?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ulcerative colitis (UC) is a chronic disease, which can result the inflammation of the rectum, mucosa of the colon, and submucosa. The active component such as polypeptide in Periplaneta americana, which is one of the most common insects in the nature, can be extracted to treat UC. However, the active components in Periplaneta americana extract (PAE) can be degraded in the stomach due to its extreme acidic environment and enzyme. In this study, we developed a pH-dependent drug delivery method using polymer cellulose acetate (Eudragit S100) as a carrier to deliver high concentration PAE to inflamed colon. Both in vitro and in vivo results showed the PAE-Eudragit-S100 could treat UC through delivering active drug components to colon without degradation.
Graphical Abstract
Eudragit S100 polymer solution was mixed with PAE ethanol solution by sonicating. Then mixture was then mixed with lecithin and glycerol monooleate in a blender . The emulsion was stirred at room temperature with a magnetic stir bar at 420 rpm. Them the microparticles were freeze-dried in a freeze-dryer. Finally, When mice were treated with this drugs. the drugs were not decomposed in stomach and small intestine but damaged in the colon, which had a good curative effeet on colitis.
1. Introduction
UC is a chronic disease, which can cause the inflammation of the rectum, mucosa of the colon, and submucosa (Rubin et al., Citation2019; Ungaro et al., Citation2019; Sarvestani et al., Citation2021). Patients who have UC are always suffered abdominal pain, diarrhea and bloody stools. The incidence of UC is related to genes mutation such as NFKBIZ, ZC3H12A, PIGIR (Nanki et al., Citation2020) and MDR1 (Dragana et al., Citation2018), infection of bacteria (Kushkevych et al., Citation2017; IvanKristýna et al., Citation2021), stress and socio-economic factors and other factors (Carbonnel et al., Citation2009; Cortot et al., Citation2009). In addition, UC also increases the risk of patients having cancer (Jonsson et al., Citation2010; Den et al., Citation2019). To treat UC, it requires patients to take drug for a long period of time which are always related to drug adverse response and poor compliance of patients. The main adverse effects that are reported after using conventional therapy of UC include fever, nausea, headache, kidney damage, myopathy, myalgia, edema, neoplasia, congestive heart failure, tuberculosis, tremor, and hirsutism (Yoko et al., Citation2014). Bronchitis, arthralgia, headache, dizziness, abdominal cramps, and minor metabolic disorders would happen when the patients were treated with 5-aminosalicylates (Patil & Moss, Citation2008; Miehlke et al., Citation2014). Corticosteroids, though effective for UC when immediate remission is required, also induce some side effects, including edema, moon face, acne, mood disturbances, adrenal suppression, congenital fetal abnormalities, cushingoid face, gastric ulceration, and osteoporosis. Moreover, the routine use of corticosteroids may cause cataract and hyperglycemia, and a huge surge in the chances of getting severe relapse (Hanauer, Citation2008; Tiago et al., Citation2012; Kondamudi et al., Citation2013).
The Periplaneta americana is the one of the most common cockroaches in US. Although the wild cockroach become a public health problem due to its ability to spread virus, fungi and bacteria to human being, the body of cockroach is rich of many kinds of nutrients such as protein, amino acids and nucleotides (Lee et al., Citation2011; Yun et al., Citation2017; Dingchun et al., Citation2018). The dry cockroach body is recorded as a traditional Chinese medicine in ‘Shennong Materia Medica Classic’, which is commonly used for infantile malnutrition, sore throat, insect and snake bite, sore carbuncle, ulcer of digestive tract, etc (Chen et al., Citation2019). Studies have shown that the Periplaneta americana extract (PAE) promotes the growth of new granulation tissue and repairing ulcer wounds (Jing et al., Citation2019; Li et al., Citation2019), which can be used to treat gastric ulcers (Ma et al., Citation2018; Lu et al., Citation2019). PAE can be delivered to treat UC intravenously or subcutaneously in mice by increasing epidermal growth factor, which significantly reduces the lesion area (Xue et al., Citation2020). However, subcutaneous and intravenous administration of drugs always induces severe infusion-related reactions and adverse reactions, which significantly limits the clinical practice of PAE (Soeters & Aus, Citation1989). To lower the risk of subcutaneous or intravenous administration without scarifying drug efficacy, oral administration of PAE is an alternative delivery approach. But most of the active ingredients, such as polypeptide and protein molecules, may be degraded in the stomach before entering the colon (Abuhelwa et al., Citation2017; Cao et al., Citation2019). To deliver PAE to the inflamed colon without being degraded in gastric environment, an advanced drug delivery system should control the release of PAE in different environments (Li et al., Citation2020).
In this study, we developed a pH-dependent drug delivery method using Eudragit S100 (chemical structure is shown in ) as a carrier to deliver the PAE to the inflammatory colon at high concentrations (So et al., Citation2007). To exam the drug release profile of PAE-S100 conjugate, the conjugate was dissolved in different pH environments which contained specific enzymes to mimic the real physiological conditions in gastric juice, small intestine and colon (see ). In addition, scanning electronic microscopic was used to observe the morphology of PAE-S100 in different physiological conditions to test the stability of the PAE-S100 microparticle. Finally, an animal study was performed to demonstrate the PAE-S100 efficacy in vivo. Mice were treated with dinitrochlorobenzene and acetic acid to induce UC. Administrated different formulations, PAE only, PAE-S100 and PAE enema, the mice were observed for changes in body weight, fecal condition, as well as pathological changes in colonic tissues. Myeloperoxidase (MPO) activity in the tissues was measured by ELISA kits to indicate tissue recovery in mice. All the information was used to evaluate the efficacy of PAE-S100 comparing to other formulations.
2. Materials and methods
2.1. Materials
Inosine, aminopropionic acid, bovine serum albumin, and hypoxanthine were supplied from Shanghai McLean Biochemical Technology Co. Ltd (Shanghai, China). Soybean lecithin was purchased from Beijing Biotechnology Co. Ltd (Beijng, China). Glyceryl monooleate was obtained from Shanghai Jiafashi Trading Co. Ltd (Shanghai, China). Methanol, ethanol, phenol reagent, ethyl ether, glacial acetic acid, NaC4H4O6, CuSO4, Na2CO3, NaOH were bought from Tianjin Fuyu Fine Chemicals Co. Ltd (Tianjin, China). Tianjin Bodi Chemical Co. Ltd (Tianjin, China) supplied Tween 80, paraffin, acetic acid, ninhydrin, ascorbic acid. Hydrochloric acid, phosphate buffer solution, acetic acid, sodium sulfate, dinitrochlorobenzene, normal saline, and formalin were purchased from Shanghai Yuanye Biotechnology Co. Ltd. (Shanghai, China). Coomassie bright blue R250 and SDS-PAGE gel preparation kit were purchased from Biyuntian Biotechnology Co. Ltd (Shanghai, China). Protein standard molecular weight marker 26610 was obtained from Beijing Solaibao Technology Co. Ltd (Beijing, China). ELISA kits were acquired from Shanghai Fanke Biotechnology Co. Ltd (Shanghai, China). Deionized water was filtered by 0.22 μm PES filter (Haining Jinzheng Filter Material Technology Co. Ltd, China) to remove any contaminants.
2.2. Mice
Animal Experiment Center in Shenyang Pharmaceutical University supplied SD mice (male, 200 ± 20 g). All animals were kept in the housing system under 24 ± 1 °C, relative humidity (40-70%), and 12-12 hours cycles of light-dark with free water and fodder access. Before the experiment, the animals were deprived of food for 12 hours but with free access to water. The protocols were performed strictly according to guidelines issued by Animal Experiment Center in Shenyang Pharmaceutical University.
2.3. Preparation of Periplaneta americana extract
The dried Periplaneta americana body was blended to fine powder and was soaked in 90 v/v % ethanol solution for 12 hours (Fu et al., Citation2021; Hua-ShengYong-Ming et al., Citation2021). Then the mixture was incubated in a water bath at 60 °C (Gongyi Yuhua Instrument Co. Ltd, Henan, China) for 1 hour and filtered by 0.22 μm PES filter. The ethanol was removed by vacuum evaporation (Shanghai Yarong Biochemical Instrument Co. Ltd, Shanghai, China) at 60 °C to obtain concentrated solution, and then the solution was filtered through 0.22 μm PES filter to remove insoluble impurities.
The macroporous adsorption AB-8 resin (Shanghai Acmec Biochemical Co. Ltd, Shanghai, China) was used to purify the PAE. The PAE was loaded into the packed column and eluted by 0.22 μm filtered deionized (DI) water at 100 mL/hour for 2 hours at room temperature. 95 v/v % ethanol was used for desorption at 100 mL/hour for 1.5 hours and the elution was collected. Finally, the PAE mixture was freeze-dried in a freeze-dryer (Ningbo Scientz Biotechnology Co. Ltd, Zhejiang, China) at −100 Pa, −40 °C for 24 hours.
2.4. Characterization of polypeptide in PAE
The protein content in PAE was detected by high performance liquid chromatooraphy (Hitachi Scientific Instruments (Beijing) Co. LTD). The mobile phase was 0.1 M Na2SO4, 0.05 M Na2HPO4 and 0.05 M NaH2PO4. The flow rate was 0.8 ml/min at 25 °C. The chromatographic column was TSKgel G3000SWxl (Yamasaki & Kato, Citation1989).(Dongcao (Shanghai) Biotechnology Co., LTD). Each injection amount is 10 µL and the peak time of protein at about 10 minutes.
2.5. Preparation of eudragit S100 PAE microparticles
6 g/L Eudragit S100 was prepared by dissolving Eudragit S100 in ethanol solution. 1 mg/mL PAE solution was prepared through dissolving PAE lyophilized cake in ethanol. Then 10 mL 6 g/L Eudragit S100 polymer solution was mixed with 1 mL 1 mg/mL PAE ethanol solution by sonicating (Ningbo Xinzhi Biotechnology Co. Ltd, Zhejiang, China) for 30 minutes to form fine emulsion. The mixture was then mixed with 0.33 g/L lecithin and 30.3 g/L glycerol monooleate in a blender (Ningbo Scientz Biotechnology Co. Ltd, Zhejiang, China) for 2 minutes to form 110 mL emulsion (Thakral et al., Citation2010). The emulsion was stirred at room temperature with a magnetic stir bar (Gongyi Yuhua Instrument Co. Ltd, Henan, China) at 420 rpm for 8 hours until the organic solvent completely evaporated. Finally, the microparticles were freeze-dried in a freeze-dryer (Ningbo Xinzhi Biotechnology, Zhejiang, China) at −100 Pa and − 40 °C for 24 hours and stored at 4 °C (Li et al., Citation2021).
2.6. Encapsulation yield rate of PAE
Polypeptide concentration was used to determine the encapsulation efficiency of PAE. 100 mg PAE was added to preparate the microparticles according to method 2.2.2. Then the solution was centrifuged (Shanghai Anting Scientific Instrument Factory, Shanghai, China) at 3000 rpm for 15 minutes, and supernatant was collected. The supernatant was used to calculate the concentration of polypeptides using method 2.3.1. The encapsulation efficiency (EE) of polypeptide microparticles was calculated according to (Thakral et al., Citation2010),
(1)
(1)
where
Ctotal was the amount of total polypeptide;
Cfree was the amount of free polypeptide that was not captured.
2.7. In vitro drug release experiments
250 mL simulate gastric solution was prepared by dissolving 0.1 mol/L HCl, and 10 g/L of pepsin into 0.22 μm filtered DI water. 250 mL simulate small intestine solution was prepared by dissolving 6.8 g/L of KH2PO4 and 10 mg/L of trypsin into filtered DI water, and 0.1 mol/L NaOH was used to adjust the pH of the solution to 6.8, and 250 mL simulate colonic fluid prepared by dissolving 0.41 g/L of KH2PO4 and 5.59 g/L K2HPO4. 4 mg/mL PAE-S100 microparticles were placed in three different solutions. They were agitated in an incubator (Jiangsu Keji Instrument Co. Ltd, Jiangsu, China) at 37 °C and 140 rpm for 20 hours, and 1 mL solution was taken out at 0.3, 0.6, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, and 20 hours, respectively (Thakral et al., Citation2010; Wang et al., Citation2016; Yuan et al., Citation2019), which were centrifuged at 5000 rpm (Shanghai Anting Scientific Instrument Factory, Shanghai, China). The supernatant was used to quantify the concentration of polypeptide. The sediment was collected, and its surface morphology was observed by SEM (Wang et al., Citation2016) (Shimadzu Corporation, Kyoto, Japan).
2.8. Drug release kinetics
To investigate the PAE release kinetics from the microspheres, the in vitro drug release data were fitted into various mathematical models as follows: zero-order (Equationequation 2(2)
(2) ), first-order (Equationequation 3
(3)
(3) ), Korsmeyer-Peppas (Equationequation 4
(4)
(4) ) (Malipeddi et al., Citation2016; Yuan et al., Citation2019),
(2)
(2)
(3)
(3)
(4)
(4)
where
Qt denoted the Cumulative release (%) of PAE at different time;
K1, K2, and K3 denoted the release-rate constants for zero-order, first-order, and Korsmeyer-Peppas;n was the release exponent.
2.9. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis
The equipment was filled with sealing glue (1 mL distilled water, 1 mL 30 w/v % Acrylamide-Bis, 1 mL 1 M Tris-HCl pH 6.8, 0.04 mL 10 w/v % ammonium persulfate, 0.004 mL N,N,N,N-tetramethylethylenediamine (TEMED)) , separating glue (2.7 mL distilled water, 3.3 mL 30 w/v % Acrylamide-Bis, 1 mL 1 M Tris-HCl pH 8.8, 0.1 mL 10 w/v % SDS, 0.1 mL 10 w/v % ammonium persulfate, 0.004 mL TEMED) and concentrating glue. (2.7 mL distilled water, 0.7 mL 30 w/v % Acrylamide-Bis, 0.5 mL 1 M Tris pH 8.8, 0.04 mL 10 w/v % SDS, 0.04 mL 10 w/v % ammonium persulfate, 0.004 mL TEMED). 4 mg/mL PAE microparticles were prepared in simulated gastric solution, simulated small intestine juice and simulated colonic juice, respectively. They were agitated in an incubator 37 °C and 140 rpm for 24 hours, and centrifugated at 12000 rpm for 15 minutes to obtain supernatant. 35 μL supernatant was mixed with 15 μL loading buffer (250 mM Tris-HCl, 10 w/v % SDS, 0.5 w/v % bromophenol blue, 50 w/v % glycerin, 5 w/v % β-mercaptoethanol) in 1.5 mL tube. Then the liquid was boiled for 5 minutes and centrifuged at 12000 rpm for 5 minutes. 30 μL protein samples and protein marker were loaded into the gel. When bromophenol blue line was closed to sealing glue, the gel was collected and stained by Coomash bright blue R-250 for 12 hours. Then the gel was de colorized by eluent, and the images of gel were recorded (Simpson, Citation2007; Brunelle & Green, Citation2014; Abedi et al., 2022).
2.10. Induction of ulcerative colitis in mice
40 mice were fed in cages for a week. Each mouse took the spinal column as the dividing line, and the skin with an area of 1 cm × 2 cm on the symmetrical part of its back was taken as the experimental area. The hair of mice was removed to expose a 1 cm × 2 cm area on its back. 2 w/v % dinitrochlorophenone solution was used to sensitize this area for 14 consecutive days. On the 15th day, the mice were fasting for 12 hours, and a silicone tube was slowly inserted into the anus for 7 ∼ 8 cm. 0.25 mL 1 w/v % dinitrochlorobenzene alcohol solution was injected into mice through the tube. The mice were gently lifted upside-down for 30 seconds to prevent the outflow of the liquid. On the 16th day, 0.7 mL 6 v/v % acetic acid solution was injected into the mice using the method described above to induce UC (Aleksandra et al., Citation2016; Wang et al., Citation2016; Rezayat et al., Citation2018).
2.11. In vivo experiment in mice
40 mice were randomly divided into five groups, model UC group, PAE treatment group, PAE-S100 treatment group, PAE enema treatment group, and control group. The control group was healthy mice. Model UC group was mice that had UC without administration. PAE-S100 treatment group was the UC mice that were treated with 0.26 g PEA microparticles which were equal to 10 mg PAE. The PAE treatment group was the UC mice that were oral administrated with 10 mg PAE. PAE enema treatment group was the UC mice that were fed with 10 mg PAE by enema. Mice in each group were given the drug every day for 14 days consecutively and were fed normally (Wang et al., Citation2016).
2.12. Disease activity index of mice
During the treatment, the disease activity index score was evaluated by changes in body weight, stool consistency, and blood in feces (Wang et al., Citation2016; Patole & Pandit, Citation2018). The specific scoring methods were shown in .
Table 1. Evaluation of disease activity index (DAI).
2.13. Histopathology of colon
After 14 days of continuous administration, all mice were killed by cervical dislocation, and the liver and colon were collected for further analysis. The colon tissue was stained by hematoxylin-eosin (HE), and its morphology was observed under the microscope Olympus BX 60 (Olympus, Japan) with eyepiece 40 and objective 10 (Wang et al., Citation2016).
2.14. Myeloperoxidase (MPO) activity
The activity of MPO in tissues was determined by MPO ELISA kits (Franck et al., Citation2009). 50 mg colon tissue was homogenized in saline. The mixture was placed in a 10 mL centrifuge tube and centrifuged at 4 °C and 10000 rpm for 10 minutes. 5 mL supernatant was collected for further analysis. 40 μL sample dilution solution and 10 μL test sample solution were added into the well on the HRP-coated plate respectively. Then 100 μL HRP-Conjugate reagent were added at the bottom of the HRP-plate, except blank well. The plate was incubated at 37 °C for 30 minutes sealed with plate cover. The solution in the well was removed and the well was washed by washing solution for 5 times. Then 50 μL solution A and solution B was added in each well, and mixture was incubated at 37 °C for 15 minutes. Finally, 50 μL termination solution was added to each well to terminate the reaction. Then, the absorbance of each well was measured at 450 nm and the concentration of MPO was calculated based on the standard calibration curve.
2.15. Statistical analysis
All experiments were carried out in triplicate. The experimental data were analyzed using SPSS version 22.0 and Origin 2021. One-way analysis of variance and Tukey's post-hoc test were used to assess the normally distributed data, and the results are reported as mean ± SD. Statistical significance was accepted at *p < 0.05.
3 Results
3.1. Results of in vitro release studies
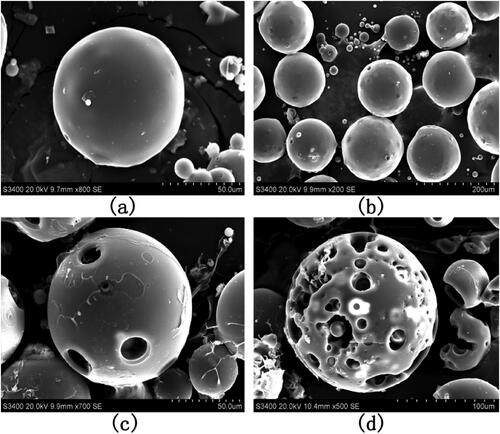
To understand the drug release profile of PAE-S100 microparticles in the digestion system, the microparticles were placed in three different solutions which mimicked environments in stomach, small intestine and large intestine. showed that protein was initially released from microparticles in all conditions and the release rate reduced over time. The microparticles stopped releasing protein after 2 hours in all conditions. Only 9% total protein was released into the bulk solution at pH 1.2, which was much lower than the released amount at pH 6.8 (about 28%) and at pH 7.8 (85%). showed the PAE-S100 microparticles before dissolving into the solutions, and these microparticles were spherical and intact. After incubating at pH 1.2 for 2 hours, the majority of PAE-S100 microparticles were still intact and in a spherical shape (see ), which was consistent with the result that only a small amount of protein was released. showed the morphology of PAE-S100 microparticles which were dissolved at pH 6.8, and only several cavities were observed on the surface of microparticles without major defect of the structure. In contrast, indicated that the structure of microparticles that were incubated at pH 7.8 started to collapse, which could cause the release of protein into bulk solution.
Figure 3. The percentage of drug released from PAE-S100 microparticles. PAE-S100 microparticles in the simulated gastric solution (pH 1.2) (■); PAE -S100 microparticles in the simulated small intestinal solution (pH 6.8) (●); PAE-S100 microparticles in the simulated colonic solution (pH 7.8) (▲).
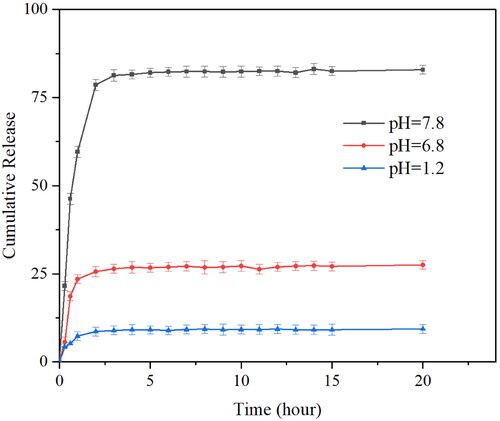
Figure 4. SEM images of PAE-S100 microparticles in different pH solutions. The morphology of (a) lyophilized microparticles; (b) the microparticles in simulated gastric solution at pH =1.2 for 18 minutes; (c) the microparticles in the simulate small intestine fluid at pH= 6.8 for 15 minutes;(d) the microparticles in simulate colonic fluid at pH= 7.8 for 2 hours.
Three drug release kinetics models were used to study the release kinetics of microparticles. The results are shown in , which indicated that the release of PAE in microparticles was followed a first-order kinetics whose R2 is 0.98, 0.97, 0.99.
Table 2. Simulation regression of drug release curve.
3.2. SDS-PAGE analysis
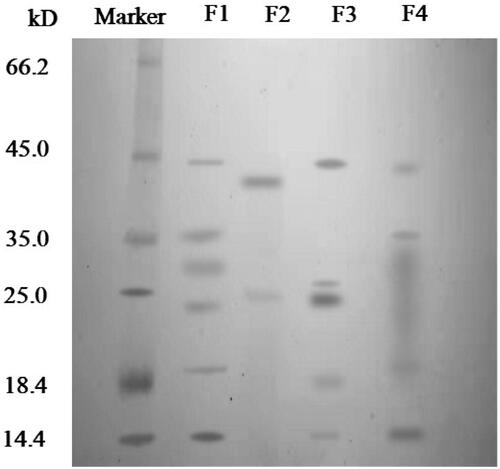
showed the major protein species in different conditions. The electrophoretic band of a protein standard sample was shown in the first column. F1 was the electrophoretic band of protein in Periplaneta americana extract dissolved in buffer, which was mainly concentrated in 14.4 kDa, 18.4 kDa, 25 kDa, 25 kDa ∼35 kDa, 35 kDa and 45 kDa. F2 was the result of protein in supernatant that was released form microparticles in simulated gastric fluid (pH 1.2) for 2 hours. Only two clear bands were observed at about 25 kDa and 35 ∼ 45 kDa. Since the microparticles were still intact (see ) and the majority of the protein was still encapsuled in the microparticle. Only some protein that was attached on the surface might release in the solution. In addition, compared to F1, a new band at 35 ∼ 45 kDa was observed, because pepsin was added into the solution to mimic the gastric fluid, whose molecular weight was 35 ∼ 45 kDa. F3 was the electrophoretic band of protein in supernatant that was released from microparticles in simulated small intestinal fluid (pH 6.8) for 2 hours. In this case, only part of microparticles have been decomposed (see ) and small amount of protein was leaked into the small intestinal fluid. So F3 band only showed 4 protein species that were similar to F1. Furthermore, we did see a new type of protein in F3, but it might be trypsin (about 25 kDa) that was used to simulate small intestine fluid. F4 was the electrophoretic band of protein in supernatant that was released from microparticles in simulated colonic fluid (pH 7.8) for 2 hours. The structure of microparticles was decomposed completely (see ), and most of components were dissolved in the solution. A wide band from 18.4 to 35 kDa was found, which indicated that some protein species were broken down by trypsin at pH 7.8.
Figure 5. SDS-PAGE gel of marker, Periplaneta americana extract (F1), protein that was released form microparticles in simulated gastric solution (pH 1.2) for 2 hours (F2), protein that was released from microparticles in simulated small intestinal fluid (pH 6.8) for 2 hours (F3), protein in supernatant that was released from microparticles in simulated colonic fluid (pH 7.8) for 2 hours (F4).
3.3. Disease activity index (DAI) of mice
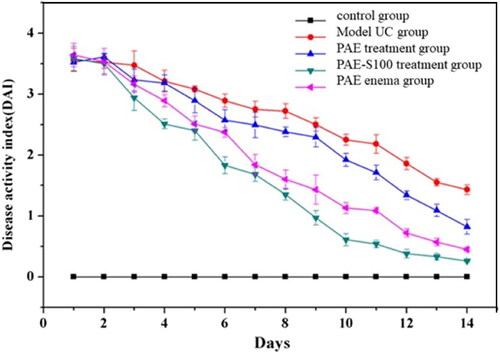
Disease activity index was visual data that could reflect the recovery of mice. All groups had high DAI scores due to UC at day 0 except the control group (see ), and DAI scores started to decline over time which indicated that mice could recover from UC. After 14 days, the model UC group had the highest DAI score and the mice still had symptoms of UC. The PAE treatment group had lower DAI score than model UC group, but the score was still higher than the other groups. The mice still suffered with UC, but they were slowly recovered over time. Because most active components in PAE was degraded during the digestion process, and only a small amount of PAE could reach colon, which significantly reduced the drug efficacy. When the PAE was delivered through enema without passing through stomach and small intestine, the mice were cure faster than oral administrated method and symptoms were gradually relieved. Even though the drug could target the colon directly using enema, the patients might suffer a lot during the operation, which limited the use of this method. The mice which were oral administrated PAE-S100 were recovered fastest among all test groups, and all symptoms were significantly reduced. This result was consistent with the observation of in vitro experiment that PAE-S100 could only release formulation at colon.
3.4. Myeloperoxidase (MPO) activity
MPO activity could be used to predict the level of inflammatory in colon. As shown in , the activity of MPO in the control group was 0.22 ± 0.06 U/g, which indicated this group of mice was healthy and had no inflammation. The model UC group had the highest MPO activity, 0.87 ± 0.153 U/g, since the colon was still injured and seriously inflamed after 14 days without any treatment. The oral administrated PAE group had similar MPO activity level to model UC group, but higher than the other two groups. Small portion of PAE might reach the colon and reduced the MPO activity, but the drug concentration was too low to treat the disease. When the PAE was delivered to colon though enema or PAE-S100 system, the MPO activity level in the colon was significant reduced. This proved that oral administrate PAE-S100 formulation could bypass the harsh digestion process and reach colon to deliver the drug.
Table 3. MPO activity in mice.
3.5. Histopathological observation of colon
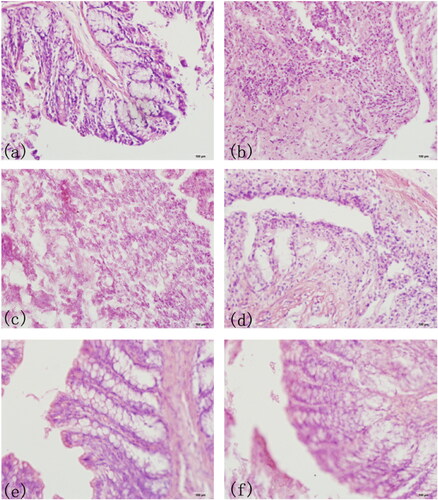
As shown in exhibited the complete villus structure and normal goblet cell morphology in normal functional colon of mice. showed colon biopsy of model UC group in the first day, and the colon lost its villus structure and epithelial cells in the tissue. showed the colon lesions of model UC group in the 14th day, and there were still many inflammatory cells, which reflected the colon injury had not been recovered. showed the PAE treatment group, PAE enema group and PAE-S100 treatment group in the 14th day, the villi had regenerated and the number of inflammatory cells had decreased. However, showed that the colon did recover from the UC when the drug was directly delivered to colon.
Figure 7. HE Staining of Mice Colon Sections. The complete villus structure and normal goblet cell morphology in normal colon of mice(a); colon biopsy of model UC group in the first day; the colon lesions of model UC group in the 14th day (c); colon of PAE treatment group in the 14th day (d); colon of PAE enema group in the 14th day; colon of PAE-S100 treatment group in the 14th day (f).
4. Discussion
Wound healing is a complex biological process involving hemostasis, inflammatory control, proliferation, and tissue remodeling (Kasuya & Tokura, Citation2014). When tissue damage occurs, the coagulation pathway is triggered, leading to the formation of a temporary fibrin matrix that allows cells to migrate to the site of injury. At the same time, platelet-derived factors attract white blood cells, activating inflammatory responses. Platelets and immune cells then secrete growth factors and cytokines that promote wound reepithelialization, extracellular matrix deposition, and angiogenesis. Active components in PAE promotes the proliferation and migration of human keratin cells, HaCaT cells (Song et al., Citation2017), and the expression of epidermal growth factor and vascular endothelial growth factor (Lekic & McCulloch, Citation1996; Velazquez, Citation2007; Li et al., Citation2019), which accelerate the rate of healing.
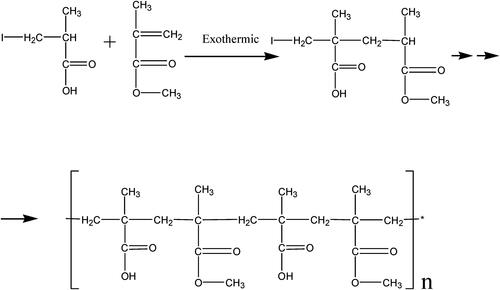
The oral administrated PAE has to pass through the gastrointestinal tract, which is composed of hydrochloric acid, protein-digesting enzymes and mucus, to access the inflammatory site in colon (Brown et al., Citation2020). However, under such highly harsh conditions, protein, one of the active components in PAE, is easily broken down into small polypeptide pieces and amino acids and lose its function (Hamman et al., Citation2005; Brown et al., Citation2020). Therefore, only a small fraction of the components can reach the colon which limited the efficacy of PAE to treat UC. Eudragit S100 (see ) is an anionic polymer formed by copolymerization of methacrylic acid with methyl methacrylate (1:2). When the microparticle which contains both Eudragit S100 and PAE arrive in the stomach, the Eudragit S100 can prevent acid and enzyme from penetrating into the core and degrading the PAE. Reaching in the colon, where the pH ranges from 5 to 8, carboxyl groups of Eudragit S100 react with alkali or amines and form water-soluble salts (Thakral et al., Citation2013). Eudragit S100 start to decompose gradually due to the pH, and PAE was released in colon. Therefore, Eudragit S100 can be used as a drug carrier to create a pH-dependent colon-targeted oral drug delivery system (Khan et al., Citation2000; Subudhi et al., Citation2015), which can prolong the residence time of the drug in the gastrointestinal tract and improve oral PAE absorption (Al-Kassas et al., Citation2021).
Myeloperoxidase (MPO), one of the most important members of the peroxidase family, mainly exists in neutrophils and mononuclear macrophages (Podrez et al., Citation2002). When inflammation occurs in the body, MPO in neutrophils is released into the circulatory system and participates in the inflammatory response of the body. Therefore, the level of MPO can be used to evaluate the degree of intestinal injury (Ogawa et al., Citation2002; Kiyosue et al., Citation2006; Li et al., Citation2008; Kim et al., Citation2016; de Oliveira et al., Citation2021). The high concentration of MPO reflects that the tissue is inflamed. When PAE-S100 microparticles arrived at colon, Eudragit S100 started to decomposed and PAE were released in the environment. Studies had shown that PAE had antimicrobial activity against bacteria (Kim et al., Citation2016) by inducing oxidative stress to activate apoptotic signals in mitochondria and promote antifungal activity (Yun et al., Citation2017). The PAE could inhibit TGF-β1, NF-κB,α-SM, and TIMP-1, thereby blocking relevant signaling pathways and preventing inflammatory responses to attenuate (Dingchun et al., Citation2018). PAE could also decrease the expression of IL-1β, IL-6, IFN-γ, and TNF-α which inhibited the PI3K/AKT/NF-κB signaling pathway, decreasing the expression of inflammatory cytokines (Ni et al., Citation2022). However, it is hard to determine which active component in PAE play a dominant role in curing UC due to abundant compositions in the PAE. In the future study, all the active components in PAE will be separated and collected to test their effectiveness in treatment of UC and investigate the potential of practical use in clinic.
5. Conclusions
In this study, we used Eudragit S100 as a drug carrier to deliver PAE in colon. PAE-S100 microparticles could research the colon without degradation in the digestion process and release PAE in colon to treat UC. Both DAI score and MPO were significantly reduced using the PAE-S100 drug delivery system. In addition, this system could be potentially used for other oral protein formulation administration.
Author contributions
All authors contributed to this paper, with Meng Li taking the supervision and writing the original draft; Hao Wu reviewing and checking the content and editing the final version of the manuscript; Jing Han, Yang Han designing the framework and research, conceptualization;Hao Wu, Meng Li, Shuang Wang, Shengshun Wu conducting the experimental operation; All authors contributed extensively to this work. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download JPEG Image (207.5 KB)Supplemental Material
Download MS Word (11.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abedi G, Talebpour Z, Aliahmadi A, et al. (2022). Identification of industrial detergent enzymes by SDS-PAGE and MALDI-TOF mass spectrometry. New J Chem 46:3939–47. https://doi.org/10.1039/D1NJ05227F
- Abuhelwa AY, Williams DB, Upton RN, et al. (2017). Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm 112:234–48. https://doi.org/10.1016/j.ejpb.2016.11.034
- Aleksandra M, Piotr C, Zygmunt W, et al. (2016). Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int J Mol Sci 17:1455. https://doi.org/10.3390/ijms17091455
- Al-Kassas R, Madni A, Buchanan C, et al. (2021). pH-sensitive nanoparticles developed and optimized using factorial design for oral delivery of gliclazide. J Pharm Innov. https://doi.org/10.1007/s12247-021-09536-7
- Brown TD, Whitehead KA, Mitragotri S. (2020). Materials for oral delivery of proteins and peptides. Nat Rev Mater 5:127–48. https://doi.org/10.1038/s41578-019-0156-6
- Brunelle JL, Green R. (2014). One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE)[J]. Methods Enzymol 541:151–9. https://doi.org/10.1016/B978-0-12-420119-4.00012-4
- Cao SJ, Xu S, Wang HM, et al. (2019). Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech 20:190. https://doi.org/10.1208/s12249-019-1325-z
- Carbonnel F, Jantchou P, Monnet E, et al. (2009). Environmental risk factors in Crohn’s disease and ulcerative colitis: an update. Gastroenterol Clin Biol 33:S145–S157. https://doi.org/10.1016/S0399-8320(09)73150-1
- Chen Z, Hu Y, Li J, et al. (2019). A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int J Pharm 571:118707. https://doi.org/10.1016/j.ijpharm.2019.118707
- Cortot A, Chambrun G, Vernier-Massouille G, et al. (2009). Inflammatory bowel disease: genetic or environmental diseases? Gastroenterol Clin Biol 33:681–91. https://doi.org/10.1016/j.gcb.2009.07.005
- de Oliveira RG, Damazo AS, Antonielli LF, et al. (2021). Dilodendron bipinnatum Radlk. extract alleviates ulcerative colitis induced by TNBS in rats by reducing inflammatory cell infiltration, TNF-α and IL-1β concentrations, IL-17 and COX-2 expressions, supporting mucus production and promotes an antioxidant effect. J Ethnopharmacol 269:113735. https://doi.org/10.1016/j.jep.2020.113735
- Den E,N, et al. (2019). Colonic mucosal transcriptomic changes in patients with long-duration ulcerative colitis revealed colitis-associated cancer pathways. J Crohn’s Colitis 13:755–63. https://doi.org/10.1093/ecco-jcc/jjz002
- Dingchun L, Wu, et al. (2018). Anti-fibrotic role and mechanism of Periplaneta americana extracts in CCl4-induced hepatic fibrosis in rats. Acta Biochim Biophys Sin 50:491–8. https://doi.org/10.1093/abbs/gmy024
- Dragana M, Irena VP, Vera M, et al. (2018). MDR1 gene polymorphisms are associated with ulcerative colitis in a cohort of Serbian patients with inflammatory bowel disease. Plos One 13:e0194536. https://doi.org/10.1371/journal.pone.0194536
- Franck T, Kohnen S, Boudjeltia KZ, et al. (2009). A new easy method for specific measurement of active myeloperoxidase in human biological fluids and tissue extracts. Talanta 80:723–9. https://doi.org/10.1016/j.talanta.2009.07.052
- Fu S, Chen J, Zhang C, et al. (2021). Gastroprotective effects of Periplaneta americana L. extract against ethanol-induced gastric ulcer in mice by suppressing apoptosis-related pathways. Front Pharmacol 12:798421. https://doi.org/10.3389/fphar.2021.798421
- Hamman JH, Enslin GM, Kotz AF. (2005). Oral delivery of peptide drugs. BioDrugs 19:165–77. https://doi.org/10.2165/00063030-200519030-00003
- Hanauer SB. (2008). Review article: evolving concepts in treatment and disease modification in ulcerative colitis. Aliment Pharmacol Ther 27:15–21. https://doi.org/10.1111/j.1365-2036.2008.03606.x
- Hua-ShengYong-Ming Z, Dai-Wei YW, et al. (2021). Small molecule constituents of Periplaneta americana and Their IL-6 inhibitory activities. Nat Prod Commun 16:1–8. https://doi.org/10.1177/1934578X211033180
- IvanKristýna K, Monika MV, et al. (2021). Intestinal microbiota and perspectives of the use of meta-analysis for comparison of ulcerative colitis studies J Clin Med 10:462. https://doi.org/10.3390/jcm10030462
- Jing J, Sun X, Zhou C, et al. (2019). Cloning, expression and effects of P. Americana thymosin on wound healing. Int J Mol Sci 20:4932. https://doi.org/10.3390/ijms20194932
- Jonsson B, Hsgren L, Andersson LO, et al. (2010). Colorectal cancer surveillance in patients with ulcerative colitis. Br J Surg 81:689–91. https://doi.org/10.1002/bjs.1800810520
- Kasuya A, Tokura Y. (2014). Attempts to accelerate wound healing. J Dermatol Sci 76:169–72. https://doi.org/10.1016/j.jdermsci.2014.11.001
- Khan MZI, Štedul HP, Kurjaković N. (2000). A pH-dependent colon-targeted oral drug delivery system using methacrylic acid copolymers. II. Manipulation of drug release using Eudragit L100 and Eudragit S100 combinations. Drug Dev Ind Pharm 26:549–54. https://doi.org/10.1081/DDC-100101266
- Kim IW, Lee JH, Subramaniyam S, et al. (2016). De Novo transcriptome analysis and detection of antimicrobial peptides of the American cockroach Periplaneta americana (Linnaeus). Plos One 11:e0155304. https://doi.org/10.1371/journal.pone.0155304
- Kiyosue M, Fujisawa M, Kinoshita K, et al. (2006). Different susceptibilities of spontaneous rhythmicity and myogenic contractility to intestinal muscularis inflammation in the hapten-induced colitis. Neurogastroenterol Motil 18:1019–30. https://doi.org/10.1111/j.1365-2982.2006.00841.x
- Kondamudi PK, Malayandi R, Eaga C, et al. (2013). Drugs as causative agents and therapeutic agents in inflammatory bowel disease. Acta Pharm Sin B 3:289–96. https://doi.org/10.1016/j.apsb.2013.06.004
- Kushkevych I, Vítězová M, Fedrová P, et al. (2017). Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet Brno 86:405–11. https://doi.org/10.2754/avb201786040405
- Lee S, Duce I, Atkins H, et al. (2011). Cockroaches and locusts: physicians’ answer to infectious diseases. Int J Antimicrob Agents 37:279–80. https://doi.org/10.1016/j.ijantimicag.2010.12.005
- Lekic P, McCulloch CAG. (1996). Periodontal ligament cell populations: the central role of fibroblasts in creating a unique tissue. Anat Rec 245:327–41. https://doi.org/10.1002/(sici)1097-0185(199606)245:2<327::aid-ar15>3.3.co;2-6
- Li H, Huo JJ, Zhang H, et al. (2021). Eudragit S100-coated halloysite nanotube/chitosan microspheres for colon-targeted release of paeoniflorin. J Drug Delivery Sci Technol 61:102258. https://doi.org/10.1016/j.jddst.2020.102258
- Li LJ, Wang MZ, Yuan TJ, et al. (2019). The crude ethanol extract of Periplaneta americana L. stimulates wound healing in vitro & in vivo. Chin Med 14:33. https://doi.org/10.1186/s13020-019-0259-4
- Li L-J, Xu X-H, Yuan T-J, et al. (2019). Periplaneta americana L. as a novel therapeutics accelerates wound repair and regeneration. Biomed Pharmacother 114:108858. https://doi.org/10.1016/j.biopha.2019.108858
- Li X, Lu C, Yang Y, et al. (2020). Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed Pharmacother 129:110486. https://doi.org/10.1016/j.biopha.2020.110486
- Li XL, Cai YQ, Qin H, et al. (2008). Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol 86:841–9. https://doi.org/10.1139/Y08-089
- Lu S, Wu D, Sun G, et al. (2019). Gastroprotective effects of Kangfuxin against water-immersion and restraint stress-induced gastric ulcer in rats: roles of antioxidation, anti-inflammation, and pro-survival. Pharm Biol 57:770–7. https://doi.org/10.1080/13880209.2019.1682620
- Ma X, Hu Y, Li X, et al. (2018). Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front Pharmacol 9:944. https://doi.org/10.3389/fphar.2018.00944
- Malipeddi VR, Dua K, Awasthi R, et al. (2016). Development and characterization of solid dispersion-microsphere controlled release system for poorly water-soluble drug. Drug Deliv Transl Res 6:540–50. https://doi.org/10.1007/s13346-016-0307-x
- Miehlke S, Madisch A, Kupcinskas L, et al. (2014). Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology 146:1222–30. https://doi.org/10.1053/j.gastro.2014.01.019
- Nanki K, Fujii M, Shimokawa M, et al. (2020). Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 577:254–6. https://doi.org/10.1038/s41586-019-1844-5
- Ni L, Lu Q, Tang M, et al. (2022). Periplaneta americana extract ameliorates dextran sulfate sodium-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacol 30:907–18. https://doi.org/10.1007/s10787-022-00955-7
- Ogawa Y, Kanatsu K, Iino T, et al. (2002). Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci 71:827–39. https://doi.org/10.1016/S0024-3205(02)01737-X
- Patil SA, Moss AC. (2008). Balsalazide disodium for the treatment of ulcerative colitis. Expert Rev Gastroenterol Hepatol 2:177–84. https://doi.org/10.1586/17474124.2.2.177
- Patole VC, Pandit AP. (2018). Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: in vitro and in vivo characterization. J Pharm Investig 48:257–67. https://doi.org/10.1007/s40005-017-0304-1
- Podrez EA, Poliakov E, Shen Z, et al. (2002). A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem 277:38517–23. https://doi.org/10.1074/jbc.M205924200
- Rezayat SM, Dehpour AR, Motamed SM, et al. (2018). Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-kB pathway. Inflammopharmacology 26:851–9. https://doi.org/10.1007/s10787-017-0409-1
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. (2019). ACG clinical guideline: ulcerative colitis in adults. Off J Am Coll Gastroenterol 114:384–413. https://doi.org/10.14309/ajg.0000000000000152
- Sarvestani SK, Signs S, Hu B, et al. (2021). Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity. Nat Commun 12:262. https://doi.org/10.1038/s41467-020-20351-5
- Simpson RJ. (2007). SDS-PAGE peptide-mapping procedure (Cleveland method). CSH Protocols 7:4591. https://doi.org/10.1101/pdb.prot4591
- So H, Nam HY, Nam J, et al. (2007). Curcumin-loaded PLGA nanoparticles coating onto metal stent by electrophoretic deposition techniques. Bull Korean Chem Soc 28:397–402. https://doi.org/10.5012/bkcs.2007.28.3.397
- Soeters R, Aus C. (1989). Hazards of injectable therapy. Trop Doctor 19:124–6. https://doi.org/10.1177/004947558901900310
- Song Q, Xie Y, Gou Q, et al. (2017). JAK/STAT3 and Smad3 activities are required for the wound healing properties of Periplaneta americana extracts. Int J Mol Med 40:465–73. https://doi.org/10.3892/ijmm.2017.3040
- Subudhi MB, Jain A, Jain A, et al. (2015). Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 8:832–49. https://doi.org/10.3390/ma8030832
- Thakral NK, Ray AR, Majumdar DK. (2010). Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci Mater Med 21:2691–9. https://doi.org/10.1007/s10856-010-4109-2
- Thakral S, Thakral NK, Majumdar DK. (2013). Eudragit®: a technology evaluation. Expert Opin Drug Deliv 10:131–49. https://doi.org/10.1517/17425247.2013.736962
- Tiago N, Manuel BDA, Ignácio MJ, et al. (2012). Oral locally active steroids in inflammatory bowel disease. J Crohn s Colitis 7:183–91. https://doi.org/10.1016/j.crohns.2012.06.010
- Ungaro R, Colombel JF, Lissoos T, et al. (2019). A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol 114:874–83. https://doi.org/10.14309/ajg.0000000000000183
- Velazquez OC. (2007). Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vascul Surg 45:A39–A47. https://doi.org/10.1016/j.jvs.2007.02.068
- Wang QS, Wang GF, Zhou J, et al. (2016). Colon targeted oral drug delivery system based on chitosan/alginate microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm 515:176–85. https://doi.org/10.1016/j.ijpharm.2016.10.002
- Xue N-n, He M, Li Y, et al. (2020). Periplaneta americana extract promotes intestinal mucosa repair of ulcerative colitis in rat1. Acta Cirurgica Bras 35:202001002. https://doi.org/10.1590/s0102-865020200100000002
- Yamasaki Y, Kato Y. (1989). Separation of intravenous IgG containing albumin by high-performance size-exclusion chromatography on TSKgel G3000SWXL. J Chromatogr 467:436–40. https://doi.org/10.1016/S0021-9673(01)93998-6
- Yoko Y, Katsuyoshi M, Taku K, et al. (2014). A large-scale, prospective, observational study of leukocytapheresis for ulcerative colitis: treatment outcomes of 847 patients in clinical practice. J Crohns Colitis 8:981–91. https://doi.org/10.1016/j.crohns.2014.01.027
- Yuan Y, Xu X, Gong J, et al. (2019). Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int J Biol Macromol 131:209–17. https://doi.org/10.1016/j.ijbiomac.2019.03.061
- Yun JE, Hwang JS, Lee DG. (2017). The antifungal activity of the peptide, periplanetasin-2, derived from American cockroach Periplaneta americana. Biochem J 474:3027–43. https://doi.org/10.1042/BCJ20170461