ABSTRACT
Due to the COVID-19 pandemic, most graduate medical education (GME) training programs conducted virtual interviews for prospective trainees during the 2020–2021 application cycle. Many internal medicine (IM) subspecialty fellowship programs hosted virtual interviews for the first time with little published data to guide best practices.
To evaluate how IM subspecialty fellowship applicants perceived the virtual interview day experience.
We designed a 38-item questionnaire that was sent via email to applicants in eight IM subspecialty programs at a single tertiary academic medical center (University of California, San Francisco) from September–November, 2020.
Seventy-five applicants completed the survey (75/244, 30.7%), including applicants from all eight fellowship programs. Most survey respondents agreed that the length of the virtual interview day (mean = 6.4 hours) was long enough to gather the information they needed (n = 65, 86.7%) and short enough to prevent fatigue (n = 55, 73.3%). Almost all survey respondents agreed that they could adequately assess the clinical experience (n = 71, 97.3%), research opportunities (n = 72, 98.6%), and program culture (n = 68, 93.2%). Of the respondents who attended a virtual educational conference, most agreed it helped to provide a sense of the program’s educational culture (n = 20, 66.7%). Areas for improvement were identified, with some survey respondents reporting that the virtual interview day was too long (n = 11) or that they would have preferred to meet more fellows (n = 10).
Survey respondents indicated that the virtual interview was an adequate format to learn about fellowship programs. These findings can inform future virtual interviews for GME training programs.
Introduction
Due to the COVID-19 pandemic, graduate medical education (GME) training programs conducted interviews for prospective trainees using a virtual format for the 2020–2021 application cycle[Citation1]. Many internal medicine (IM) subspecialty fellowship programs hosted virtual interviews for the first time with little published data to guide best practices.
Interviews are an important component of the subspecialty fellowship application process, often affecting the final rank order of both programs and applicants [Citation2,Citation3]. Previously, most GME training programs conducted in-person interviews at the site(s) of the training program[Citation4], A small number of programs previously reported their experiences with virtual interviews [Citation4–11]. In these studies, applicants reported that they were able to present themselves to their satisfaction [Citation10,Citation11], gain a satisfactory understanding of the program[Citation10], ask questions of fellows and faculty[Citation11], and had an overall positive virtual interview experience [Citation8,Citation11]. In contrast, some applicants reported that virtual interviews were less effective than an on-site interview[Citation6], indicated a preference for interviewing in person[Citation11], or reported that the virtual interview experience had an unfavorable impact on their rank position of the program[Citation10], although reasons for these views were not fully explored. However, these were all studies from single fellowship programs with a small number of respondents and they did not evaluate specific aspects of the virtual interview day (e.g., the optimal number and length of interviews, how to structure interactions with fellows, whether or not to have applicants observe didactics, etc.). Moreover, as most studies did not report the demographic data of the applicants, it was not known whether the experience differed among demographic groups. As such, best practices for constructing a virtual interview day are unknown.
We aimed to address this gap by conducting a comprehensive, multi-program evaluation of the virtual interview applicant experience at our institution. We surveyed applicants from eight internal medicine (IM) subspecialty fellowship programs about the elements and effectiveness of the virtual interview day, allowing for comparisons between interview structures and fellowships.
Methods
Setting and participants
Eight IM subspecialty fellowship programs at the University of California, San Francisco (UCSF) participated in this study: clinical informatics, endocrinology, gastroenterology, geriatrics, hematology/oncology, hospice and palliative medicine, infectious diseases, and rheumatology. Each of these 8 programs conducted only virtual interviews, with no in-person interviews offered to any applicant. Features of the planned virtual interview day for each fellowship program are shown in Supplementary . Fellowship applicants from all eight programs (n = 244) were emailed an anonymous survey link on the day they completed their UCSF fellowship interview. Participation was voluntary, and no incentives were provided. The email and survey text explicitly stated that the survey results would not be examined until after the National Fellowship Resident Matching Program (NRMP) Match Day. Survey responses were collected between September and November, 2020.
Table 1. Demographic information about survey respondents
Survey design and outcomes measured
We designed a 38-item questionnaire with Qualtrics Survey Software (Provo, UT; Seattle, WA) using Artino’s survey design process[Citation12]. See full survey instrument in Supplemental Data. The survey included quantitative and qualitative sections. Item types included multiple choice, sliding scale, 5-point Likert scale, and open-ended questions. Both complete (70) and incomplete (5) responses were included in calculating the response rate, and questions that were answered on incomplete surveys were included in the data analysis.
Demographic data definitions
We used the Association of American Medical Colleges (AAMC) definition of Underrepresented in Medicine (UIM)[Citation13], which is defined as individuals who self-identify as African American or Black, Hispanic or Latino, American Indian or Alaska Native, or Native Hawaiian or Pacific Islander[Citation14].
Data analysis
Quantitative data were analyzed as received, including entries that may have been omitted, in order to maintain data integrity. For Likert scale questions, ‘strongly agree’ and ‘somewhat agree’ were considered favorable responses (combined as ‘agree’ throughout the results section) and ‘neither agree nor disagree’, ‘somewhat disagree’, and ‘strongly disagree’ were considered unfavorable responses. Data was analyzed using Prism Software (GraphPad; San Diego, CA). We used descriptive statistics to summarize numeric responses. Comparisons between groups were made using the unpaired t-test or two-sided Fisher’s exact test, as appropriate. A p value of <0.05 was considered statistically significant.
Qualitative survey data was analyzed using inductive content analysis to identify themes[Citation15]. One investigator (LAH) coded all data and two other investigators (GH, JMB) each reviewed 50% of the coded responses for agreement. Differences in coding were discussed and reconciled.
IRB statement
The study was deemed exempt by the UCSF Institutional Review Board.
Results
Survey respondent characteristics
We invited 244 applicants from eight IM subspecialty fellowship programs to participate. 75 applicants responded, yielding an overall response rate of 30.7% (). Of the 75 respondents, 33 identified as female (44.0%) and most were in their third year of residency training (n = 48, 64.0%). 15 respondents self-identified as UIM (20.0%). The survey response rate varied by subspecialty (Supplementary ). Demographics of survey respondents vs. non-respondents are shown in Supplementary .
Table 2. Overall strengths and weaknesses of the virtual interview day experience: Results from content analysis of responses to open-ended items
Table 3. Recommended considerations for structuring an effective virtual interview day
Structure of the virtual interview day
Survey respondents reported the virtual interview day lasted an average of 6.4 hours () with an average of 3.7 breaks () and 4.3 individual interviews (). Most applicants agreed that the virtual interview day was long enough to gather the necessary information (n = 65, 86.7%) but short enough to prevent fatigue (n = 55, 73.3%) (). The respondents who indicated that they felt fatigued reported a longer average interview day (7.4 hours) than respondents who did not report fatigue (6.0 hours) (p < 0.01). Most respondents agreed that there were adequate breaks (n = 68, 90.7%, ). Respondents who indicated that there were an inadequate breaks reported fewer average breaks (2.3 breaks) than respondents who felt that the number of breaks was adequate (4.1 breaks) (p = 0.03). 16 out of 75 applicants (21.3%) reported technical difficulties during the virtual interview day (). Most technical issues were resolved in less than 10 minutes (n = 15, 93.8%). Despite these technical issues, almost all applicants agreed that the technical experience went smoothly (n = 71, 94.7%, ).
Figure 1. Features of the virtual interview day
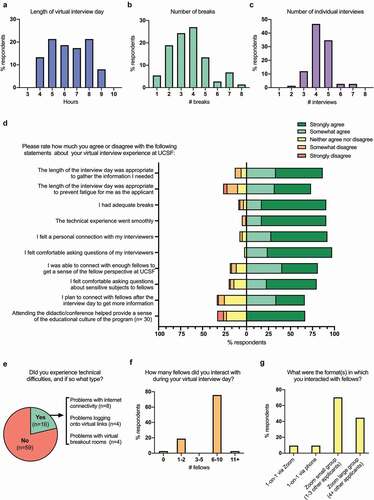
Individual interviews
Most survey respondents reported that their average individual interview lasted 16–30 minutes (n = 68, 90.7%); a minority reported a 31–45 minute average interview time (n = 7, 9.3%). Most applicants agreed that they felt a personal connection with their interviewers (n = 69, 92.0%) and felt comfortable asking questions (n = 73, 97.3%) (). Of note, there was no statistically significant difference in the number of respondents who agreed that they felt a personal connection with their interviewer or felt comfortable asking questions based on gender, year in training (R2/R3 vs. post-residency), or UIM status (Supplementary Table 4).
Interactions with fellows
Most respondents reported that they interacted with 6–10 fellows during their virtual interview day (n = 56, 75.7%, ). Most fellow interactions occurred during the virtual interview day (n = 68, 91.9%) and/or the night prior (n = 11, 14.9%). Respondents connected with fellows in virtual large groups with 4+ other applicants (n = 33, 44.6%), virtual small groups with 1–3 other applicants (n = 52, 70.3%), one-on-one via a virtual platform (n = 7, 9.5%) and/or one-on-one via telephone (n = 7, 9.5%) (). Most respondents agreed that they had an opportunity to meet enough fellows to get a sense of the fellow perspective (n = 60, 81.1%, ). Most respondents agreed that they felt comfortable asking fellows about ‘sensitive subjects’ (e.g., having a family, being a UIM trainee) (n = 59, 79.7%, ), and responses to this question did not differ based on gender, year in training (R2/R3 vs. post-residency), or UIM status (Supplementary Table 4).
Attending a virtual educational conference
30 applicants reported that they attended an educational conference during the virtual interview day (41.7%) and 8 indicated that they were given information about how to access a conference at a later time (11.1%). Of the latter group, 4 planned to attend at a later time (50.0%) and 4 did not (50.0%). Of the 30 applicants who attended during the virtual interview day, two-thirds agreed that attending the virtual conference helped provide a sense of the program’s educational culture (n = 20, 66.7%, ).
Assessing educational opportunities
Most respondents agreed that the virtual interview day gave them an adequate sense of the educational opportunities at the fellowship program, including the clinical experiences (n = 71, 97.3%), research experiences/opportunities (n = 72, 98.6%), opportunities for additional coursework/degrees (n = 65, 89.0%), formal teaching/curriculum (n = 68, 93.2%), and mentorship (n = 66, 90.4%) ().
Figure 2. Ability to assess the fellowship components and culture, and overall assessment
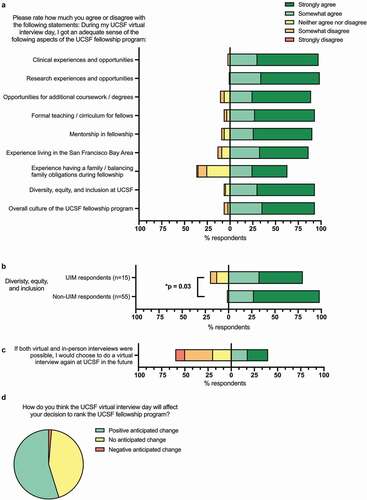
Assessing the surrounding city and fellowship culture
Most applicants agreed that the virtual interview gave them an adequate sense of the fellow experience living in the surrounding city (San Francisco) (n = 63, 86.3%, ). Most applicants agreed that they were able to get an adequate sense of the overall culture of the fellowship program based on their virtual interview day (n = 68, 93.2%, ). Almost two-thirds of respondents agreed that they were able to adequately assess the experience balancing family obligations during fellowship (n = 46, 63.0%, ). Responses to this question did not differ by gender, year in training, or UIM status (Supplementary Table 4). Most respondents agreed that they were able to get an adequate sense of the program’s support for diversity, equity, and inclusion (DEI) (n = 68, 93.2%, ). Respondents who identified as UIM were less likely to agree that they had an adequate sense of the support for DEI compared to non-UIM respondents (p = 0.03, , Supplementary Table 4).
Overall impression
Most survey respondents agreed that they were able to get an adequate sense of the overall culture of the UCSF fellowship program based on their virtual interview experience (n = 68, 93.2%). Responses to this question did not vary based on gender, year in training, UIM status, or interview length (Supplementary Tables 4 and 5). Survey respondents were divided about whether they would choose to interview virtually again if both virtual and in-person interviews were possible: 39.7% agreed that they would choose to interview virtually again (n = 29), 20.5% neither agreed nor disagreed (n = 15), and 50.7% disagreed (n = 37) (). Responses to this question also did not vary based on gender, year in training, or UIM status (Supplementary Table 4).
Most applicants indicated that there were areas that they hoped to explore more fully with an in-person visit (n = 53, 72.6%). Free-text responses to explore these areas fell into four broad categories: the opportunity for in-person fellow interaction (n = 27), the opportunity to observe faculty/fellow interactions (n = 16), the opportunity to tour the hospital (n = 29), and/or the opportunity to visit the host city (n = 15). Respondents were also given the opportunity to highlight overall strengths and weaknesses of the virtual interview day experience using free-text responses, and representative responses are provided in . The most frequently cited strengths were the strong organization of the virtual interview day (n = 24), the welcoming faculty/fellows (n = 15), and time/money saved (n = 14). The most common areas for improvement included the long length of the virtual interview day (n = 11) and the desire to meet more fellows, particularly in small groups or one-on-one (n = 10). Of note, several comments referred to issues that were not addressed elsewhere in the survey, such as challenges with having a common virtual waiting room and abrupt interview cut offs due to automatic closing of the interview rooms.
Finally, when asked whether the virtual interview day experience impacted their anticipated rank position of the fellowship program, 32 applicants indicated an anticipated positive change (43.8%), 40 indicated no anticipated change (54.8%), and 2 indicated an anticipated negative change (2.7%) ().
Discussion
Our evaluation of virtual interviews demonstrated that this format can be effective way for applicants to evaluate fellowship training programs. Strengths of the virtual interview days included adequate length, number of breaks, organization/technical delivery, connection with interviewers, and the ability to assess the educational and cultural components of the program. There were also opportunities for improvement, such as the suggestion to shorten the length of the virtual interview day and the desire to meet more fellows, particularly in smaller groups or one-on-one. Based on our quantitative and qualitative survey data, we created a list of considerations for how to best design an effective virtual interview day to meet the needs of the applicant ().
Unlike a prior study which indicated that the virtual interview experience had a negative impact on applicant’s rank order[Citation10], our survey respondents reported that the virtual interview had a neutral or positive effect on anticipated rank order. This may reflect the fit of the program or personal factors more than the virtual interview itself, and may also be viewed differently during an all-virtual interview year. Interestingly, applicants expressed mixed responses about whether they would choose to interview virtually again if both virtual and in-person interview are offered. It is possible that the perception of virtual interviews will continue to improve as programs make changes and applicants become more familiar with this format. In fact, it may be that virtual interviews actually enhance the interview experience in certain ways (e.g., cost savings, decreased travel time, updated supplementary videos and materials, ability to engage faculty for optimal interview pairings given ease of virtual interviews, etc.). Alternatively, there may be certain features of the in-person experience that cannot be fully captured virtually despite best efforts.
After the COVID-19 pandemic, programs may choose to offer both virtual and in-person interviews to meet the needs of all applicants. In this case, it will be critical to evaluate the experience and outcomes of a hybrid approach to ensure equity[Citation16]. For example, if a program offers virtual interviews and optional in-person visits, the latter component could be coordinated by a faculty/staff member not involved in the applicant selection process and after program rank lists are determined.
This study has a number of notable strengths. First, it is one of the largest surveys of GME virtual interview applicant experiences published to date. Second, the 38-item survey comprehensively evaluates the structure of the virtual day and the ability to evaluate program culture using multiple-question types to collect both quantitative and qualitative data. Of note, responses to the Likert questions sometimes differed from the free-text responses. For example, most fellows reported that they were able to get an adequate sense of the surrounding city, but many fellows still indicated a preference to see the city in person. The detail provided by free-text comments offers an important perspective[Citation17], and may highlight areas that were adequately shown virtually but are still desired areas for improvement. Third, although this was a single-center study, it included applicants from eight subspecialty fellowship programs, each of which had a different format, allowing for comparisons between subspecialties and a more comprehensive picture of applicant experiences. Therefore, the results of this survey are generalizable across most IM subspecialties, and likely to residency programs as well.
This survey also has some limitations. First, the response rate was 30.7% (75/244), so there may have been response bias along applicants who had either a positive or negative experience. However, our qualitative data allowed us to gain a rich understanding of the virtual interview applicant experience, thus bolstering our survey conclusions. The response rate among applicants who identified as females and UIM were lower than the total number of applicants in each demographic group, although some survey respondents preferred not to provide demographic information (, Supplementary ). Second, we chose to send the survey immediately after each interview day and prior to the Fellowship NRMP Match Day. Although we emphasized that the results of the survey would not be viewed prior to Match Day, it is possible that some applicants did not respond due to concern for lack of anonymity and/or that applicants may have responded more positively. Third, there were more respondents in certain subspecialties, such as hematology/oncology and infectious diseases, so the experiences of applicants in these subspecialties were over-represented. Fourth, we did not ask applicants about their home residency institution or familiarity with the UCSF fellowship program prior to the virtual interview day, so it is possible that applicants who trained at UCSF may have had a better baseline understanding of the fellowship program and city. Finally, since all interviews were conducted virtually this year, there was no in-person interview comparison group. In the future, when both virtual and in-person interviews are possible it will be important to study the unique experiences of each to better compare the interview formats.
Conclusions
In summary, this study provides a comprehensive assessment of the virtual interview experience from the applicant perspective across eight IM subspecialty fellowship programs at our institution, highlighting strengths of this format and areas for improvement. The findings from this study can inform future virtual interviews for GME training programs, which is particularly relevant in the era of COVID-19 and may continue as a common practice after the pandemic.
Disclosures
LAH, LF, JAF, LSG, AQ, BSS, EW, CZ, GH, JMB have no relevant disclosures
NE receives funding from Finch Therapeutics, Federation Bio, Assembly Biosciences and Freenome for microbiome-related research.
ECH serves in a volunteer capacity on the registry advisory board of the International Fibrodysplasia Ossificans Progressiva Association, the International Clinical Council on FOP, and on the Fibrous Dysplasia Foundation Medical Advisory Board. ECH receives clinical trials research support through his institution from Clementia Pharmaceuticals Inc., an Ipsen company, and Neurocrine Biosciences, Inc. ECH received prior clinical trials funding through his institution from Regeneron Pharmaceuticals.
RRK has received licensing income from Voalte, Inc, which is unrelated to the current study.
Supplemental Material
Download MS Word (34.1 KB)Acknowledgments
We thank the members of the UCSF Department of Medicine GME office and Fellowship Programs, including the Fellowship coordinators.
Supplemental data
Supplemental data for this article can be accessed here.
Disclosure statement
·LAH, LF, JAF, LSG, AQ, BSS, EW, CZ, GH, JMB have no relevant disclosures.
·NE receives funding from Finch Therapeutics, Federation Bio, Assembly Biosciences and Freenome for microbiome related research.
·ECH serves in a volunteer capacity on the registry advisory board of the International Fibrodysplasia Ossificans Progressiva Association, the International Clinical Council on FOP, and on the Fibrous Dysplasia Foundation Medical Advisory Board. ECH receives clinical trials research support through his institution from Clementia Pharmaceuticals Inc., an Ipsen company, and Neurocrine Biosciences, Inc. ECH received prior clinical trials funding through his institution from Regeneron Pharmaceuticals.
·RRK has received licensing income from Voalte, Inc, which is unrelated to the current study..
References
- Conducting Interviews During the Coronavirus Pandemic. AAMC. Accessed June 24, 2020. https://www.aamc.org/what-we-do/mission-areas/medical-education/conducting-interviews-during-coronavirus-pandemic
- Kim RH, Gilbert T, Suh S, et al. General surgery residency interviews: are we following best practices? Am J Surg. 2016;211(2):476–481.e3.
- Makdisi G, Takeuchi T, Rodriguez J, et al. How we select our residents—a survey of selection criteria in general surgery residents. J Surg Educ. 2011;68(1):67–9.
- Huppert LA, Hsiao EC, Cho KC, et al. Virtual interviews at graduate medical education training programs: determining evidence-based best practices. Published online. Acad Med. 2020 December 8
- Pasadhika S, Altenbernd T, Ober RR, et al. Residency interview video conferencing. Ophthalmology. 2012;119(2):426–426.e5.
- Shah SK, Arora S, Skipper B, et al. Randomized evaluation of a web based interview process for urology resident selection. J Urol. 2012;187(4):1380–1384.
- Edje L, Miller C, Kiefer J, et al. Using skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503–505.
- Daram SR, Wu R, Tang S-J. Interview from anywhere: feasibility and utility of web-based videoconference interviews in the gastroenterology fellowship selection process. Am J Gastroenterol. 2014;109(2):155–159.
- Vadi MG, Malkin MR, Lenart J, et al. Comparison of web-based and face-to-face interviews for application to an anesthesiology training program: a pilot study. Int J Med Educ. 2016;7:102–108.
- Healy WL, Bedair H. Videoconference interviews for an adult reconstruction fellowship: lessons learned. JBJS. 2017;99(21):e114.
- Vining CC, Eng OS, Hogg ME, et al.Ann Surg Oncol. 2020 May 18;1–5. Published online.
- Artino AR, La Rochelle JS, Dezee KJ, et al. Developing questionnaires for educational research: AMEE guide no. 87. Med Teach. 2014;36(6):463–474.
- American of Medical Colleges. Underrepresented in medicine definition. Accessed January 8, 2021. https://www.aamc.org/what-we-do/diversity-inclusion/underrepresented-in-medicine
- Medical Minority Applicant Registry (Med-MAR). Accessed January 14, 2021. https://students-residents.aamc.org/choosing-medical-career/article/medical-minority-applicant-registry-med-mar/
- Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288.
- Marbin J, Y-v H, Schaeffer S. Avoiding the virtual pitfall: Identifying and mitigating biases in graduate medical education videoconference interviews. Acad Med J Assoc Am Med Coll. 2021 January 12. Published online.
- Riiskjær E, Ammentorp J, Kofoed P-E. The value of open-ended questions in surveys on patient experience: number of comments and perceived usefulness from a hospital perspective. Int J Qual Health Care. 2012;24(5):509–516.
