sudberg, K. Patel, J. Pineda, and B. Fine. 2010. Fingerprint analysis and the application of HPTLC to the determination of identity and quality of botanicals, from an industry perspective. Journal of AOAC International 93 (5):1367–75. doi: 10.1093/jaoac/93.5.1367.
- Sullivan, R., J. E. Smith, and N. J. Rowan. 2006. Medicinal mushrooms and cancer therapy: translating a traditional practice into western medicine. Perspectives in Biology and Medicine 49 (2):159–70. doi: 10.1353/pbm.2006.0034.
- Thangavel, K, and K. Dhivya. 2019. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Engineering in Agriculture, Environment and Food 12 (2):264–9.
- Tiralongo, E., S. S. Wee, and R. A. Lea. 2016. Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 8 (4):182. doi: 10.3390/nu8040182.
- Ullah, M. I., M. Akhtar, Z. Iqbal, M. Shahid, and M. M. Awais. 2015. Immunomodulating and antiprotozoal effects of different extracts of the oyster culinary-medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) against coccidiosis in broiler. International Journal of High Risk Behaviors & Addiction 17 (3):309–17.
- UNICEF. 2022. Easy, affordable and healthy eating tips during COVID-19. https://www.unicef.org/coronavirus/easy-affordable-and-healthy-eating-tips-during-coronavirus-disease-covid-19-outbreak.
- Upton, R., B. David, S. Gafner, and S. Glasl. 2020. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochemistry Reviews 19 (5):1157–77. doi: 10.1007/s11101-019-09625-z.
- United States Code. 2006. 21 U.S. Code § 342 - Adulterated food. Accessed September 14, 2022. https://www.law.cornell.edu/uscode/text/21/342.
- Vaagt, F., I. Haase, and M. Fischer. 2013. Loop-mediated isothermal amplification (LAMP)-based method for rapid mushroom species identification. Journal of Agricultural and Food Chemistry 61 (8):1833–40. doi: 10.1021/jf304824b.
- Valizadeh, H., S. Abdolmohammadi-Vahid, S. Danshina, M. Ziya Gencer, A. Ammari, A. Sadeghi, L. Roshangar, S. Aslani, A. Esmaeilzadeh, M. Ghaebi, et al. 2020. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. International Immunopharmacology 89 (Pt B):107088. doi: 10.1016/j.intimp.2020.107088.
- Van, Q., B. N. Nayak, M. Reimer, P. J. H. Jones, R. G. Fulcher, and C. B. Rempel. 2009. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. Journal of Ethnopharmacology 125 (3):487–93. doi: 10.1016/j.jep.2009.06.026.
- Viapiana, A, and M. Wesolowski. 2016. HPLC fingerprint combined with quantitation of phenolic compounds and chemometrics as an efficient strategy for quality consistency evaluation of Sambucus nigra berries. Natural Product Communications 11 (10):1449–54. doi: 10.1177/1934578X1601101009.
- Vidal-Casanella, O., N. Nuñez, S. Sentellas, O. Núñez, and J. Saurina. 2021. Characterization of turmeric and curry samples by liquid chromatography with spectroscopic detection based on polyphenolic and curcuminoid contents. Separations 7 (2):23. doi: 10.3390/separations7020023.
- Villani, T. S., H. Patel, J. Zhen, A. R. Koroch, and J. E. Simon. 2015. Microscopy for quality assessment of bilberry fruit (Vaccinium myrtillus L.). Journal of Medicinally Active Plants 4 (1):8–15.
- Vlachojannis, C., B. F. Zimmermann, and S. Chrubasik-Hausmann. 2015. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytotherapy Research: PTR 29 (4):561–5. doi: 10.1002/ptr.5284.
- Wang, J., L. Kan, S. Nie, H. Chen, S. W. Cui, A. O. Phillips, G. O. Phillips, Y. Li, and M. Xie. 2015. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured cordyceps sinensis. LWT - Food Science and Technology 63 (1):2–7. doi: 10.1016/j.lwt.2015.03.109.
- Wei, X., J. Yao, F. Wang, D. Wu, and R. Zhang. 2022. Extraction, isolation, structural characterization, and antioxidant activity of polysaccharides from elderberry fruit. Frontiers in Nutrition 9:947706. doi: 10.3389/fnut.2022.947706.
- Wieland, L. S., V. Piechotta, T. Feinberg, E. Ludeman, B. Hutton, S. Kanji, D. Seely, and C. Garritty. 2021. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complementary Medicine and Therapies 21 (1):112. doi: 10.1186/s12906-021-03283-5.
- World Health Organization. 2022. Nutrition advice for adults during the COVID-19 outbreak. WHO, Regional Office for the Eastern Mediterranean. Accessed February 16, 2022. http://www.emro.who.int/nutrition/news/nutrition-advice-for-adults-during-the-covid-19-outbreak.html.
- Wu, D.-T., Y. Deng, L.-X. Chen, J. Zhao, A. Bzhelyansky, and S.-P. Li. 2017. Evaluation on quality consistency of Ganoderma lucidum dietary supplements collected in the United States. Scientific Reports 7 (1):7792. doi: 10.1038/s41598-017-06336-3.
- Xiaokang, W., N. P. Brunton, J. G. Lyng, S. M. Harrison, S. T. Carpes, and K. Papoutsis. 2020. Volatile and non-volatile compounds of shiitake mushrooms treated with pulsed light after twenty-four hour storage at different conditions. Food Bioscience 36:100619. doi: 10.1016/j.fbio.2020.100619.
- Xiaokang, W., J. G. Lyng, N. P. Brunton, L. Cody, J.-C. Jacquier, S. M. Harrison, and K. Papoutsis. 2020. Monitoring the effect of different microwave extraction parameters on the recovery of polyphenols from shiitake mushrooms: Comparison with hot-water and organic-solvent extractions. Biotechnology Reports (Amsterdam, Netherlands) 27:e00504. doi: 10.1016/j.btre.2020.e00504.
- Yap, N., and M. L. Ng. 2003. Immunopotentiating properties of lentinan (1®3)-b-D-glucan extracted from culinary–medicinal shiitake mushroom Lentinus edodes (Berk.) Singer (Agaric). International Journal of Medicinal Mushrooms 5 (4):20.
- You, H., H. Gershon, F. Goren, F. Xue, T. Kantowski, and L. Monheit. 2022. Analytical strategies to determine the labelling accuracy and economically-motivated adulteration of “natural” dietary supplements in the marketplace. Food Chemistry 370:131007. doi: 10.1016/j.foodchem.2021.131007.
- You, H., B. Ireland, M. Moeszinger, H. Zhang, L. Snow, S. Krepich, and V. Takagawa. 2019. Determination of bioactive nonvolatile ginger constituents in dietary supplements by a rapid and economic HPLC method: Analytical method development and single-laboratory validation. Talanta 194:795–802. doi: 10.1016/j.talanta.2018.10.075.
- You, H., B. Ireland, M. Moeszinger, H. Zhang, L. Snow, S. Krepich, and V. Takagawa. 2020. Determination of select nonvolatile ginger constituents in dietary ingredients and finished dosage forms, first action 2018.04. Journal of AOAC International 103 (1):124–31. doi: 10.5740/jaoacint.19-0004.
- Zakay-Rones, Z., E. Thom, T. Wollan, and J. Wadstein. 2004. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. The Journal of International Medical Research 32 (2):132–40. doi: 10.1177/147323000403200205.
- Zakay-Rones, Z., N. Varsano, M. Zlotnik, O. Manor, L. Regev, M. Schlesinger, and M. Mumcuoglu. 1995. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. The Journal of Alternative and Complementary Medicine 1 (4):361–9. doi: 10.1089/acm.1995.1.361.
- Zhou, Y., Y. G. Gao, and M. M. Giusti. 2020. Accumulation of anthocyanins and other phytochemicals in american elderberry cultivars during fruit ripening and its impact on color expression. Plants 9 (12):1721. doi: 10.3390/plants9121721.
- Zielińska-Wasielica, J., A. Olejnik, K. Kowalska, M. Olkowicz, and R. Dembczyński. 2019. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods (Basel, Switzerland) 8 (8):326. doi: 10.3390/foods8080326.