Abstract
Current literature lacks structured methodologies for analyzing medical technologies’ impact from the patient-centered care perspective. This study introduces, applies and validates ‘Patient-Centered Care Impact Analysis’ (PCIA) as a method for identifying patient-centered care associated demands and expectations for a particular technology and assessing its compliance with these demands. PCIA involves five stages: (1) demand identification, (2) ranking demands’ impact magnitude, (3) scoring demand compliance (DC), (4) demand priority (DP) assignment based on impact magnitude and compliance, (5) generating a summative impact priority number (IPN). PCIA was performed as a comparative assessment of two central nervous system (CNS) drug-delivery platforms; SipNose, a novel noninvasive Direct-Nose-to-Brain (DNTB), vs. the standard-of-care invasive intrathecal/intracerebroventricular injection (Invasive I/I). Study participants included a ranking team (RT) without experience with the SipNose technology that based their scoring on experimental data; and a validation team (VT) experienced with the SipNose platform. All had experience with, or knowledge of, InvasiveI/I. Demand identification and impact magnitude were performed by one content and one assessment expert. Each participant assessed each technology’s DC. DP scores, IPN’s and IPN DNTB:InvasiveI/I ratios were generated for each technology, for each team, based on DC and summative DP scores, respectively. Both teams assigned DNTB higher DC scores, resulting in higher DNTB DP, IPN scores and DNTB:InvasiveI/I IPN ratios. Lack of difference between team assessments of DP and IPN ratio validate PCIA as an assessment tool capable of predicting patient-centered clinical care quality for a new technology. The significant differences between the platforms highlight SipNose’s patient-care centered advantages as an effective CNS drug-delivery platform.
Introduction
The issue of patient-centered care, with an emphasis on human factors and quality of treatment, is of paramount importance among pharmaceutical and technological companies. In fact, patient-centered care is considered a significant issue in medical technology assessment (MTA), although practically there is no structured method that analyzes the medical platforms’ and technologies’ impact from the patient-centered care perspective.
MTA evaluates medical technology based on medical efficacy as well as economic, socio-cultural, legal, ethical, and organizational factors (Parsons, Citation2021). The United States Food and Drug Administration (FDA) and European Commission’s notified bodies also evaluate medical technologies by focusing on technologies’ safe and effective performance. They rely on clinical trials and risk analyses, which are powerful tools for evidence based medical product safety and efficacy evaluation (Sherman et al., Citation2016; Grennan & Town, Citation2020). Additionally, both the FDA’s and European Commission’s notified bodies require that medical products undergo quality assessment via the International Organization for Standardization (ISO) 13485 certified quality management systems. The ISO 13485 specifies the requirements for medical device quality management systems for the purpose of regulatory and consumer oversight. However, regulatory oversight and quality assessment do not incorporate the patient-centered care perspective and thus do not guarantee the production of high-quality medical devices from this crucial perspective. In contrast, the principles of ‘quality’ in industry and marketing emphasize meeting the customer requirements and expectations, or in the case of healthcare systems, meeting the patient and/or patient’s family requirements and expectations. According to the Institute of Medicine (IOM), medical care should be ‘respectful of, and responsive to individual patient preferences, needs, and values, and ensures that patient values guide all clinical decisions’ (National Research Council, Citation2001).
The concept of patient-centered care, which according to the IOM is one of the ‘fundamental approaches to improving the quality of U.S. health care’ (National Research Council, Citation2001), encourages health care systems to shift their focus away from treating solely medical conditions or producing higher quality devices to complying with patients’ expectations and satisfaction (Michael et al., Citation2012). In other words, health care systems need to focus on human factors and quality of clinical treatment. This shift has positive impact at both the clinical and organizational levels (Chue, Citation2006; Glickman et al., Citation2010; Bertakis & Azari, Citation2011; Farrell et al., Citation2015; Tevis et al., Citation2015). For example, there is a positive correlation between patients’ positive experiences and improvement in clinical incidents, reduced repeated hospitalization rates (Farrell et al., Citation2015), reduction in health service consumption (i.e. clinical tests, referrals) (Bertakis & Azari, Citation2011), and even hospital profitability (Glickman et al., Citation2010; Tevis et al., Citation2015).
Having said that, it should be taken in account that patient-centered care approach that is predominantly based on subjective patient experience through surveys and questionnaires which is common by health organizations worldwide, is inadequate and in most cases will not lead to the optimized therapeutic outcome. Organization process assessment exclusively through surveys and questionnaires influenced by patient expectations, communication barriers, patient health conditions and cultural gaps, somehow disregards the fundamental issue of the quality of clinical care received (Glickman et al., Citation2010; Boulding et al., Citation2011; Wolf et al., Citation2012; DeRosier et al., Citation2002, Cheng et al., Citation2012). Moreover, most patients lack the medical knowledge needed to balance the personal experience and frustrations with the clinical quality of the care received (Manary et al., Citation2013). Thus, patient-centered care should not be guided only by subjective patient satisfaction, but also by other dimensions such as clinical quality of treatment assessed by clinicians.
Currently, there is no structured method that analyzes the impact of medical platforms and technologies from the perspective of patient-centered care.
This paper is the first presentation of ‘Patient-Centered Care Impact Analysis’ (PCIA); a novel structured method that analyzes medical platforms’ and technologies’ impact from the patient-centered care perspective. PCIA provides a comprehensive simultaneous analysis of a variety of factors that directly and indirectly affect a particular medical technology/platform’s impact on the therapeutic outcomes and patient experience.
Herein we report on PCIA’s implementation via a comparative assessment of SipNose, a novel noninvasive Direct Nose-to-Brain (DNTB) delivery platform that delivers drugs to the central nervous system (CNS), versus intrathecal and intracerebroventricular injection (Invasive I/I) as the standard-of-care invasive technology for CNS drug delivery. Both latter methods are well-known, widely used, invasive treatment modalities for the management of central nervous system (CNS) disorders. These well-established modes of invasive drug delivery assume that effective delivery of therapeutics to the brain can only be achieved via a platform that invasively crosses the blood-brain-barrier (BBB). This is deemed necessary either due to most drugs’ inability to penetrate the BBB, or in the case of BBB penetrating drugs (less than 2% of existing drugs), due to these methods’ allowing for low dose of drug to be delivered near the site of action. This direct delivery to the target site reduces drug adverse effects and their severity (Delhaas & Huygen, Citation2020). Conversely, the noninvasive DNTB technology takes advantage of the physiological structure of the nasal cavity and its proximity to the olfactory and trigeminal nerve pathways, to allow for efficient direct drug absorption and delivery from the upper nasal cavity to the CNS along these neuronal pathways, thereby bypassing the BBB (Chen et al., Citation1998; Dhuria et al., Citation2010; Gomez et al., Citation2012). Direct nose to brain drug transport allows for an enormous range of neurotherapeutic molecular sizes to be delivered noninvasively to the CNS (Chapman et al., Citation2013; Kosyakovsky et al., Citation2021).
Method
Setting
This study presents ‘Patient-Centered Care Impact Analysis’ (PCIA) and its validation, by analyzing the impact of two technologies that deliver drugs directly to the CNS: the widely-used invasive intrathecal/intracerebroventricular, (Invasive I/I) and the novel SipNose Direct Nose to Brain platform, (Noninvasive DNTB).
Participants
Study participants included two independent expert teams: a ranking team and a validation team.
Ranking team participants had no clinical experience with the SipNose platform, whereas validation team participants had real-time clinical experience with the SipNose platform through their participation as clinicians in prior SipNose clinical studies. All participants had experience with, or medical knowledge of, the invasive treatment.
The ranking team included: Hilel Frankenthal, MD, a physician specialist in pediatric critical care; Aziz Darawsha, MD, a physician specialist in internal medicine, cardiology, and emergency medicine; and Professor Izhar Ben Shlomo, MD, a physician specialist in obstetrics and gynecology.
The validation team included: Professor Avraham Karasik MD MHA- former head of the institute of endocrinology, Sheba Medical Center, Tel HaShomer, Israel; Professor Tamir Ben Hur, MD, Ph.D. head, division of medical neurosciences, Hadassah Medical Center, Jerusalem, Israel; Dr. Dana Ekstien, MD, Ph.D. head, department of neurology, Hadassah Medical Center, Jerusalem, Israel; Dr. Adit Zohar Beja, Ph.D. clinical dietitian specialist in the treatment of eating disorders, Sheba Medical Center, Tel HaShomer; Dr. Lisa Amir, MD, MPH, deputy director, department of emergency medicine and chair of hospital resuscitation committee, Schneider Medical Center, Israel.
Patient-Centered Care Impact Analysis (PCIA) terms and approach
For each technology, PCIA utilizes technology associated demands and expectations that are patient-centered care focused to assess the specific technology. We use the term ‘demands’ to refer to these demands and expectations.
Study design
Study design focused on the PCIA model implementation and validation. Implementation was achieved through PCIA’s application as an assessment tool for the Invasive I/I treatment and the noninvasive DNTB. This was performed by the ranking and validation teams independently through ranking and prioritizing demands in terms of their patient-care centered impact. The ranking team used clinical and pre-clinical SipNose data, literature reviews, and their clinical experience and knowledge. The validation team used their real-time clinical experience with the SipNose delivery technology, and their medical knowledge and literature for the Invasive I/I.
The PCIA model
PCIA has three goals:
To identify the most relevant and essential patient-centered care associated demands and expectations for a particular technology.
To evaluate the extent to which the technology in question meets each of these demands.
To provide a summative patient-centered care assessment value for a particular technology.
The PCIA model aims to achieve these goals through five steps:
Demand identification
The first step involves identifying the demands relevant to patient-centered care, in terms of clinical quality of treatment and human factors. This is achieved through expert brainstorming and literature review followed by editing and approval by a ranking team. In this study, DS, and IS utilized brainstorming and literature review (Story, Citation2012) to identify the demands relevant to patient-centered care, in terms of clinical quality of treatment and human factors, in direct drug delivery to the CNS therapeutic field. The demands were approved and edited by the ranking team.
Demand impact magnitude ranking
In the second step, an impact magnitude score is assigned to each identified demand. The term impact magnitude refers to the magnitude that a demand has on patient centered care. The rank order of demand impact magnitude is as follows: I—major impact, II—minor impact.
In this paper, AKG and IBS ranked the demand impact for the demands identified in the prior step.
Demand compliance ranking
The third step evaluates the extent to which the technology in question fulfills each demand. For the purposes of PCIA, we term this fulfillment ‘compliance.’ For each demand, the degree of compliance is assigned as a rank value (see ).
Table 1. The grading scale: the technology/platform’s compliance with specified demand.
In contradistinction to widely used commercially available ‘shelf’ technologies, new products lack longstanding multi-user familiarity and work experience. Therefore, different approaches were used to determine demand compliance ranking for new vs. known technology, respectively.
For new technology, the demand compliance ranking is determined based on clinical and pre-clinical trial reports that related to each demand. In this study, the ranking team, used this approach to rank the SipNose platform’s demand compliance. The validation team based its scoring on self-experience with the SipNose technology.
The demand compliance ranking for well known ‘shelf’ products is determined based on clinical expertise and literature review (Delhaas & Huygen, Citation2020). In this study, this approach was used by the ranking and validation teams, to rank Invasive I/I treatments’ demand compliance. The general ranking scale for new and ‘shelf’ technologies can be seen in .
Demand prioritization
The fourth step incorporates the results of both previous steps, the impact magnitude and demand compliance ranking, to generate a priority value for each demand and a summative priority value for each assessed technology. Prioritization ranking can be High (H), Medium (M), or Low (L). The demand prioritization scoring method is depicted in .
Table 2. Prioritization matrix.
A demand with Major impact (I) and full compliance (A) is assigned a High (H) priority value. A demand with Major impact (I) and Moderate compliance (B) is assigned a Medium (M) priority value. A demand with Major impact (I) and Minor compliance (C) is assigned a Low (L) priority value. PCIA focuses on demands with major impact to evaluate the demands most relevant and essential to patient-centered care, in terms of treatment quality and human factors. Therefore, all demands with Minor impact (II) are assigned a Low (L) priority value regardless of compliance ranking.
Subsequently, a summative prioritization value is assigned to technology in query. This value is generated with the following conversion. Each demand’s priority value (H, M, L) is converted to a numerical value, such that H = 3, M = 2, L = 1. The numerical sum of all the demands provides the summative prioritization value for the technology in question.
In this study, each team was studied in isolation. Each technology’s summative prioritization value was determined for each team by counting the sum of H, M, L numerical values of all participants, for all demands, for invasive treatment and SipNose treatment. Since there were different numbers of participants in the ranking and validation teams, this number was normalized as reflected in step 5 below.
Impact priority number (IPN)
The impact priority number (IPN) is a measure factor in the number of evaluators and provides a numerical value that represents a technology’s overall evaluation with regard to ‘Patient-Centered Care.’ The summative prioritization value for each team and each technology, determined in the previous step, is divided by the number of participants. In this study there were three participants in the ranking team and five participants in the validation team.
PCIA model validation
In this study, the ranking team lacked clinical experience, especially with regard to the new Noninvasive DNTB technology. They determined their PCIA evaluation for the new technology solely based on research data. In contrast, the validation team had clinical experience using the new technology. As such, the ranking team team’s evaluation represents an anticipated pre-use evaluation whereas the validation team’s evaluation represents a post-use evaluation. PCIA model validation was achieved by comparing demand prioritization for the Noninvasive DNTB and the Invasive I/I treatments, as calculated by the ranking and validation teams, respectively. Further validation testing included calculating the ratio between the impact priority number (IPN) assigned for both technologies by each team. The ratio for each team was then compared statistically.
Outcome measures
Primary outcomes included a comparison of each demand’s ranking by the expert and validation teams’ in terms of the demand compliance and prioritization, and a comparison of the impact priority numbers (IPN (for each team. A statistical lack of significance for each of these validates the PCIA model in terms of evaluation group correlation.
Secondary outcomes relate to the specific technologies evaluated. Statistically significant differences between demand compliance, prioritization and IPN provide an evaluative comparison between Invasive I/I treatment and Noninvasive DNTB with regards to Patient-Centered Care. Furthermore, strong correlation between the ranking teams theoretical ranking (based on clinical, pre-clinical data and literature) and the validation team’s experienced ranking validates the model’s ability to serve as a retrospective assessment tool for well-known technologies and a prospective assessment tool for new technologies, even before their actual widespread use.
Statistical analysis
We used a paired t-test to compare between: IPN Validation team vs. ranking team; IPN noninvasive DNTB treatment vs. Invasive I/I treatment; prioritization of the technology by validation team vs. ranking team, invasive and noninvasive treatment. p-Values below .05 indicate statistical significance.
Results
Step 1: demand identification
The Patient-Centered Care demands identified for the technologies assessed in this study:
Drug delivery to the brain/CNS
Control of dose accuracy
Enabling short time to effect
Ability to deliver treatment on as-needed basis (PRN).
Minimal user training and retraining. Applicable for Self-administration (non-dependence on skilled staff)
Device ease of use
Ability of drug administration without patient cooperation (unconscious or when the patient resists treatment)
Efficacy independent of patient position for administration (for invasive, relates to initial administration)
Low risk of administration error
Administration does not cause pain, anxiety, or trauma to the patient in the short term
Administration does not cause pain, anxiety, or trauma to the patient in the long term
Avoidance of contamination (complications) resulting from the treatment procedure
Avoidance of local/systemic toxicity (complications)
Avoidance of CNS complications
Treatment does not require additional interventions/tests (more complex procedures, more trained staff, etc.)
No contamination between patients (resulting usually from reusable parts, wrong procedure of discarding equipment, etc.)
Flexibility in treatment location/site (home, clinic, etc.)
Minimal disruption to patient-daily functioning
Easy-to-carry devices (patient)
Step 2: demand impact magnitude ranking
All the demands chosen for this study and listed above, were determined to have major (I) impact on technology outcomes in the field of Brain Delivery.
Steps 3 and 4: demand compliance ranking and prioritization
The PCIA implementation by the ranking team is presented in under ‘Compliance with Demand’ for both Invasive and SipNose technologies, along with demand impact intensity., The relevant clinical and pre-clinical trials (used by the ranking team) are detailed for each demand, and impact prioritization for each technology is defined. At the bottom of the table, the summative prioritization for each technology is calculated by counting the number of H, M, and L scores of all ranking team participants, for all demands, for the Invasive I/I treatments and Noninvasive DNTB treatments, respectively. The ranking team overall assigned Invasive I/I treatments 10 High, 15 Moderate and 32 Low prioritizations. The ranking team assigned Noninvasive DNTB 42 High, 15 Moderate and 0 Low prioritizations.
Table 3. The PCIA implementation by the ranking team: the impact intensity of the demands, compliance to demands, clinical and pre-clinical trials (for SipNose ranking), demand prioritization.
The clinical and pre-clinical trials, according to which the ranking was determined by the ranking team, are summarized in . The full details of the reports are available in the supplementary material.
Table 4. The clinical and pre-clinical trials, according to which the grading was determined by the ranking team.
The PCIA implementation by the validation team is presented in under ‘Compliance with Demand’ for both Invasive and SipNose technologies, along with demand impact intensity, and impact prioritization for each technology is defined. At the bottom of the table, the summative prioritization for each technology is calculated. by counting the sum of H, M, and L scores of all validation team participants, for all demands, for the Invasive I/I treatment and Noninvasive DNTB treatments, respectively. The validation team overall assigned Invasive I/I treatments 23 High, 24 Moderate and 48 Low prioritizations. The validation team assigned Noninvasive DNTB 88 High, 7 Moderate and 0 Low prioritizations. presents this information in bar graph format.
Figure 1. The prioritization of invasive treatment (Invasive I/I) vs. SipNose (Noninvasive DNTB), derived from the grading of the validation team (experience-based).
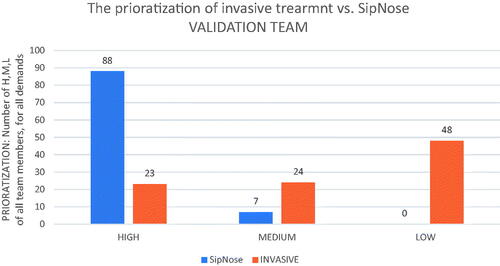
Table 5. The PCIA implementation by the validation team: the impact intensity of the demands, compliance to demand, demand prioritization.
presents the summative prioritization for each technology by the validation team vs. ranking team, along with statistical comparison. The differences between both teams’ prioritization assessments for Noninvasive DNTB is 0.53, and for Invasive I/Is is 0.08, thus no statistic significant difference between the ranking and validation groups for both technologies.
Table 6. The comparison of prioritization of the technology by validation team vs. ranking team, separately for Invasive and SipNose.
Step 5: impact priority number (IPN)
The impact priority number (IPN) assigned by both teams, and the proportion between the IPN of Noninvasive DNTB and Invasive I/I for each team can be seen in and are presented visually in bar graph format in .
Figure 2. IPN Validation team vs. ranking team; IPN SipNose Noninvasive DNTB vs. Invasive I/I Abbreviations: NS: not significant.
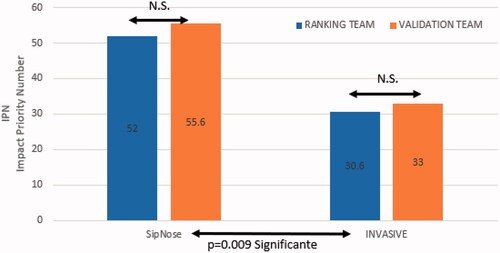
Table 7. The impact priority number (IPN) of both validation and ranking teams, the proportion between the IPN of SipNose/Invasive, and p value (validation team vs. ranking team; SipNose vs. Invasive; NS: not significant).
Bothe ranking and validation teams gave much higher impact scoring to the noninvasive DNTB technology when compared to the Invasive I/I technology. The ranking team assigned Noninvasive DNTB and Invasive I/I IPN’s of 52 and 30.6 respectively. The validation team assigned Noninvasive DNTB and Invasive I/I IPN’s of 55.6 and 33 respectively. The overall IPN differences between Noninvasive DNTB vs. Invasive I/I had a significant p-value (p = .009). The IPN proportions were very similar between both teams: 1.69 for the ranking team and 1.68 for the validation team with the difference showing non-significant difference between the groups (p = .15).
Discussion
This study aimed to design a tool for technology assessment in a ‘patient-centered evaluation’ manner. In terms of primary outcomes, the model showed strong correlation between the ranking team and the validation team assessments in terms of major demand compliance, prioritization and IPN. Its strong results introduce a validated method for ‘patient-centered evaluation’ of medical platforms/devices. This model can be applied with minor adaptations for technology evaluation in many medical fields. Additionally, its strong correlation between the ranking team evaluation and the validation team evaluation demonstrates the models’ applicability to new technology assessments prior to widespread clinical implementation.
In terms of secondary outcomes, the model showed significant higher Patient-Centered Care Impact in favor of the new technology, the Noninvasive DNTB SipNose platform, in comparison with the well-known Invasive I/I intrathecal or intracerebroventricular platforms for CNS drug delivery.
Our findings, indicate that the PCIA method is reliably capable of predicting the patient-centered clinical care quality anticipated for a new patient care technology. In other words, it allows prospective prediction of the full scope of device/platform acceptability and usability in real life. PCIA is novel in the sense that, in addition to considering technical elements and patient post-treatment reports, it combines all aspect into one comprehensive evaluation tool.
The choice of the technologies for evaluating a new method, was driven by the clinical need in the area of CNS therapeutics. Until recently, only highly invasive treatment devices and procedures were available for administering low or no-BBB penetrable molecules, due to the challenge of crossing the BBB. The recent advent of Noninvasive Direct Nose to Brain delivery availed a new approach of CNS drug delivery. This approach, represented by the SipNose DNTB delivery platform was evaluated and compared to the currently accepted invasive method for direct drug delivery to the CNS.
This study’s outcomes highlight the qualitative clinical advantages of a novel delivery method of pharmaceuticals to the brain, the SipNose Noninvasive Direct Nose To Brain (DNTB) delivery method. Both ranking and validation teams assigned significantly higher prioritization values to Noninvasive DNTB vs. Invasive I/I methods. The ratios between the IPN of the Noninvasive DNTB to the Invasive I/I in both ranking and validation teams, reflects a much higher Impact score of the Noninvasive DNTB SipNose method, about 70% greater than that of the Invasive I/I. This method scored high in meeting patient-centered care demands because it is highly effective, reproducible, and presents very high Human Factor and Quality of Treatment scoring. It achieves this through providing a highly safe solution for noninvasive drug delivery to the CNS. The SipNose platform is suitable for delivering a large variety of molecules (small molecules, high molecular weight proteins and macromolecules, etc.), with flexible chemical nature (hydrophilic and hydrophobic), as either liquid or dry powder formulations.
The ranking team members scored the two demands: (2) Control over dose accuracy; and (3) Enabling short time to effect; as ‘A’ for the Invasive I/I method (marked in ** in ). However, they raised concerns that although the clinical literature indicates assigning A for these demands, it is over tolerant of catheter obstruction. In their clinical experience, intracerebroventricular or intrathecal catheter obstruction results in poor drug delivery and significant adverse patient outcomes.
The validation team members Noninvasive DNTB scoring for demand (1) Delivery to the brain/CNS (marked in *** in ); four participants scored A for this demand, and one participant scored B. This score is based on uncertainty of delivering macromolecules that have yet to be tested. Otherwise, all pre-clinical and clinical data that was established up to this point, showed clear delivery to the CNS.
This study presents and validates Patient-Centered Care Impact Analysis, a new method for technology quality assessment from a patient-centered care perspective in terms of treatment quality and human factors. This method is applicable for assessing both well-known and new technologies, both retrospectively after gaining clinical experience with its use and prospectively to predict its patient-centered care clinical impact. The study further demonstrates the benefits that the new SipNose, a Noninvasive Direct Nose to Brain drug delivery method, adds to patient care, and thus should be considered as an alternate therapy to the invasive intrathecal and intracerebroventricular modalities.
The study’s limitations that we can address are related to the fact that all the demands listed above were determined to have major impact in the technologies assessed in this study. We recommend that future studies will also define demands with minor impact. Also, the fact that the members of the validation team were required to have prior experience with the technology in order to validate the PCIA model, resulted in having two of the validation team members associated with SipNose.
Author contributions
AKG & IS – Designed the study, oversaw the implementation, and wrote the manuscript.
HF, AD, AK, AZB, TBH, DE, LA, DS, IBS, WHF – Participation in the implementation and review of the manuscript
All authors approved the final manuscript version being submitted for publication.
Supplemental Material
Download PDF (2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Manary MP, Boulding W, Staelin R, Glickman SW. (2013). The patient experience and health outcomes. N Engl J Med 368:1754–3.
- Bertakis KD, Azari R. (2011). Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 24:229–39. 100170.
- Boulding W, Glickman SW, Manary MP, et al. (2011). Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care 17:41–8.
- Chapman CD, Frey WH, Craft S, et al. (2013). Intranasal treatment of central nervous system dysfunction in humans. Pharm Res 30:2475–84.
- Chen XQ ,Fawcett JR, Rahman YE, et al. (1998). Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis 1:35–44. IOS Press.
- Cheng CH, Chou CJ, Wang PC, et al. (2012). Applying HFMEA to prevent chemotherapy errors. J Med Syst 36:1543–51.
- Chue P. (2006). The relationship between patient satisfaction and treatment outcomes in schizophrenia. J Psychopharmacol 20:38–56.
- Delhaas EM, Huygen FJPM. (2020). Complications associated with intrathecal drug delivery systems. BJA Educ 20:51–7.
- DeRosier J, Stalhandske E, Bagian JP, et al. (2002). Using health care hazard and effect analysis: the VA National Center for Patient Safety’s prospective risk analysis system. J Comm J Qual Improv 28:248–67..
- Dhuria SV, Hanson LR, Frey WH II. (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–73.
- Farrell TW, Tomoaia-Cotisel A, Scammon DL, et al. (2015). Impact of an integrated transition management program in primary care on hospital readmissions. J Healthc Qual 37:81–92.
- Glickman SW, Boulding W, Manary M, et al. (2010). Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes 3:188–95.
- Gomez D, Martinez JA, Hanson LR, et al. (2012). Intranasal treatment of neurodegenerative diseases and stroke. Front Biosci (Schol Ed) 4:74–89.
- Grennan M, Town RJ. (2020). Regulating innovation with uncertain quality: information, risk, and access in medical devices. Am Econ Rev 110:120–61.
- Kosyakovsky J, Fine JM, Frey WH, Hanson LR. (2021). Mechanisms of intranasal deferoxamine in neurodegenerative and neurovascular disease. Pharmaceuticals 14:95.
- LaVela SL, Gallan A. (2014). Evaluation and measurement of patient experience (2014). Patient Exp J 1:28–36. Available at: https://ssrn.com/abstract=2643249
- Michael J, Barry MD, Susan Edgman-Levitan PA. (2012). Shared decision making — the pinnacle of patient-centered care. N Engl J Med 366:780–1.
- National Research Council. (2001). Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academies Press.
- Parsons JE. (2021). Communicating the findings of health technology assessments: considering uncertainty. Med Writing 30:56–9.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. (2016). Real-world evidence - what is it and what can it tell us?. N Engl J Med 375:2293–7.
- Story MF. (2012). FDA perspective in human factor in device development. RAPS Webinar June, 7, 2012.
- Tevis SE ,Kennedy GD, Kent KC. (2015). Is there a relationship between patient satisfaction and favorable surgical outcomes? Adv Surg 49:221–33.
- Wolf A, Olsson LE, Taft C, et al. (2012). Impacts of patient characteristics on hospital care experience in 34,000 Swedish patients. BMC Nurs 11:8.
