Abstract
Lung cancer is the second most common type of cancer after breast cancer. It ranks first in terms of mortality rate among all types of cancer. Lung cancer therapies are still being developed, one of which makes use of nanoparticle technology. However, conjugation with specific ligands capable of delivering drugs more precisely to cancer sites is still required to enhance nanoparticle targeting performance. Monoclonal antibodies are one type of mediator that can actively target nanoparticles. Due to the large number of antigens on the surface of cancer cells, monoclonal antibodies are widely used to deliver nanoparticles and improve drug targeting to cancer cells. Unfortunately, these antibodies have some drawbacks, such as rapid elimination, which results in a short half-life and ineffective dose. As a result, many of them are formulated in nanoparticles to minimize their major drawbacks and enhance drug targeting. This review summarizes and discusses articles on developing and applying various types of monoclonal antibody ligand nanoparticles as lung cancer target drugs. This review will serve as a guide for the choice of nanoparticle systems containing monoclonal antibody ligands for drug delivery in lung cancer therapy.
1. Introduction
The lungs are an important organ in the human body, particularly in the respiratory system. Damage to this organ can endanger lives and perhaps result in death. Lung cancer is a form of cancer that affects the human lungs (Bade & Dela Cruz, Citation2020). This malignancy is the second most common after breast cancer and has the greatest fatality rate of any type of cancer (International Agency for Research on Cancer (IARC), Citation2020). It is reported that this cancer has a mortality rate of 1,796,144 or 18% of the total number of cancer deaths and an incidence rate of 2,206,771 which is 11.4% of all cancer incidences worldwide both in women and men (Globocan, Citation2020). There are currently three options for cancer treatment: surgery, radiation therapy, and chemotherapy (Abbas & Rehman, Citation2018). Stage I or II Non-Small Cell Lung Cancer ‘NSCLC’ treatment is surgical resection of the tumor followed by adjuvant therapy. When the cancer progresses to stage III or IV, the treatments are chemotherapeutic and/or radiation therapy. Since the cancer invaded surrounding tissues, metastases can occur through the circulatory system or lymphatic system (Huang et al., Citation2015). Chemotherapy is a form of cancer treatment that employs medications. As a result of the drug’s inability to target specific cells, this therapy is often associated with severe adverse effects (Ohnoshi et al., Citation1992; Partridge et al., Citation2001; Sun et al., Citation2005; Aslam et al., Citation2014). It has inspired the development of cancer medicines, one of which is the use of nanoparticles.
The particle size of nanoparticles in drug delivery systems ranges from 1 to 1000 nanometers (1–1000 nm) (Jeevanandam et al., Citation2018; Naito et al., Citation2018; Khan et al., Citation2019). Nanoparticles are often utilized in the healthcare domain for diagnostic and therapeutic applications (Jiang et al., Citation2008; Sukhanova et al., Citation2018). This approach has a number of benefits in cancer therapy, including improving drug bioavailability through increased dissolution rate (Li et al., Citation2015; Jafari & McClements, Citation2017), minimizing pharmacological adverse effects via modest dosages, and maintaining constant drug levels in plasma (Park et al., Citation2009). As a diagnostic agent, nanoparticles possess qualities such as size, optical properties, photodynamic magnetic properties, and others that can aid in the diagnosis of cancer (Al-Jamal & Kostarelos, Citation2007; Fang & Zhang, Citation2010; Wu et al., Citation2019).
Nanoparticles as a passive delivery route for cancer therapy, particularly lung cancer, have been widely studied (Ye et al., Citation2008; Bazak et al., Citation2014; Clemons et al., Citation2018). Alternative nanoparticle delivery strategies for lung cancer have also been developed. Based on the interaction of certain ligands and receptors that are abundantly expressed in cancer cells, this targeting mechanism is able to specifically identify cancer cells (You et al., Citation2008; Bazak et al., Citation2015; Costa et al., Citation2019). Up to this point, there have been no therapies that transport drug directly to the tumor site (Stella et al., Citation2000; Steinhauser et al., Citation2006; Xu et al., Citation2019). In this active nanoparticle delivery technique, monoclonal antibodies are one of the ligands used for this purpose.
Monoclonal antibodies are currently being used extensively to deliver nanoparticles to multiple antigens on the surface of cancer cells. This antigen is what distinguishes cancerous cells from healthy ones, because their expression is higher in cancer cells, drug-targeting therapies can take advantage of this. Due to enhanced drug targeting, the systemic toxicity of the drug treatment is reduced by conjugating monoclonal antibodies to the nanoparticles (Trail & Bianchi, Citation1999; Adams & Weiner, Citation2005). Generally, different receptor expressions were found in cancer cells. Monoclonal antibodies are aimed solely to target receptors such as the Epidermal Growth Factor Receptor, EpCAM receptor, NSE receptor, and other receptors that are abundantly expressed in lung cancer (Tseng et al., Citation2007; Wang & Zhou, Citation2015; Chen et al., Citation2021). Unfortunately, it has a major drawback, including fast clearance from circulation, which results in a short half-life and renders the dosage ineffective. As a result, monoclonal antibodies are still synthesized in nanoparticles to improve their targeting ability and reduce their disadvantages (Silvestre et al., Citation2020).
Monoclonal antibodies have been conjugated in several nanoparticle systems, including lipid-based nanoparticles, gold nanoparticles, super magnetic iron oxide nanoparticles, and other materials for active lung cancer targeting. The objective of this linkage is to explore remedies for this type of cancer. Therefore, this review summarizes and discusses the deployment of several kinds of nanoparticles in combination with ligand monoclonal antibodies to target lung cancer cells.
2. Methodology
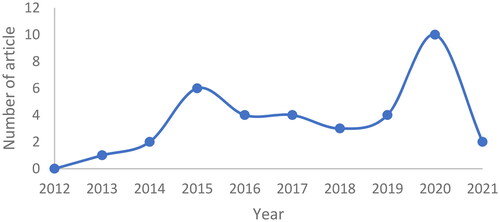
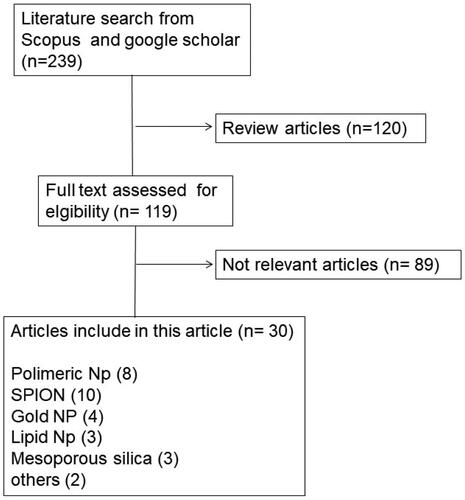
The publications were identified using the keywords ‘monoclonal antibody nanoparticle for lung cancer’, ‘anti-EpCAM nanoparticle for lung cancer’, ‘anti-EGFR nanoparticle for lung cancer’, in Scopus and Google Scholar databases. The selection of these journals was subjected to inclusion and exclusion criteria. Articles published in the recent 10 years (2012–2021) were included in the study, while review articles were excluded. There were 239 articles in our initial search. Some, such as the formation of nanoparticles with monoclonal antibody ligands for lung cancer treatment, were removed since they might not meet the criteria. As a result, the total number of articles utilized in this evaluation is 36, with the most articles being published in 2020. (). The process is depicted in () as a flowchart.
3. Targeted drug delivery
3.1. Passive targeting drug delivery strategy
As a strategy, passive targeting relies on the tumor microenvironment for improved permeability and retention effects. Through the retention effects of nanoparticles in the circulation, it is possible to be localized within cancer tissue, thus facilitating the accumulation of drugs into cancer tissue, avoiding systemic metabolism, which is widely utilized in cancer therapy (Won et al., Citation2012; Wakaskar, Citation2017).
The passive targeting of nanocarriers is based on their biodistribution in the body, where these nanoparticles can be rapidly cleared from the body due to being rapidly opsonized and engulfed by macrophages. However, when the rapid cleaning of the nanoparticles is minimized, there is a significant increase in their bioavailability. Accumulation of nanoparticles in solid tumors through the phenomenon called increased permeability and retention effect (Szczepanowicz et al., Citation2016).
Enhanced permeability and retention (EPR) occurs because a limited amount of fluid is supplied to the lymphatic circulation, blood capillaries in injured tissues become more permeable [39–40]. Nanoparticles can deliver the medicine to the tumor site in a concentrated form since they are tiny and small enough to travel (400 nm or smaller). A variety of angiogenesis-regulating substances, such as vascular endothelial growth factor (VEGF), which are widely expressed in tumors, have the capacity to physiologically alter tumor vessel shape as well as increase vascular permeability (Kreuter, Citation2007; Alavi & Hamidi, Citation2019).
3.2. Active targeting drug delivery strategy
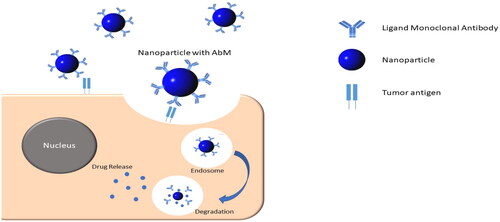
The drug delivery system utilizes two distinct mechanisms: passive targeting based on increased permeability and retention, and active targeting based on the ligand binding to its receptor (Salahpour Anarjan, Citation2019). The active targeting system is one of the drug targeting strategies using the help of a ligand in which this ligand will bind to its specific receptor (Byrne et al., Citation2008). The ligand will be conjugated on the surface of the nanoparticles resulting in increased cellular uptake by receptor-mediated endocytosis, hence increased drug accumulation in cancer cells (Li et al. Citation2013). This mechanism relies on the interaction between conjugated ligands on the surface of nanoparticles and cell surface receptors or antigens on the surface of cancer cells (Muhamad et al., Citation2018; Yao et al., Citation2020). The inclusion of ligands to this targeting mechanism allows nanoparticles to be delivered to precisely identified cells or even subcellular locations, and therefore decreasing cytotoxic drug systemic exposure (Maeda & Matsumura, Citation1989; Yu et al., Citation2010).
There are several biological ligands that have been found which are known to improve active nanoparticle targeting systems. When a ligand binds to a receptor on the cell surface, the amount of drug accumulated increases, as does the effectiveness of the treatment. Ligands have been identified to be proteins, polysaccharides, nucleic acids, peptides, and small compounds. Nanoparticles and ligands will interact in two distinct ways, namely at the stage after nanoparticle fabrication, chemical conjugation, or physical entrapment of nanoparticles, and the second at a stage prior to nanoparticle production, through binding to nanoparticle components such as polymers (Yoo et al., Citation2019).
The most important challenge in active targeting systems is determining the best agent(s) to be used to deliver the nanoparticle system to cancer tissue while avoiding toxicity. Furthermore, the targeted agent or ligand must have high affinity for the cancer cell surface to trigger endocytosis. This interaction will transport the therapeutic agent to the cancerous site (Wakaskar, Citation2017).
This targeted delivery technique is categorized into three parts: organ-targeted, cell-targeted, and subcellular-targeted. The organ-targeted delivery method is designed to distribute the drug into an organ by taking use of the organ’s unique characteristics. For example, the liver possesses tissue properties that allow macromolecules and microparticles that easily infiltrate it, ensuring that the drug does not damage adjacent tissues due to massive connections. The cell-targeted delivery system includes a recognizable substance that binds to corresponding antigens and receptors on the cell surface. The subcellular delivery system delivers drugs to specific locations within the cells, such as the nuclei of cells () (Winarti, Citation2013).
4. Receptor of monoclonal antibody on lung cancer
Cancer cells are cells with a high rate of proliferation. This property causes the expression of several receptors that activate proliferative activity in these cells much more than in normal cells. Researchers have exploited this receptor overexpression in developing active targeted drug delivery systems.
Several receptors, including the Epidermal Growth Factor (EGFR) receptor, are overexpressed in lung cancer, including HER-1, HER-2/neu, HER-3, and HER-4. Furthermore, cell membrane receptors with intrinsic tyrosine kinase activity can change proliferative signals in response to various binding ligands. EGFR overexpression is associated with increased tumor proliferation, poor differentiation, a high probability of metastasis, and a poor prognosis in these cancers (Seyhan et al., Citation2010). The expression of this receptor in ‘NSCLC’ is estimated at 32% (Rusch et al., Citation1997).
Another widely expressed receptor on lung cancer cells is the Epithelial Cell Adhesion Molecules (EpCAM) receptor, which is a receptor responsible for mediating the adhesion of epithelial-specific, Ca2+-independent homotypic cells and represents the first tumor-associated antigen discovered in humans (Pak et al., Citation2012). The expression of these receptors is associated with an increase in tumor progression. Recently, the EpCAM-specific therapeutic drug was licensed for clinical usage in cancer patients (Spizzo et al., Citation2011). Expression of this receptor in NSCLC was reported as 51.3% in adenocarcinoma tissue (Kim et al., Citation2009).
The NSE receptor or Neuron Specific Enolase is another receptor that is also overexpressed in lung cancer (Tiseo et al., Citation2008). Previously, this receptor was considered as a biomarker for small cell lung cancer ‘SCL’. The expression of this NSE receptor is associated with the process of cancer metastasis, with the outcomes of functional study revealing that overexpression of this NSE receptor increases the migration and invasion of SCL cells (Zha et al., Citation2021). Other receptors are also found in lung cancer such as disialoganglioside receptors of GD2, GD3, and so on (Tivnan et al., Citation2012).
5. Nanoparticle with monoclonal antibody for lung cancer
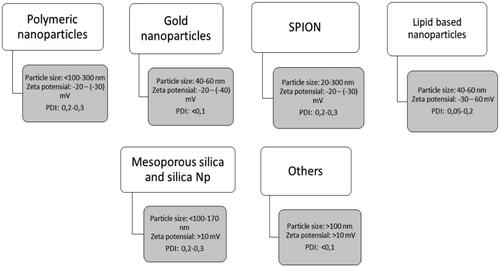
Polymeric nanoparticle systems, lipid-based nanoparticles, dendrimers, etc have been utilized to prepare nanoparticles containing ligand monoclonal antibodies for lung cancer therapy. The utilization of these various types of nanoparticles for lung cancer is presented in .
Table 1. Applying Nanoparticles with Ligand Monoclonal Antibodies for Lung Cancer.
5.1. Polymeric nanoparticle
Polymeric nanoparticles are nanoparticle systems in which polymers are used to entrap drugs (Jeevanandam et al., Citation2018). These polymeric nanoparticles have been widely developed for application of passive and active targeted drug delivery systems, particularly for cancer treatment fields (Deng et al., Citation2014; Li et al., Citation2018; Zhang et al., Citation2018; Lin et al., Citation2019; Malhotra et al., Citation2021). There have also been several developments in polymeric nanoparticles for active targeted delivery systems for the treatment of lung cancer (), one of which makes use of ligands monoclonal antibodies. The extracellular domain of epithelial cell adhesion molecules was targeted using an EpCAM in the study by Alibolandi et al. (Citation2015) using PLGA-b-PEG nanopolymersomes and an RNA aptamer. The SK-MES-1 and A549 cell lines were used to test these nanoparticles. Results demonstrated an increase in the amount of drug absorbed by cells and a rise in the toxicity that drug produced. In-vivo results revealed that tumor development was inhibited and decreased tumor volume, size, and density. It demonstrates that EpCAM antibodies can increase the targeting of nanoparticles to lung cancer cells (Alibolandi et al., Citation2015).
Demethoxycurcumin was entrapped in chitosan nanoparticles coated with anti-EGFR ligands. These nanoparticles were evaluated in vitro on the A549 cell line and in vivo on rats. The nanoparticles had a particle size of 200 nm as well as a controlled release system. These nanoparticles were effective at delivering drugs to EGFR receptors, resulting in an eight - fold reduction in tumor mass in experimental animals compared to the control group (Huang et al., Citation2015). PLGA nanoparticles were also successfully formulated to entrap docetaxel with the cetuximab ligand to actively deliver the drug to the EGFR receptor. These nanoparticles were formulated by solvent evaporation method and resulted in nanoparticles with a particle size of 128.4 nm and zeta potential value of −31 mV. In vitro results showed that these nanoparticles were able to release 25% of the drug at a pH of 5.5 after 48 hours. In addition, these nanoparticles were able to decrease the viability of A549 cell line and induce apoptosis. As for the in vivo result on mice with tumor volume 150 mm3, these nanoparticles showed a significant reduction in tumor growth, and the tumor volume decreases about 81% (Patel et al., Citation2018).
Doxorubicin is encapsulated in polymeric nanoparticles comprised of amphipathic cationic starch and hyaluronic acid. The inclusion of erlotinib, apatinib, and icotinib, which are anti-EGFR ligands, were conducted to be evaluated on A549, NCI-H1975, and PC9 cells line. Rats were used as experimental animals for in vivo investigations. When compared to other ligands, the data showed that icotinib was the most effective. Icotinib nanoparticles had a particle size of 65.7 nm, which increased cytotoxic activity and prevented cancer cell types from migrating. The in vivo test findings revealed an increase in the concentration of nanoparticles at the target region and enhanced drug selectivity compared to the normal cells (Li et al., Citation2020). Anti-EGFR was also used to deliver paclitaxel palmitate nanoparticles. Cetuximab administration in this system increased A549-luc-C8 absorption, internalization, and therapeutic efficacy in vitro and in vivo in metastasis lung tumor (Karra et al., Citation2013).
Wang, Liu, et al. (Citation2015) conducted another study in which they used anti-EGFR to deliver gemcitabine nanoparticles to patients with NSCLC. In vitro test results performed on A549 cells showed an increase in cellular uptake, observed by measuring the fluorescence intensity of cells treated with anti-EGFR nanoparticles compared to cells treated without-anti-EGFR nanoparticles (Wang & Zhou, Citation2015). LFC131 is likewise a monoclonal antibody against the CXCR4 receptor, which is highly expressed in lung cancer. LFC131 can enhance the accumulation of doxorubicin nanoparticles made of PLGA polymer and can efficiently deliver drugs to A549 cells (Chittasupho et al., Citation2014). In another study, Tn antigen was also used in nanoparticle formulation with chitosan polymer to deliver doxorubicin. The results revealed that cellular absorption increased while cell viability decreased. Tn antigen is widely used as antibodies specific and a lung cancer-specific antigen (Castro et al., Citation2021).
5.2. Gold nanoparticle
Gold nanoparticles (AuNP) are an example of nanocarriers exhibiting favorable size, shape, stability, and biocompatibility. Gold nanoparticles’ adjustable surface and distance-dependent optical properties reveal their enormous potential to be used in a variety of scientific domains (Wang et al., Citation2019). Due to their surface charge or potential zeta value, gold nanoparticles have been used as drug carriers, supported by their physicochemical properties, stability, and incorporation into cellular processes, as well as their further accumulation. The level of toxicity assigned to AuNPs is highly dependent on the surface charge of the particles, with positively charged gold nanoparticles causing cell death at much lower concentrations, while neutrally charged particles cause cell death at much higher concentrations (Corsi et al., Citation2020).
Gold nanoparticles have been widely developed for drug delivery in lung cancer. Conjugation of gold nanoparticles with ligand monoclonal antibodies for lung cancer has been widely carried out to obtain a precisely targeted delivery system. Asthon et al. (Citation2018) used two distinct ligands to create gold nanoparticles that target EGFR: the cetuximab ligand; and a single-domain llama-derived anti-EGFR antibody with a lower binding affinity than cetuximab. Cetuximab-containing nanoparticles exhibited the lowest residence time in vitro, and all nanoparticles accumulated at the tumor site more than control nanoparticles (without ligand). Cetuximab nanoparticles accumulated at a significantly greater rate than any other (Ashton et al., Citation2018).
The findings of the in vitro experiments revealed that the inclusion of ligand improves the uptake of the A549 cell line by 14.9 times when compared to the formulation without ligand. In vivo studies performed to determine the biodistribution in rat with tumor volume about 229 mm3, revealed that the nanoparticles with cetuximab ligand actively carried the medicine to the experimental animals generated cancer sites (Kao et al., Citation2014).
The two new redox-active species of gold-decorated polyaniline derivatives (Au-PANI derivatives), poly-gold(o-aminophenol) (Au-PoAP) and poly-gold(p-phenylenediamine) (Au-PpPD), were synthesized as nanoparticles by a one-pot method utilizing oxidants in the form of chloroauric acid and monomers in the form of o-aminophenol The gold nanoparticles exhibited an immunosensor with a wide linearity range from 0.01 to 100 ng/mL and a detection limit of 6.3 pg/mL for CEA, 8.5 pg/mL for CYFRA21-1, and 7.9 pg/mL for NSE. Furthermore, the findings from this immunosensor were compatible with those from the enzyme-linked immunosorbent test (ELISA), suggesting that nanoparticles containing lung cancer biomarkers may detect the presence of lung cancer (Wang, Liu, et al., Citation2015).
The use of gold nanoparticles with other monoclonal antibody ligands was carried out by Crous and Abrahamse (Citation2020), who succeeded in formulating a photosensitizer (PS) (AlPcS4Cl), AuNPs and Abs with several antibodies used (Ig Abs, CD133 Ab, CD56 Monoclonal Anti-N Cam and CD44 Ab). The results showed that the nanoparticles were well localized in homeostatic cells, and showed good cytotoxicity and cell death activity in AlPcS4Cl-AuNP-Ab A549 cell line compared to AlPcS4Cl (Crous & Abrahamse, Citation2020). In addition, the formulation of tetraethyl orthosilicate, aminopropyl triethoxysilane, macrocyclic chelator DOTAGA anhydride, GD3+ in the form of gold nanoparticles with anti-MUC1-C ligand suggested that there was an increase in retention in vivo and by administering anti-MUC1-C/NPs with XRT (radiation therapy), it was possible to significantly increased the inhibition of tumor growth and to prolong overall animal survival (Detappe et al., Citation2020). Another conjugation using Fe3O4/Au with monoclonal antibody EGFR (scFv) showed that scFv was able to deliver Fe3O4/Au to NSCLC in vivo and increased the localization of these nanoparticles to the target site (Lu et al., Citation2021).
5.3. Spion
Super magnetic iron oxide nanoparticles (SPION) are a type of nanoparticle composed of magnetite (Fe3O4) crystals with a face-centered cubic lattice and an oxygen-based solid foundation. It has octahedral sites parallel to the external magnetic field in ferrous (Fe2+) and iron (Fe3+) ions. In the tetrahedral area opposing the external magnetic field, Fe3+ occupies a tetrahedral area. (Kowalik et al., Citation2020) SPION’s physicochemical features include high sensitivity, low toxicity, and the ability to more readily change the surface. (Lammers et al., Citation2015) SPION has a hydrodynamic diameter of 5-300 nm and is frequently utilized for diagnostic and therapeutic purposes (Lammers et al., Citation2015; Yan et al., Citation2018). SPION can be used to deliver and treat lung cancer by passive or active administration employing ligand monoclonal antibodies ().
Several studies on active targeted delivery of nanoparticles involving this ligand have been widely used (anti-EPCAM and anti-EGFR). Wang, Ye, et al. (Citation2015) developed a method for simultaneous CTC (Circulating Tumor Cells) trapping and detection by merging a silicon nanowire (SiNW) microfluidic array with multifunctional magnetic conversion nanoparticles. These nanoparticles were coupled with anti-EpCAM, allowing them to precisely detect tumor cells in blood samples from metastasis tumor patients when exposed to an external magnetic field. The results demonstrated that this method enabled for the sensitive identification of a small number of tumor cells, which could then be collected for further investigation and re-culture. These nanoparticles have been used to detect CTCs in clinical blood samples of lung cancer patients by monitoring UCL (up conversion luminescence) signals and clinical outcomes of lung cancer metastases. The findings were consistent (Wang, Ye, et al., Citation2015).
Abdi and Shahbazi-Gahrouei (Citation2021) report on the performance of superparamagnetic iron oxide (SPION) nanoparticles combined with EGFR receptor antibodies for the detection of lung cancer using Magnetic Resonance Imaging (MRI). The study was conducted on C57BL/6 experimental mice utilizing LLC1cell line. The results showed that employing ligand antibodies increased SPION absorption in cancer cells, as evaluated by an increase in atomic absorption spectrophotometry intensity (AAS). It reveals that tagging SPION with a ligand can identify cancerous cells (Abdi & Shahbazi-Gahrouei, Citation2021).
SPION has also been produced in nanometric complexes with docetaxel and dicarboxylic acid-terminated polyethylene-glycol (PEG). The complex generated metal-hybrid nanoparticles after sodium borohydride (NaBH4) reduction, with the active ingredient protected in a gold core contained in the polymer chain. These nanoparticles were coupled with a human anti-EGFR antibody to target the overexpressed hERG1 channel on human lung cancer cell membranes, which increased anticancer efficacy. The findings revealed that three-dimensional (3 D) spheroids formed on the Air-Liquid Interface, mimicing tissues in vitro. It was observed that encapsulating docetaxel in the gold core had a substantial impact on pharmacological efficacy, with a large rise in the therapeutic index when docetaxel was conjugated with Au and explicitly targeted against EGFR (Haddada et al., Citation2020).
SPION conjugation with an anti-EGFR monoclonal antibody has also been accomplished and published. C57BL/6 mice were used to execute ex vivo and in vivo cytotoxicity studies on Lewis lung cancer cells (LLC1). The findings indicated that these nanoparticles exhibited spherical shapes of 20 and 80 nm in the nanoparticles and SPION-EGFR, respectively. The results of cell viability after 24 hours of incubation with various nanoprobe concentrations indicated just a 20% decrease in cell viability. The nanoprobe was developed and given by systemic injection into C57BL/6 mice (tumor diameter about 4–6 mm) revealed a great absorption in tumors as well as appropriate imaging signal intensity in both ex vivo and in vivo conditions. The nanoprobe concentration was higher in SPION-EGFR compared to SPION without EGFR and control. It recommends that the nanoprobe be given specifically to the tumor site (Shahbazi-Gahrouei et al., Citation2020).
SPION conjugated with anti-EGFR was also successfully formulated by Wang, Tang, et al. (Citation2017) who expected these nanoparticles to be conjugated via the epidermal growth factor receptor (EGFR). The results of these nanoparticles showed a greater increased in cell death indicated by a significant decrease in the signal intensity of the H460 cell line on T2WI compared to nanoparticles without ligands. These nanoparticles demonstrated that higher intracellular iron (Fe) of the nanoparticles was observed in the H460 cell line compared to the nanoparticles without the ligand. These results suggest that the anti-EGFR ligands can target the nanoparticles to the overexpressed EGFR in the H460 cell line in vitro (Wang, Tang, et al., Citation2017). The subsequent development of SPION with active targeting enhances imaging sensitivity and energy deposition efficiency when used with a clinical MRgFUS (Magnetic Resonance guided Focused Ultrasound) system. For lung cancer targeted delivery with EGFR overexpression, the surface of these PEGylated SPION nanoparticles has been coated with an anti-EGFR. These nanoparticles were studied in vitro and in vivo in a human lung cancer xenograft mouse model (H460). This study found that, as compared to SPION without ligand, SPION with ligand had superior targeting ability against H460 tumor cells. Furthermore, SPION in combination with ligands may considerably improve the efficiency of ultrasonic energy deposition in MRgFUS in in vivo model (Wang, Qiao, et al., Citation2017).
The formulation using magnetic albumin immuno-nanospheres (MAINs) loaded simultaneously with SPION to entrap plasmid-survivin/shRNA (pshRNA) in the form of an anticancer gene, and modified by the anti-EGFR cetuximab to improve the targeting mechanism has been studied. The in vitro release profile test used in the research demonstrated that nanospheres had an impact on pshRNA release. According to the results of the agglutination test and immunofluorescence analysis, the immunonanosphere sustained Cetuximab’s immunological reactivity. By manipulating magnetic albumin nanospheres, the MAIN significantly increased the adhesion and absorption of GLC-82 lung cancer cells overexpressing EGFR (MANs). The MANs formulation with pshRNA outperformed equimolar doses of Cetuximab, and single magnetic targeting with pshRNA or a single monoclonal antibody targeting with pshRNA in the treatment of GLC-82 lung cancer cells was more effective than without the ligand (Hou et al., Citation2016). Other albumin nanospheres were co-loaded in SPION as a vector of anticancer genes, modified by adding the anti-EGFR ligand monoclonal antibody with cetuximab as a targeting agent for adsorption of pDONR233-IFNG, aiming at the lung cancer cell line GLC-82. In this study, photo transfection and agarose gel electrophoresis were employed to demonstrate albumin nanosphere encapsulation. The Cell Counting Kit-8 test demonstrated that the combination therapy group had a more significant therapeutic impact on GLC-82 cells than the other treatment groups, notably a result of the increase in apoptosis and the ability to distribute and target efficiently (Zhang et al., Citation2015).
To improve the efficacy of doxorubicin in lung cancer, SPION was synthesized containing the chemotherapeutic agent doxorubicin and the anti-EGFR ligand cetuximab. Doxorubicin and cetuximab were conjugated using Fe3O4 magnetic nanoparticles to a previously conjugated dextran. In vitro studies on the A549 cells revealed that these nanoparticles dramatically suppressed cell growth more efficiently compared to NPs without anti-EGFR (Zhang et al., Citation2019). Other anti-EGFRs were used to target SPION in lung cancer. The Fe3O4 was loaded in PLGA-PEG-aldehyde nanoparticles synthesized by double emulsion method (water-in-oil-in water) and anti-EGFR conjugated on the surface by aldehyde-amine reaction. It was reported that these solid nanoparticles detect lung cancer efficently through magnetic resonance (Salehnia et al., Citation2019). Other SPION formulations were conjugated with oleic acid and carboxymethyl dextran and CD44v6 monoclonal antibody with Fe/carboxymethyl dextran ratio of 1/1 and 2/1 (w/w). The results indicated that these nanoparticles were strongly associated with A549 cell line in vitro and improved detection on magnetic resonance imaging (MRI) (Wan et al., Citation2016).
5.4. Lipid based nanoparticle
Lipid-based nanoparticles are nanoparticles composed of lipids which are usually in the form of liposomes, (Ninomiya et al., Citation2014) solid lipid nanoparticles, (Pindiprolu et al., Citation2019) or nanostructured lipid carriers (Haron et al., Citation2018). These nanoparticles are getting a lot of interest in drug development and cancer treatment. Some of these nanoparticles can transport both hydrophobic and hydrophilic compounds with extremely low or no toxicity. Furthermore, by possessing a prolonged half-life and regulated drug release, these nanoparticles can enhance the therapeutic action time. Lipid nanoparticles have been widely utilized as therapeutic carriers, particularly in cancer treatment. These nanoparticles have also been produced for active targeted distribution by conjugating them with ligand monoclonal antibodies that are selective for specific receptors.
To overcome hypoxia induced medication resistance in lung cancer, Li et al. (Citation2017) developed complex liposomes that can transport oxygen and molecular targeted drugs. To co-administer erlotinib and PFOB (Perfluorooctyl bromide) against the EGFR overexpressed in NSCLC, anti-EGFR aptamer-conjugated chitosan-immobilized liposomes were constructed. Controlled drug release and anti-EGFR ligands were found to potently inhibits cell proliferation, induces apoptosis and improve cellular absorption when compared to nanoparticles without ligands (Li et al., Citation2017).
The synthesis of lipid-based nanoparticles was modified by utilizing an anti-EGFR aptamer to distribute erlotinib and surviving-shRNA. Chloroquine (CQ) was used with AP/ES (anti-EGFR Aptamers- modified polyamidoamine) to restore tumor vasculature to provide optimal drug/gene delivery and overcome treatment resistance in NSCLC cells. These nanoparticles showed good biostability, a controlled release profile, and a remarkable selectivity to EGFR, which is widely expressed in NSCLC. The improved drug delivery may increase the efficiency of the nanoparticles against lung cancer cell types. Both in vitro and in vivo, the combination with chloroquine displayed promising benefits against erlotinib-resistant cancer cells. In this study, the results showed that the IC50 of the nanoparticles in PC9 cells was much lower compared to H1975 cells, consistent with previous reports that erlotonib may affects this molecular targets and inhibits EGFR-mutant PC9 cells but fails to achieve good results NSCLC cells with EGFR T790M mutation are obtained (Lv et al., Citation2018).
Lipid nanoparticles encapsulating PD-L1 ligand antibodies were created using double emulsion and thin-film dispersion techniques to entrap Adriamycin. A549 cells were used for in vitro and in vivo studies. The results showed that at the same Adriamycin concentration, the intracellular derived fluorescence of the nanoparticles with this ligand was much larger than that of the nanoparticles without the ligand. The findings showed that nanoparticles containing an anti-PD-L1 ligand decreased tumor volume in the experimental animals more than the Adriamycin-only group (Xing et al., Citation2020). Other formulations of lipid-based nanoparticles using hyaluronic acid (HA) nanoparticles hybrid pH sensitive lipid-polymer with erlotinib and bevacizumab ligands for lung cancer targeting NSCLC exhibits stable nanoparticle. This formulation did not show dramatic changes in appearance, visible aggregation or precipitation, and no major changes were seen in particle size, zeta potential, and entrapment efficiency. Compared to nanoparticles without ligands, these nanoparticles showed an increase of accumulated particles in tumor tissue and low toxicity. The tumor volume in experimental animals was smaller in the group treated with ligand-containing nanoparticles (229.2 ± 13.1 mm3) than with ligand-free nanoparticles (437.3 ± 25.3 mm3). Therefore, this system is promising for NSCLC therapy (Pang et al., Citation2020).
5.5. Mesoporous silica and silica nanoparticle
Mesoporous silica is a type of nanoparticle with chemically and thermally stable nanomaterials with controlled morphology and porosity (Trewyn et al., Citation2007). This nanoparticle has been widely utilized and developed in drug delivery for lung cancer therapy. The addition of ligands to these nanoparticles can improve the system’s selectivity. The synthesis of silica nanoparticles was achieved by conjugating them with monoclonal antibodies. It was created by the self-assembly of mesoporous silica on reduced graphene oxide nano sheets with nanogap-aligned gold nanoparticles (AuNPs). It was then encapsulated and distributed within the nano pores of the mesoporous silica layer. Rhodamine 6 G (R6G) was then encapsulated into nano networks with anti-EGFR conjugated on the surface of the nanocomposite, along with PEG (polyethylene glycol). CPSS was a porous carbon silica nanofilm. When compared to the conventional cell line, the results revealed that these nanoparticles targeted overexpressed EGFR lung cancer cells (A549) with great specificity compared to (MRC-5) cells. The synergistic effect of linked AuNPs and nanosheets enables good photothermal therapeutic efficiency with a low power density (0.5 W cm2) of near-infrared laser. These findings imply that these nanoparticles constitute a one-of-a-kind theragnostic nano system with exceptional cell targeting and cell tracking capabilities, as well as photothermal therapeutic potential (Chen et al., Citation2016).
To detect lung cancer, silica nanoparticles containing anti-EGFR were coupled with NIRF (near-infrared fluorescent dye) and MB (methylene blue). This Anti-EGFR enhanced the cellular absorption in vitro and in vivo in experimental mice with tumor volume about 80–100 mm3 developed using A549 cells. It reveals that when anti-EGFR are used, these nanoparticles have excellent targeting capabilities (Wan et al., Citation2017). The manufacture of mesoporous silica in combination with anti-EGFR in the form of cetuximab was also carried out to deliver siRNA against polo-like kinase 1 (PLK1), which is critical in lung cancer mitosis. This EGFR response has been shown to be exhibited in up to 50% of lung cancer patients. In vivo experiments on A549 cancer-inolculated rats with tumor size of 120 mm3 indicated a reduction in tumor formation as well as the ability to function as an NSCLC targeted agent (Reda et al., Citation2019).
5.6. Others
The synthesis of functionally assembled supramolecular nanoparticles was performed for RNAi agent loading and tumor target treatment. The adamantane-grafted poly(ethylene glycol) molecule was modified with the specific binding ligand EGFR GE11 or the pH-sensitive fusogenic peptide GALA, which was then used for self-assembly with the cyclodextrin-grafted branched polyethyleneimine (CD-PEI), adamantane-grafted polyamidoamine dendrimer (Ad-PAMAM), and DNA. These nanoparticles showed that beneficial peptides can enhance targeted cell binding, internalization, and endosome release. Furthermore, it leads to enhanced reporter gene expression and effective target gene silencing. Systemic administration can effectively lower intratumoral VEGF protein levels, limit angiogenesis, and considerably suppress tumor development in A549 xenografts (Lu et al., Citation2020).
The CD146 antibody ligand in combination with 1,4,7-triazacyclononane-triacetic acid has been studied. PET imaging was employed as an experimental animal to examine tumor biodistribution and uptake of these nanoparticles in lung cancer-induced nude mice (A549, NCI-H358, NCI-H522, HCC4006, H23, and NCI-H460 cell lines). The relationship between CD146 expression and nanoparticle uptake was studied using graphical tools. Ex vivo biodistribution and immunohistochemical assays were performed to corroborate the correctness of the PET results and the spatial expression of CD146. When compared to other lung cancer cell lines, the H460 and H23 cell lines showed the highest expression of CD146, resulting in much greater cellular uptake in these cell lines (Sun et al., Citation2016).
Another nanoparticle development for lung cancer therapy was carried out by Abd-Rabou and Ahmed (Citation2019), who successfully formulated doxorubicin, bevacizumab and CCR2 inhibitor in the form of nanoparticles. The results of this study showed a decrease in the viability of A549 cells and was able to increase the level of apoptosis in cell lines (Abd-Rabou & Ahmed, Citation2019). The formulation using an active substance in the form of gemcitabine which was formulated into nanoparticles with anti-EGFR ligands (SDP-GEM/PEI-PEG-anti-EGFR) indicated an increase in the eradication of cancer cells in vitro. This system can deliver drugs to the appropriate target for 3 hours in vivo, besides that it is able to extend lifetime and reduce tumor volume (Tang et al., Citation2020).
6. Author perspective
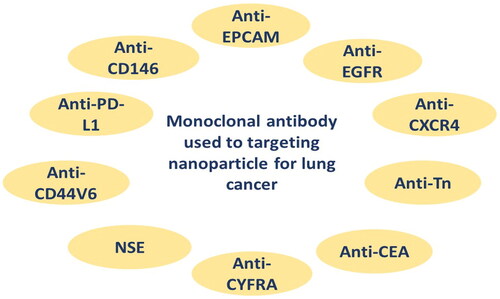
A monoclonal antibody is a kind of ligand utilized in active targeted drug delivery systems, particularly at cancer areas. Many cancer cells express antigens on their surfaces, which can be utilized as markers to deliver medications to the target area via specific antibodies. Nanoparticles containing this ligand have been created to treat cancer, especially lung cancer. Monoclonal antibody that has been used for targeting lung cancer are summarized in . Monoclonal antibodies are used as ligands to help target nanoparticles to a specific population of cells, through exploiting the presence of specific antigens that are widely distributed on the surface of lung cancer cells (Tiseo et al., Citation2008). The binding reaction between the ligand and its antigen triggers endocytosis within the cell, resulting in rapid entry of the nanoparticles into cancer cells, as shown in .
Several types of nanoparticles have been used, including lipid-based, polymeric, SPION, dendrimers, mesoporous silica, and others (). When conjugated to monoclonal antibody, anticancer drugs are most often attached to carboxyl or amino groups. The steps involved in conjugating chemotherapeutic drugs to monoclonal antibody often involve neutral binding, which can reduce antibody solubility and lead to aggregation and precipitation. Attention should also be paid to the number of drug residues attached to each monoclonal antibody. Ideally, the ratio of residual drug per monoclonal antibody should be maximized while maintaining acceptable levels of monoclonal antibody activity and specificity (Pietersz et al., Citation1987).
Many of the applications of these nanoparticles with monoclonal antibodies are still limited to cancer detection tests, while others have not yet been used to deliver specific active chemicals. Moreover, production of nanoparticles with antibodies to target the specific antigen is still of little use for positively targeting nanoparticles to lung cancer, such as Anti-EpCAM, anti-NSE, anti-ganglioside, etc. As a result, continued development of nanoparticles containing monoclonal antibodies is predicted to be successful as a medication delivery mechanism for lung cancer.
7. Conclusion
Lung cancer-specific ligand monoclonal antibodies are often used to produce polymeric nanoparticles, SPION nanoparticles, lipid-based nanoparticles, and others. Using these nanoparticles, drug is released in a controlled manner. Nanoparticles can also increase therapeutic effectiveness by enhancing drug accumulation at the target site. Recently, nanoparticles have been widely used for cancer. They are being used for more than just drug delivery; they are also being used to diagnose cancer and track its progression throughout the body. Targeted delivery systems in lung cancer therapy have been found to deliver drugs selectively, increase selectivity, and reduce the side effects of treatment. Therefore, the novel formulation of nanoparticles containing monoclonal antibodies as a targeted drug delivery system for lung cancer is interesting, and are continually developed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbas Z, Rehman S. (2018). An overview of cancer treatment modalities. Neoplasm 1:139–157.
- Abdi N, Shahbazi-Gahrouei D. (2021). Assessment of superparamagnetic iron oxide nanoparticles conjugated with anti-epidermal growth factor receptor antibody for the detection of lung cancer by Magnetic Resonance Imaging (MRI) in C57BL/6. J Isfahan Med Sch 38:1038–42.
- Abd-Rabou AA, Ahmed HH. (2019). Bevacizumab and CCR2 inhibitor nanoparticles induce cytotoxicity-mediated apoptosis in doxorubicin-treated hepatic and non-small lung cancer cells. Asian Pac J Cancer Prev 20:2225–38.
- Adams GP, Weiner LM. (2005). Monoclonal antibody therapy of cancer. Nat Biotechnol 23:1147–57.
- Alavi M, Hamidi M. (2019). Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther 34:1–8.
- Alibolandi M, Ramezani M, Abnous K, et al. (2015). In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release 209:88–100.
- Al-Jamal WT, Kostarelos K. (2007). Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine (Lond) 2:85–98.
- Ashton JR, Gottlin EB, Patz EF, et al. (2018). A comparative analysis of EGFR-targeting antibodies for gold nanoparticle CT imaging of lung cancer. PLoS One 13(11):1–20.
- Aslam MS, Naveed S, Ahmed A, et al. (2014). Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J Cancer Ther 05:817–22.
- Bade BC, Dela Cruz CS. (2020). Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med 41:1–24.
- Bazak R, Houri M, Achy S, et al. (2014). Passive targeting of nanoparticles to cancer: a comprehensive review of the literature. Mol Clin Oncol 2:904–08.
- Bazak R, Houri M, El Achy S, et al. (2015). Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol 141:769–84.
- Byrne JD, Betancourt T, Brannon-Peppas L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–26.
- Castro A, Berois N, Malanga A, et al. (2021). Docetaxel in chitosan-based nanocapsules conjugated with an anti-Tn antigen mouse/human chimeric antibody as a promising targeting strategy of lung tumors. Int J Biol Macromol 182:806–14.
- Chen S, Zhang S, Wang Y, et al. (2021). Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy. Asian J Pharm Sci 16:598–611.
- Chen YW, Liu TY, Chen PJ, et al. (2016). A high-sensitivity and low-power theranostic nanosystem for cell SERS imaging and selectively photothermal therapy using anti-EGFR-conjugated reduced graphene oxide/mesoporous silica/AuNPs nanosheets. Small 12:1458–68.
- Chittasupho C, Lirdprapamongkol K, Kewsuwan P, Sarisuta N. (2014). Targeted delivery of doxorubicin to A549 lung cancer cells by CXCR4 antagonist conjugated PLGA nanoparticles. Eur J Pharm Biopharm 88:529–38.
- Clemons TD, Singh R, Sorolla A, et al. (2018). Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir 34:15343–9.
- Corsi I, Bergami E, Grassi G. (2020). Behavior and bio-interactions of anthropogenic particles in marine environment for a more realistic ecological risk assessment. Front Environ Sci 8:1–21.
- Costa SA, Mozhdehi D, Dzuricky MJ, et al. (2019). Active targeting of cancer cells by nanobody decorated polypeptide micelle with bio-orthogonally conjugated drug. Nano Lett 19:247–54.
- Crous A, Abrahamse H. (2020). Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int J Mol Sci 21:1–23.
- Deng X, Cao M, Zhang J, et al. (2014). Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 35:4333–44.
- Detappe A, Mathieu C, Jin C, et al. (2020). Anti-MUC1-C antibody–conjugated nanoparticles potentiate the efficacy of fractionated radiation therapy. Int J Radiat Oncol Biol Phys 108:1380–9.
- Fang C, Zhang M. (2010). Nanoparticle-based theragnostics: integrating diagnostic and therapeutic potentials in nanomedicine. J Control Release 146:2–5.
- Globocan. 2020. Lung fact sheet. Obs Glob do Câncer 419:1–2. https://gco.iarc.fr/today
- Haddada MB, Movia D, Prina-Mello A, Spadavecchia J. (2020). Docetaxel gold complex nanoflowers: a chemo-biological evaluation for their use as nanotherapeutics. Colloids Surf B Biointerfaces 194:111172 (1–18).
- Haron AS, Syed Alwi SS, Saiful Yazan L, et al. (2018). Cytotoxic effect of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) on liver cancer cell integrated with hepatitis B genome, Hep3B. Evidence-Based Complement Altern Med 2018:1–13.
- Hou X, Zhang H, Li H, Zhang D. (2016). Magnetic albumin immuno-nanospheres as an efficient gene delivery system for a potential use in lung cancer: preparation, in vitro targeting and biological effect analysis. J Drug Target 24:247–56.
- Huang WT, Larsson M, Wang YJ, et al. (2015). Demethoxycurcumin-carrying chitosan-antibody core-shell nanoparticles with multitherapeutic efficacy toward malignant a549 lung tumor: from in vitro characterization to in vivo evaluation. Mol Pharm 12:1242–249.
- International Agency for Research on Cancer (IARC). 2020. Indonesia - Global Cancer Observatory. Globocan. 858.
- Jafari SM, McClements DJ. (2017). Nanotechnology approaches for increasing nutrient bioavailability. Adv Food Nutr Res 81:1–30.
- Jeevanandam J, Barhoum A, Chan YS, et al. (2018). Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–074.
- Jiang W, Kim BYS, Rutka JT, Chan WCW. (2008). Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145–50.
- Kao HW, Lin YY, Chen CC, et al. (2014). Biological characterization of cetuximab-conjugated gold nanoparticles in a tumor animal model. Nanotechnology 25:295102.
- Karra N, Nassar T, Ripin AN, et al. (2013). Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: efficacy and biofate in a lung cancer mouse model. Small 9:4221–36.
- Khan I, Saeed K, Khan I. (2019). Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–31.
- Kim Y, Hyo SK, Zheng YC, et al. (2009). Clinicopathological implications of EpCAM expression in adenocarcinoma of the lung. Anticancer Res 29:1817–822.
- Kowalik P, Mikulski J, Borodziuk A, et al. (2020). Yttrium-doped iron oxide nanoparticles for magnetic hyperthermia applications. J Phys Chem C 124:6871–883.
- Kreuter J. (2007). Nanoparticles-a historical perspective. Int J Pharm 331:1–10.
- Lammers T, Mertens ME, Gremse F, et al. (2015). Theranostic tissue engineering: MR imaging of uspio-labeled collagen scaffolds and vascular grafts. Mol Imaging Biol 17:1–23.
- Li F, Mei H, Gao Y, et al. (2017). Co-delivery of oxygen and erlotinib by aptamer-modified liposomal complexes to reverse hypoxia-induced drug resistance in lung cancer. Biomaterials 145:56–71.
- Li J, Huang P, Chang L, et al. (2013). Tumor targeting and pH-responsive polyelectrolyte complex nanoparticles based on hyaluronic acid-paclitaxel conjugates and Chitosan for oral delivery of paclitaxel. Macromol Res 21:1331–37.
- Li J, Zhen X, Lyu Y, et al. (2018). Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano 12:8520–30.
- Li K, Zhan W, Jia M, et al. (2020). Dual loading of nanoparticles with doxorubicin and icotinib for the synergistic suppression of non-small cell lung cancer. Int J Med Sci 17:390–402.
- Li Z, Jiang H, Xu C, Gu L. (2015). A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocolloids 43:153–64.
- Lin W, Ma G, Yuan Z, et al. (2019). Development of zwitterionic polypeptide nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir 35:1273–83.
- Lu S, Bao X, Hai W, et al. (2020). Multi-functional self-assembled nanoparticles for pVEGF-shRNA loading and anti-tumor targeted therapy. Int J Pharm 575:118898 (1–37).
- Lu Y, Huang J, Li F, et al. (2021). EGFR-specific single-chain variable fragment antibody-conjugated Fe3O4/Au nanoparticles as an active MRI contrast agent for NSCLC. Magn Reson Mater Phys. 34:581–91.
- Lv T, Li Z, Xu L, et al. (2018). Chloroquine in combination with aptamer-modified nanocomplexes for tumor vessel normalization and efficient erlotinib/Survivin shRNA co-delivery to overcome drug resistance in EGFR-mutated non-small cell lung cancer. Acta Biomater 76:257–74.
- Maeda H, Matsumura Y. (1989). Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst 6:193–210.
- Malhotra S, Dumoga S, Joshi A, et al. (2021). Polymeric micelles coated with hybrid nanovesicles enhance the therapeutic potential of the reversible topoisomerase inhibitor camptothecin in a mouse model. Acta Biomater 121:579–91.
- Muhamad N, Plengsuriyakarn T, Na-Bangchang K. (2018). Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review. Int J Nanomedicine 13:3921–35.
- Naito M, Yokoyama T, Hosokawa K, Nogi K. 2018. Nanoparticle technology handbook. Amsterdam: Elsevier.
- Ninomiya K, Kawabata S, Tashita H, Shimizu N. (2014). Ultrasound-mediated drug delivery using liposomes modified with a thermosensitive polymer. Ultrason Sonochem 21:310–16.
- Ohnoshi T, Ueoka h, Hino N, et al. (1992). Treatment of small cell lung cancer in the elderly: the progress and limitation of chemotherapy. Jpn J Thorac Dis 30:216–23.
- Pak MG, Shin DH, Lee CH, Lee MK. (2012). Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J Surg Onc 10:1–8.
- Pang J, Xing H, Sun Y, et al. (2020). Non-small cell lung cancer combination therapy: hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed Pharmacother 125:109861.
- Park J, Fong PM, Lu J, et al. (2009). PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed Nanotechnol Biol Med 5:410–18.
- Partridge AH, Burstein HJ, Winer EP. (2001). Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 30:135–42.
- Patel J, Amrutiya J, Bhatt P, et al. (2018). Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J Microencapsul 35:204–17.
- Pietersz GA, Kanellos J, Smyth MJ, et al. (1987). The use of monoclonal antibody conjugates for the diagnosis and treatment of cancer. Immunol Cell Biol 65:111–25.
- Pindiprolu SKSS, Chintamaneni PK, Krishnamurthy PT, Ratna Sree Ganapathineedi K. (2019). Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev Ind Pharm 45:304–13.
- Reda M, Ngamcherdtrakul W, Gu S, et al. (2019). PLK1 and EGFR targeted nanoparticle as a radiation sensitizer for non-small cell lung cancer. Cancer Lett 467:9–18.
- Rusch V, Klimstra D, Venkatraman E, et al. (1997). Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor α is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 3:515–22.
- Salahpour Anarjan F. (2019). Active targeting drug delivery nanocarriers: ligands. Nano-Struct Nano-Objects 19:100370 (17).
- Salehnia Z, Shahbazi-Gahrouei D, Akbarzadeh A, et al. (2019). Synthesis and characterisation of iron oxide nanoparticles conjugated with epidermal growth factor receptor (EGFR) monoclonal antibody as MRI contrast agent for cancer detection. IET Nanobiotechnol 13:400–6.
- Seyhan EC, Altin S, Çetinkaya E, et al. (2010). Prognostic value of epidermal growth factor receptor expression in operable non-small cell lung carcinoma. Multidiscip Respir Med 5:1–7.
- Shahbazi-Gahrouei D, Abdi N, Shahbazi-Gahrouei S, Hejazi SH, et al. (2020). In vivo study of anti-epidermal growth factor receptor antibody-based iron oxide nanoparticles (anti-EGFR-SPIONs) as a novel MR imaging contrast agent for lung cancer (LLC1) cells detection. IET Nanobiotechnol 14:369–74.
- Silvestre ALP, Oshiro-Júnior JA, Garcia C, et al. (2020). Monoclonal antibodies carried in drug delivery nanosystems as a strategy for cancer treatment. Curr Med Chem 28:401–18.
- Spizzo G, Fong D, Wurm M, et al. (2011). EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol 64:415–20.
- Steinhauser I, Spänkuch B, Strebhardt K, Langer K. (2006). Trastuzumab-modified nanoparticles: optimisation of preparation and uptake in cancer cells. Biomaterials 27:4975–83.
- Stella B, Arpicco S, Peracchia MT, et al. (2000). Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci 89:1452–64.
- Sukhanova A, Bozrova S, Sokolov P, et al. (2018). Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett 13:1–21.
- Sun CC, Bodurka DC, Weaver CB, Rasu R, et al. (2005). Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13:219–27.
- Sun H, England CG, Hernandez R, et al. (2016). ImmunoPET for assessing the differential uptake of a CD146-specific monoclonal antibody in lung cancer. Eur J Nucl Med Mol Imaging 43:2169–79.
- Szczepanowicz K, Bzowska M, Kruk T, et al. (2016). Pegylated polyelectrolyte nanoparticles containing paclitaxel as a promising candidate for drug carriers for passive targeting. Colloids Surfaces B Biointerfaces 143:463–71.
- Tang J, Zheng F, Zhao J, Zhao J. (2020). Self-assembled multifunctional nanotheranostics loading GEM for targeted lung cancer therapy. Mater Sci Eng C Mater Biol Appl 112:110786.
- Tiseo M, Ardizzoni A, Cafferata MA, Loprevite M, et al. (2008). Predictive and prognostic significance of neuron-specific enolase (NSE) in non-small cell lung cancer. Anticancer Res 28:507–13.
- Tivnan A, Orr WS, Gubala V, et al. (2012). Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS One 7:e38129.
- Trail PA, Bianchi AB. (1999). Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol 11:584–88.
- Trewyn BG, Slowing II, Giri S, et al. (2007). Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc Chem Res 40:846–53.
- Tseng CL, Wang TW, Dong GC, et al. (2007). Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 28:3996–4005.
- Wakaskar RR. (2017). Passive and active targeting in tumor microenvironment. Int J Drug Dev Res 9:37–41.
- Wan J, Wu W, Zhang R, et al. (2017). Anti-EGFR antibody conjugated silica nanoparticles as probes for lung cancer detection. Exp Ther Med 14:3407–12.
- Wan X, Song Y, Song N, et al. (2016). The preliminary study of immune superparamagnetic iron oxide nanoparticles for the detection of lung cancer in magnetic resonance imaging. Carbohydr Res 419:33–40.
- Wang C, Ye M, Cheng L, et al. (2015). Simultaneous isolation and detection of circulating tumor cells with a microfluidic silicon-nanowire-array integrated with magnetic upconversion nanoprobes. Biomaterials 54:55–62.
- Wang L, Liu N, Ma Z. (2015). Novel gold-decorated polyaniline derivatives as redox-active species for simultaneous detection of three biomarkers of lung cancer. J Mater Chem B 3:2867–72.
- Wang X, Wu JR, Liang F, Yang YW. (2019). Situ gold nanoparticle synthesis mediated by a water-soluble leaning pillar [6] arene for self-assembly, detection, and catalysis. Org Lett 21:5215–18.
- Wang XB, Zhou HY. (2015). Molecularly targeted gemcitabine-loaded nanoparticulate system towards the treatment of EGFR overexpressing lung cancer. Biomed Pharmacother 70:123–28.
- Wang Z, Qiao R, Tang N, et al. (2017). Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 127:25–35.
- Wang Z, Tang N, Wang H, et al. (2017). Preparation of anti-EGFR-PEG-SPIO molecular probe and its targeting MRI for lung adenocarcinoma cells. Chin J Med Imaging Technol 33:1797–801.
- Winarti L. 2013. Sistem penghantaran obat tertarget, macam, jenis-jenis sistem penghantaran, dan aplikasinya. Stomatognatic (J K G Unej).
- Won YW, Yoon SM, Lim KS, Kim YH. (2012). Self-assembled nanoparticles with dual effects of passive tumor targeting and cancer-selective anticancer effects. Adv Funct Mater 22:1199–208.
- Wu X, Yang H, Yang W, et al. (2019). Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J Mater Chem B 7:4734–50.
- Xing J, Xin B, Xia H. (2020). Preparation of programmed death-ligand 1 antibody nanoparticles and their lung cancer targeting therapeutic effects. J Nanosci Nanotechnol 20:6033–9.
- Xu W, Wang H, Dong L, et al. (2019). Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int J Nanomed 14:4649–66.
- Yan L, Amirshaghaghi A, Huang D, et al. (2018). Protoporphyrin IX (PpIX)-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusters for magnetic resonance imaging and photodynamic therapy. Adv Funct Mater 28:1707030 (1-8).
- Yao Y, Zhou Y, Liu L, et al. (2020). Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7:1–14.
- Ye J, Wang Q, Zhou X, Zhang N. (2008). Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm 352:(1–2.
- Yoo J, Park C, Yi G, et al. (2019). Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel) 11:640.
- You J, Li X, De Cui F, et al. (2008). Folate-conjugated polymer micelles for active targeting to cancer cells: preparation, in vitro evaluation of targeting ability and cytotoxicity. Nanotechnology 19:045102.
- Yu B, Tai HC, Xue W, et al. (2010). Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol 27:286–98.
- Zha Z, Li D, Zhang P, et al. (2021). Neuron specific enolase promotes tumor metastasis by activating the Wnt/β-catenin pathway in small cell lung cancer. Transl Oncol 14:101039 (1-11).
- Zhang F, Ni Q, Jacobson O, et al. (2018). Polymeric nanoparticles with a glutathione-sensitive heterodimeric multifunctional prodrug for in vivo drug monitoring and synergistic cancer therapy. Angew Chemie - Int Ed 57:7184–88.
- Zhang H, Hou X, Lin M, et al. (2015). The study on the preparation and characterization of gene-loaded immunomagnetic albumin nanospheres and their anti-cell proliferative effect combined with magnetic fluid hyperthermia on GLC -82 cells. Drug Des Devel Ther 9:6445–60.
- Zhang Q, Liu Q, Du M, et al. (2019). Cetuximab and Doxorubicin loaded dextran-coated Fe 3 O 4 magnetic nanoparticles as novel targeted nanocarriers for non-small cell lung cancer. J Magn Magn Mater 481:122–28.