Abstract
Findings regarding the role of sex in follicular lymphoma (FL) are contradictory and the prognostic value of sex among patients with early progression of disease (POD) remains unclear. We collected real-life data from nine hospitals in Finland and Spain including 1020 FL patients to study the influence of sex on disease outcome. The median follow-up duration was 67 months (range 0–226 months). Female patients showed better progression-free survival (PFS) (hazard ratio [HR], 0.720; 95% confidence interval [CI], 0.588–0.881), disease-specific survival (DSS) (HR, 0.653; 95% CI, 0.448–0.951), and overall survival (OS) (HR, 0.653; 95% CI, 0.501–0.853) than male patients. However, there were no significant sex differences in prognosis in patients with early POD. This study strengthens the understanding that male sex is an adverse prognostic factor for FL. However, this difference does not apply to patients with early POD.
Keywords:
Introduction
Follicular lymphoma (FL) is the most common indolent lymphoma in Western countries. Although it is still incurable in the advanced stage, its prognosis has improved greatly over the last 20 years [Citation1–3]. This improvement is attributed to therapeutic advances, among which the introduction of rituximab is the most significant. FL is a heterogeneous disease with a varying prognosis. The choice of treatment for FL is highly dependent on the patient and disease characteristics. Various clinical, pathological, treatment-related, and genetic prognostic biomarkers have been reported [Citation4,Citation5]. Follicular Lymphoma-specific International Prognostic Index (FLIPI) is the most established tool for prognostic purposes [Citation6]. Model includes five prognostic factors: age >60 years, elevated LDH, stage III-IV, > 4 nodal sites and hemoglobin level <12 g/dL. It is, however, based on retrospective survival data from patients diagnosed in the preimmunotherapy era.
Contradictory data are available regarding the role of sex in the prognosis of FL. In studies before the widespread use of rituximab, male sex was reported to be associated with poor clinical outcome [Citation6–9]. These studies also included the original datasets on which the FLIPI model was based, although male sex was not included in the final five factors of FLIPI [Citation6]. Only a few recent studies from the rituximab era have studied the role of sex as a prognostic factor in FL. Most of them show that male sex is an independent adverse prognostic factor, especially among elderly men [Citation1,Citation10–12]. However, in a study by Provencio et al., the standardized mortality ratio (SMR) was higher in females [Citation13]. Some studies have reported no difference in prognosis according to sex [Citation8,Citation14].
Currently it is widely accepted that 20% of the FL patients who have progression of disease (POD) within 2 years of the first-line treatment have poor survival [Citation13,Citation15–17]. However, no data are available about the role of sex in these patients.
We collected comprehensive real-life data from nine hospitals in Finland and Spain including more than 1000 FL patients and studied the influence of sex on disease outcome among these patients.
Materials and methods
Patients
Clinical data for this retrospective registry study were collected from four university hospitals and three central hospitals in Finland and from two hospitals in Spain. The data included all patients with FL diagnosed between 1997 and 2016. Altogether, 1045 patients were identified including 344 from Spain and 701 from Finland. The collected information included demographic, pathological and prognostic factors; the type of treatment; and treatment outcomes. The baseline information included age, sex, serum lactate dehydrogenase (LDH) and hemoglobin levels, Ann Arbor stage, B-symptoms (unexplained weight loss, fever, night sweats), and FLIPI-score. Treatment information included the type of therapy, response to therapy, patient survival status (assessed in months), disease progression, retreatment, and deaths (classified as deaths due to FL or deaths due to other causes). Patients with composite lymphoma, patients who were lost to follow-up, and patients with no information about sex were excluded. Finally, 1020 patients were deemed evaluable. In the group of patients with first-line watchful waiting, any type of therapy for FL received by the patients was considered the first-line treatment.
The study was reviewed and approved by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District. The principles of the Declaration of Helsinki were followed throughout the study.
Statistical analysis
Survival estimates were calculated using the Kaplan-Meier method. The log-rank test was used to calculate the statistical significance between subgroups. p-values <0.05 were considered statistically significant. Pearson’s chi-squared test was used to evaluate the differences in the baseline information between males and females. Cox regression analysis was used to calculate the hazard ratio (HR), and in the multivariate analyses, where age, histology grade, hemoglobin, lactate dehydrogenase, B-symptoms, and FLIPI-score, were taken into account as covariates. Disease-specific survival (DSS) was calculated from the date of diagnosis to the date of lymphoma-related death or the last follow-up. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or the last follow-up. Progression-free survival 1 (PFS1) was calculated from the first day of the treatment to the date of first relapse, the date of death from any cause, or the last date of follow-up, whichever occurred first. PFS2 was calculated from the first day of the second-line treatment to the date of second relapse, the date of death from any cause, or the last follow-up date. Details about the second-line treatments were collected for all the patients who received any second-line treatment. However, only the patients whose date of treatment was available were included in the survival analysis. While analyzing the impact of the first remission on disease outcome, patients were divided into two subgroups depending on the duration of the response to the first-line treatment. Early POD was defined as progression within 24 months from the start of the therapy (also referred to as POD24), and late POD was defined as progression more than 24 months after the initial treatment. Statistical analyses were performed using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Patient characteristics are presented in . Among the 1020 evaluable patients, 494 were males and 526 were females. Patient characteristics except age and hemoglobin levels were similar between males and females. The median age of female patients was higher than that of male patients (60 vs. 59 years, p = 0.003). The hemoglobin level at diagnosis was lower in females (19.1% of the females with hemoglobin <12 g/dL vs. 11.2% of the males, p = 0.001).
Table 1. Patient characteristics according to sex.
Treatments
Details of the first-line treatments are presented in . In the whole study population, no statistically significant differences were observed in the selection of first-line treatment between males and females (p = 0.853). Among the whole study population, 69.6% of the males and 68.3% of the females received rituximab plus chemotherapy (p = 0.633), 3.2% of the males and 3.0% of the females received rituximab monotherapy (p = 0.857), and 28.9% of the males and 24.5% of the females received maintenance rituximab (p = 0.110).
Table 2. First-line treatments according to sex.
When we compared the first-line treatment selection between countries, there were no differences between males and females in Finland. In Spain, males received more maintenance rituximab than females (36.7% vs. 24.4%, p = 0.015). Moreover, males tended to receive more anthracycline-containing regimens (58.9% vs. 48.9%, p = 0.067), while females tended to receive more less-intensive therapies (13.9% vs. 22.2%, p = 0.052) ().
Among all patients, 399 (39.1%) showed disease progression during the follow-up period (210 males and 189 females, p = 0.031) and 321 patients (31.5%) received second-line treatment. No difference was observed in receiving a second-line treatment after relapse between males and females (p = 0.437). Among patients who received second-line treatment, 62.8% of the males and 61.7% of the females received rituximab (p = 0.847), 16.9% of the males and 26.8% of the females received anthracycline-containing regimens (p = 0.030), 20.9% of the males and 18.8% of the females received bendamustine (p = 0.633), 12.2% of the males and 14.1% of the females received fludarabine-containing regimens (p = 0.618), and 15.1% of the males and 20.8% of the females received maintenance rituximab (p = 0.183).
Survival in the entire study population
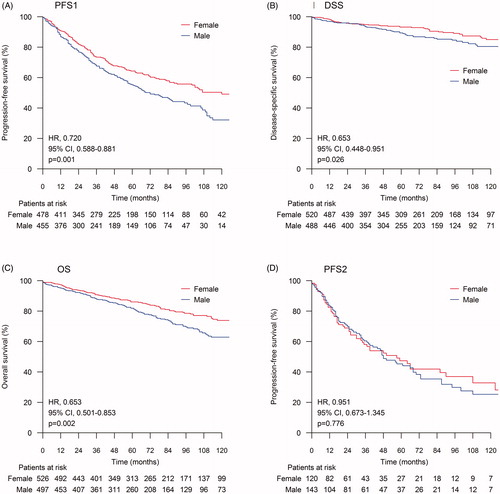
The median follow-up duration was 67 months (range: 0–226 months). Females showed better PFS1 (HR, 0.720; 95% confidence interval [CI], 0.588–0.881; p = 0.001), DSS (HR, 0.653; 95% CI, 0.448–0.951; p = 0.026), and OS (HR, 0.653; 95% CI, 0.501–0.853, p = 0.002) than males (). The median PFS1 was 69 months for males and 120 months for females and the 5-year PFS rates were 55.1% and 64.3%, respectively (p = 0.001). The median DSS and OS were not reached at the time of analysis, but the 5-year DSS and 5-year OS were better in the female population (89.4% vs. 93.2%, p = 0.025 and 82.1% vs. 86.0%, p = 0.002; respectively). Nonetheless, the difference in survival disappeared after the first relapse, as there was no statistically significant difference in PFS2 between males and females (p = 0.776) ().
Figure 1. Sex differences in survival. The log-rank test was used to calculate the statistical difference between subgroups. (A) Progression-free survival after first-line treatment (PFS1); (B) Disease-specific survival (DSS); (C) Overall survival (OS); (D) Progression-free survival after second-line treatment (PFS2).
Prognostic impact of sex among patients with early and late progression
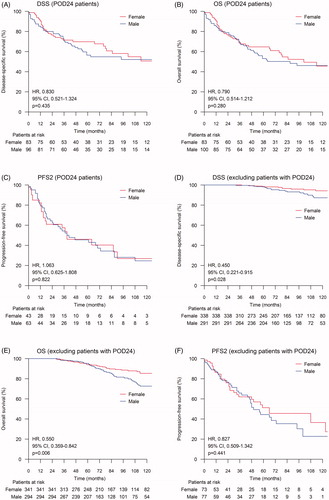
When the patients were divided according to the time of POD (early vs. late), male patients were more likely to exhibit early POD when compared with females (25.3% vs. 19.6%, p = 0.049). No differences were observed in OS, DSS, or PFS2 between males and females in the early POD group (). In the late POD group, the differences in OS and DSS between males and females remained significant, but there was no difference in PFS2 ().
Figure 2. Sex differences in disease-specific survival (DSS), overall survival (OS), and progression-free survival after second-line therapy (PFS2) according to the time of progression of disease (POD). The log-rank test was used to calculate the statistical difference between subgroups. PFS1 = Progression-free survival after first-line treatment. (A) DSS for patients with POD24; (B) OS for patients with POD24; (C) PFS2 for patients with POD24; (D) DSS for patients with PFS1 lasting over 24 months; (E) OS for patients with first PFS1 lasting over 24 months; (F) PFS2 for patients with PFS1 lasting over 24 months.
Impact of country of residence
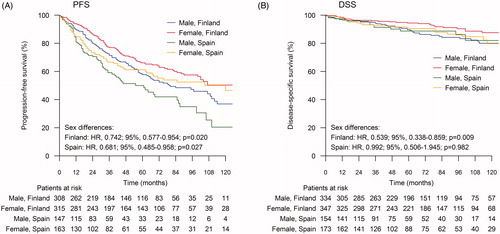
When the survival was compared between males and females according to the country of residence (Finland vs. Spain), females showed better PFS1 in both the countries (Finland: HR, 0.742; 95% CI, 0.577–0.954; p = 0.020 and Spain: HR, 0.681; 95% CI, 0.485–0.958; p = 0.027) (). In the Finnish population, females showed better OS and DSS, but in the Spanish population no differences were observed in OS and DSS between males and females. The probabilities for DSS are depicted in . Hemoglobin levels <12 g/dL at diagnosis were observed more often in females than in males in both the countries. Significant difference in age was observed between males and females only in the Finnish population (females were older than males).
Figure 3. Sex differences in progression free survival after first-line treatment (PFS) and in disease-specific survival (DSS) according to the country of residence. The log-rank test was used to calculate the statistical difference between males and females. (A) PFS; (B) DSS.
In the multivariate analysis, sex remained an independent prognostic factor for PFS, OS, and DSS as shown in .
Table 3. HR and adjusted HR for Sex Difference in PFS, DSS and OS.
Discussion
In the present study, we observed that male sex was an independent adverse prognostic factor of PFS, OS, and DSS in FL. However, among patients with early POD this difference disappeared, suggesting that FL with early POD is biologically different from FL with late POD. Notably, females had a better prognosis, although they were older and their hemoglobin levels were lower at diagnosis when compared with males.
Although conflicting results have been published regarding the role of sex in FL, several studies have reported results consistent with our results. A large study from the US with 2652 FL patients reported that females showed better PFS and females aged <60 years showed better OS and lymphoma-related mortality [Citation12]. Their data included a minor difference in treatment selection between males and females. A Finnish study that included 110 FL patients and 217 patients with diffuse large B-cell lymphoma (DLBCL) analyzed the role of sex in B-cell lymphomas treated with immunochemotherapy (ICT). Females showed better PFS than males, but no difference was observed in OS [Citation10]. A population-based registry study from Sweden examined the role of rituximab in survival. They found that with increasing rituximab use, male sex emerged as an adverse factor in OS analysis among patients aged 60 years and older [Citation1]. Surprisingly, in a comprehensive SEER analysis from the US that included data of over 18 000 FL patients, females treated between 1992 and 2000 showed superior OS compared to males, but those treated between 2001 and 2009 did not show this difference [Citation8]. A Spanish study with 1074 FL patients reported even higher SMR in females [Citation13].
In the present study, when the study population was divided into two groups depending on the country of origin, the sex differences in favor of females remained in the Finnish population regardless of the fact that women were older than men, and age is known to be associated with a poorer prognosis in FL [Citation6,Citation14]. In the Spanish population, survival difference according to sex was observed only in PFS1. No prognostic difference between males and females or even a difference in favor of males was also observed in a previously reported Spanish study [Citation13]. This difference cannot be explained by general differences in life expectancy, as females have better life expectancy than males in both Finnish and Spanish populations [Citation18]. In the present study, Spanish males received more maintenance rituximab and tended to receive more intensive therapies than females. We believe that conflicting results among different studies may be attributed to cultural differences associated with treatment selection or ethical issues. Possibly, women might be more reluctant to receive treatments causing hair loss. In order to fully understand the role of sex in diseases such as FL that have multiple treatment options, differentiating the true biological impact of sex from sociocultural issues is of utmost importance.
It is hypothesized that females may benefit more from rituximab-containing regimens than males due to the higher serum levels of rituximab. Data published especially from the DLBCL studies support this hypothesis [Citation19–21]. In our data, the difference in PFS1 between males and females was also observed in the subgroup that did not receive rituximab-containing regimens. Among these patients, we observed a trend toward differences in DSS and OS. However, the results were not statistically significant possibly due to the small number of patients (data not shown). The role of estrogen has also been studied in lymphomas. Estrogen seems to play a protective role in the pathogenesis and progression of lymphoma [Citation22,Citation23]. Normal B-lymphocytes as well as B-cell lymphomas express estrogen receptors. Particularly, B-cell lymphomas express estrogen receptor b, which has been shown to have antiproliferative effect [Citation24]. Postmenopausal estrogen replacement therapy seems to decrease the risk of lymphoma and blocking the estrogen synthesis via inhibition of the aromatase pathways is shown to accelerate lymphoma growth [Citation25,Citation26]. Moreover, estrogen receptor agonists inhibit tumor growth both in vivo and in vitro [Citation24,Citation27,Citation28]. Further investigations are needed to clarify whether these results indicate that circulating estrogens in females are able to slow down the growth of lymphoma or whether the lymphomas arising under the influence of estrogens are biologically distinct. It is unclear whether lymphomas showing early progression have lost their sensitivity to estrogen.
The median PFS1 in our study (5.8 years for males and 10 years for females) was fairly high when compared with other published data. In our study, over two-thirds (68.9%) of the patients received ICT, which may partly explain the good results. In a similar study by Nabhan et al., median PFS was 6.3 years for males and 6.9 years for females, but only around a half of the patients received ICT [Citation12]. Moreover, in our study, 26.7% of the patients received maintenance rituximab. In the PRIMA study, which focused on the possible benefits of maintenance rituximab, patients received R-CHOP, R-CVP, and R-FCM regimens (approximately 75%, 22% and 3%, respectively). Subsequently, they were randomly assigned to the 2-year maintenance rituximab group or the observation group. The median PFS was 10.5 years in the rituximab maintenance arm and 4.1 years in the observation arm. However, no benefit of rituximab maintenance was observed regarding OS [Citation29]. Thus, the role of rituximab maintenance is controversial [Citation30].
Although the prognosis of FL is generally good, many studies have shown that the prognosis of patients with early POD is considerably worse [Citation13,Citation15,Citation16]. The frequency of early POD is remarkably consistent across studies and it is probable that a different kind of disease biology may be present in this group of patients. Our results underline these biological differences and suggest that the protective effect of estrogen is absent in these cases due to unknown reason. In addition, various gene alterations have predictive value for FL prognosis. It is known that TP53 mutations are associated with a worse OS [Citation31,Citation32]. These mutations are found in 5–6% of the patients at diagnosis. However, they are more frequent in patients with early POD, which might be one of the reasons for worse survival [Citation32,Citation33]. The present study showed that sex had no impact on prognosis in the subgroup of patients with early POD. To the best of our knowledge, this is the first study to examine the role of sex in patients with early POD.
The strengths of this multicenter study were the real-life setting and the large number of patients included from both academic and nonacademic hospitals. The data included all FL patients diagnosed between 1997 and 2016 in the nine participating hospitals. Inclusion of hospitals from two different countries enabled us to perform comparisons between different nationalities. In this setting, our study provided a potential explanation for the contradictory results presented in the literature [Citation1,Citation8,Citation10,Citation12,Citation13]. Our study also has several limitations. The follow-up time was quite short and the therapies were heterogeneous. In our data, grade 3 lymphomas were not classified into A and B categories. Thus, they were analyzed as a single entity. Unfortunately, we did not have a pathological review or information regarding the possible transformations to aggressive lymphoma.
In conclusion, we have reported large, two-country, real-life data regarding sex differences in the outcome of FL. We observed that male sex was an adverse prognostic factor. However, this finding was restricted to patients whose first remission lasted for at least 24 months from the beginning of the first-line therapy. Our results also suggest that sociocultural factors may obscure biological differences in retrospective data.
Disclosure statement
Juan-Manuel Sancho has received honoraria from Roche, Gilead, Janssen, Novartis, Celgene, Takeda and Incyte, and been consultant or participant in advisory boards for Roche, Gilead, Janssen, Novartis, Celgene and Incyte. Other authors report no conflicts of interest.
References
- Junlén HR, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry Study. Leukemia. 2015;29(3):668–676.
- Mozas P, Nadeu F, Rivas-Delgado A, et al. Patterns of change in treatment, response, and outcome in patients with follicular lymphoma over the last four decades: a single-center experience. Blood Cancer J. 2020;10(3):31.
- Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122(6):981–987.
- Matasar MJ, Luminari S, Barr PM, et al. Follicular lymphoma: recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist. 2019;24(11):e1236–e1250.
- Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v83–v90.
- Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265.
- Kondo E, Ogura M, Kagami Y, et al. Assessment of prognostic factors in follicular lymphoma patients. Int J Hematol. 2001;73(3):363–368.
- Nabhan C, Aschebrook-Kilfoy B, Chiu BCH, et al. The impact of race, age, and sex in follicular lymphoma: a comprehensive SEER analysis across consecutive treatment eras. Am J Hematol. 2014;89(6):633–638.
- Federico M, Vitolo U, Zinzani PL, et al. Prognosis of follicular lymphoma: A predictive model based on a retrospective analysis of 987 cases. Blood. 2000;95:783–789.
- Riihijärvi S, Taskinen M, Jerkeman M, et al. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol. 2011;86(2):124–128.
- Nabhan C, Byrtek M, Rai A, et al. Disease characteristics, treatment patterns, prognosis, outcomes and lymphoma-related mortality in elderly follicular lymphoma in the United States. Br J Haematol. 2015;170(1):85–95.
- Nabhan C, Zhou X, Day BM, et al. Disease, treatment, and outcome differences between men and women with follicular lymphoma in the United States. Am J Hematol. 2016;91(8):770–775.
- Provencio M, Royuela A, Torrente M, et al. Prognostic value of event-free survival at 12 and 24 months and long-term mortality for non-Hodgkin follicular lymphoma patients: a study report from the Spanish Lymphoma Oncology Group. Cancer. 2017;123(19):3709–3716.
- Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562.
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–2522.
- Morrison VA, Shou Y, Bell JA, et al. Treatment patterns and survival outcomes in patients with follicular lymphoma: a 2007 to 2015 humedica database study. Clin Lymphoma, Myeloma Leuk. 2019;19(4):e172–e183.
- Seymour JF, Marcus R, Davies A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica. 2019;104(6):1202–1208.
- Eurostat the statistical office of the EU. Life expectancy by age and sex [Internet]. Eurostat, Stat. Off. Eur. Union; 2020 [cited 2020 May 18]. Available from: https://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=sdg_03_10&plugin=1.
- Jäger U, Fridrik M, Zeitlinger M, et al. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97(9):1431–1438.
- Müller C, Murawski N, Wiesen MHJ, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119(14):3276–3284.
- Pfreundschuh M, Müller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123(5):640–646.
- Nelson BRA, Levine AM, Bernstein L. Reproductive factors and risk of intermediate- or high-grade B-cell non-hodgkin's lymphoma in women. J Clin Oncol. 2001;19(5):1381–1387.
- Ladikou EE, Kassi E. The emerging role of estrogen in B cell malignancies. Leuk Lymphoma. 2017;58(3):528–539.
- Yakimchuk K, Iravani M, Hasni MS, et al. Effect of ligand-activated estrogen receptor Β on lymphoma growth in vitro and in vivo. Leukemia. 2011;25(7):1103–1110.
- Talaber G, Yakimchuk K, Guan J, et al. Inhibition of estrogen biosynthesis enhances lymphoma growth in mice. Oncotarget. 2016;7(15):20718–20727.
- Kane EV, Bernstein L, Bracci PM, et al. Postmenopausal hormone therapy and non-hodgkin lymphoma: a pooled analysis of interlymph case-control studies. Ann Oncol. 2013;24(2):433–441.
- Yakimchuk K, Hasni MS, Guan J, et al. Inhibition of lymphoma vascularization and dissemination by estrogen receptor β agonists. Blood. 2014;123(13):2054–2061.
- Pierdominici M, Maselli A, Locatelli SL, et al. Estrogen receptor β ligation inhibits Hodgkin lymphoma growth by inducing autophagy. Oncotarget. 2017;8(5):8522–8535.
- Bachy E, Seymour JF, Feugier P, et al. Sustained progression-free survival benefit of rituximab maintenance in patients with follicular lymphoma: Long-term results of the PRIMA study. J Clin Oncol. 2019;37(31):2815–2824.
- Friedberg JW. Progress in advanced-stage follicular lymphoma. J Clin Oncol. 2018;36(23):2363–2365.
- Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111–1122.
- O'Shea D, O'Riain C, Taylor C, et al. The presence of TP53 mutation at diagnosis of Follicular Lymphoma identifies a high-risk group of patients with shortened time to disease progression and poorer overall survival. Blood. 2008;112(8):3126–3129.
- Sorigue M, Sancho JM. Current prognostic and predictive factors in follicular lymphoma. Ann Hematol. 2018;97(2):209–227.
