Abstract
Approval of midostaurin, a multikinase inhibitor, in combination with chemotherapy for the treatment of adults with newly diagnosed FLT3 mutation–positive acute myeloid leukemia, was based on the phase 3 RATIFY trial results. RADIUS-X (NCT02624570) was an expanded access program providing access to midostaurin during regulatory review and extending the understanding of the safety and tolerability of midostaurin. Patients aged ≥18 years received midostaurin with 1–2 cycles of induction therapy (cytarabine plus daunorubicin or idarubicin) and ≤4 cycles of high-dose cytarabine consolidation chemotherapy or as single-agent maintenance therapy. The study enrolled 103 patients. No new safety events were observed; toxicities were not influenced by age, anthracycline choice, or coadministration of CYP3A4 inhibitors. The most common adverse events (AEs) were febrile neutropenia, nausea, and diarrhea. During maintenance, 46% of patients reported AEs. Midostaurin demonstrated a manageable safety profile and was associated with high transplant and low on-treatment relapse rates.
Introduction
Patients with acute myeloid leukemia (AML) harboring mutations in the fms-like tyrosine kinase 3 (FLT3) gene typically have poor outcomes with available treatment [Citation1–4]. FLT3 mutations are among the most common mutations in AML, affecting approximately 30% of adult patients with newly diagnosed AML and leading to constitutive activation of the receptor [Citation1,Citation5,Citation6]. The most common FLT3 mutations are known as internal tandem duplications (ITDs) and comprise approximately 75% of FLT3 mutations in AML [Citation1,Citation7–9]. ITDs consist of in-frame duplications of 3 to ≥400 base pairs, typically in the juxtamembrane domain [Citation5,Citation10,Citation11]. Expression of FLT3-ITD relative to the wild-type FLT3 allele varies between patients, and the FLT3-ITD-to-wild-type ratio impacts prognosis [Citation12]. A high allelic ratio (generally ≥ 0.5) has been found to be a negative prognostic factor for patients with FLT3-ITD-mutated AML [Citation3,Citation12–15].
Mutations in the tyrosine kinase domain (TKD) are the second most prevalent FLT3 mutations, comprising approximately 25% of FLT3 mutations in AML; these mutations typically occur within the activating loop of the FLT3 kinase [Citation1,Citation5,Citation8,Citation9,Citation16]. The debate surrounding the prognostic impact of FLT3-TKD mutations is ongoing; it is likely that the impact of FLT3-TKD mutations depends on the cytogenetic background and the presence of other mutations [Citation1,Citation16–18]. Mutations of FLT3 generally lead to poor overall survival due to high relapse rates [Citation3,Citation4,Citation14].
Midostaurin, a multikinase inhibitor with targets that include FLT3 and other kinases [Citation19,Citation20], was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency’s Committee for Medicinal Products for Human Use for the treatment of adult patients with newly diagnosed FLT3 mutation–positive (mut+) AML in combination with standard induction and consolidation chemotherapy and (in certain regions) as single-agent maintenance therapy [Citation21,Citation22]. Regulatory approval was largely based on the results from the large, randomized, phase 3, placebo-controlled RATIFY/CALGB 10603 trial [Citation9]. The trial evaluated standard induction and consolidation chemotherapy in combination with midostaurin 50 mg twice daily (bid) or placebo on days 8–21 of each 28-day cycle followed by single-agent midostaurin 50 mg bid or placebo maintenance therapy for ≤ 12 cycles in patients aged 18–59 years with newly diagnosed FLT3-mut + AML. Patients receiving midostaurin experienced significant clinical benefits vs placebo, with improved overall and event-free survival.
RADIUS-X (NCT02624570) was an expanded access program (EAP) designed to provide access to midostaurin for eligible patients during the FDA review process and to expand the understanding of the safety and tolerability profile of midostaurin in adult patients with newly diagnosed FLT3-mut + AML.
Materials and methods
Study design
This open-label, single-arm EAP was designed to mirror the active treatment arm of the RATIFY study, while allowing for broader inclusion (e.g. older patients and those with therapy-related AML) and the possibility of gaining insights into the safety of midostaurin as a single agent and in combination with alternative anthracyclines (e.g. idarubicin or daunorubicin), with patients receiving midostaurin in combination with chemotherapy during induction and consolidation followed by single-agent midostaurin maintenance therapy for up to 1 year. Patients (aged ≥ 18 years) with newly diagnosed FLT3-mut + AML who were fit to receive intensive induction and consolidation chemotherapy received standard induction chemotherapy as defined by the V1.2015 National Comprehensive Cancer Network Guidelines for AML plus midostaurin (50 mg bid on days 8–21 of each 28-day cycle) [Citation23]. Patients with secondary AML, therapy-related AML, or a history of antecedent treatment for myelodysplasia were also eligible. Patients received ≤ 4 cycles of high-dose cytarabine consolidation chemotherapy (dosing per investigator’s discretion on days 1, 3, and 5 of each 28-day cycle) plus midostaurin (50 mg bid on days 8–21 of each 28-day cycle), followed by ≤ 12 months of single-agent midostaurin (50 mg bid on days 1–28).
Study details
Key inclusion criteria were AML diagnosis, documented FLT3 mutation (ITD and/or TKD), Eastern Cooperative Oncology Group performance status of ≤ 2, and adequate organ function. Standard chemotherapy could be initiated at the investigator’s discretion during screening. Patients could enroll in the program and initiate midostaurin therapy at any time prior to completing 2 cycles of consolidation chemotherapy. Patients achieving complete remission (CR) or CR with incomplete hematologic recovery (CRi) after induction proceeded to consolidation; patients who maintained a response were eligible to proceed to maintenance. CRi was defined as complete remission with an absolute neutrophil count of ≤ 109/L with or without a platelet count of < 100 × 109/L and with or without transfusion dependence. Failure to achieve CR/CRi led to study discontinuation.
Similar to RATIFY, RADIUS-X allowed allogeneic hematopoietic stem cell transplant (alloHSCT) to be performed per the investigator’s discretion, but midostaurin treatment was discontinued before beginning alloHSCT conditioning. Patients were followed for ≤ 30 days after discontinuing study treatment.
The primary endpoints were safety and tolerability of midostaurin per Common Terminology Criteria for Adverse Events v4.0 [Citation24]. Dose modifications of midostaurin were allowed for grade ≥ 3 pulmonary toxicity, grade ≥ 3 nonhematologic toxicities possibly related to midostaurin, and corrected QT (QTc) interval of > 470 ms during induction, consolidation, and maintenance therapy. Dose modifications of midostaurin were allowed for hematologic toxicity of grade 4 neutropenia during maintenance therapy; no dose modifications of midostaurin were allowed for hematologic toxicity during induction or consolidation therapy. Drug holidays of ≤ 28 days were allowed for persistent grade 1 or 2 toxicities during maintenance therapy. Missed doses of midostaurin were not made up.
The study was designed and reported in accordance with the International Council for Harmonization Good Clinical Practice guidelines, consistent with the principles of the Declaration of Helsinki, and was approved by the institutional review board at each participating institution. All patients provided written informed consent.
Statistical analysis
No statistical hypotheses were tested in this EAP. The estimated proportion of grade 3 or 4 serious adverse events (AEs) and 95% CI using the normal approximation to the binomial were calculated.
Results
Patients
Between 12 February 2016, and 28 April 2017, 111 patients were screened and 103 patients were enrolled in the study: 47 during induction (46%) and 56 during consolidation (54%) (). Patients were enrolled at 33 study sites, including 16 academic centers and 17 community practices. Of 47 patients enrolled during induction, 15 received daunorubicin (60–90 mg/m2/day) and 32 received idarubicin (12 mg/m2/day) as the anthracycline. Of 35 patients who completed consolidation and entered maintenance, 12 had completed the protocol treatment.
Figure 1. Patient disposition. AE: adverse event; CR: complete remission. aAmong the 9 patients who were initially identified as meeting eligibility criteria and entered the study during induction cycle 2, 1 patient was later found to have relapsed disease during induction treatment prior to starting midostaurin therapy, which made the patient ineligible to continue on study. bIncludes 56 patients who entered during consolidation and 29 who completed induction and then went on to receive consolidation. cIncludes 35 patients who completed consolidation and then went on to receive maintenance. dRelapse events during induction occurred following completion of induction therapy but prior to initiation of consolidation therapy. eDeaths occurring up to 30 days after the last dose are all included.
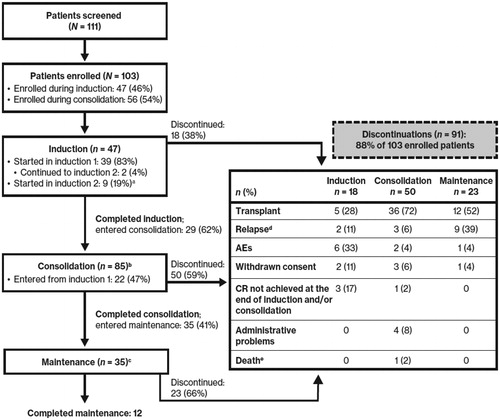
Baseline patient characteristics are shown in . The median age was 58 years (range, 19–79 years). All patients were FLT3 mut+, with 75 patients (73%) having FLT3-ITD mutations, 23 patients (22%) having FLT3-TKD mutations, and 4 patients (4%) possessing both FLT3-ITD and -TKD mutations.
Table 1. Baseline patient characteristics.
Safety
Nearly all patients (99%) experienced ≥ 1 any-grade AE, mostly during induction and/or consolidation (). The most common AEs occurring in ≥ 20% of patients were febrile neutropenia (53%), nausea (42%), diarrhea (37%), anemia (36%), platelet count decreased (31%), fatigue (23%), headache (22%), and vomiting (22%). AEs were also generally similar regardless of age. In general, the most common nonhematologic AEs during induction were similar between patients receiving idarubicin and those receiving daunorubicin ().
Table 2. Common nonhematologic AEs (occurring in ≥ 10% of patients overall) in each treatment phase overall and by age.
Table 3. Common nonhematologic AEs (occurring in ≥15% of patients overall) by induction anthracycline.
Hematologic AE rates were notably lower than would be expected for patients receiving intensive chemotherapy. Reporting of hematologic events may have been impacted by the nature of this study and the timing of patient enrollment. Because patients could enroll up to the second cycle of consolidation, hematologic AEs for patients already experiencing cytopenia due to being midcycle in intensive chemotherapy may not have been reported. During maintenance, the rates of hematologic AEs were generally low and included platelet count decreased (n = 3 [9%]), neutrophil count decreased (n = 2 [6%]), and anemia, leukocytosis, pancytopenia, thrombocytopenia, and white blood cell count decreased (all n = 1 [3%]).
Serious AEs occurred in 50% of patients overall, most commonly febrile neutropenia (37%) (). One serious AE (leukocytosis) not deemed related to midostaurin was reported during the maintenance phase in a patient who relapsed. One patient discontinued the study due to a serious AE (respiratory failure) and died due to disease progression during the 30-day safety follow-up period. Overall, dose adjustment or interruption occurred due to AEs in 26 patients, most commonly due to febrile neutropenia (n = 8) and gastrointestinal disorders (n = 6).
Table 4. Serious AEs regardless of study drug attribution (occurring in ≥ 2% of patients overall) in each treatment phase.
The median duration of midostaurin exposure was 30 days (range, 3–425 days). The most common reason for study discontinuation was proceeding to transplant (overall, 51%; induction, 28%; consolidation, 72%; maintenance, 52%) (). Nine patients (15%) discontinued due to AEs: 6 during induction (respiratory failure, total bilirubin increased, prolonged QTc interval, febrile neutropenia, renal dysfunction, oromandibular rhizomucor infection [n = 1 each], 2 during consolidation (alanine aminotransferase increased and skin lesions [n = 1 each]), and 1 during maintenance (leukocytosis).
Overall, 73 patients received cytochrome P450 3A4 (CYP3A4) inhibitors. Moderate and strong CYP3A4 inhibitors were coadministered in 48% and 16% of patients, respectively (), with voriconazole, posaconazole, and ketoconazole being the most frequently coadministered strong CYP3A4 inhibitors. Dose adjustment or interruption occurred due to concomitant administration of CYP3A4 inhibitors in 12 patients (6 adjustments, 6 interruptions) overall and in 11 of 22 patients who received strong inhibitors (all received a dose reduction of midostaurin to 25 mg every other day). The most common nonhematologic AEs reported in patients receiving CYP3A4 inhibitors were nausea, diarrhea, and fatigue; these AEs were observed at a similar rate in patients who did not receive CYP3A4 inhibitors ().
Frederica-corrected QT (QTcF) interval increases of > 30 ms were reported in 6 patients (6%). There were 11 total QT prolongation events; none of these events had QTcF intervals of > 500 ms. Two QTcF events occurred during the maintenance phase. One patient (1%) experienced a QTcF interval increase of > 60 ms from 401 ms to 463 ms. Concomitant strong CYP3A4 inhibitors were administered in 3 of the 6 patients with QTcF interval increases of > 30 ms. QTc intervals normalized when the midostaurin dose was reduced to 50 mg once daily. No QTc interval increases were associated with clinically significant events.
Twenty of 35 patients (57%) reported any-grade AEs with midostaurin monotherapy. The majority of events were grade 1/2. The most common any-grade and grade 3/4 AEs occurring in > 1 patient were platelet count decreased (11% and 3%), nausea (9% and 0%), and oropharyngeal pain (6% and 0%).
Efficacy
Per protocol, CR/CRi rates were collected to assess patient eligibility to continue to the next phase of therapy. Patients in RADIUS-X were allowed to start treatment at any point up to their second cycle of consolidation. Among the 47 patients who started on study during induction therapy, 35 (74%) achieved a complete response (CR/CRi) and 10 went on to receive all 3 phases of treatment (i.e. induction, consolidation, and single-agent maintenance) (). Regardless of the time of study entry (e.g. induction or consolidation), 12 of the 35 patients (34%) who entered maintenance completed all 12 cycles of maintenance therapy ().
Figure 2. Time to first response and duration on study in patients who started induction. AE: adverse event; CR: complete remission; CRi: complete remission with incomplete hematologic recovery. Gray bars represent days when patients did not receive midostaurin. Day 0 represents the patient’s first dose of midostaurin. cTreatment duration completed as per protocol. dReason for discontinuation: administrative problems (physician decision). eReason for discontinuation: administrative problems (patient decision to receive treatment at an alternate facility). fPatient was later found to have relapsed disease during induction treatment prior to starting midostaurin therapy, which made the patient ineligible to continue on study.
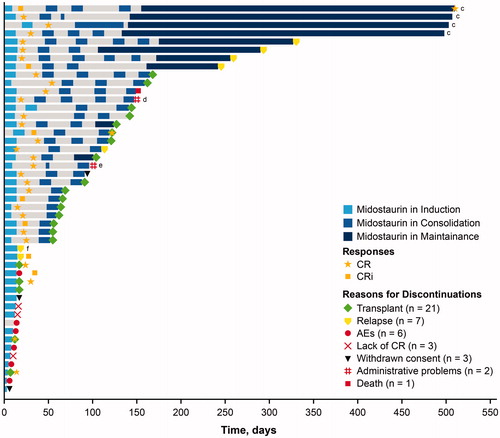
Twenty-one patients who entered the study during induction discontinued the study to receive an alloHSCT; the majority of these transplants (76%) occurred during consolidation or maintenance (). Overall, 51% of patients discontinued the study to receive an alloHSCT.
Of the patients who began the study during induction, 7 relapsed and came off study (1 patient was later found to have relapsed disease during induction treatment prior to starting midostaurin therapy, which made the patient ineligible to continue on study) (); overall, 14% of patients relapsed across all 3 phases of treatment (). Additional reasons for discontinuation for patients who entered the study during induction included AEs (n = 6 [13%]; 9% overall), withdrawn consent (n = 3 [6%]; 6% overall), lack of CR at the end of induction or consolidation (n = 3 [6%]; 4% overall), administrative problems (n = 2 [4%]; 4% overall), or death (n = 1 [2%]; 1% overall) ( and Citation2).
Discussion
RADIUS-X was designed to provide access to midostaurin for patients with newly diagnosed FLT3-mut + AML during the FDA review process and to gather additional safety and tolerability data in this patient population. Although this EAP had a similar design as the active arm of the RATIFY study, there were notable differences, which provided an opportunity to expand upon the known safety profile of midostaurin. In RADIUS-X, a broader patient population had access to midostaurin than in RATIFY, including patients ≥ 60 years, patients with therapy-related AML, and patients with myelodysplastic syndromes previously treated with cytotoxic chemotherapy (including hypomethylating agents). Additionally, patients were able to receive either idarubicin or daunorubicin during induction. In the previously investigated population and with these protocol expansions (i.e. idarubicin induction and patients ≥ 60 years), midostaurin continued to demonstrate a manageable safety profile consistent with that in the phase 3 RATIFY trial of midostaurin [Citation9].
No new safety signals were identified. There was no increase in overall toxicity with age or coadministration of CYP3A4 inhibitors; however, if possible, alternative therapies to CYP3A4 inhibitors should be considered [Citation21,Citation22]. While these data provide insight into the coadministration of midostaurin with moderate and strong CYP3A4 inhibitors, it is important to note that, as an expanded access program, this study was not designed to definitively address the safety of concomitant therapy, and the overall number of patients was relatively small. Also, data showing AEs before and after concomitant administration of CYP3A4 inhibitors and midostaurin were not collected. The data from RADIUS-X demonstrate that the observed AE profile was consistent regardless of anthracycline choice (idarubicin vs daunorubicin) or concomitant CYP3A4 inhibition (yes vs no).
Approximately one-third of all patients in RADIUS-X received maintenance therapy with midostaurin monotherapy. Maintenance therapy was well tolerated, and no new safety signals were observed. Midostaurin therapy was associated with high rates of alloHSCT and low rates of relapse while on treatment. The CR/CRi rate during induction therapy (74%) was comparable to that reported in other studies evaluating midostaurin in combination with intensive induction chemotherapy [Citation25]. Only 1 death was reported during treatment.
The RADIUS-X EAP allowed patients to enroll through the second cycle of consolidation to provide this novel therapy to patients with limited treatment options. Considering patients who started midostaurin therapy during induction, the number of on-treatment relapses was low (n = 7), and many patients went on to receive an alloHSCT (n = 21). For those who were unable to receive an alloHSCT, they were able to continue treatment with single-agent maintenance. These data continue to support the long-term benefit of midostaurin that was demonstrated in the large RATIFY trial [Citation9].
GLAL-2020-0075-File004.docx
Download MS Word (38.1 KB)Acknowledgments
We thank the patients and the investigators who participated in the RADIUS study. Medical editorial assistance was provided by JoAnna Anderson, PhD, of ArticulateScience LLC, and was supported by Novartis Pharmaceuticals Corporation.
Disclosure statement
G J Roboz discloses consultancy for AbbVie, Amphivena, Argenx, Astex, Bayer, Celgene, Celltrion, Daiichi Sankyo, Eisai, Janssen, Jazz, Novartis, Orsenix, Otsuka, Pfizer, Roche/Genentech, and Sandoz and research funding from Cellectis; S A Strickland discloses consultancy for AbbVie, Astellas, Jazz, Kite, Novartis, and Pfizer and research funding from Sunesis; M R Litzow and A Dalovisio have nothing to disclose; A E Perl discloses consultancy for Arog, Astellas, and Daiichi Sankyo and advisory board membership for AbbVie, Agios, Actinium, Novartis, NewLink Genetics, Pfizer, and Takeda; G Bonifacio, A Barbera, and D Purkayastha disclose employment with Novartis; K Haines discloses former employment with Novartis and current employment with Regeneron; K Sweet discloses consultancy for Agios, Astellas, Novartis, and Pfizer and research funding from Incyte, honoraria from or served as an advisory board member for Astellas, Bristol-Myers Squibb, Jazz, Novartis, Pfizer, and Stemline and serves on the speakers bureau for Celgene, Jazz, and Novartis.
Additional information
Funding
References
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221.
- Gregory TK, Wald D, Chen Y, et al. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J Hematol Oncol. 2009;2:23
- Pratcorona M, Brunet S, Nomdedéu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738.
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759.
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3(9):650–665.
- Murphy K, Weaver C, Mowat A, et al. Janeway’s immunobiology. Quart Rev Biol. 2018;93(1):59–59.
- Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089.
- Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993–2003.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464.
- Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–2392.
- Patnaik MM. The importance of FLT3 mutational analysis in acute myeloid leukemia. Leuk Lymphoma. 2018;59(10):2273–2286.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335.
- Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784.
- Koszarska M, Meggyesi N, Bors A, et al. Medium-sized FLT3 internal tandem duplications confer worse prognosis than short and long duplications in a non-elderly acute myeloid leukemia cohort. Leuk Lymphoma. 2014;55(7):1510–1517.
- Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters-an analysis of 3082 patients. Blood. 2008;111(5):2527–2537.
- Boddu P, Kantarjian H, Borthakur G, et al. Co-occurrence of FLT3-TKD and NPM1 mutations defines a highly favorable prognostic AML group. Blood Adv. 2017;1(19):1546–1550.
- Santos FP, Jones D, Qiao W, et al. Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer. 2011;117(10):2145–2155.
- Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19(5):1485–1492.
- Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–443.
- Rydapt [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation; 2020.
- Rydapt [summary of product characteristics]. Basel, Switzerland: Novartis Pharmaceuticals AG; 2018.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Acute Myeloid Leukemia. V1. 2015.
- Common terminology criteria for adverse events v4.03. Bethesda (MD): National Cancer Institute; 2010.
- Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851.
