?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Invasive alien plant species (IAPs) represent one of the main biological threats to biodiversity worldwide. Information about their phenotypic plasticity are needed to increase awareness about their future invasive potential. A study about phenotypic plasticity in response to contrasting light regimes and its quantification by a plasticity index (PI) of two IAPs (Ailanthus altissima and Robinia pseudoacacia) inside a Strict Nature Reserve was conducted. R. pseudoacacia showed a 70% higher PI, with a strongly greater value at morphological leaf level, associated with a greater ability to survive and grow in forest understory, explaining its greater widespread. Otherwise, A. altissima showed its highest PI at physiological level, which was associated with the ability to colonize and grow in environments with high-light regimes. Based on these results, the conservative management has limited the presence of A. altissima by its lower ability to grow in forest understory. In fact, the small-scale gaps in the forest infrastructure, that could allow its recruitment, are originated only from the death of a single tree or small group of trees. Regarding R. pseudoacacia, it is critical to maintain this type of management because any disturbances resulting in large openings could further promote its presence inside the Reserve.
Introduction
The invasion of alien species is among the main biological threats to biodiversity worldwide (Pauchard & Shea, Citation2006). Once established, invasive alien plant species (IAPs) may affect native plant communities by reducing their diversity and abundance (Vilà et al., Citation2011). These effects may be caused directly by allelopathy and competition for resources (Maron & Marler, Citation2008) or indirectly by modifying the environment to the detriment of native species in their own benefit (Niu, Liu, Wan, & Liu, Citation2007). In particular, the effects that IAPs can have on the environment range from changing fire and hydrological regimes (Le Maitre, Van Wilgen, Chapman, & McKelly, Citation1996) to the alteration of carbon and nitrogen cycle (Ehrenfeld, Citation2003) by affecting several processes including nitrogen fixation, soil nitrogen mineralization (Hawkes, Wren, Herman, & Firestone, Citation2005), plant nutrient uptake, and nutrient transfer to soil through litterfall (Lindsay & French, Citation2005).
Plant traits involved in allowing invasive species to become successful invaders remains a challenging question in invasion ecology (van Kleunen, Weberm, & Fischer, Citation2010). Previous studies together with exhaustive meta-analysis (Davidson, Jennions, & Nicotra, Citation2011; Pigliucci & Preston, Citation2004; Richards, Bossdorf, Muth, Gurevitch, & Pigliucci, Citation2006) emphasized how a high phenotypic plasticity in some particular functional traits can be considered as a potential factor in promoting invasion success since helps these species to express advantageous phenotypes over a broad range of environments (Matesanz, Gianoli, & Valladares, Citation2010). Actually, according to the original definition by Bradshaw (Citation1965) phenotypic plasticity can be explained as the change in phenotypic expression of a genotype in response to environmental factors. Among the functional traits, morphological leaf traits (e.g. specific leaf area) and physiological leaf traits (e.g. photosynthetic rates) differ between invasive and noninvasive ⁄ native species and are usually associated with invasiveness (Godoy, Valladares, & Castro-Díez, Citation2012). Specifically, IAPs compared to noninvasive plant species seem to have a high plastic response to light levels (Yamashita, Ishida, Kushima, & Tanaka, Citation2000); that, among the environmental factors, play a key role in plant survival and development (Nascimento, Pastorini, Romagnolo, & de Souza, Citation2015). In fact, the ability of plants to capture and utilize light is an important determinant of species growth, recruitment, and fitness, which is of utmost importance for introduced plants invading forest ecosystems (Standish, Robertson, & Williams, Citation2001). Changes in light availability may therefore induce differentiation in the plant architectural, morphological, anatomical, and physiological traits that affect plant survival in various environmental conditions (Oguchi, Hikosaka, & Hirose, Citation2003). In particular, leaf traits are highly plastic (Chen, Zeng, Fahey, Yao, & Yu, Citation2010) and being the leaf the main light-harvesting organ it requires a great acclimation capacity to enable carbon gain optimization under different light environmental conditions (Guo, Li, Gao, & Yang, Citation2019; Nascimento et al., Citation2015). Thus, a better understanding of the invasive ability and future invasive potential of introduced species can be achieved through information on phenotypic plasticity (Wei, Tang, Pan, & Li, Citation2017). In order to achieve this, a set of common methods for the quantitative estimation of phenotypic plasticity has been defined, depending on the purpose of the study (Valladares, Sanchez-Gomez, & Zavala, Citation2006).
In such context, the objective of this research was to analyze the phenotypic plasticity of several leaf traits at anatomical, morphological, and physiological level of two IAPs in response to contrasting light conditions (understory vs. opening). The selected species were the tree of heaven (Ailanthus altissima (Mill.) Swingle) and the black locust (Robinia pseudoacacia L.) considered among the 100 worst invasive species in Europe (Nentwig, Bacher, Kumschick, Pyšek, & Vilà, Citation2018) and included in the Italian watch-list of the invasive species. They are two of the most invasive plant species growing in the Strict Nature Reserve “Bosco Siro Negri” (Italy) which represents one of the best conserved relicts of the original alluvial forests that in the past largely covered the banks of the Ticino river (Motta, Nola, & Berretti, Citation2009). To date, the alluvial forest ecosystems are one of the most threatened natural environments of southern Europe because they have been greatly reduced in size and are now represented exclusively by fragmented remnants (Schnitzler, Hale, & Alsum, Citation2007). These two IAPs were introduced into the Ticino Regional Park at the end of the 19th century (Motta et al., Citation2009); and are now widespread contributing to significant ecological problems because they impact biodiversity and forest ecosystem functioning (Motta Fré & Motta, Citation2000). R. pseudoacacia is native to the south-eastern United States, and it is recognized as one of the most problematic invaders in Europe (Kleinbauer, Dullinge, Peterseil, & Essl, Citation2010), including Italy (Celesti Grapow, Pretto, Brundu, Carli, & Blasi, Citation2009). A. altissima is native to northeastern China expanding on all continents except Antarctica (Kowarik & Säumel, Citation2007). The analysis of morphological, anatomical, and physiological changes in response to contrasting light and quantitative evaluation through a phenotypic plasticity index could allow a better awareness of the capacity of the two IAPs to expand inside the Strict Nature Reserve. Therefore, these information could provide more sustain to the current integrated management of the Reserve in view of maintaining a sustainable forest. In particular, among all the common methods used to quantify the phenotypic plasticity, we applied the phenotypic plasticity index based on maximum and minimum means, which has been used in others previous studies to compared the phenotypic plastic response to contrasting light regimes (among others, Catoni, Granata, Sartori, Varone, & Gratani, Citation2015a; Catoni, Gratani, Sartori, Varone, & Granata, Citation2015b; Valladares et al., Citation2002; Xiao, Wang, Liu, Wang, & Du, Citation2015). Currently, inside the Strict Nature Reserve R. pseudoacacia is more widespread in the closed-canopy forest (Granata, Gratani, Bracco, Sartori, & Catoni, Citation2016; Motta et al., Citation2009), compared to A. altissima. Otherwise, both the IPAs are widespread in the forest edge characterized by high-light regime, confirming that this environment is more susceptible to the invasion than forest interior (H.-W. Lee & Lee, Citation2006; Radtke et al., Citation2013). Thus, our research hypothesis was that the greater invasion success of R. pseudoacacia in the forest understory arises from a higher acclimatization capacity to light environment variations resulting in a greater plasticity index compared to A. altissima.
Materials and methods
Study area and plant material
The study was carried out in the broadleaf deciduous forest within the Strict Nature Reserve “Bosco Siro Negri” (45°12ʹ39́’’N; 09°03ʹ26ʹ’E, 74 m a.s.l., Italy) in the period of May – October 2017.
The Reserve extending over 10 ha and no logging was carried out since its establishment in 1970 (Castagneri, Garbarino, & Nola, Citation2013). The Reserve belongs to a Site of Community Importance (IT 2080014, “Bosco Siro Negri and Moriano”) which covers an area of 1,352 ha.
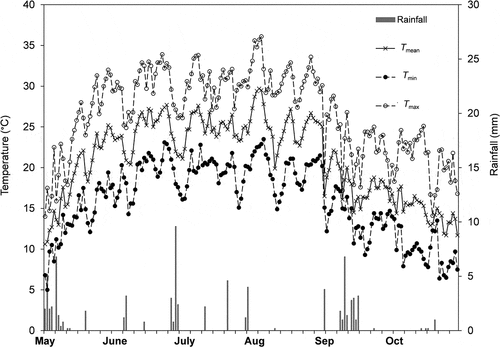
The climate of the area is characterized by a total annual rainfall of 654 mm most of it falling in autumn and winter. The mean minimum air temperature (Tmin) of the coldest month (January) is – 0.2 ± 1.8°C, the mean maximum air temperature (Tmax) of the hottest month (July) 30.1 ± 1.3°C and the mean annual temperature (Tm) 13.7 ± 8.2°C. Floods occurred sporadically every 5–10 years during the last 40 years, with water levels up to 1.50 m height in the forest during exceptional events (Castagneri et al., Citation2013; Motta et al., Citation2009). On average, groundwater level is around – 4.50 m in winter reaching – 3.50 m in summer due to irrigation in the surrounding area (Lombardia Regional Agency for Environmental Protection, Meteorological Station of Pavia, Ponte Ticino SS35, data for the period 2002–2016). During the study period total rainfall was 83 mm, Tm 21.1 ± 4.8°C and Tmax (August) 31.3 ± 2.2°C (Lombardia Regional Agency for Environmental Protection, Meteorological Station of Pavia, Ponte Ticino SS35, data for the period May – October 2017) ().
Figure 1. Trend of mean air temperature (Tmean), minimum air temperature (Tmin), maximum air temperature (Tmax) and rainfall during the study period (May–October).
Five representative juvenile plants of R. pseudoacacia and of A. altissima growing outside the forest, in high-light conditions (sun plants) and five representative juvenile plants of each species growing in the understory of the forest (shade plants) were randomly chosen. All the considered morphological, anatomical, and physiological leaf traits are summarized in .
Table 1. List of the analyzed leaf traits at morphological, anatomical, and physiological level in Ailanthus altissima and Robinia pseudoacacia.
Soil and light regime measurements
Soil samples (500 g each, three samples for sun and shade conditions) were collected in July in the area of the selected plants, at least 5 days after the last rainfall, by a hand auger at 40-cm depth. Soil analysis was performed according to Violante (Citation2000): pH, soil water content (SWC), total soil nitrogen content (N), soil organic matter content (SOM), and soil organic carbon content (C) were determined. Soil samples were air-dried and then passed through a 2-mm sieve. The pH (in H2O) was measured by a glass electrode pH meter (Corning Model 220 pH-meter, Labequip Ltd., Canada) on a 1:2.5 soil – water suspension. N was determined by the Kjeldahl method, and C by the oxidation method using K2Cr2O7- H2SO4 (Sims & Haby, Citation1971). The ratio between carbon and nitrogen content (C/N) was calculated. SWC was determined on soil samples (500 g each) as fresh soil minus dry soil divided by dry soil, calculated after oven dried at 90ºC to a constant mass.
The photosynthetic photon flux density (PPFD, µmol photon m−2 s−1) was measured monthly, during the study period, from 09.00 to 12.00 h, in the forest understory (shade condition) and in the open area (sun condition) by a quantum radiometer photometer (LI-185B, Licor, USA).
Morphological and anatomical measurements
Fully expanded leaves (n = 10 per each sun and shade plant per species) from the external medium portion of the crown of the considered plants were collected at the end of May. Leaf samples were sealed in plastic bags and transported immediately to the laboratory for measurements. Measurements included leaf surface area (LA), obtained by the image analysis system (Winfolia Software) and leaf dry mass (DM), determined by drying leaves at 80°C to a constant mass. The specific leaf area (SLA) was calculated by the ratio of LA and DM.
Fresh leaf sections from fully expanded leaves (n = 10 per each sun and shade plant and species) were hand cut and analyzed by light microscopy using an image analysis system (Axiovision AC software). The following parameters were measured: total leaf thickness (LT), palisade and spongy parenchyma thickness (PP and SP, µm, respectively), thickness of the adaxial and abaxial cuticle and epidermis (CETad and CETab, µm, respectively). All measurements were restricted to vein-free areas, according to B.F. Chabot and Chabot (Citation1977).
Gas-exchange and relative chlorophyll content measurements
Gas-exchange measurements were carried out in the period of May to October 2017 (three leaves per each sun and shade plant per species). Net photosynthetic rate (PN), stomatal conductance (gs), leaf transpiration (E), leaf temperature (Tl), and sub-stomatal CO2 concentration (Ci) were measured by an infrared gas analyzer (LCPro+, ADC, UK) equipped with a leaf chamber (PLC, Parkinson Leaf Chamber, UK). Measurements were carried out on cloud-free days in the morning from 9.00 to 11.00 h. In particular, measurements in sun were carried out when PPFD was ≥ 1,200 μmol photon m–2 s–1 to ensure that the maximum rates were measured. CO2 concentration in the leaf chamber (Ca) was set at 400 µmol CO2 mol−1 air, while air temperature ranged 25–33 ◦C and the relative humidity of the incoming air ranged between 40 and 60%. On each sampling occasion, leaf respiration (RD) was measured after PN measurements (on the same leaves) as CO2 efflux by darkening the leaf chamber with a black paper, according to Cai, Slot, and Fan (Citation2005) for 30 min prior to each measurement, to avoid the release of CO2 transient post irradiation bursts. The shown RD and PN represented the mean values of three days of measurements per month characterized by the same weather conditions. The ratio between RD and PN was calculated as indicative of the leaf carbon balance according to Catoni and Gratani (Citation2014).
The relative chlorophyll content (RCc, SPAD unit) was determined by means of SPAD-502 chlorophyll meter (Minolta, Japan). Measurements were carried out on the same leaves used for gas-exchange and chlorophyll fluorescence measurements (three leaves per each sun and shade plant per species).
Chl fluorescence measurements
Chlorophyll fluorescence measurements including maximum PSII photochemical efficiency (Fv/FM), actual quantum yield of photosynthesis of light-adapted leaves (ɸPSII) and electron transportation rate (ETR) were carried out by a portable modulated fluorometer (OS5p, Opti-Sciences, USA) on fully expanded leaves (three leaves per each sun and shade plant per species). For measurements of Fv/FM, leaves were first dark-adapted for 30 min by leaf clips then a saturating pulse was applied to measure initial (F0) and maximum (FM) fluorescence. FV/FM was estimated as:
ɸPSII was calculated on light-adapted leaves, according to Genty, Briantais, and Baker (Citation1989) as:
where FM´ was the maximum fluorescence obtained with a light-saturating pulse (∼8000 µmol m−2 s−1) and Fs was the steady-state fluorescence of illuminated leaves (1600 µmol m−2 s−1).
ETR (µmol e- m−2s−1) was calculated according to Krall and Edwards (Citation1992) as:
Leaf traits plasticity
A phenotypic plasticity index was calculated for each of the considered anatomical (PIa), morphological (PIm), and physiological (PIp) leaf traits measured in May, since previous studies have demonstrated that longer-term foliage traits versus light relationships are stable during most of the growing season (Hallik, Niinemets, & Kull, Citation2012; Niinemets, Kull, & Tenhunenet, Citation2004).
The index was calculated as the difference between the minimum and the maximum mean value between sun and shade leaves divided by the maximum mean value, according to Valladares, Wright, Lasso, Kitajima, and Pearcy (Citation2000). Higher PI values closer to 1 indicate that the variable is more plastic (Valladares et al., Citation2005). The mean plasticity index (PI) was calculated by averaging the plasticity index for all the considered anatomical, morphological, and physiological leaf traits. The PI had the advantage that variable with different units and contrasting ranges can be compared (Peperkorn, Werner, & Beyschlag, Citation2005; Valladares et al., Citation2000).
Statistical analysis
Differences in morphological, anatomical, and physiological leaf traits were analyzed by one-way analysis of variance (ANOVA). Repeated measure ANOVA was performed on physiological variables to test for significant difference (p ≤ 0.05) among months (i.e. main factor) and sampling days (i.e. within effect) in sun and shade plant and species. Simple regression analysis was carried out among the considered leaf traits. All statistical tests were performed using a statistical software (Statistica, Statsoft, USA).
Results
Soil and light regime measurements
Soil characterization in sun and shade conditions are shown in . The results showed a pH value of 5.15 ± 0.03 and 5.50 ± 0.05 in sun and shade, respectively, and a soil water content (SWC) of 17.0 ± 1.0% and 25.2 ± 3.3% in sun and shade, respectively. The soil N content was 47% higher in sun than in shade, while the ratio C/N was 93% higher in shade than in sun, soil organic matter (SOM) was 3.3 ± 0.3% and 5.2 ± 0.3% in sun and shade, respectively. During the study period, the average PPFD value in sun condition was 1,536 ± 100 µmol photon m–2 s–1 (mean value) and in shade condition was 124 ± 20 µmol photon m–2 s–1 (mean value).
Table 2. Soil characterization in sun and shade conditions. SWC – soil water content; N – total soil nitrogen content; C/N – ratio between carbon and nitrogen content; SOM – soil organic matter content. Mean values (± SE) are shown (n = 3).
Morphological and anatomical responses to contrasting light regimes
Morphological and anatomical leaf traits for sun and shade conditions are shown in .
Table 3. Morphological and anatomical leaf traits of sun and shade conditions in Ailanthus altissima and Robinia pseudoacacia. CETad – adaxial cuticle and epidermis thickness; CETab – abaxial cuticle and epidermis thickness. Mean values (± SE) are shown (n = 50). Different letters indicate significant differences between sun and shade conditions within each species (T-test, p < 0.05).
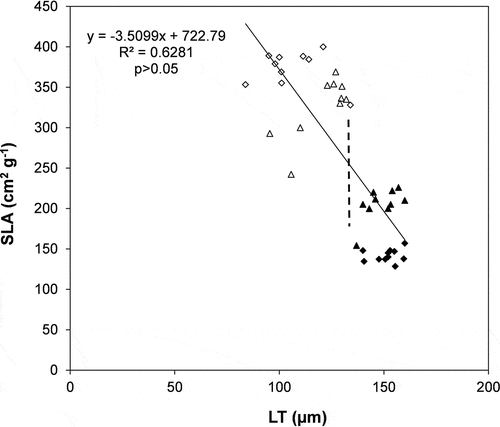
Leaf area was 45% lower (mean value of the two species) in sun than in shade condition, with the greater variation observed in R. pseudoacacia (47.5 ± 1.2 and 203.2 ± 5.5 cm2, sun and shade conditions, respectively). The higher LA in shade resulted, on an average, in a more than 100% higher SLA, with R. pseudoacacia having the highest value in shade condition (344.7 ± 18.8 cm2 g−1). At anatomical level, leaves in sun condition showed a higher leaf thickness and palisade parenchyma (by 37 and 82%, respectively) compared to shade. Among the two species, R. pseudoacacia had the highest LT in sun (150.5 ± 2.2 µm), while A. altissima the highest value in shade leaves (130.8 ± 4.7 µm). On the contrary, spongy parenchyma thickness was 8% higher (mean value of the two species) in shade than in sun condition. The palisade-to-spongy parenchyma ratio resulted 43% lower (mean value) in shade than in sun. CETad and CETab were 17 and 11%, respectively, higher in sun than in shade condition, both showing a higher value in A. altissima. The association between higher SLA with lower LT resulted in a significant (p < 0.05) negative correlation between these two variables, showing that a 63% of SLA variations was explained by variations at anatomical level. The broken line in defined a clear separation between sun and shade condition, with this last placed in the upper leaf part.
Figure 2. Regression analysis between specific leaf area (SLA) and total leaf thickness (LT). Regression equation, determination coefficient (R2) and significance level (p) are shown. (∆ = Ailanthus altissima shade condition; ◊ = Robinia pseudoacacia shade condition; ▲ = Ailanthus altissima sun condition; ♦ = Robinia pseudoacacia sun condition). The perpendicular, broken line separates the values of shade leaves (upper left part) and sun leaves (lower right part).
Physiological response to contrasting light regimes
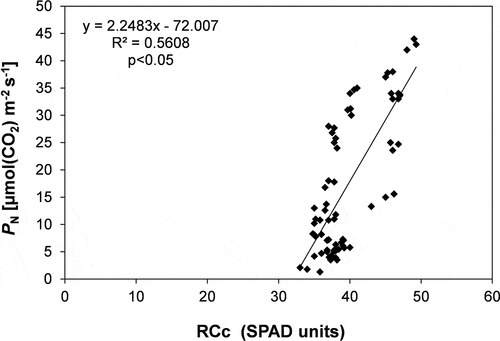
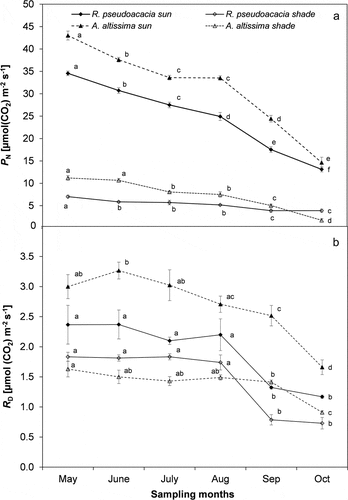
Trend of PN during the study period for sun and shade condition is shown in ). During the study period, leaves in sun condition had, on an average, more than 100% higher PN compared to shade, with the highest rates in May (34.7 ± 2.3 and 42.0 ± 1.9 µmol m−2 s−1, in R. pseudoacacia and A. altissima, respectively) decreasing by 64% (mean value) in October due to the approaching senescence phase. On an average, leaf respiration (RD) had a 63% higher value in sun than in shade, with the highest rates reached in May either for sun and shade condition in both the species (). Due to the lowest PN rates, leaves in shade condition showed a more than 100% higher RD/PN value than sun leaves. RCc was on an average 14% higher in sun than in shade, with A. altissima showing the highest value in sun (45.6 ± 0.6 SPAD units, mean value of the study period). RCc showed the same PN trend during the study period as attested by the significant (p < 0.05) positive relationship between these two variables ().
Figure 3. Trend of (a) net photosynthetic rates (PN), (b) leaf dark respiration (RD) during the study period in sun and shade conditions for Ailanthus altissima and Robinia pseudoacacia. Each point is the mean (±S.E.) of 15 leaves in three sampling days per month. Different letters indicates significant differences (ANOVA, p ≤ 0.05) during the study period.
Chl fluorescence response to contrasting light regimes
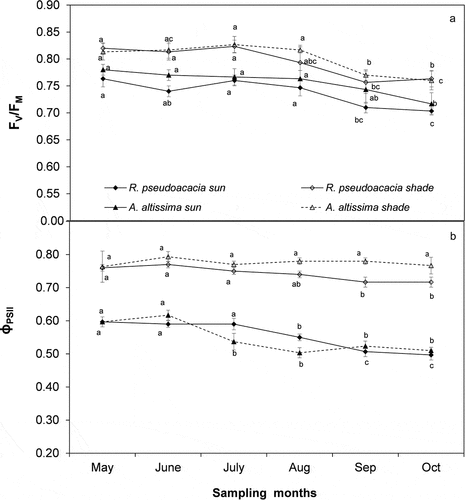
The mean value of FV/FM was higher in shade than in sun for both the species (0.80 ± 0.01 and 0.75 ± 0.01, respectively, mean value of the study period) ()). The same behavior was observed for ɸPSII with the highest rates in shade (0.76 ± 0.02, mean value during the study period) than in sun leaves (0.55 ± 0.01, mean value) ()). On the contrary, ETR was more than 100% higher in sun than in shade leaves.
Figure 5. Trend of A) maximum PSII photochemical efficiency (FV/FM) B) quantum yield of photosynthesis of light-adapted leaves (ɸPSII) during the study period in sun and shade conditions for Ailanthus altissima and Robinia pseudoacacia. Each point is the mean (±S.E.) of 15 leaves in three sampling days per month. Different letters indicates significant differences (ANOVA, p ≤ 0.05) during the study period.
Plasticity index
Values of phenotypic plasticity index for all the considered anatomical, morphological, and physiological leaf traits are show in . The comparison between the two investigated species resulted in a greater mean PI in R. pseudoacacia (0.46) compared to A. altissima (0.27), with this last showed the lowest PI for all the three analyzed levels of plasticity (i.e. PIa, PIm and PIp). Both the species showed the lowest value for the anatomical leaf traits (0.08 and 0.29 in A. altissima and R. pseudoacacia, respectively), and among the considered anatomical leaf traits, palisade parenchyma thickness had the largest variation (0.40, mean value). The highest PI was found for morphological traits in R. pseudoacacia, with LA having the largest variation between sun and shade conditions (PI = 0.77). A. altissima showed its highest PI at physiological level (PIp = 0.45), with a greater variation in ETR values (PI = 0.81).
Table 4. Plasticity index for the anatomical, morphological, and physiological leaf traits of Ailanthus altissima and Robinia pseudoacacia. The anatomical (PIa), morphological (PIm), physiological (PIp) plasticity index, are shown. PP – palisade parenchyma thickness; SP – spongy parenchyma thickness; CETad – adaxial cuticle and epidermis thickness; CETab – abaxial cuticle and epidermis thickness; LT – total leaf thickness; LA – leaf area; DM – dry mass; SLA – specific leaf area; E – transpiration rate; gs – stomatal conductance; PN – net photosynthetic rate; RD – respiration rate; RD/PN – ratio between RD and PN; RCc – relative chlorophyll content; FV/FM – maximal quantum yield of PSII photochemistry; ΦPSII – effective quantum yield of PSII photochemistry; ETR – electron transport rate. Bold font indicates leaf traits that significantly differed (p < 0.05) between sun and shade conditions within each species.
Discussion
The considered forest in the Strict Nature Reserve is characterized by a high light extinction at soil level, quantify by a value of the relative intercept irradiance by the canopy of 0.77 ± 0.12%, which is in the range of the typical closed-canopy forests (0.5–5%; Chazdon & Percy, Citation1991; Granata et al., Citation2016). In this type of environment, the low-light level in the understory and the ability of the trees species to acclimatize to shade conditions may be a driver for the invasion by the IAPs (Martin, Canham, & Marks, Citation2009). Several leaf traits at anatomical, morphological, and physiological level are involved in determine the ability of a species to grow and survive under low-light regime. Considering the need of preventing and limiting the presence of IAPs in forest ecosystem, a better knowledge of how the latter behave in these environments could be important in understanding their future potential spread.
Analysis of leaf traits in response to contrasting light regimes
Overall, A. altissima and R. pseudoacacia develop the well characterized changes at anatomical, morphological and physiological leaf level that enable them to adjust to the contrasting light regimes (sun vs shade); although these changes result more evident in R. pseudoacacia as will be seen through the plasticity index. In particular, both the IAPs under low-light show a larger SLA due to the lower total leaf thickness, as confirmed by the regression analysis between these two variables, that returns also a clear split along the regression line between sun and shade condition, confirming that SLA is one of the main morphological trait which changes in response to light variations (Puglielli, Varone, Gratani, & Catoni, Citation2017). In fact, its change, according to the light regimes, is an adaptation mechanism to maximize light harvesting per unit of resources invested in construction of photosynthetic tissue (Lusk, Reich, Montgomery, Ackerly, & Cavender Bares, Citation2008). Besides this, in shade condition the lower PP/SP scatters irradiance internally resulting in an increase in light absorption (Sack, Melcher, Liu, Middleton, & Pardee, Citation2006). Otherwise, in high-light conditions the thicker PP allows a greater light penetration, and the arrangement of chloroplasts on the cellular surface avoids the absorption of excess light through the shading mechanism (Pereira, Barros, & Scarano, Citation2009). In the same condition, a higher CET (by 14%, mean value) protects mesophyll layer against the excess of irradiance. Moreover, the thicker epidermal cell wall may induce a more humid microclimate, reducing the gradient of water diffusion between leaf and air (Tenberge, Citation1992). The wider cuticle and epidermis can be seen as a possibly response to both soil water shortage and to higher exposure to light, since the biosynthetic pathway for lignin and cuticle production is directly dependent on light (Hrazdina & Jensen, Citation1992). Furthermore at physiological level, the lower PN rates observed at low light regime, due to anatomical constraints that limit, among other, the amount of CO2 that reaches carboxylation sites in chloroplasts (Oguchi, Hikosaka, Hiura, & Hirose, Citation2006) are associated with a RD decrease. This is necessary to maintain a positive leaf carbon balance (expressed by the RD/PN ratio) allowing the growth under low light according to the “carbon gain hypothesis” (Valladares & Niinemets, Citation2008). The RCc increases in response to the high-light level in forest edge, suggesting an increased concentration of chlorophyll and resulting a common characteristic among light tolerant species (Lambers, Chapin III, & Pons, Citation1998). Moreover, another typical response to the high-light regime is to reduce the variable fluorescence ratio (FV/FM) leading to a slow recovery process, allowing an enhanced photosynthetic capacity (Azevedo & Marenco, Citation2012). In fact, the lower FV/FM in sun leaves (0.75 ± 0.01, mean value of the two species during the study period) respect to shade leaves (0.80 ± 0.01, mean value) highlights an efficient functioning of the photo-protection mechanism without oxidative damage to the photosynthetic machinery, as suggested by Thiele, Krause, and Winter (Citation1998). In fact, it should be considered that the PPFD of full sunlight exceeds the energy input that is exploitable in plant metabolism; as a consequence, excess light energy must be safely dissipated (Dietz, Citation2015), otherwise the plants are vulnerable to stress (Demmig- Adams & Adams, Citation1992). According, also the lower ɸPSII in high-light (by 27%, mean value) compared to shade condition highlights their higher capacity to dissipate the excess of the excitation energy as heat (Björkman & Demmig Adams, Citation1994), and it is associated with a more than 100% higher ETR (mean value) according to the results of Yang, Sun, Zhang, Cochard, and Cao (Citation2014).
Comparative analysis of leaf traits plasticity
Plants may have high or low plasticity at morphological, anatomical, and physiological levels, which enable their acclimatization, survival, and growth under different light regimes in the forest (Valladares et al., Citation2000). The comparative analysis of the phenotypic plasticity index allows us to better understand the response of the two IAPs to contrasting light environment, and then to obtain information also about the future capacity to widespread in the Strict Nature Reserve. According to our working hypothesis, based on the widespread presence of R. pseudoacacia in the forest interior, it shows a 70% higher PI, and, among the three level of analyzed plasticity index, it has a strongly greater PIa and PIm (more than 100% higher compared to A. altissima). In particular, R. pseudoacacia shows the highest value at morphological level, which can be associated with a greater ability to survive and grow in the forest understory (Catoni et al., Citation2015a; Niinemets & Valladares, Citation2004; Valladares et al., Citation2002). Otherwise, A. altissima shows its highest plasticity index at physiological level (PIp = 0.45), which is associated with the ability to colonize and grow in environments with high-light regime (Catoni et al., Citation2015b; Niinemets & Valladares, Citation2004; Valladares et al., Citation2002), because it ensures adjustments of gas exchange in response to stress factors changes in the short term (Zunzunegui et al., Citation2009). In particular, among the considered physiological traits, A. altissima shows a higher photosynthetic plasticity which determines a larger photosynthetic capacity to use full sunlight, which results in a more efficient avoidance of photo-inhibition (Valladares et al., Citation2002).
Conclusion
The results highlight a greater capacity of both the species to grow in the forest edge, characterize by a high-light conditions, by preventing photo-inhibition of photosynthesis through several adaptations at anatomical, morphological and physiological level (i.e. a higher CETab, a lower LA, lower FV/FM and ɸPSII) according to Celesti-Grapow et al. (Citation2009).
At the same time, we found that R. pseudoacacia shows a higher capacity to acclimatize to low-light conditions in the forest understory as expressed by its higher PI and indeed its presence in the closed-canopy forest is well known (Catoni et al., Citation2015b; Granata, Gratani, Bracco, & Catoni, Citation2019; Granata et al., Citation2016; Motta et al., Citation2009). Moreover, our data support the traditional description of A. altissima as highly shade-intolerant species (Kobe, Pacala, Silander, & Canham, Citation1995). In particular, Knapp and Canham (Citation2000) analyzed the invasion of A. altissima in an old-growth forest in New York concluding that the species is gap-obligate rather than gap-facultative. It is able to establish a ramet bank in the forest understory that can persist until gap open in the forest canopy and then, once established and exposed to light in a gap, A altissima is capable to growing in the upper forest canopy (Knapp & Canham, Citation2000). Due to these considerations and taking into account its high growth rates, high reproductive capacity and dispersal potency of its seeds associated with its early maturation (Swearingen, Citation2006) it is an imperative to maintain closed undisturbed forest conditions. Thus, the conservative management of the Reserve carried out since its establishment has limited the presence of A. altissima because the small-scale gaps arise in the forest infrastructure, which could allow the A. altissima recruitment, are originated from death of a single tree or small group of trees (Motta et al., Citation2009). Concerning R. pseudoacacia it is critical to maintain this type of management in the future because any disturbances resulting in large openings could further promote its spread inside the closed-canopy forest.
We aware that this study concerned only two IAPs and for that reason it cannot be generalized in other threatened forest ecosystems. Nevertheless, our finding together with a general superior physiological performance of invasive alien species over the native co-existing ones (Le, Tennakoon, Metali, & Sukri, Citation2019), as observed in previous works carried out inside the Reserve (Catoni et al., Citation2015b; Granata et al., Citation2019) further reinforce the imperative to guarantee this type of management in order to maintain over time the ecosystem integrity of this Reserve.
Acknowledgments
This work was supported by the “Natural Reserve Siro Negri” funded by the Ministry of the Environmental and Protection of Land and Sea of Italy.
References
- Azevedo, G. F. C., & Marenco, R. A. (2012). Growth and physiological changes in saplings of Minquartia guianensis and Swietenia macrophylla during acclimation to full sunlight. Photosynthetica, 50, 86–94. doi:10.1007/s11099-012-0001-2
- Björkman, O., & Demmig-Adams, B. (1994). Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In E. D. Schulze & M. M. Caldwell (Eds.), Ecophysiology of Photosynthesis (pp. 17–47). Berlin, Germany: Springer-Verlag.
- Bradshaw, A. D. (1965). Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics, 13, 115–155.
- Cai, Z. Q., Slot, M., & Fan, Z. X. (2005). Leaf development and photosynthetic properties of three tropical tree species with delayed greening. Photosynthetica, 43, 91–98. doi:10.1007/s11099-005-1098-3
- Castagneri, D., Garbarino, M., & Nola, P. (2013). Host preference and growth patterns of ivy (Hedera helix L.) in a temperate alluvial forest. Plant Ecology, 214, 1–9. doi:10.1007/s11258-012-0130-5
- Catoni, R., Granata, M. U., Sartori, F., Varone, L., & Gratani, L. (2015a). Corylus avellana responsiveness to light variations: Morphological, anatomical, and physiological leaf trait plasticity. Photosynthetica, 53, 35–46. doi:10.1007/s11099-015-0078-5
- Catoni, R., & Gratani, L. (2014). Variations in leaf respiration and photosynthesis ratio in response to air temperature and water availability among Mediterranean evergreen species. Journal of Arid Environments, 102, 82–88. doi:10.1016/j.jaridenv.2013.11.013
- Catoni, R., Gratani, L., Sartori, F., Varone, L., & Granata, M. U. (2015b). Carbon gain optimization in five broadleaf deciduous trees in response to light variation within the crown: Correlations among morphological, anatomical and physiological leaf traits. Acta Botanica Croatica, 74, 71–94. doi:10.1515/botcro-2015-0010
- Celesti-Grapow et al. (2009). A thematic contribution to the National Biodiversity Strategy. In Plant invasion in Italy, an overview. Ministry for the Environment Land and Sea Protection, Nature Protection Directorate, Rome, IT: Palombi & Partner S.r.l., 32 p.
- Chabot, B. F., & Chabot, J. F. (1977). Effects of light and temperature on leaf anatomy and photosynthesis in Fragaria vesca. Oecologia, 26, 363–377. doi:10.1007/BF00345535
- Chazdon, R. L., & Pearcy, R. W. (1991). The Importance of sunflecks for forest understory plants. BioScience, 41, 760–766. doi:10.2307/1311725
- Chen, F. S., Zeng, D. H., Fahey, T. J., Yao, C. Y., & Yu, Z. Y. (2010). Response of leaf anatomy of Chenopodium acuminatum to soil resource availability in a semi-arid grassland. Plant Ecology, 209, 375–382. doi:10.1007/s11258-010-9778-x
- Davidson, A. M., Jennions, M., & Nicotra, A. B. (2011). Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters, 14, 419–431. doi:10.1111/ele.2011.14.issue-4
- Demmig-Adams, B., & Adams, W. W. I. I. I. (1996). Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plants species. Planta, 198, 460–470.
- Dietz, K.-J. (2015). Efficient high light acclimation involves rapid processes at multiple mechanistic levels. Journal of Experimental Botany, 66, 2401–2414. doi:10.1093/jxb/eru505
- Ehrenfeld, J. G. (2003). Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems, 6, 503–523. doi:10.1007/s10021-002-0151-3
- Genty, B., Briantais, J. M., & Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica Et Biophysica Acta, 990, 87–92. doi:10.1016/S0304-4165(89)80016-9
- Godoy, O., Valladares, F., & Castro-Díez, P. (2012). The relative importance for plant invasiveness of trait means, and their plasticity and integration in a multivariate framework. New Phytologist, 195, 912–922. doi:10.1111/j.1469-8137.2012.04205.x
- Granata, M. U., Gratani, L., Bracco, F., & Catoni, R. (2019). Carbon dioxide sequestration capability of an unmanaged old-growth broadleaf deciduous forest in a Strict Nature Reserve. Journal of Sustainable Forestry, 38, 85–92. doi:10.1080/10549811.2018.1504685
- Granata, M. U., Gratani, L., Bracco, F., Sartori, F., & Catoni, R. (2016). Carbon stock estimation in an unmanaged old-growth forest: A case study from a broad-leaf deciduous forest in the northwest of Italy. International Forestry Review, 18, 444–451. doi:10.1505/146554816820127587
- Guo, Q. Q., Li, H. E., Gao, C., & Yang, R. (2019). Leaf traits and photosynthetic characteristics of endangered Sinopodophyllum hexandrum (Royle) Ying under different light regimes in Southeastern Tibet Plateau. Photosynthetica, 57(2), 548–555. doi:10.32615/ps.2019.080
- Hallik, L., Niinemets, Ü., & Kull, O. (2012). Photosynthetic acclimation to light in woody and herbaceous species: A comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biology, 14, 88–99. doi:10.1111/j.1438-8677.2011.00544.x
- Hawkes, C. V., Wren, I. F., Herman, D. J., & Firestone, M. K. (2005). Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecological Letters, 8, 976–985. doi:10.1111/j.1461-0248.2005.00802.x
- Hrazdina, G., & Jensen, R. A. (1992). Spatial organization of enzymes in plant metabolic pathways. Annual Review of Plant Physiology and Molecular Biology, 43, 241–267. doi:10.1146/annurev.pp.43.060192.001325
- Kleinbauer, I., Dullinge, S., Peterseil, J., & Essl, F. (2010). Climate change must drive the invasive tree Robinia pseudoacacia into nature reserve and endangered habitats. Biological Conservation, 143, 382–390. doi:10.1016/j.biocon.2009.10.024
- Knapp, L. B., & Canham, C. D. (2000). Invasion of an old-growth forest in New York by Ailanthus altissima: Sapling growth and recruitment in canopy gaps. The Journal of the Torrey Botanical Society, 127, 307–315. doi:10.2307/3088649
- Kobe, R. K., Pacala, S. W., Silander, J. A., & Canham, C. D. (1995). Juvenile tree survivorship as a component of shade tolerance. Ecological Applications, 55, 517–532. doi:10.2307/1942040
- Kowarik, I., & Säumel, I. (2007). Biological flora of central Europe: Ailanthus altissima (Mill.) Swingle. Perspectives in Plant Ecology, Evolution and Systematics, 8, 207–237. doi:10.1016/j.ppees.2007.03.002
- Krall, J. P., & Edwards, G. E. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum, 86, 180–187. doi:10.1111/ppl.1992.86.issue-1
- Lambers, H., Chapin, F. S., III, & Pons, T. L. (1998). Plant physiological ecology. New York, NY: Springer-Verlag.
- Le Maitre, D. C., Van Wilgen, B. W., Chapman, R. A., & McKelly, D. H. (1996). Invasive plants and water resources in the Western Cape Province, South Africa: Modelling the consequences of a lack of management. Journal of Applied Ecology, 33, 161–172. doi:10.2307/2405025
- Le, Q. V., Tennakoon, K. U., Metali, F., & Sukri, R. S. (2019). Photosynthesis in co-occurring invasive Acacia spp. and native Bornean heath forest trees at the post-establishment invasion stage. Journal of Sustainable Forestry. doi:10.1080/10549811.2018.1530602
- Lee, H.-W., & Lee, C.-S. (2006). Environmental factors affecting establishment and expansion of the invasive alien species of tree of heaven (Ailanthus altissima) in Seoripool Park, Seoul. Integrative Biosciences, 10(1), 27–40. doi:10.1080/17386357.2006.9647281
- Lindsay, E. A., & French, K. (2005). Litterfall and nitrogen cycling following invasion by Chrysanthemoides monilifera ssp. rotundata in coastal Australia. Journal of Applied Ecology, 42, 556–566. doi:10.1111/j.1365-2664.2005.01036.x
- Lusk, C. H., Reich, P. B., Montgomery, R. A., Ackerly, D. D., & Cavender Bares, J. (2008). Why are evergreen leaves so contrary about shade? Trends in Ecology and Evolution, 23, 299–303. doi:10.1016/j.tree.2008.02.006
- Maron, J. L., & Marler, M. (2008). Field-based competitive impacts between invaders and natives at varying resource supply. Journal of Ecology, 96, 1187–1197. doi:10.1111/jec.2008.96.issue-6
- Martin, P. H., Canham, C. D., & Marks, P. L. (2009). Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Frontiers in Ecology and the Environment, 7, 142–149. doi:10.1890/070096
- Matesanz, S., Gianoli, E., & Valladares, F. (2010). Global change and the evolution of phenotypic plasticity in plants. Annals of the New York Academy of Sciences, 1206, 35–55. doi:10.1111/nyas.2010.1206.issue-1
- Motta Fré, V., & Motta, R. (2000). Selvicoltura e ciliegio tardivo (Prunus serotina Ehrh.) nella Riserva Naturale Orientata “La Fagiana” (Magenta -MI). Sherwood, 6, 5–14.
- Motta, R., Nola, P., & Berretti, R. (2009). The rise and fall of the black locust (Robinia pseudoacacia L.) in the »Siro Negri« Forest Reserve (Lombardy, Italy): Lessons learned and future uncertainties. Annals of Forest Science, 66, 410–419. doi:10.1051/forest/2009012
- Nascimento, K. C., Pastorini, L. H., Romagnolo, M. B., & de Souza, L. A. (2015). Do Eugenia hiemalis seedling leaves under different light conditions develop phenotypic plasticity? Plant Ecology, 216, 1571–1581. doi:10.1007/s11258-015-0540-2
- Nentwig, W., Bacher, S., Kumschick, S., Pyšek, P., & Vilà, M. (2018). More than “100 worst” alien species in Europe. Biological Invasions, 20, 1611–1621. doi:10.1007/s10530-017-1651-6
- Niinemets, Ü., Kull, O., & Tenhunen, J. D. (2004). Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell and Environment, 27, 293–313. doi:10.1111/j.1365-3040.2003.01143.x
- Niinemets, Ü., & Valladares, F. (2004). Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: Optimality and constraints. Plant Biology, 6, 254–268. doi:10.1055/s-2004-817881
- Niu, H., Liu, W., Wan, F., & Liu, B. (2007). An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant and Soil, 294, 73–85. doi:10.1007/s11104-007-9230-8
- Oguchi, R., Hikosaka, K., & Hirose, T. (2003). Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell and Environment, 26, 505–512. doi:10.1046/j.1365-3040.2003.00981.x
- Oguchi, R., Hikosaka, K., Hiura, T., & Hirose, T. (2006). Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate forest. Oecologia, 149, 571–582. doi:10.1007/s00442-006-0485-1
- Pauchard, A., & Shea, K. (2006). Integrating the study of non-native plant invasions across spatial scales. Biological Invasions, 8, 399–413. doi:10.1007/s10530-005-6419-8
- Peperkorn, R., Werner, C., & Beyschlag, W. (2005). Phenotypic plasticity of an invasive acacia versus two native Mediterranean species. Plant Functional Biology, 32, 933–944. doi:10.1071/FP04197
- Pereira, D. C., Barros, C. F., & Scarano, F. R. (2009). In situ variation in leaf anatomy and morphology of Andira legalis (Leguminosae) in two neighboring but contrasting light environments in a Brazilian sandy coastal plain. Acta Botanica Brasilica, 23, 267–273. doi:10.1590/S0102-33062009000100028
- Pigliucci and Preston (2004). Eds. Phenotypic integration: Studying the ecology and evolution of complex phenotypes. New York, NY: Oxford University Press., 437 p.
- Puglielli, G., Varone, L., Gratani, L., & Catoni, R. (2017). Specific leaf area variations drive acclimation of Cistus salvifolius in different light environments. Photosynthetica, 55, 31–40. doi:10.1007/s11099-016-0235-5
- Radtke, A., Ambraß, S., Zerbe, S., Tonon, G., Fontana, V., & Ammer, C. (2013). Traditional coppice forest management drives the invasion of Ailanthus altissima and Robinia pseudoacacia into deciduous forests. Forest Ecology and Management, 291, 308–317. doi:10.1016/j.foreco.2012.11.022
- Richards, C. L., Bossdorf, O., Muth, N. Z., Gurevitch, J., & Pigliucci, M. (2006). Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters, 9, 981–993. doi:10.1111/ele.2006.9.issue-8
- Sack, L., Melcher, P. J., Liu, W. H., Middleton, E., & Pardee, T. (2006). How strong is intra canopy leaf plasticity in temperate deciduous trees? American Journal of Botany, 9, 829–839. doi:10.3732/ajb.93.6.829
- Schnitzler, A., Hale, B. W., & Alsum, E. M. (2007). Examining native and exotic species diversity in European riparian forests. Biological Conservation, 138, 146–156. doi:10.1016/j.biocon.2007.04.010
- Sims, J. R., & Haby, V. A. (1971). Simplified colorimetric determination of soil organic matter. Soil Science, 112, 137–141. doi:10.1097/00010694-197108000-00007
- Standish, R. J., Robertson, A. W., & Williams, P. A. (2001). The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. Journal of Applied Ecology, 38, 1253–1263. doi:10.1046/j.0021-8901.2001.00673.x
- Swearingen, J. M. (2006). Tree-of-Heaven. Ailanthus altissima (Mill.) Swingle. Quassia family (Simaroubaceae). Retrieved from: www.nps.gov/plants/alien/fact/aial1.htm.
- Tenberge, K. B. (1992). Ultrastructure and development of the outer epiderm wall of spruce (Picea abies) needles. Canadian Journal of Botany, 70, 1467–1487. doi:10.1139/b92-185
- Thiele, A., Krause, G. H., & Winter, K. (1998). In situ study of photoinhibition of photosynthesis and xanthophyll cycle activity in plants growing in natural gaps of the tropical forest. Australian Journal of Plant Physiology, 25, 189–195.
- Valladares, F., Arrieta, S., Aranda, I., Lorenzo, D., Sánchez-Gómez, D., Tena, D., . …., & Alberto Pardos, J. (2005). Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental-Mediterranean sites. Tree Physiology, 25, 1041–1052. doi:10.1093/treephys/25.8.1041
- Valladares, F., Chico, J. M., Aranda, I., Balaguer, L., Dizengremel, P., Manrique, E., & Dreyer, E. (2002). The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees, 16, 395–403.
- Valladares, F., & Niinemets, U. (2008). Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology and Evolution, S 39, 237–257. doi:10.1146/annurev.ecolsys.39.110707.173506
- Valladares, F., Sanchez-Gomez, D., & Zavala, M. A. (2006). Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology, 94, 1103–1116. doi:10.1111/jec.2006.94.issue-6
- Valladares, F., Wright, S. J., Lasso, E., Kitajima, K., & Pearcy, R. W. (2000). Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology, 81, 1925–1936. doi:10.1890/0012-9658(2000)081[1925:PPRTLO]2.0.CO;2
- van Kleunen, M., Weber, E., & Fischer, M. (2010). A meta-analysis of trait differences between invasive and non-invasive plant species. Ecological Letters, 13, 235–245. doi:10.1111/j.1461-0248.2009.01418.x
- Vilá, M., Espinar, J. L., Hejda, M., Hulme, P. E., Jarosík, V., Maron, J. L., . …. …., …. Pysek, P. (2011). Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecological Letters, 14, 702–708. doi:10.1111/j.1461-0248.2011.01628.x
- Violante, P. (2000). Metodi di analisi chimica del suolo. [Chemical Methods of Soil Analysis]. Milan, IT: Franco Angeli editore, 536 p.
- Wei, C., Tang, S., Pan, Y., & Li, X. (2017). Plastic responses of invasive Bidens frondosa to water and nitrogen addition. Nordic Journal of Botany, 35, 232–239. doi:10.1111/njb.2017.v35.i2
- Xiao, H., Wang, C., Liu, J., Wang, L., & Du, D. (2015). Insights into the differences in leaf functional traits of heterophyllous Syringa oblata under different light intensities. Journal of Forestry Research, 26(3), 613–621. doi:10.1007/s11676-015-0100-6
- Yamashita, N., Ishida, A., Kushima, H., & Tanaka, N. (2000). Acclimation to sudden increase in light favouring an invasive over native trees in subtropical islands, Japan. Oecologia, 125, 412–419. doi:10.1007/s004420000475
- Yang, S., Sun, M., Zhang, Y., Cochard, H., & Cao, K. (2014). Strong leaf morphological, anatomical, and physiological responses of a sub tropical woody bamboo (Sinarundinaria nitida) to contrasting light environments. Plant Ecology, 215, 97–109. doi:10.1007/s11258-013-0281-z
- Zunzunegui, M., Ain-Lhout, F., Díaz Barradas, M. C., Álvarez-Cansino, L., Esquivias, M. P., & García Novo, F. (2009). Physiological, morphological and allocation plasticity of a semi-deciduous shrub. Acta Oecologiae, 35, 370–379. doi:10.1016/j.actao.2009.02.004