?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
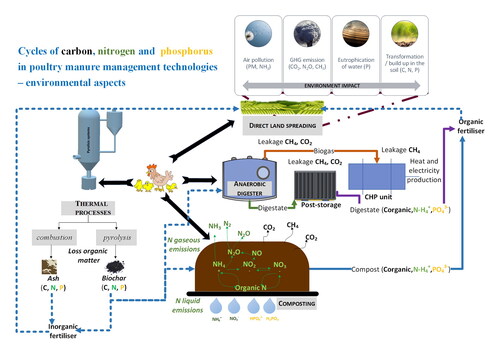
Poultry manure (PM) has become a serious environmental problem due to large scale of industrial production and unsustainable management, causing emissions of greenhouse gases (GHGs), odors, leakage of nutrients as well as inorganic, organic and biological pollutants. The main goal of this review was to get a better understanding of carbon, nitrogen, and phosphorous cycles in processing of poultry manure with the most common technologies such as composting, anaerobic digestion, or thermal processes, e.g. pyrolysis. The carbon contained in organic matter (ca. 31%) is mineralized and humified under aerobic conditions (matter recovery) and/or converted into biogas under anaerobic conditions (energy recovery). PM as a feedstock for pyrolysis to obtain biochar may effectively store and prevent C, thus contributing to climate change abatement. During composting, nitrogen is reduced from the compost mixture by leachates in the form of NH4+, NO3− or gaseous emissions of NH3, N2O, N2. The C/N ratio is also decisive parameter. Most environmental threats of unmanaged PM result from ammonia and nitrous oxide emissions, being higher for PM compared to cattle and cow manure independently of technological processes. The phosphorous in PM is mainly inorganic (32-84%). Using untreated manure as a fertilizer does not allow taking up high doses of phosphorus contained in poultry manure, so the excess accumulates in the soil and then leaches into groundwater. Biochar and struvite are an alternative to storage and source of high concentrations of phosphorous. Cycles of carbon, nitrogen and phosphorus are an integral effect of technologies used for PM management.
GRAPHICAL ABSTRACT
HANDLING EDITORS:
1. Introduction
Poultry production is estimated at 40% of the total meat consumption with the biggest producers in 2020: USA (23 million Mg), China (20 million Mg) and Brazil (16 million Mg), (USDA, Citation2021). Almost 20% of poultry meat within EU-27 countries is produced in Poland (Eurostat, 2021).
Poultry manure (PM) contains about 3-5% nitrogen, 0.9-3.5% phosphorus, and 1.5-3% potassium (Jędrczak et al., Citation2014). Moreover, the content of calcium, magnesium and sulfur is much higher than in cattle or pig manure (Bolan et al., Citation2010; Brassard et al., Citation2018). PM can be directly spreading on soil or treated by different processes to produce added-value products (compost, digestate, biochar). Soil application of PM initiates a series of reactions such as decomposition, hydrolysis, ammonia volatilization, nitrification, denitrification, fermentation and finally could be important source of GHGs: ammonia (NH3), methane (CH4), nitrous oxide (N2O), nitric oxide (NO) and carbon dioxide (CO2), Li at al. (2012). Moreover, can lead to soil/groundwater contamination by heavy metals - Zn, Cu (Liu et al., Citation2020). The buffer capacity of the ecosystem may be exceeded and the fertilizer or long-term stored PM become an environmental hazard (Kabelitz et al., Citation2021). Therefore, any PM processing technology should be based on a thorough analysis of the nutrients (especially C/N/P, properly calibrated application methods based on biogeochemical cycles to prevent GHGs emissions and efficiently use the nutrients for soil improvement. Composting and anaerobic digestion (AD) are biotic-based, however strictly controlled by abiotic factors as temperature, moisture, redox potential, pH, substrate concentration gradient (Li et al., Citation2012). Biogas production from PM and use of the digestate as an organic fertilizer as renewable energy production and substitution of the mineral fertilizers led to the decrease of GHGs emissions (Kreidenweis et al., Citation2021). However, improperly processing results in an increase GHGs emissions, mainly due to high ammonia volatilization in the initial step of process.
Thermal conversion of PM is also considered as renewable energy source. The energy obtained is CO2 neutral and saves fossil fuels (Billen et al., Citation2015). The life cycle assessments shown that thermal processing of PM generates less GHGs than direct land application and AD (He et al., Citation2022). Moreover, the solid post-process residues (ash, biochar) enable their environmental use as soil improvement (Piash et al., Citation2022).
The overall goal of this study was to analyze the current state of the art on biogeochemical cycles of carbon, nitrogen and phosphorus in the most common technologies poultry manure management such as land spreading, composting, anaerobic fermentation, combustion, gasification and pyrolysis in the context of environmental threats and climate changes mitigation.
2. Technologies for management
2.1. Mechanical
2.1.1. Landspreading
Traditionally PM has been used directly as a soil fertilizer (Table S1, supplementary material). Proper use led to the increase of biomass production and crop quality due to the content of minerals, such nitrogen and phosphorus. However, inappropriate use affected soil and plant quality and contaminated surface and groundwater by potentially toxic elements (PTEs), including cadmium, chromium, nickel, cobalt, copper, barium, lead, (Muhammad et al., Citation2020). Moreover PM could be a significant source of pathogens. In Australia 100% of broiler litter was contaminated with Actinobacillus and Salmonella and in the US - with Escherichia coli resistant to amoxicillin, ceftiofur, tetracycline, and sulfonamide (Kyakuwaire et al., Citation2019).
Table 1. Selected biochar properties described by (Novak et al., Citation2009, Song & Guo, Citation2012, Srinivasan et al., Citation2015, Khan et al., Citation2016, Qi et al., Citation2017, Li et al., Citation2018, Bavariani et al., Citation2019, Wystalska et al., Citation2021).
2.2. Biological
2.2.1. Composting
During PM composting, mineralization, nitrification, incomplete denitrification, and the release of gaseous occur. It follows various phases, i.e. mesophilic, thermophilic (dynamic biological processes involving microorganisms), cooling, and finally the compost maturation. The process requires adequate moisture, a C/N ratio, and the porosity of the compost mixture. To meet these conditions, the manure must be mixed with appropriate bulking materials such as sawdust, grasses, various types of straw, wood shavings, and chips. The temperature of the compost mixture should exceed 55°C and reach 70°C for the hygienization (EU Regulation No 142/2011). This applies, inter alia, to the degradation of live eggs of Ascaris sp., Trichuris sp., Toxocara sp., bacteria Salmonella and Escherichia coli. The pathogens number can be reduced after reaching high temperatures however requires strict monitoring (Lim et al., Citation2020). Aeration of the compost mixture is crucial and the minimum 15-20% O2 is necessary. Lower values may cause the formation of anaerobic zones and contribute to the putrefaction of the mixture, thus hindering the proper functioning of microorganisms. The processing time may be 4-40 weeks, and the final weight of the compost will be reduced by 40-50% in relation to the initial weight. During composting nitrogen, carbon and phosphorous are partially lost: 13-70% for nitrogen, 42-62% for carbon and 28-50% for phosphorus (Hao & Benke, Citation2008; Harrison, Citation2008). Nitrogen is lost through leaching of NH4+, NO3- or releasing gaseous emissions of NH3, N2O, N2. Carbon is lost in the form of CO2, CH4 and phosphorus mainly in the leachate of HPO42- and H2PO4.
Bacteria and fungi are essential for composting. The most common bacteria in the compost mixture includes Pseudomonas, Xanthomonas, Actinobacteria, Nocardia, Streptomyces which can break down organic matter. Nitrosomonas and Nitrospira specializing in nitrogen transformation are especially important for composting poultry manure. The fungi such Aspergillus, Acremonium, Chrysosporium, Fusarium, Mortierella, Penicillium and Trichoderma occur partially in the first and dominate the last stages of composting. Fungi occur in the mesophilic phase and during compost maturation where the temperature is not as high as in the active phase during which it can reach 50-60 °C (Sánchez et al., Citation2017).
Compost from PM has a positive effect on reducing soil evaporation, enriches soil with nitrogen, improves the release of nutrients, plant biomass and quality (Al-Bataina et al., Citation2016; Faucette et al., Citation2004). For example, the increase starch content of cassava as well as the oil and protein from mustard seeds was observed when plants were cultivated on soil fertilized by PM compost (Amanullah et al., Citation2010).
The mineralization causes changes in the organometallic complex of heavy metals. The ligands are transformed into forms of ligands with oxygen, i.e., carboxylic or hydroxyl complexes, which affect the mobility of particles and the possibility of their accumulation in living organisms.
2.2.2. Anaerobic processes
Anaerobic digestion (AD) is a complex biochemical process of macromolecular organic substances decomposition contained under anaerobic conditions (ORP<-200mV). Transformations allow for the conversion of biodegradable organic matter mainly into methane and carbon dioxide (Gallert & Winter, Citation2005) and reduction of nitrate and sulfate to hydrogen sulfide and sulfide, as well as anaerobic ammonification. The overall process of AD can be described by the Equationequation (1)(1)
(1) proposed in 1952 by Buswell (Anukam et al., Citation2019; Meegoda et al., Citation2018):
(1)
(1)
Based on the biochemical reaction formula and the elementary composition of raw chicken manure (C (35.16%), H (4.83%), O (30.12%), S (0.84%), and N (5.44%)), Qioa et al. estimated that degradation of 1 kg of volatile solids (VS) of chicken manure produced
0.74 m3 biogas, 0.42 m3 methane and 72 g of ammonia nitrogen (87 g free ammonia nitrogen (FAN)) (Shapovalov et al., Citation2020).
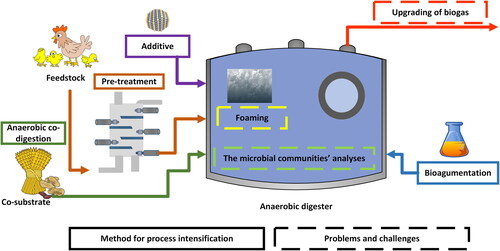
AD is often limited by low C/N ratio, high content of nitrogen, hydrogen sulfide in biogas as well as problem with foaming (). Optimization and improvement of anaerobic digestion efficiency is achieved by feedstock pretreatment before introduction to the digestion chambers and/or co-digestion of poultry manure with other organic waste (Dróżdż et al., Citation2020; Khoshnevisan et al., Citation2021; Kreidenweis et al., Citation2021). Bioaugmentation and the use of additives in the form of nanoparticles or trace elements supplementation also seem to be an promising option (Aguilar-Moreno et al., Citation2020; Hassanein et al., Citation2019; Shah et al., Citation2021) Furthermore, to reduce the risk of failure of the process due to ammonia accumulation, the following solutions can be applied: i) stripping (Guštin & Marinšek-Logar, Citation2011); ii) addition of ion exchange/adsorption materials such as e.g., zeolite (Ziganshina et al., Citation2015), natural chabazite (Lin et al., Citation2016) or biochar (Ma et al., Citation2021; Pan et al., Citation2019); iii) membrane separation (Wang et al., Citation2018); d) dilution of the feedstock (Duan et al., Citation2018). Table S2 (supplementary materials) summarizes some results of AD of poultry manure within the above-mentioned solutions.
Figure 1. Key issues of research regarding AD of poultry manure, based on (Khoshnevisan et al., Citation2021).
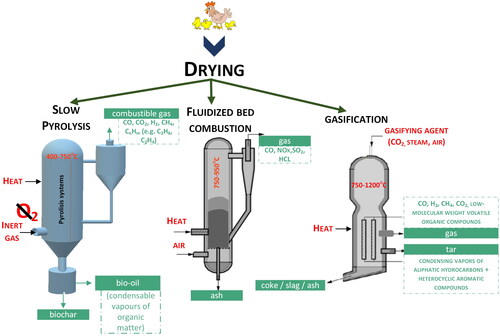
2.3. Thermal
2.3.1. Combustion
The mostly used PM thermal process is combustion and co-combustion (Junga et al., Citation2017, Florin et al., Citation2009), where organic compounds are oxidized with the release of large amounts of heat (). The process produces gases such as sulfur dioxide, nitrogen oxides, carbon dioxide, hydrogen chloride and ash. The use of the scaffold technology requires good technical solutions due to high ash content in PM and its low melting point (Kelleher et al., Citation2002). While the fluidized bed technology enables the reduction of nitrogen oxide emissions (Florin et al., Citation2009). Usually, before combustion, gasification or pyrolysis, manure is pre-prepared by drying (Tańczuk et al., Citation2019a) which increase its caloric value from 2.6 to 13.5 GJ Mg−1 (Kelleher et al., Citation2002).
The ash generated by fluidized combustion is odorless, sterile and dry. It can be used as a fertilizer or a soil improver due to the desired phosphorus and potassium content (Billen et al., Citation2015, Kaikake et al., Citation2009, Luyckx et al., Citation2020). Therefore, it can also be used as additive for fertilizers or even chicken feed (Blake & Hess, Citation2014).
2.3.2. Gasification
PM gasification () is usually carried out in 1000°C in presence of oxidizing agents (e.g. air, oxygen, water vapor). In result, gaseous products mainly hydrogen and carbon monoxide, methane, carbon dioxide, nitrogen and water vapor are creating (Equationequations 2-9) (Wielgosiński, Citation2016):
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
(7)
(7)
(8)
(8)
(9)
(9)
Thermal decomposition can be summarized (Equationequation 10(10)
(10) ):
(10)
(10)
These chemical reactions are both exothermic and endothermic. Solid residues in the form of ash or slag are also produced. The PM gasification is often analyzed in the option of co-gasification with biomass of plant origin. However, process is rather limited to laboratory and small-scale applications (Tańczuk et al., Citation2019a) due to technical problems and low quality of the gas produced (Tańczuk et al., Citation2019b). However, comparing to the gasification of fossil fuels the negative environmental impacts of this process (i.e. greenhouse effect) is reduced (Jeswani et al., Citation2019).
2.3.3. Pyrolysis
Pyrolysis is a process of transformation of organic matter under anaerobic conditions at high temperatures up to 1000° C (Wielgosiński, Citation2016) (Equationequation 11(11)
(11) ):
(11)
(11)
The liquid products of pyrolysis are mainly a mixture of oils, tars and water with dissolved simple aldehydes, alcohols and organic acids (). Moreover, gases, such hydrogen, methane, ethane and their homologues, carbon monoxide and dioxide, and compounds such as hydrogen sulfide, ammonia, hydrogen chloride, and hydrogen fluoride are produced (Wielgosiński, Citation2016). Solid products containing mainly carbon (Wielgosiński, Citation2016) are referred to as biochar. Pyrolytic technologies are not as widely used on an industrial scale as is the case of combustion. In the effect the pyrolysis of PM biochar which can be used as a soil improver is produced (Mickan et al., Citation2016). Moreover, bio-oils can be a potential substrate for bio-grease production. The so-called slow pyrolysis (Ibarrola et al., Citation2012) during which metals are immobilized and phosphorus is made available, is recommended for the production of biochar (Roberts et al., Citation2017, Kleemann et al., Citation2017). Process efficiency is determined by the type of substrate being converted and the temperature of pyrolysis (Bavariani et al., Citation2019).
PM thermal processing ensures the sanitary safety of post-process products (Maj et al., Citation2022), () and eliminate potential risk of infection during direct land application (He et al., Citation2022). The presence of antibiotics or heavy metals in the PM does not interfere with thermal processes. However, the high content of chlorine and alkali metals in the ash can generate many problems, such as high-temperature corrosion, deposit formation on boiler heating surfaces and bed agglomeration in fluidized bed boilers (Maj et al., Citation2022).
3. Carbon cycle
3.1. Mineralization and humification under aerobic conditions
The 31% of PM is organic matter (Morvan et al., Citation2021). Mineralization and humification are fundamental for carbon, nitrogen and phosphorous cycles under aerobic conditions. Firstly organic matter is fragmented by invertebrates, chemically degraded, and organic substances are leached (Morgado et al., Citation2018). Then, during chemical reactions, microorganisms converted the C structures of complex organic molecules toward simpler generally soluble compounds (e.g., carbohydrates, proteins, amino acids) (Morgado et al., Citation2018). Some of them immediately transform during the primary mineralization process into soluble inorganics and/or gaseous (Figure S1, supplementary material). Simultaneously, the slower humification begins and the formation of complex molecules of colloidal nature and dark color called humus. During humification through several oxidation and hydrolysis reactions - humic acids, fulvic acids, hymatomelanic acids, and humins are created. C:N ratio of initial feedstock material play a crucial role in the process of carbon fixation associated with enzymatic activity and microbial variation. Products contain higher C, H and lower O compared to the original fresh residues, thus are more stable and resistant to decomposition (Morgado et al., Citation2018). The C/N value of 17.3 significantly enhanced the composting efficiency and the plant germination index (23.7%) compared to the C/N ratio 9.61, (Liu et al., Citation2020). Moreover, the suitable C/N ratio increased the relative abundance of Bacilli which played an important role during the mesophilic and thermophilic phases. Bacilli abundance was related to cellulose and β-glycosidase activities, thus improved fiber degradation and humification.
During the secondary mineralization the humus can be partially lost. Addition of thermally treated montmorillonite (M-) and illite (I-) improved the humus formation (Pan et al., Citation2021) . Furthermore, structural equation model and variance partitioning analysis demonstrated that M- and I- treatments promoted precursors to synthesize humic acids (HA) by coordinated regulation of biotic and abiotic pathway and to increase of HA content in the M- and I- treatments mainly through the abiotic pathway.
Microbially dependent mineralization is divided into three stages (Islam et al., Citation2021): i) an initial with typically present bacteria such Bacillus, Clostridium, Proteus and lactic acid bacteria; ii) an intermediate stage with more specialized Bacillus, Pseudomonas, Acinetobacter, Arthrobacter, Enterobacter and iii) last stage when the one-carbon-compound-oxidizing bacteria and other specialists release CO2 from the most refractory of the organic residues. Kinetics of PM mineralization in soil depends on the type of manure. Higher CO2 emissions (800 mg C kg−1) were found in soils amended with hen poultry manure (PMH) as compare to broiler poultry manure (PMB) − 600 mg C kg−1 (Martín José et al., Citation2012). Moreover, application of different doses of solid and liquid fermented poultry manure significantly influenced the CO2 emission from treated soils (Sathya & Maheswari, Citation2017).
3.2. Conversion into biogas under anaerobic conditions
Anaerobic digestion (AD) generally is divided into four phases such as hydrolysis, acidogenesis, acetogenesis (acetogenesis), and methanogenesis (Figure S2, supplementary materials). During technical scale, AD is divided into two phases: acidic (includes hydrolysis and acidogenesis) and methane (acetogenesis and methanogenesis) (Anukam et al., Citation2019; Issah et al., Citation2020).
Phase I - hydrolysis - in which water-insoluble organic polymers such as proteins, lipids, and carbohydrates are decomposed into simple, soluble monomers by extracellular enzymes (hydrolases e.g., proteases, lipases, cellulases, amylases) released by hydrolytic bacteria. The products of hydrolysis are amino acids, simple sugars, hydroxy-alcohols (main glycerol), and long-chain fatty acids (LCFAs) (Sahota et al., Citation2018). However, only 50% of the organic matter contained in the feedstock is broken down in this phase. This is linked with a lack of suitable enzymes for their degradation. (Meegoda et al., Citation2018).
The presence of lignocellulosic material in PM (e.g., straw, wood shavings, rice husk) is the bottleneck of this stage. Generally, lignocelluloses consists of three polymers: cellulose, hemicellulose, lignin and various inorganic materials (Bhatnagar et al., Citation2022). The proportion of each component depends on the manure type, the region and, consequently, the digestibility of fiber by the animals live in different parts of the world (Orlando & Borja, Citation2020, Table S2 supplementary material). Hemicellulose fractions are easily hydrolyzed into simple sugars. However cellulose and lignin are more difficult to degrade (Ferraro et al., Citation2020; Matheri et al., Citation2018). Lignin hydrolysis is a complicated process mainly due to the numerous carbon and ether bonds between phenolic alcohol derivatives (the basic monomers are trans-p-coniferyl, trans-p-coumaryl and trans-p-sinapyl alcohol). Furthermore, lignin surrounds hemicellulose and cellulose and forms a barrier that limits enzyme accessibility. The breakdown of polysaccharides become hampered (Cai et al., Citation2021; Orlando & Borja, Citation2020; Paritosh et al., Citation2021; Shrestha et al., Citation2017). Therefore, a pretreatment is essential to solubilization and degradation of the substrate with a significant content of recalcitrant lignin, such as PM (Figure S3, suplementary material). To accelerate the hydrolysis process after pretreatment of animal manure can be used: i) repture the crystalline structure of cellulose fraction; ii) increase the digestibility of the feedstocks owing to the reduction of particle size; iii) increase of lignocellulose materials porosity; iv) solubilization of hemicellulose; v) distruption of hemicellulose; vi) depolymerization of hemicellulose; vii) deacylation of hemicellulose; viii) alteration of lignin structure; ix) breakdown of lignin; x) hydrolization of fibrus proteins (e.g., keratin); xi) split complex polimer (Orlando & Borja, Citation2020, Reyes et al., Citation2021). Also, pretreatment of poultry manure before AD, via enzymes such as peroxidase and laccase enhance the rate of hydrolysis (Bhatnagar et al., Citation2022).
Figure 3. Flow chart of the anaerobic digestion of poultry manure, based on (Khoshnevisan et al., Citation2021).
Phase II – acidogenesis - in which, facultative and obligate acidogenic bacteria convert the products of the hydrolysis to low-molecular organic compounds, mainly volatile fatty acids VFAs (formic, acetic, propionic, butyric, valerian, caproic), alcohols (methanol, ethanol), aldehydes, and gaseous products (carbon dioxide and hydrogen). The key factor impact on the products obtained in the phase is the hydrogen partial pressure formed during the process. At high pressure of the gas, less reduced metabolites such as lactate, butyrate, fatty acids with at least 3 carbon atoms in the chain (C3) are produced. On the other hand, under stable operating conditions (depends on the feedstock and species of bacteria), acetates, carbon dioxide, and hydrogen are produced, which are precursors for the production of methane in the fourth stage of AD (Anukam et al., Citation2019; Deublein & Steinhauser, Citation2011). This phase is characterized by a low pH (5-6) and an intense odor. In a balanced system, most of the organic matter is converted into readily available products for methanogens (acetate, hydrogen, and carbon dioxide), while the remainder (about 30%) is broken down into volatile fatty acids (VFAs) or alcohols (Anukam et al., Citation2019; Issah et al., Citation2020).
Phase III – acetogenesis - in which, organic acids and some aromatic compounds (e.g., benzoate) are converted into acetic acid, hydrogen, and carbon dioxide (Table S3, supplementary material). For thermodynamic reasons (ΔG0 < 0), the necessary condition for the decomposition of the compounds is keeping the concentration of hydrogen formed during the process at a low level (Figure S3) (Anukam et al., Citation2019; Deublein & Steinhauser, Citation2011; Khanal, Citation2011).
In a properly functioning system, the partial pressure of hydrogen should be in the range from 10−4 Ba (a necessary condition for the reduction of carbon dioxide) to 10−6 Ba (oxidation of propionic acid) (Jędrczak, Citation2007; Ramirez et al., Citation2009).Therefore, acetogens exist in close symbiosis with other bacterial species between which the so-called interspecies hydrogen transfer (IHR) takes place, (Deublein & Steinhauser, Citation2011; Figure S.4. supplementary material). The generated hydrogen can not only be utilized by methanogens so-called hydrogenotrophs (EquationEquation 12(12)
(12) ), but also by sulfate-reducing bacteria (SRB) e.g., Desulfovibrio desulfuricans (EquationEquation 13
(13)
(13) ). It is worth emphasizing the fact that sometimes there can be competition for hydrogen between the mentioned groups of microorganisms. In digesters, hydrogen can also be consumed by homooacetogenic bacteria such as Acetobacterium woodii and Clostridium thermoaceticum (EquationEquation 14
(14)
(14) ) (Anukam et al., Citation2019; Deublein & Steinhauser, Citation2011; Gallert & Winter, Citation2005; Khanal, Citation2011).
(12)
(12)
(13)
(13)
(14)
(14)
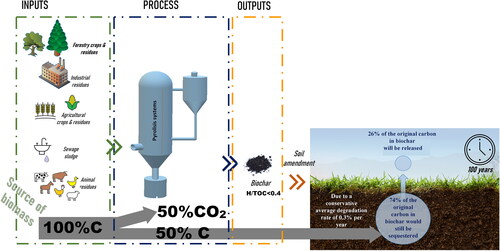
Figure 4. Scheme of transformation of C contained in biomass into C-sinks, based on (European Biochar Certficate 2020a).
At high ammonia concentrations, synthetic acetate oxidation (SAO) can also occur in this phase (Ramirez et al., Citation2009). This is a two-step process and involves oxidation by SAOB (syntrophic acetate oxidizing bacteria) of acetates to hydrogen and carbon dioxide (EquationEquation 15(15)
(15) ) and for thermodynamic reasons the conversion of the resulting products to methane by hydrogenotrophic methanogens (Buhlmann et al., Citation2019). As reported by Ramirez et al. (Citation2009) the effect of altering the biochemical pathway of acetate degradation results in the reduction of biogas production.
(15)
(15)
However, the enzymes involved in SAO require trace elements (especially cobalt, selenium, tungsten) for their proper function. Lack of supplementation of TE during AD can lead to SAO inhibition, and accumulation of VFAs in the fermentation broth. Molaey et al. (Citation2018) indicated that supplementation of selenium during AD of PM has a critical role in the stable digestion performance and improves methane production at high organic loading rate and TAN levels.
Phase IV – methanogenesis − 70% of the methane is produced by the decomposition of acetic acid by heterotrophic bacteria (acetotrophic methanogens) (EquationEquation 16(16)
(16) ). The remaining amount is generated by carbon dioxide reduction, of which only 5 to 6% is obtained from dissolved hydrogen. This phenomenon is explained by interspecies hydrogen transfer, during which hydrogen is not dissolved in the fermentation broth, but transferred directly from acetogens to methanogens (Deublein & Steinhauser, Citation2011; Jędrczak, Citation2007).
(16)
(16)
Methanogens can also produce methane from the following substrates, (Frąc & Ziemiński, Citation2012): HCOOH, CH3OH, CO, (CH3)3N, (CH3)3NH, (CH3)3NH2, (CH3)2S, Me0. Some methanogens can only convert one substrate e.g., Methanobrevibacter arboriphilus or Methanosaeta - H2/CO2 and acetate, respectively. While others are more versatile e.g., Methanospirillum hungatei and Methanobacterium formicicum and capable of taking up formate, or genus Methanosarcina can convert a wide range of compounds e.g., H2/CO2, acetate, methanol, methylamines, acetic acid, carbon monoxide (Sousa et al., Citation2007).
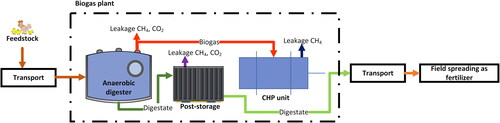
However, determining the environmental impact of AD and analyzing the carbon cycle during this process requires a broader view. Apart the biogas production, loses of methane during production and transmission, losses of gases during digestate storage as well as emission from co-generation units should also be taken into account (). Table S4 (supplementary material) summarizes the formulas that can be used to estimate gas emissions during the AD (Kreidenweis et al., Citation2021).
A separate issue is the fate of micropollutants and emerging contaminants during AD. The removal of antibiotics, antibiotic resistance genes (ARGs), and heavy metals under anaerobic conditions are debatable (Riaz et al., Citation2020). Zhang et al. (Citation2019) demonstrated that microwave pretreatment of manure and activated carbon enhanced ARGs removal and methane production during mesophilic AD of PM. Enrofloxacin in dose 8 mg kg−1·TS promoted the AD, while higher than 16 mg kg−1·TS inhibited the biogas production rate (Du et al., Citation2022). However lack is explanation why most of these pollutants are still present in the digestate (Gurmessa et al., Citation2020). Riaz et al. (Citation2020) and Gurmessa et al. (Citation2020) suggested that following factors play important role in the degradation: i) the operational parameters of the AD (e.g. temperature, hydraulic retention time, etc.); ii) microbial community structure; iii) the physicochemical character of the substrate; iv) genetic elements. Despite that the digestate before application to the soil is treated (e.g., composting or solid-liquid separation (SLS) which reduce manure contaminants (Congilosi & Aga, Citation2021) still the attention must be put to optimization of post-treatment processes as well as aspects related to feedstock preparation (Gurmessa et al., Citation2020).
3.3. Biochar production and carbon sequestration potential
Pyrolysis temperature and biochar substrates have impact on the stability of C in biochar and determine its application ().
selected biochar properties described by (Novak et al., Citation2009, Song & Guo, Citation2012, Srinivasan et al., Citation2015, Khan et al., Citation2016, Qi et al., Citation2017, Li et al., Citation2018, Bavariani et al., Citation2019, Wystalska et al., Citation2021)
The C content (86.70%) in biochar from PM is significantly higher, than in other biochar types produced at similar temperatures, due to a high proportion of litter material in the substrate converted into biochar (Srinivasan et al., Citation2015). Presence of stable C cause that biochar can also be used to sequester carbon in the soil. Using pyrolysis, more than half of the C of biomass can be converted into permanent C-sinks instead of lost as CO2 (European Biochar Certficate Citation2020a) (). Application of biochar to soil or feed additives average degradation rate is about 0.3% per year. This means that 100 years after biochar application to soil, biochar would still sequester about 74% of the original carbon (European Biochar Certficate Citation2020a). Moreover, the use of biochar on cultivated soils reduced CH4 emissions and improved the soil ability to absorb greenhouse gases. The use of biochar from chicken manure for maize cultivation caused reduction in CO2 of 3.2 kg per 1 kg of biochar (Panwar et al., Citation2019)
Biochar with a high aromatic carbon content is probably suitable for long-term sequestration of C (Novak et al., Citation2009). The stability of biochar depends on the O/C molar ratio, the lower its value, the potentially longer the half-life period of biochar and the higher its usefulness for sequestration of C in soil. The O/C ratio of biochar studied by Srinivasan et al. (Citation2015) was low and equal to 0.12, similarly in the study reported by Novak et al. (Citation2009), the O/C ratio was 0.14 and < 0.01. Higher O/C in biochar, i.e. 0.46 and 0.48 was reported by Li et al. (Citation2018). The O/C > 0.6 indicates the half-life of the biochar <100 years. A range of O/C values of 0.2-0.6 may indicate a half-life of 1000-100 years(Srinivasan et al., Citation2015, Zhang et al., Citation2017). The O/C < 0.2 extends the half-life > 1000 years. In turn, high values of H/C ratio suggest that biochar will degrade quicker in the soil which will result in an increased emission of CO2 (Van Zwieten et al., Citation2010). The greatest potential for sequestration of C is displayed by biochar with the H/Corg below 0.4 (Brassard et al., Citation2018) and may achieve the value H/C ratio of 0.013 (Srinivasan et al., Citation2015). The low H/C ratio, indicating the high aromaticity of the analyzed biochar, affects the potential of C sequestration. Among biochar produced from different substrates at 680°C, only biochar from pine sawdust had a higher potential to longer storage in the soil than poultry manure biochar.
For the assessment of C sequestration potential, the overall carbon balance should include all direct and indirect emissions from e.g., biomass production (planting, cultivation, harvesting, transport, crushing), the processing of biomass into biochar and its final treatment (e.g. grinding, mixing, packaging), transport, and soil application (European Biochar Certificate, Citation2020b), ().
CO2 equivalent (CO2eq) covers all GHGs in effect of production and treatment of the biomass, its conversion in a pyrolytic installation, and the processing of biochar (European Biochar Certificate, Citation2020a). The calculation example for the C-sink potential based on guidelines of EBC (i.e. European Biochar Certificate, Citation2020b) is shown in Table S5 (supplementary material). If renewable energy is used in the production of biochar, CO2eq is considered to be zero and similarly when electricity corresponding to at least its consumption is generated in the production of biochar in the pyrolysis process (European Biochar Certificate, Citation2020b). Only biochar produced from C-neutral biomass, e.g., biomass that is waste or by-product that meets certain requirements (European Biochar Certificate, Citation2020b), is eligible for C-sink certification.
4. Nitrogen cycle
4.1. Forms of nitrogen in poultry manure
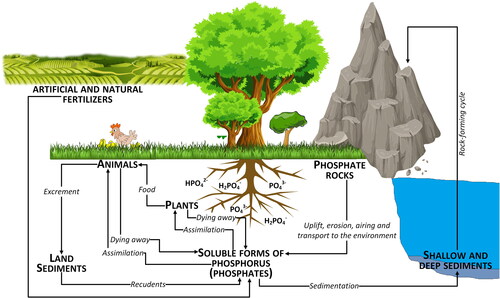
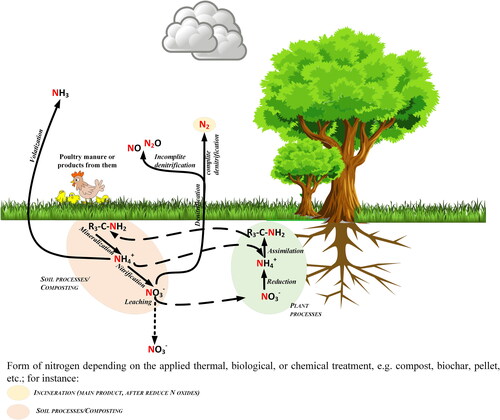
High nitrogen content (3-5% PM dry weight) in the form of uric acid (40-70%), feed protein (10-40%), urea (4-12%) and ammonium (4-20%) (Bhatnagar et al., Citation2022) depends primarily on animal diet, type and age (Nahm, Citation2003) when applied to soil undergoes a number of transformations (). During PM storage organic N is converted into ammonia which leads to volatilization and N loss into the air. When poultry manure is applied to soil for plant uptake N from organic forms is converted through mineralization to N mineral forms. This depends mainly on pH, temperature and moisture content.
Figure 5. Transformation of nitrogen based on poultry manure in soil based on (Ros et al., Citation2020).
4.2. Ammonia emission
Effect of poultry production is gaseous emissions of carbon dioxide, ammonia, hydrogen sulfide, methane, nitrogen oxides, dust and others. Ammonia emission from poultry manure is higher than from cattle and cow manure and during the three-month storage period was 2.94 kg Mg−1, pig manure 2.40 kg Mg−1 and cow's 0.45 kg Mg−1, respectively. It also depends on the type of poultry, feed and a breeding system. The highest amount of ammonia is obtained from turkey manure - over 950 kg/year per 1000 birds, from ducks at 680 kg/year from 1000 heads goose at 350 kg/year per 1000 birds whereas smallest broiler chickens generated about 220 kg ammonia/year per 1000 birds. Stable ventilation of farm buildings, can reduce the amount of ammonia by about 20% by decrease the interior temperature, thus slowing down the uncontrolled processes of organic matter decomposition (Bittman et al., Citation2014). The decrease of ammonia generation is also achieved by acidification of the liquid part of the manure, e.g. with sulfuric acid to a pH of 3.5-5.5.
During composting too fast or absent thermophilic phase and an unfavorable C:N ratio (range 40-50: 1) results in the formation of slow-acting organic combinations of nutrients. During this time, the mesophilic microflora develops excessively, which allows for the maintenance of the chemical activity of urease causing excessive ammonia emissions (Krawczyk & Walczak, Citation2016).
Ammonium ion (NH4+) and/or free ammonia nitrogen (FAN) are the main inorganic products of decomposition of organic nitrogen fraction in phase II – acidogenesis of anaerobic digestion (AD). The equilibrium state is established between the forms mentioned above of nitrogen, and their share in total ammonia nitrogen (TAN – sum FAN and NH4+) depends on the pH. As the pH increases, free ammonia nitrogen (FAN) increases. At neutral pH, the dissociated form prevails; only 0.5% of nitrogen is in the form of free ammonia, while at pH equal to 8, its (FAN) share increases to 5% (Deublein & Steinhauser, Citation2011). Accumulation of TAN in the digester can lead to inhibition of the process. The inhibition mechanism caused by ammonia can be related to: i) inhibition of specific enzymatic reactions (blocking enzymes); ii) pH imbalance inside the cell and thus the disturbance of the acid-base balance and/or potassium deficiency; iii) the accumulation of VFAs (there is an interaction between FA, pH and VFAs; disturbance of the process balance leads to accumulation of VFAs in the chamber, resulting in a decrease in pH and thus decrease FAN concentration). Moreover, ammonia inhibits the process of acetic acid uptake (Jiang et al., Citation2019, Fuchs et al., Citation2018). It is difficult to indicate the TAN limit value for inhibition of the AD of PM because it depends on the characteristics of feedstock, inoculum characteristics, process conditions (temperature, pH), form of nitrogen (FAN is more toxic than ammonium ions, process inhibition for FAN is observed at concentrations higher than 45-100 mg l−1and for ammonium ions higher than 1700 mg l−1) and a degree of adaptation of bacteria to TAN (generally, a gradual increase of ammonia concentration promotes adaptation to it). For this reason, a wide range of inhibiting TAN concentrations has been reported in the literature (from 1.7 g l−1to 14 g l−1) (Duan et al., Citation2018; Reyes et al., Citation2021; Yin et al., Citation2021) However, it is important to note that adaptation of microorganisms to high ammonia concentrations can result in high ammonia concentrations in the digestate. According to Ortakci et al. (Citation2019) the concentration of ammonia nitrogen in such a digestate can be higher than 5000 mg dm−1.
4.3. Nitrous oxide and nitric oxide emission
Nitrous oxide (N2O) and nitric oxide (NO) can be emitted as a result of nitrification where NH4+ is a substrate for aerobic respiration or alternatively – denitrification when oxygen is limited and NO3− is used for anaerobic respiration as an electron acceptor by microorganisms.
N2O has a global warming potential of 298 times that of CO2 (IPCC, 2019) and can be the main GHG contributor (Ziganshina et al., Citation2015; Zhu et al., Citation2020). Meta-analysis results showed that PM considerably increased CO2, CH4, and N2O emissions than pig and cattle manure (Shakoor et al., Citation2021). Soil application of PM during autumn season resulted in N2O emission at the level of 1.52% total N applied (Thorman et al., Citation2020). Indirect N2O emissions may arise from the re-deposition of volatilized NH3 and NOx onto nearby soil, providing a substrate for nitrification and denitrification. The important factor is C:N value (Charles et al., Citation2017). Nitrogen are lost to surface water via leaching of NO3− which is later denitrified (Thorman et al., Citation2020).
Moreover, many other factors influenced emission, such as soil properties (texture, drainage, bulk density, water holding capacity), SOC, Ntot., crop rotation, duration of the land management and climatic factors. Soil nitrogen cycle is closely related to soil C cycle and therefore increased SOC sequestration might even cause increase N2O emission. The process can be mitigate by biochar or non-pyrogenic C amendment application (Janczak et al., Citation2017, Guenet et al., Citation2021).
4.4. Leaching and runoff lost
Nitrogen losses from livestock production through leaching and runoff loss can reach up to about 50%. Nitrogen losses through leaching occur mostly in the form of nitrates (NO3-) which is favored by high soil moisture, heavy rainfall, excessive irrigation, and poorly developed root systems of plants. Uncontrolled losses of NO3 to groundwater can be harmful to human health in dose higher than 50 mg l−1 NO3. Light soils, i.e. fawn, podzolic, and rusty podzolic soils, created on the basis of sands, are very permeable, airy, and quickly heat up, contributing to the rapid leaching of nitrates and their seepage into the soil profile and further into the groundwater. On the other hand, heavy soils, i.e. mainly clays and loams, poorly permeate water and oxygen to the deeper parts of the soil profile. They limit the leaching of nitrates, but plants growing on such soils have a problem with the properly growth through a too compact structure. A soil with a sand and clay fraction is optimal, which makes it good for plant growth and does not allow significant amounts of nitrogenous compounds to pass into groundwater (Soroko & Strzelczyk, Citation2009).
5. Phosphorus cycle
5.1. Forms of phosphorus in poultry manure
The content of phosphorus in PM is 2 to 4 times higher than in other manure and ranges from13.6 to 25.4 g P2O5 kg−1dm (Table S.6, supplementary material). Because in cereals and others (barley, wheat bran, soybean, rapeseed meal), phosphorous occurs in form of inaviable phytin, hence, the phytic form is present in significant amounts in poultry excreta (Toth et al., Citation2011, Ashworth et al., Citation2020). Available phosphorus sources for birds are mineral supplements added to feed (Sharpley et al., Citation2007). The inorganic phosphorus in PM is in the range of 32-84% wt (Othake & Tsuneda, Citation2019) and organic in the range of 14-68% wt, with the potential to be mineralized to a plant-available form (He et al., Citation2008). Pagliari and Laboski (Citation2014) found that the solid phase of phosphorus is not entirely organic, but is combination of minerals and organic compounds. The inorganic phosphate fraction includes calcium phosphate, dibasic calcium phosphate, and poorly bound water-soluble phosphorus (He et al., Citation2016). Both fractions of total phosphorus (orthophosphates and organic phosphorus) are essentially immediately labile. Phytates are an exception. Compared to other animal manures, poultry manure contains proportionally more of the stable form of phosphorus, which ranges from 22 to 58% of total phosphorus (Dail et al., Citation2007).
Between 12 and 20% of the phosphorus in PM is in a water-soluble form and is washed away in rainfalls after being applied to the soil (Guo et al., Citation2009). Combustion of poultry manure to produce nutrient-rich ash for agricultural applications is the most environmentally friendly methods (Fahimi et al., Citation2020; Kaikake et al., Citation2009). The phosphorus content in ash ranges from 8.3 to 13% and is comparable to that of some natural phosphate rocks (Desmidt et al., Citation2015). The amorphous phase of phosphorus in poultry ash is a source of a bioavailable form of phosphorus, and depends on the combustion temperature (Vance, Citation2019). The main mineral phase of the ash is potassium-sodium-calcium phosphate (K,Na)Ca2(PO4)2 and in the form of calcium phosphates (hydroxyapatite and whitlockite) - from 8.8 to 14.8% P2O5. Bioavailable forms in ash may achieve to 31% d.w.P2O5 of total content (Table S7, supplementary materials) Song and Guo (Citation2012) demonstrated that the conversion of poultry manure to biochar by slow pyrolysis at ≥ 350 °C reduced the proportion of water-soluble phosphorus from 19.5% to less than 6.9%. This will help maintain a steady and long-term supply of nutrients in the soil preventing rapid nutrient loss during runoff. Such an effect can be achieved by converting poultry manure by pyrolysis into biochar and its use as a soil additive (Guo et al., Citation2020; Haider et al., Citation2021). Wang et al. (Citation2015) found that pyrolysis immobilized the water-extractable phosphorus contained in raw PM by converting it into more stable, less available minerals (Mg and Ca phosphates) present in biochar (Figure S6, supplementary materials). Phosphorus content in biochar from PM was higher than that of unprocessed manure and increased with pyrolysis temperature from 3.391% in 200 °C to 6.38% in 500 °C (Table S8, supplementary material), Bavariani et al. (Citation2019).
Experiments on sequential leaching of biocarbon from raw poultry manure produced at 600 °C showed that phosphorus was released slowly but in much higher (43.2 mg g−1) amounts compared to other biochars of animal and lignocellulose origin (Table S9, supplementary material), (Hadroug et al., Citation2021). The total amount of released phosphorus was about 95% of its original content in the biochar.
Negassa and Leinweber (Citation2009) evaluated the balance of nutrients (including phosphorus) in poultry litter used as a fertilizer for five different treatments: direct application on the ground; composting; composting and pelleting; co-digestion with feed corn; thermal conversion. Final products of all the treatment methods contained the same amount of phosphorus (in the form of P2O5) per Mg of processed litter. In the case of ash, the annual efficiency of P2O5, depending on the crop, varies from 37% to even 100%. In contrast, it is around 70% for other products. The effectiveness of P2O5, depends on the crop and soil type where poultry litter ash is used as a fertilizer. Figure S5 (Nusselder et al., Citation2020, supplementary materials) shows the fertilizer equivalents available in the first year to plants, delivered from 1 Mg of poultry litter, treated using various methods.
5.2. Poultry manure struvite
Struvite (MgNH4PO4·6H2O), i.e. hydrated ammonium-magnesium phosphate can be an alternative source of phosphorus contain the two macronutrients (phosphorus and nitrogen) needed for plant growth and development. Struvite is formed by the following reaction (17):
(17)
(17)
According to low solubility (Rech et al., Citation2018) the fertilizing components are released slowly and the plants themselves stimulate the intensity of release which reduces the need for frequent fertilization. No leaching is observed, either into surface water or to deeper soil layers where phosphorus could become unavailable to plants. Struvite is as effective as compound fertilizer, providing plants with the right doses of nutrients N, P, K, Ca, and Mg (Krishnamoorthy et al., Citation2021; Zhang et al., Citation2020).
Struvite produced from the acidified PM ash extract after supplementing with Mg and adjusting the pH to about 8.5 contained 90% of the phosphorus originally present in fresh chicken manure (Poblete-Grant et al., Citation2020). The process yielded a multi-nutrient fertilizer composed of struvite-NH4+, struvite - K, and significant amounts of potassium sulfate and hydroxyapatite, with a high content of macroelements (P, K, Mg, S), low content of microelements, and lower levels of heavy metals compared to fresh manure (Poblete-Grant et al., Citation2020).
Poultry manure is a good source of phosphorus for the production of universal agricultural fertilizer (Rivera et al., Citation2022; Sarvi et al., Citation2021). Yilmazel and Demirer (Citation2013) confirmed the recovery of nutrients (N, P) by precipitation of struvite from poultry manure using HCl. However, it further led to the dissolution of metals along with nutrients and crystallization of other minerals along with struvite. It was also found that high concentrations of Ca2+ ions allow complete recovery of phosphorus from a solution due to calcium phosphate precipitation.
5.3. The mobility of phosphorous in environment
The biogeochemical cycle of phosphorus is very sensitive to agricultural and industrial activity, especially to poorly planned or implemented agrotechnical procedures that modify the natural P cycle ().
The content of total phosphorus in soil ranges from 100 to 3000 mg P kg−1 (Sönmez et al., Citation2016), but is mostly in the range of 500-800 mg P kg−1 soil and depends on the type of bedrock, the degree of weathering, and the content of organic matter. This amount exceeds many times the average nutritional needs of plants. However, only 0.03-0.5 mg P kg−1 is actually available to plants, as most of the phosphorus binds in the soil with various chemical forms of other elements (e.g. 2- and 3-valent metal cations, i.e. Mg, Ca, Fe and Al), forming hardly soluble compounds. Phosphorus content decreases with the depth of the soil profile. The active phosphorus in the soil solution is phosphorus in the form of phosphate ions (H2PO4-, HPO42-), taken up directly by plant roots. When the amount of active phosphorus decreases due to intensive plant uptake, mobile phosphorus is converted to its bioavailable form, making up for the deficiency. Another form of phosphorus is reserve phosphorus in the form of hard to dissolve minerals, i.e. apatite, phosphate rock and variscite (Mulgueen et al., 2004). Therefore, the phosphorus cycle in the soil is influenced by its biological activity and high enzyme production/activity associated with organic phosphorus hydrolysis. The following extracellular phosphatases are mainly involved in phosphorus metabolism in the soil: acid phosphomonoesterase, alkaline phosphomonoesterase, and phosphodiesterases, (Waldrip et al., Citation2011). Manure application increases the concentration of total and soluble phosphorus in the soil and the concentration of stable organic groups of this element (Waldrip-Dail et al., Citation2009).
Depending on the form of occurrence (type of binding) and variation of soil and water conditions (i.e. soil reaction, the organic matter, Fe and Al), phosphorus may be released to soil solution and ground water and as a consequence, through leaching, move deep into the soil profile or undergo the process of leaching to surface waters (McDowell et al., Citation2011). The following mechanisms are responsible for phosphorus transfer from soil to water: i) chemical dissolving; ii) physical separation and removal of soil particles through erosion; iii) incidental migration from soil to water after application of phosphorus fertilizer or manure in conditions that are not conducive to its permeating into the soil structure, (Roy, Citation2017).
The release of phosphorus from the soil in the presence of organic carbon, is associated with leaching of its soluble forms into groundwater which with high mineralization efficiency and low phosphorus utilization by plants results in contamination of groundwater (Figure S7, supplementary material). Phosphorus bound to mineral and organic soil particles accounts for 60 to 90% of phosphorus transported through surface runoff. Dissolved forms include phosphorus desorbed from the soil, leached from plant residues, and released from sources such as mineral fertilizers and manure. In catchments in agricultural areas, the level of phosphorus leaching may be about 0.5 kg ha−1. The process of phosphorus leaching is a type of the flow through privileged pathways of underground flow, i.e. the networks of regular cracks and macropores, huge cracks in the soil, chasms and channels carved by the soil fauna, root channels and wedges, lenses and layers that are characterized by improved filtration parameters than other parts of the soil. These migration pathways are responsible for the intensity and time of solution transfer (including phosphorus) during precipitation and thawing (Weiler & McDonnell, Citation2007).
Excessive or inappropriate application of manure and litter can result in increased accumulation of phosphorus in the soil and subsequent increase in surface runoff, leading to eutrophication of reservoirs, (Tiessen et al., Citation2011). Transport of phosphorus contained in PM after its application in the soil shown that dry mass of the manure decreased over time, but the concentrations of phosphorus in the manure were essentially constant over time, meaning that it was removed from the manure at the same rate as it decomposed Vadas et al. (Citation2007). Numerous studies have also examined the effects of drying conditions on phosphorus content in PM (He et al., Citation2006). For broiler manure, freeze-drying or oven-drying (105 °C) yielded lower concentrations of inorganic and total phosphorus than for air drying (Akinremi et al., Citation2003). The drying process can cause the relatively stable phosphorus in PM to shift to more labile, or conversely, insoluble forms (Dail et al., Citation2007). Crops are unable to absorb the high doses of phosphorus contained in PM, so the excess accumulates in the soil. The P availability for plants can be increased by mixing with calcium-rich opoka rock (Kacprzak & Sobik-Szołtysek, Citation2022). Other possibility is adding sulfur-phosphate mixtures. This results in the oxidation of sulfur to sulfuric acid through the activity of Thiobacillus spp. bacteria. The reaction with acid led to the partial decomposition of phosphates and the formation of mono- and dicalcium phosphates, which are more readily available to plants (Figure S7, supplementary material).
6. Conclusion
Poultry manure is historically used as a fertilizer, however positive impact of application on the increase of the plant yield or the improvement of soil properties depends on many factors, such dose, soil type or temperature. Generally, increase of organic matter content, nitrogen ammonification, pH value, content of P, K, Cu, Mn, Fe and Zn available to plants is noted. However, PM can affect enzyme activity and introduce contaminations (e.g. pathogens; antibiotics, heavy metals). Moreover, ammonia, NOx emission or unbalanced C:N ratio in soil poses serious environmental problem.
Technological processes of PM has both positive and negative effect on carbon, nitrogen and phosphorous transformations and gases emissions. During aerobic processes (composting) nitrogen is lost by leachate of NH4+, NO3- or by emissions of NH3, N2O, N2; carbon by emissions of CO2, CH4 and phosphorus by the leachate in the form of HPO42-, H2PO4, respectively. In turn, the main challenge of anaerobic digestion is optimization toward high methane production. PM is not an easily biodegradable substrate, hence modification of technological parameters and co-substrates additives is required to increase the process efficiency and to obtain a digestate can be used as valuable fertilizer. Finally, thermal treatment requires energy for PM drying and monitoring of specific emissions. However from other hand products resulting from thermal processes have great potential for carbon (biochar) and phosphorous (ash) long-storage.
Regardless of the technology used, PM can be a suitable substrate for the production of valuable products that have a positive impact on the environment.
Supplemental Material
Download MS Word (433.4 KB)Additional information
Funding
References
- Aguilar-Moreno, G. S., Navarro-Cerón, E., Velázquez-Hernández, A., Hernández-Eugenio, G., Aguilar-Méndez, M. Á., & Espinosa-Solares, T. (2020). Enhancing methane yield of chicken litter in anaerobic digestion using magnetite nanoparticles. Renewable Energy, 147, 204–213. https://doi.org/10.1016/j.renene.2019.08.111
- Akinremi, O. O., Armisen, N., Kashem, M. A., & Janzen, H. H. (2003). Evaluation of analytical methods for total phosphorus in organic amendments. Communications in Soil Science and Plant Analysis, 34(19–20), 2981. https://doi.org/10.1081/CSS-120025220
- Al-Bataina, B. B., Young, T. M., & Ranieri, E. (2016). Effects of compost age on the release of nutrients. International Soil and Water Conservation Research, 4(3), 230–236.https://doi.org/10.1016/j.iswcr.2016.07.003
- Amanullah, M., Sekar, S., & Muthukrish, P. (2010). Prospects and potential of poultry manure. Asian Journal of Plant Sciences, 9(4), 172–182. https://doi.org/10.3923/ajps.2010.172.182
- Anukam, A., Mohammadi, A., Naqvi, M., & Granström, K. (2019). A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes, 7(8), 504. https://doi.org/10.3390/pr7080504
- Ashworth, A. J., Chastain, J. P., & Moore, J. P. (2020). Nutrient characteristics of poultry manure and litter. In H. M. Waldrip, P. H. Pagliari, & Z. He (Eds.), Animal manure: production, characteristics, environmental concerns, and management. Book Series ASA Special Publications.
- Bavariani, M. Z., Ronaghi, A., & Ghasemi, R. (2019). Influence of pyrolysis temperatures on FTIR analysis, nutrient bioavailability, and agricultural use of poultry manure biochars. Communications in Soil Science and Plant Analysis, 50(4), 402–411. https://doi.org/10.1080/00103624.2018.1563101
- Bhatnagar, N., Ryan, D., Murphy, R., & Enright, A. M. (2022). A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential–Global and Irish perspective. Renewable and Sustainable Energy Reviews, 154, 111884. https://doi.org/10.1016/j.rser.2021.111884
- Billen, P., Costa, J., Van der Aa, L., Van Caneghem, J., & Vandecasteele, C. (2015). Electricity from poultry manure: a cleaner alternative to direct land application. Journal of Cleaner Production, 96, 467–475. PagesISSN 09596526https://doi.org/10.1016/j.jclepro.2014.04.016
- Bittman, S., Dedina, M., Howard, C. M., Oenema, O., & Sutton, M. A. (2014). Options for ammonia mitigation: Guidance from the UNECE task force on reactive nitrogen. Centre for Ecology and Hydrology (CEH). ISBN: 978-1-906698-46-1
- Blake, J., & Hess, J. (2014). Suitability of poultry litter ash as a feed supplement for broiler chicken. Journal of Applied Poultry Research, 23(1), 94–100. https://doi.org/10.3382/japr.2013-00836
- Bolan, N. S., Szogi, A. A., Chuasavathi, T., Seshadri, B., Rothrock, M. J., Jr., & Panneerselvam, P. (2010). Uses and management of poultry litter. World's Poultry Science Journal, 66(4), 673–698. https://doi.org/10.1017/S0043933910000656
- Brassard, P., Godbout, S., Palacios, J. H., Jeanne, T., Hogue, R., Dubé, P., Limousy, L., & Raghavan, V. (2018). Effect of six engineered biochars on GHG emissions from two agricultural soils: A short-term incubation study. Geoderma, 327, 73–84. https://doi.org/10.1016/j.geoderma.2018.04.022
- Buhlmann, C. H., Mickan, B. S., Jenkins, S. N., Tait, S., Kahandawala, T. K., & Bahri, P. A. (2019). Ammonia stress on a resilient mesophilic anaerobic inoculum: Methane production, microbial community, and putative metabolic pathways. Bioresource Technology, 275, 70–77. https://doi.org/10.1016/j.biortech.2018.12.012
- Cai, Y., Zheng, Z., Schäfer, F., Stinner, W., Yuan, X., Wang, H., Cui, Z., & Wang, X. (2021). A review about pretreatment of lignocellulosic biomass in anaerobic digestion: achievement and challenge in Germany and China. Journal of Cleaner Production, 299, 126885. https://doi.org/10.1016/j.jclepro.2021.126885
- Charles, A., Rochette, P., Whalen, J. K., Angers, D. A., Chantigny, M. H., & Bertrand, N. (2017). Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agriculture, Ecosystems and Environment, 236, 88–98. https://doi.org/10.1016/j.agee.2016.11.021
- Congilosi, J. L., & Aga, D. S. (2021). Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. Journal of Hazardous Materials, 405, 123634.
- Dail, H. W., He, Z., Erich, M. S., & Honeycutt, C. W. (2007). Effect of drying on phosphorus distribution in poultry manure. Communications in Soil Science and Plant Analysis, 38(13–14), 1879–1895. https://doi.org/10.1080/00103620701435639
- Desmidt, E., Ghyselbrecht, K., Zhang, Y., Pinoy, L., Van der Bruggen, B., Verstraete, W., Rabaey, K., & Meesschaert, B. (2015). Global phosphorus scarcity and full-scale Precovery techniques: a review. Critical Reviews in Environmental Science and Technology, 45(4), 336–384. https://doi.org/10.1080/10643389.2013.866531
- Deublein, D., & Steinhauser, A. (2011). Biogas from waste and renewable resources: An introduction (pp. 578). John Wiley & Sons.
- Dróżdż, D., Wystalska, K., Malińska, K., Grosser, A., Grobelak, A., & Kacprzak, M. (2020). Management of poultry manure in Poland – Current state and future perspectives. Journal of Environmental Management, 264, 110327. https://doi.org/10.1016/j.jenvman.2020.110327
- Du, T., Feng, L., & Zhen, X. (2022). Microbial community structures and antibiotic biodegradation characteristics during anaerobic digestion of chicken manure containing residual enrofloxacin. Journal of Environmental Science and Health, Part B, 57(2), 102–112. https://doi.org/10.1080/03601234.2022.2026124
- Duan, N., Ran, X., Li, R., Kougias, P. G., Zhang, Y., Lin, C., & Liu, H. (2018). Performance evaluation of mesophilic anaerobic digestion of chicken manure with algal digestate. Energies, 11(7), 1829. https://doi.org/10.3390/en11071829
- European Biochar Certificate. (2020a). Certification of the carbon sink potential of biochar. Ithaka Institute. Version 10E of 1st June 2020. Retrieved February 10, 2021, from http://European-biochar.org
- European Biochar Certificate. (2020b). Certification of the carbon sink potential of biochar. Ithaka Institute. Version 10E of 1st June 2021. Retrieved February 10, 2021, from http://European-biochar.org
- Eurostat. (2021). Slaughtering in slaughterhouses - annual data. Retrieved August 27,2021, from http://aphttp://appsso.eurostat.ec.europa.eu/nui/show.do?query=BOOKMARK_DS-056118_QID_-7A1EB2C7_UID_-3F171EB0&layout=TIME,C,X,0;GEO,L,Y,0;MEAT,L,Z,0;MEATITEM,L,Z,1;UNIT,L,Z,2;INDICATORS,C,Z,3;&zSelection=DS-056118UNIT,THS_T;DS-056118MEAT,B7000;DS-056118MEATITEM,SL;DS-056118INDICATORS,OBS_FLAG;&rankName1=MEAT_1_2_-1_2&rankName2=UNIT_1_2_-1_2&rankName3=MEATITEM_1_2_-1_2&rankName4=INDICATORS_1_2_-1_2&rankName5=TIME_1_0_0_0&rankName6=GEO_1_2_0_1&sortC=ASC_-1_FIRST&rStp=&cStp=&rDCh=&cDCh=&rDM=true&cDM=true&footnes=false&empty=false&wai=false&time_mode=ROLLING&time_most_recent=false&lang=EN&cfo=%23%23%23%2C%23%23%23.%23%23%23
- Fahimi, A., Bilo, F., Assi, A., Dalipi, R., Federici, S., Guedes, A., Valentim, B., Olgun, H., Ye, G., Bialecka, B., Fiameni, L., Borgese, L., Cathelineau, M., Boiron, M. C., Predeanu, G., & Bontempi, E. (2020). Poultry litter ash characterization and recovery. Waste Management, 111, 10–21. https://doi.org/10.1016/j.wasman.2020.05.010
- Faucette, L. B., Risse, L. M., Nearing, M. A., Gaskin, J. W., & West, L. T. (2004). Runoff, erosion, and nutrient losses from compost and mulch blankets under simulated rainfall. Journal of Soil and Water Conservation, 59(4), 154–160.
- Ferraro, A., Massini, G., Miritana, V. M., Rosa, S., Signorini, A., & Fabbricino, M. (2020). A novel enrichment approach for anaerobic digestion of lignocellulosic biomass: Process performance enhancement through an inoculum habitat selection. Bioresource Technology, 313, 123703. https://doi.org/10.1016/j.biortech.2020.123703
- Florin, N. H., Maddocks, A. R., Wood, S., & Harris, A. T. (2009). High-temperature thermal destruction of poultry derived wastes for energy recovery in Australia. Waste Management, 29(4), 1399–1408. https://doi.org/10.1016/j.wasman.2008.10.002
- Frąc, M., & Ziemiński, K. (2012). Methane fermentation process for utilization of organic waste. International Agrophysics, 26(3), 317–330. https://doi.org/10.2478/v10247-012-0045-3
- Fuchs, W., Wang, X., Gabauer, W., Ortner, M., & Li, Z. (2018). Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renewable and Sustainable Energy Reviews, 97, 186–199. https://doi.org/10.1016/j.rser.2018.08.038
- Gallert, C., & Winter, J. (2005). Bacterial metabolism in wastewater treatment systems. In H. J. Jordening & J. Winter (Eds.), Environmental biotechnology, concepts and applications (pp.1–48). Weinheim: Wiley-VCH. https://doi.org/10.1002/3527604286.ch1
- Guenet, B., Gabrielle, B., Chenu, C., Arrouays, D., Balesdent, J., Bernoux, M., Bruni, E., Caliman, J.-P., Cardinael, R., Chen, S., Ciais, P., Desbois, D., Fouche, J., Frank, S., Henault, C., Lugato, E., Naipal, V., Nesme, T., Obersteiner, M., … Zhou, F. (2021). Can N2O emissions offset the benefits from soil organic carbon storage? Global Change Biology, 27(2), 237–256. https://doi.org/10.1111/gcb.15342
- Guo, X., Liu, H., & Zhang, J. (2020). The role of biochar in organic waste composting and soil improvement: A review. Waste Management (New York, N.Y.), 102, 884–899. https://doi.org/10.1016/j.wasman.2019.12.003
- Guo, M., Tongtavee, N., & Labreveux, M. (2009). Nutrient dynamics of field-weathered Delmarva poultry litter: implications for land application. Biology and Fertility of Soils, 45(8), 829–838. https://doi.org/10.1007/s00374-009-0397-4
- Gurmessa, B., Pedretti, E. F., Cocco, S., Cardelli, V., & Corti, G. (2020). Manure anaerobic digestion effects and the role of pre-and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. The Science of the Total Environment, 721, 137532. https://doi.org/10.1016/j.scitotenv.2020.137532
- Guštin, S., & Marinšek-Logar, R. (2011). Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the Anaerobic digestion. Process Safety and Environmental Protection, 89(1), 61–66. https://doi.org/10.1016/j.psep.2010.11.001
- Hadroug, S., Jellali, S., Jeguirim, M., Kwapinska, M., Hamdi, H., James, J., Leahy, J. J., & Kwapinski, W. (2021). Static and dynamic investigations on leaching/retention of nutrients from raw poultry manure biochars and amended agricultural soil. Sustainability, 13(3), 1212. https://doi.org/10.3390/su13031212
- Haider, F. U., Coulter, J. A., Cheema, S. A., Farooq, M., Wu, J., Zhang, R., Shuaijie, G., & Liqun, C. (2021). Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicology and Environmental Safety, 214, 112112. https://doi.org/10.1016/j.ecoenv.2021.112112
- Hao, X., & Benke, M. B. (2008). Nitrogen transformation and losses during composting and mitigation. Dynamic Soil, Dynamic Plant 2, (Special issue 1), 10–18.
- Harrison, R. B. (2008). Composting and formation of humic substances. Encyclopedia of Ecology, 713–719. https://doi.org/10.1016/b978-008045405-4.00262-7
- Hassanein, A., Lansing, S., & Tikekar, R. (2019). Impact of metal nanoparticles on biogas production from poultry litter. Bioresource Technology, 275, 200–206. https://doi.org/10.1016/j.biortech.2018.12.048
- He, Z., Honeycutt, C. W., Cade-Menun, B. J., Senwo, Z. N., & Tazisong, I. A. (2008). Phosphorus in poultry litter and soil: Enzymatic and nuclear magnetic resonance characterization. Soil Science Society of America Journal, 72(5), 1425–1433. https://doi.org/10.2136/sssaj2007.0407
- He, X., Hu, Q., Chen, J., Leong, W. Q., Dai, J., & Wang, C.-H. (2022). Energy and environmental risk assessments of poultry manure sustainable solution: An industrial case study in Singapore. Journal of Cleaner Production, 339, 130787. https://doi.org/10.1016/j.jclepro.2022.130787
- He, Z., Pagliari, P. H., & Waldrip, H. M. (2016). Applied and environmental chemistry of animal manure: A review. Pedosphere, 26(6), 779–816. https://doi.org/10.1016/S1002-0160(15)60087-X
- He, Z., Senwo, Z. N., Mankolo, R. N., & Honeycutt, C. W. (2006). Phosphorus fractions in poultry litter characterized by sequential fractionation coupled with phosphatase hydrolysis. Journal of Food, Agriculture and Environment, 4, 304–312.
- Hergoualc’h, K., Akiyama, H., Bernoux, M., Chirinda, N., del Prado, A., Kasimir, Å., MacDonald, J. D., Ogle, S. M., Regina, K., & van der Weerden, T. J., IPCC. (2019). “Chapter 11: N2O emissions from managed soils, and CO2 emissions from lime and urea application,” in Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Retrieved September 9, 2021, from https://www.ipcc-nggip.iges.or.jp/public/2019rf/pdf/4_Volume4/19R_V4_Ch11_Soils_N2O_CO2.pdf
- Ibarrola, R., Shackley, S., & Hammond, J. (2012). Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Management (New York, NY), 32(5), 859–868. https://doi.org/10.1016/j.wasman.2011.10.005
- Islam, M. R., Bilkis, S., Hoque, T. S., Uddin, S., Jahiruddin, M., Rahman, M. M., Siddique, A. B., Hossain, M. A., Danso Marfo, T., Danish, S., & Datta, R. (2021). Mineralization of farm manures and slurries under aerobic and anaerobic conditionsfor subsequent release of phosphorus and sulphur in soil. Sustainability, 13(15), 8605. https://doi.org/10.3390/su13158605
- Issah, A. A., Kabera, T., & Kemausuor, F. (2020). Biogas optimisation processes and effluent quality: A review. Biomass and Bioenergy, 133, 105449. https://doi.org/10.1016/j.biombioe.2019.105449
- Janczak, D., Malińska, K., Czekała, W., Cáceres, R., Lewicki, A., & Dach, J. (2017). Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Management (New York, NY), 66, 36–45. https://doi.org/10.1016/j.wasman.2017.04.033
- Jędrczak, A. (2007). Biologiczne przetwarzanie odpadów. Wydawnictwo Naukowe PWN. ISBN 978-83-01-15166-9.
- Jędrczak, A., Królik, D., Sądecka, Z., Myszograj, S., Suchowska-Kisielewicz, M., & Bojarski, J. (2014). Testing of co-fermentation of poultry manure and corn silage. Civil and Environmental Engineering Reports, 13(2), 31–47. https://doi.org/10.2478/ceer-2014-0013
- Jeswani, H. K., Whiting, A., Martin, A., & Azapagic, A. (2019). Environmental and economic sustainability of poultry litter gasification for electricity and heat generation. Waste Management (New York, NY), 95, 182–191. https://doi.org/10.1016/j.wasman.2019.05.053
- Jiang, Y., McAdam, E., Zhang, Y., Heaven, S., Banks, C., & Longhurst, P. (2019). Ammonia inhibition and toxicity in anaerobic digestion: A critical review. Journal of Water Process Engineering, 32, 100899. https://doi.org/10.1016/j.jwpe.2019.100899
- Junga, R., Knauer, W., Niemiec, P., & Tańczuk, M. (2017). Experimental tests of co-combustion of laying hens manure with coal by using thermogravimetric analysis. Renewable Energy, 111, 245–255. https://doi.org/10.1016/j.renene.2017.03.099
- Kabelitz, T., Biniasch, O., Ammon, C., Nübel, U., Thiel, N., Janke, D., Swaminathan, S., Funk, R., Münch, S., Rösler, U., Siller, P., Amon, B., Aarnink, A. J. A., & Amon, T. (2021). Particulate matter emissions during field application of poultry manure - The influence of moisture content and treatment. The Science of the Total Environment, 780, 146652. https://doi.org/10.1016/j.scitotenv.2021.146652
- Kacprzak, M., & Sobik-Szołtysek, J. (2022). The opoka-rock in N and P of poultry manure management according to circular economy. Journal of Environmental Management, 316(2022), 115262. https://doi.org/10.1016/j.jenvman.2022.115262
- Kaikake, K., Sekito, T., & Dote, Y. (2009). Phosphate recovery from phosphorus-rich solution obtained from chicken manure incineration ash. Waste Management, 29(3), 1084–1088. https://doi.org/10.1016/j.wasman.2008.09.008
- Kelleher, B. P., Leahy, J. J., Henihan, A. M., O'Dwyer, T. F., Sutton, D., & Leahy, M. J. (2002). Advances in poultry litter disposal technology – a review. Bioresource Technology, 83(1), 27–36. https://doi.org/10.1016/S0960-8524(01)00133-X
- Khan, N., Clark, I., Sanchez-Monedero, M. A., Shea, S., Meier, S., Qi, F., Kookana, R. S., & Bolan, N. (2016). Physical and chemical properties of biochars co-composted with biowastes and incubated with a chicken litter compost. Chemosphere, 142, 14–23. https://doi.org/10.1016/j.chemosphere.2015.05.065
- Khanal, S. K. (2011). Anaerobic biotechnology for bioenergy production: principles and applications. John Wiley & Sons. ISBN: 978-0-813-82346-1
- Khoshnevisan, B., Duan, N., Tsapekos, P., Awasthi, M. K., Liu, Z., Mohammadi, A., Angelidaki, I., Tsang, D. C. W., Zhang, Z., Pan, J., Ma, L., Aghbashlo, M., Tabatabaei, M., & Liu, H. (2021). A critical review on livestock manure biorefinery technologies: Sustainability, challenges, and future perspectives. Renewable and Sustainable Energy Reviews, 135, 110033. https://doi.org/10.1016/j.rser.2020.110033
- Kleemann, R., Chenoweth, J., Clift, R., Morse, S., Pearce, P., & Saroj, D. (2017). Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Management (New York, NY), 60, 201–210. https://doi.org/10.1016/j.wasman.2016.10.055
- Krawczyk, W., & Walczak, J. (2016). Redukcja emisji gazowych z kurników poprzez zastosowanie biofiltracji powietrza. Rocz. Nauk. Zoot., T, 43(2), 267–275.
- Kreidenweis, U., Breier, J., Herrmann, C., Libra, J., & Prochnow, A. (2021). Greenhouse gas emissions from broiler manure treatment options are lowest in well-managed biogas production. Journal of Cleaner Production, 280, 124969. https://doi.org/10.1016/j.jclepro.2020.124969
- Krishnamoorthy, N., Dey, B., Unpaprom, Y., Ramaraj, R., Maniam, G. P., Govindan, N., Sivaraman Jayaraman, S., Arunachalam, T., & Paramasivan, B. (2021). Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. Journal of Environmental Chemical Engineering, 9(5), 105579. https://doi.org/10.1016/j.jece.2021.105579
- Kyakuwaire, M., Olupot, G., Amoding, A., Nkedi-Kizza, P., & Basamba, T. A. (2019). How safe is chicken litter for land application as an organic fertilizer? A Review. International Journal of Environmental Research and Public Health, 16(19), 3521. https://doi.org/10.3390/ijerph16193521
- Li, J., Cao, L., Yuan, Y., Wang, R., Wen, Y., & Man, J. (2018). Comparative study for microcystin-LR sorption onto biochars produced from various plant- and animal- wastes at different pyrolysis temperatures: Influencing mechanisms of biochar properties. Bioresource Technology, 247, 794–803. https://doi.org/10.1016/j.biortech.2017.09.120
- Lim, S. C., Knight, D. R., Moono, P., Foster, N. F., & Riley, T. V. (2020). Clostridium difficile in soil conditioners, mulches and garden mixes with evidence of a clonal relationship with historical food and clinical isolates. Environmental Microbiology Reports, 12(6), 672–680. https://doi.org/10.1111/1758-2229.12889
- Lin, H., Wu, X., & Zhu, J. (2016). Kinetics, equilibrium, and thermodynamics of ammonium sorption from swine manure by natural chabazite. Separation Science and Technology, 51(2), 202–213. https://doi.org/10.1080/01496395.2015.1086379
- Li, C., Salas, W., Zhang, R., Krauter, C., Rotz, A., & Mitloehner, F. (2012). Manure-DNDC: a biogeochemical process model for quantifying greenhouse gas and ammonia emissions from livestock manure systems. Nutrient Cycling in Agroecosystems, 93(2), 163–200. https://doi.org/10.1007/s10705-012-9507-z
- Liu, H., Huang, Y., Wang, H., Shen, Z., Qiao, C., Li, R., & Shen, Q. (2020). Enzymatic activities triggered by the succession of microbiota steered fiber degradation and humification during co-composting of chicken manure and rice husk. Journal of Environmental Management, 258, 110014. https://doi.org/10.1016/j.jenvman.2019.110014
- Luyckx, L., de Leeuw, G. H. J., & Van Caneghem, J. (2020). Characterization of poultry litter ash in view of its valorization. Waste and Biomass Valorization, 11(10), 5333–5348. https://doi.org/10.1007/s12649-019-00750-6
- Ma, J., Chen, F., Xue, S., Pan, J., Khoshnevisan, B., Yang, Y., Liu, H., & Qiu, L. (2021). Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresource Technology, 325, 124697. https://doi.org/10.1016/j.biortech.2021.124697
- Maj, I., Kalisz, S., & Ciukaj, S. (2022). Properties of animal-origin ash - A valuable material for circular economy. Energies, 15(4), 1274. https://doi.org/10.3390/en15041274
- Martín José, V., Miralles de Imperial, R., Calvo, R., Garcia, M. C., Leon-Cófreces, C., & Delgado, M. M. (2012). Carbon mineralisation kinetics of poultry manure in two soils. Soil Research, 50(3), 222–228. https://doi.org/10.1071/SR11170
- Matheri, A. N., Ntuli, F., Ngila, J. C., Seodigeng, T., Zvinowanda, C., & Njenga, C. K. (2018). Quantitative characterization of carbonaceous and lignocellulosic biomass for anaerobic digestion. Renewable and Sustainable Energy Reviews, 92, 9–16. https://doi.org/10.1016/j.rser.2018.04.070
- McDowell, R. W., Sharpley, A. N., Condron, L. M., Haygarth, P. M., & Brookes, P. C. (2011). Processes controlling soil phosphorus release to runoff and implications for agricultural management. W: Phosphorus in action. Biological processes in soil phosphorus cycling. In Pr. zbior. Red. E.K. Buenemann, A. Oberson, E. Frossard. Series: Soil Biology (Vol. 100, pp. 269–284). Springer-Verlag s.
- Meegoda, J. N., Li, B., Patel, K., & Wang, L. B. (2018). A review of the processes, parameters, and optimization of anaerobic digestion. Int. International Journal of Environmental Research and Public Health, 15(10), 2224. https://doi.org/10.3390/ijerph15102224
- Mickan, B. S., Abbott, L. K., Stefanova, K., & Solaiman, Z. M. (2016). Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza, 26(6), 565–574. https://doi.org/10.1007/s00572-016-0693-4
- Molaey, R., Bayrakdar, A., Sürmeli, R. Ö., & Çalli, B. (2018). Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass and Bioenergy, 108, 439–446. https://doi.org/10.1016/j.biombioe.2017.10.050
- Morgado, R. G., Loureiro, S., & González-Alcaraz, M. N. (2018). Changes in soil ecosystem structure and functions due to soil contamination. In A. C. Duarte, A. Cachada, & T. Rocha-Santos (Eds.), Soil pollution (pp. 59–87). Academic Press. ISBN 9780128498736, https://doi.org/10.1016/B978-0-12-849873-6.00003-0
- Morvan, T., Gogé, F., Oboyet, T., Carel, O., & Fouad, Y. (2021). A dataset of the chemical composition and near-infrared spectroscopy measurements of raw cattle, poultry and pig manure. Data in Brief, 39, 107475. https://doi.org/10.1016/j.dib.2021.107475
- Muhammad, J., Khan, S., Lei, M., Khan, M. A., Nawab, J., Rashid, A., Ullah, S., & Khisro, S. B. (2020). Application of poultry manure in agriculture fields leads to food plant contamination with potentially toxic elements and causes health risk. Environmental Technology & Innovation, 19, 100909. https://doi.org/10.1016/j.eti.2020.100909
- Nahm, K. H. (2003). Evaluation of the nitrogen content in poultry manure. World's Poultry Science Journal, 59(1), 77–88. https://doi.org/10.1079/WPS20030004
- Negassa, W., & Leinweber, P. (2009). How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: a review. Journal of Plant Nutrition and Soil Science, 172(3), 305–325. https://doi.org/10.1002/jpln.200800223
- Novak, J. M., Lima, I., Xing, B., Gaskin, J. W., Steiner, C., Das, K. C., Ahmedna, M., Rehrah, D., Watts, D. W., Busscher, W. J., & Schomberg, H. (2009). Characterizaton of designer biochar produced at different temperatures and their effects on a loamy sand. Annals of Environmental Science, 3, 195–206.
- Nusselder, S., de Graaff, L. G., Odegard, I. Y. R., Vandecasteele, C., & Croezen, H. J. (2020). Life cycle assessment and nutrient balance for five different treatment methods for poultry litter. Journal of Cleaner Production, 267, 121862. https://doi.org/10.1016/j.jclepro.2020.121862
- Orlando, M. Q., & Borja, V. M. (2020). Pretreatment of animal manure biomass to improve biogas production: A review. Energies, 13(14), 3573. https://doi.org/10.3390/en13143573
- Ortakci, S., Yesil, H., & Tugtas, A. E. (2019). Ammonia removal from chicken manure digestate through vapor pressure membrane contactor (VPMC) and phytoremediation. Waste Management (New York, N.Y.), 85, 186–194. https://doi.org/10.1016/j.wasman.2018.12.033
- Othake, H., & Tsuneda, S. (2019). Phosphorus recovery and recycling. Springer Nature. https://link.springer.com/book/101007/978-981-10-8031-9
- Pagliari, P. H., & Laboski, C. A. M. (2014). Effects of manure inorganic and enzymatically hydrolyzable phosphorus on soil test phosphorus. Soil Science Society of America Journal, 78(4), 1301–1309. https://doi.org/10.2136/sssaj2014.03.0104
- Pan, J., Ma, J., Liu, X., Zhai, L., Ouyang, X., & Liu, H. (2019). Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresource Technology, 275, 258–265. https://doi.org/10.1016/j.biortech.2018.12.068
- Panwar, N. L., Pawar, A., & Salvi, B. L. (2019). Comprehensive review on production and utilization of biochar. SN Applied Sciences, 1(2), 168. https://doi.org/10.1007/s42452-019-0172-6
- Pan, C., Zhao, Y., Zhao, L., Wu, J., Zhang, X., Xie, X., Kang, K., & Jia, L. (2021). Modified montmorillonite and illite adjusted the preference of biotic and abiotic pathways of humus formation during chicken manure composting. Bioresource Technology, 319, 124121. https://doi.org/10.1016/j.biortech.2020.124121
- Paritosh, K., Yadav, M., Kesharwani, N., Pareek, N., Karthyikeyan, O. P., Balan, V., & Vivekanand, V. (2021). Strategies to improve solid state anaerobic bioconversion of lignocellulosic biomass an overview. Bioresource Technology, 331, 125036. https://doi.org/10.1016/j.biortech.2021.125036
- Piash, M. I., Iwabuchi, K., & Itoh, T. (2022). Synthesizing biochar-based fertilizer with sustained phosphorus and potassium release: Co-pyrolysis of nutrient-rich chicken manure and Ca-bentonite. The Science of the Total Environment, 822, 153509. https://doi.org/10.1016/j.scitotenv.2022.153509
- Poblete-Grant, P., Suazo-Hernández, J., Condron, L., Rumpel, C., Demanet, R., Sparkle, L., Malone, S. L., de, L. L., & Mora, M. (2020). Soil available P, soil organic carbon and aggregation as affected by long-term poultry manure application to Andisols under pastures in Southern Chile. Geoderma Regional, 21, e00271. https://doi.org/10.1016/j.geodrs.2020.e00271
- Qi, F., Yan, Y., Lamb, D., Naidu, R., Bolan, N. S., Liu, Y., Ok, Y. S., Donne, S. W., & Semple, K. T. (2017). Thermal stability of biochar and its effects on cadmium sorption capacity. Bioresource Technology, 246, 48–56. https://doi.org/10.1016/j.biortech.2017.07.033
- Ramirez, I., Mottet, A., Carrère, H., Déléris, S., Vedrenne, F., & Steyer, J. P. (2009). Modified ADM1 disintegration/hydrolysis structures for modeling batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge. Water Research, 43(14), 3479–3492. https://doi.org/10.1016/j.watres.2009.05.023
- Rech, I., Withers, P., Jones, D., & Pavinato, P. (2018). Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability, 11(1), 134. https://doi.org/10.3390/su11010134
- Reyes, Y. A., Barrera, E. L., & Cheng, K. K. (2021). A review on the prospective use of chicken manure leachate in high-rate anaerobic reactors. Journal of Environmental Chemical Engineering, 9(1), 104695. https://doi.org/10.1016/j.jece.2020.104695
- Riaz, L., Wang, Q., Yang, Q., Li, X., & Yuan, W. (2020). Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. The Science of the Total Environment, 718, 137414. https://doi.org/10.1016/j.scitotenv.2020.137414
- Rivera, R., Chagnes, A., Cathelineau, M., & Boiron, M.-C. (2022). Conditioning of poultry manure ash for subsequent phosphorous separation and assessment for a process design. Sustainable Materials and Technologies, 31, e00377. https://doi.org/10.1016/j.susmat.2021.e00377
- Roberts, D. A., Cole, A. J., Whelan, A., de Nys, R., & Paul, N. A. (2017). Slow pyrolysis enhances the recovery and reuse of phosphorus and reduces metal leaching from biosolids. Waste Management (New York, N.Y.), 64, 133–139. https://doi.org/10.1016/j.wasman.2017.03.012
- Ros, M., Hendriks, C. M. J., Sigurnjak, I., Robles, A., Aguilar, E., Meers, E., Michels, Z., Hajdu, J., Prado, H., Pose Guerra, D., Fangueiro., & J. P., Lesschen. (2020). Report: D.1.4 Effects of current techniques and management systems on CNP flows in Europe, project Nutri2Cycle. https://www.nutri2cycle.eu/wp-content/uploads/2020/03/D1.4-Nutri2Cycle-techniques-and-management-systems.pdf
- Roy, E. D. (2017). Phosphorus recovery and recycling with ecological engineering: A review. Ecological Engineering, 98, 213–227. https://doi.org/10.1016/j.ecoleng.2016.10.076
- Sahota, S., Shah, G., Ghosh, P., Kapoor, R., Sengupta, S., Singh, P., Vijay, V., Sahay, A., Kumar, V., & Shekhar, I. (2018). Bioresource technology reports review of trends in biogas upgradation technologies and future perspectives. Bioresource Technology Reports, 1, 79–88. https://doi.org/10.1016/j.biteb.2018.01.002
- Sánchez, Ó. J., Ospina, D. A., & Montoya, S. (2017). Compost supplementation with nutrients and microorganisms in composting process. Waste Management, 69, 136–153. https://doi.org/10.1016/j.wasman.2017.08.012
- Sarvi, M., Hagner, M., Velmala, S., Soinne, H., Uusitalo, R., Keskinen, R., Ylivainio, K., Kaseva, J., & Kimmo Rasa, K. (2021). Bioavailability of phosphorus in granulated and pyrolyzed broiler manure. Environmental Technology & Innovation, 23, 101584. https://doi.org/10.1016/j.eti.2021.101584
- Sathya, V., & Maheswari, M. (2017). Nutrient mineralization during the application of poultry manure. Nature Environment and Pollution Technology, 16(3), 905–909.
- Shah, G. A., Ahmed, J., Iqbal, Z., Hassan, F. u., & Rashid, M. I. (2021). Toxicity of NiO nanoparticles to soil nutrient availability and herbage N uptake from poultry manure. Scientific Reports. 11, 1–13. https://doi.org/10.1038/s41598-021-91080-y
- Shakoor, A., Shakoor, S., Rehman, A., Ashraf, F., Abdullah, M., Shahzad, S. M., Farooq, T. H., Ashraf, M., Manzoor, M. A., Altaf, M. M., & Altaf, M. A. (2021). Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. Journal of Cleaner Production, 278, 124019. https://doi.org/10.1016/j.jclepro.2020.124019
- Shapovalov, Y., Zhadan, S., Bochmann, G., Salyuk, A., & Nykyforov, V. (2020). Dry anaerobic digestion of chicken manure: A review. Applied Sciences, 10(21), 7825. https://doi.org/10.3390/app10217825
- Sharpley, A. N., Herron, S., West, C., & Daniel, T. C. (2007). Overcoming the challenges of phosphorus-based management in poultry farming. Journal of Environmental Quality, 62, 375–389.
- Shrestha, S., Fonoll, X., Khanal, S. K., & Raskin, L. (2017). Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: current status and future perspectives. Bioresource Technology, 245(Pt A), 1245–1257. https://doi.org/10.1016/j.biortech.2017.08.089
- Song, W., & Guo, M. (2012). Quality variations of poultry litter biochar generated at different pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis, 94, 138–145. https://doi.org/10.1016/j.jaap.2011.11.018
- Sönmez, O., Turan, V., & Kaya, C. (2016). The effects of sulfur, cattle, and poultry manure addition on soil phosphorus. Turkish Journal of Agriculture and Forestry, 40, 536–541. https://doi.org/10.3906/tar-1601-41
- Soroko, M., & Strzelczyk, M. (2009). Zawartość azotu mineralnego w wodach gruntowych i powierzchniowych na obszarach narażonych gnojowicą. Woda-Środowisko-Obszary Wiejskie, 93(27), 179–186.
- Sousa, D. Z., Alcina Pereira, M., Stams, A. J. M., Alves, M. M., & Smidt, H. (2007). Microbial communities involved in anaerobic degradation of unsaturated or saturated long-chain fatty acids. Applied and Environmental Microbiology, 73(4), 1054–1064. https://doi.org/10.1128/AEM.01723-06
- Srinivasan, P., Sarmah, A. K., Smernik, R., Das, O., Farid, M., & Gao, W. (2015). A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: production, characterization and potential application. The Science of the Total Environment, 512-513, 495–505. https://doi.org/10.1016/j.scitotenv.2015.01.068
- Tańczuk, M., Junga, R., Kolasa-Więcek, A., & Niemiec, P. (2019a). Assessment of the energy potential of chicken manure in Poland. Energies, 12(7), 1244. https://doi.org/10.3390/en12071244
- Tańczuk, M., Junga, R., Werle, S., Chabiński, M., & Ziółkowski, Ł. (2019b). Experimental analysis of the fixed bed gasification process of the mixtures of the chicken manure with biomass. Renewable Energy, 136, 1055–1063. https://doi.org/10.1016/j.renene.2017.05.074
- Thorman, R. E., Nicholson, F. A., Topp, C. F. E., Bell, M. J., Cardenas, L. M., Chadwick, D. R., Cloy, J. M., Misselbrook, T. H., Rees, R. M., Watson, C. J., & Williams, J. R. (2020). Towards country-specific nitrous oxide emission factors for manures applied to arable and grassland soils in the UK. Frontiers in Sustainable Food Systems, 4, 62. https://doi.org/10.3389/fsufs.2020.00062
- Tiessen, H., Ballester, M. V., & Salcedo, I. (2011). Phosphorus and global change. W: Phosphorus in action. Biological processes in soil phosphorus cycling Series: Soil Biology. K. Red, E. Buenemann, A. Oberson, & E. Frossard (Eds.). (vol. 100, pp. 459–471). Springer-Verlag.
- Toth, J. D., Dou, Z., & He, Z. (2011). Solubility of manure phosphorus characterized by selective and sequential extractions. In Z. He (Ed.), Environmental chemistry of animal manure (pp. 227–251). Nova Science Publishers
- USDA. (2021). United States Department of Agriculture Foreign Agricultural Service July 12, 2021 Livestock and Poultry: World Markets and Trade. Retrieved August 26, 2021, from https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf
- Vadas, P. A., Harmel, R. D., & Kleinman, P. J. A. (2007). Transformations of soil and manure phosphorus after surface application of manure to field plots. Nutrient Cycling in Agroecosystems, 77(1), 83–99. https://doi.org/10.1007/s10705-006-9047-5
- Van Zwieten, L., Kimber, S., Morris, S., Downie, A., Berger, E., Rust, J., & Scheer, C. (2010). Infuence of biochar on flux of N2O and CO2 from ferrosol. Soil Research, 48(7), 555–568. https://doi.org/10.1071/SR10004
- Vance, C. L. (2019). [ LSU Digital commons using poultry litter ash as a fertilizer source for Bermudagrass (Cynodon dactylon) Establishment and loblolly pine (Pinus taeda) plantation ]. [LSU Doctoral Dissertations]. 5099. Louisiana State University and Agricultural and Mechanical College. From https://digitalcommons.lsu.edu/gradschool_dissertations/5099/
- Waldrip, H., He, Z., & Erich, S. M. (2011). Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biology and Fertility of Soils, 47(4), 407–418. https://www.ars.usda.gov/research/publications/publication/?seqNo115=259647 https://doi.org/10.1007/s00374-011-0546-4
- Waldrip-Dail, H., He, Z., Erich, S. M., & Honeycutt, W. C. (2009). Soil phosphorus dynamics in response to poultry manure amendment. Soil Science, 174(4), 195–201. https://pubag.nal.usda.gov/download/30149/PDF https://doi.org/10.1097/SS.0b013e31819cd25d
- Wang, X., Gabauer, W., Li, Z., Ortner, M., & Fuchs, W. (2018). Improving exploitation of chicken manure via two-stage anaerobic digestion with an intermediate membrane contactor to extract ammonia. Bioresource Technology, 268, 811–814. https://doi.org/10.1016/j.biortech.2018.08.027
- Wang, Y., Lin, Y., Chiu, P. C., Imhoff, P. T., & Guo, M. (2015). Phosphorus release behaviors of poultry litter biochar as a soil amendment. The Science of the Total Environment, 512-513, 454–463. https://doi.org/10.1016/j.scitotenv.2015.01.093
- Weiler, M., & McDonnell, J. J. (2007). Conceptualizing lateral preferential flow and flow networks and simulating the effects on gauged and ungauged hillslopes. Water Resources Research, 43(3), W03403.1–W03413.13. https://doi.org/10.1029/2006WR004867
- Wielgosiński, G. (2016). Termiczne przekształcanie odpadów komunalnych – wybrane zagadnienia. Wydawnictwo „Nowa Energia”, Racibórz. https://nowa-energia.com.pl/wydawnictwa-ksiazkowe/
- Wystalska, K., Malińska, K., & Barczak, M. (2021). Poultry manure derived biochars – the impact of pyrolysis temperature on selected properties and potentials for further modifications. Journal of Sustainable Development of Energy, Water and Environment Systems, 9(1). https://doi.org/10.13044/j.sdewes.d8.0337
- Yilmazel, Y. D., & Demirer, G. N. (2013). Nitrogen and phosphorus recovery from anaerobic co-digestion residues of poultry manure and maize silage via struvite precipitation. Waste Management & Research: The Journal of the International Solid Wastes and Public Cleansing Association, ISWA, 31(8), 792–804. https://doi.org/10.1177/0734242X13492005
- Yin, D. M., Mahboubi, A., Wainaina, S., Qiao, W., & Taherzadeh, M. J. (2021). The effect of mono-and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresource Technology, 330, 124992. https://doi.org/10.1016/j.biortech.2021.124992
- Zhang, H., Chen, C., Gray, E. M., & Boyd, S. E. (2017). Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass and Bioenergy, 105, 136–146. https://doi.org/10.1016/j.biombioe.2017.06.024
- Zhang, C., Clark, G. J., Patti, A. F., Bolan, N., Cheng, M., Sale, P. W. G., & Tang, C. (2015). Contrasting effects of organic amendments of phytoextraction of heavy metals in a contaminated sediment. Plant and Soil, 397(1-2), 331–345. https://doi.org/10.1007/s11104-015-2615-1
- Zhang, T., He, X., Deng, Y., Tsang, D. C. W., Jiang, R., Becker, G. C., & Kruse, A. (2020). Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. The Science of the Total Environment, 698, 134240. https://doi.org/10.1016/j.scitotenv.2019.134240
- Zhang, L., Loh, K. C., & Zhang, J. (2019). Jointly reducing antibiotic resistance genes and improving methane yield in anaerobic digestion of chicken manure by feedstock microwave pretreatment and activated carbon supplementation. Chemical Engineering Journal, 372, 815–824. https://doi.org/10.1016/j.cej.2019.04.207
- Zhu, Z., Li, L., Dong, H., & Wang, Y. (2020). Ammonia and greenhouse gas emissions of different types of livestock and poultry manure during storage. Transactions of the ASABE, 63(6), 1723–1733. https://doi.org/10.13031/trans.14079
- Ziganshina, E. E., Belostotskiy, D. E., Ilinskaya, O. N., Boulygina, E. A., Grigoryeva, T. V., & Ziganshin, A. M. (2015). Effect of the organic loading rate increase and the presence of zeolite on microbial community composition and process stability during anaerobic digestion of chicken wastes. Microbial Ecology, 70(4), 948–960. https://doi.org/10.1007/s00248-015-0635-2