Abstract
Three different Lactuca sativa L. varieties (Iceberg, Romaine and Baby head) were analysed in order to determine differences in the antioxidant activity, both hydrophilic and lipophilic, and in the total phenolic content of different leaves (stem, inner, medium and outermost leaves). Romaine showed the highest level of hydrophilic and lipophilic antioxidant activity, and its phenolic content was also higher than that of Iceberg and Baby head. According to leaf position, lipophilic antioxidant activity increased sharply from stem to outermost leaves, suggesting a protective role for the lipophilic antioxidant in mature or light-exposed leaves, while hydrophilic antioxidant activity shows a non-specific distribution. The phenolic content also increased in Romaine from stem to outermost leaves, although no significant changes were observed in Iceberg or Baby head lettuces in this respect.
Introduction
Lettuce (Lactuca sativa L.) is a widely used as minimally processed food product in the preparation of salads and ready-to-eat foodstuffs because it has a long shelf life storage and is beneficial for human health.[Citation1,Citation2] Many phenolic compounds are antioxidants that may contribute to reducing human diseases, including the risk of cancer and heart diseases. The beneficial effect of eating a diet rich in fruits and vegetables has, in part, been attributed to the increased consumption of phenolic compounds with antioxidant properties.[Citation3,Citation4] These compounds reduce the oxidative damage that has been linked to arteriosclerosis, brain disorder and cancer.[Citation5–8] In addition to polyphenols, several authors have reported an important role for other plant secondary metabolites in preventing diseases.Citation9,Citation10] For example, since carotenoids are not synthesized by human tissues, these molecules have to be obtained from foods. They are also used as antioxidantCitation11,Citation12] in order to restore natural levels in processed foods or to obtain fortified products. The growth pattern of lettuce head, where the outermost leaves enwrap the inner ones, thus determining the metabolism of the latter, which are less green due to undeveloped chloroplasts, represents an ideal model for investigating the possible gradient of antioxidants in leaves. It has previously been found that the synthesis of polyphenols and other secondary metabolites are directly related with the photosynthetic activity of tissues and the mediation of phytochrome and/or cryptochrome.Citation13,Citation14]
Despite the importance of antioxidants for human health, information regarding variations in the overall antioxidant activity of plant tissues is poor or indirect. Furthermore the data that are available mostly refer to measurements of particular antioxidants, which do not provide information on the total antioxidant activity. Any evaluation of this property must take into account the methodology used and the relative importance of the food matrix.[Citation15] There are several methods currently used for determining antioxidant activity, which are based on the generation of radical species.[Citation16–18] Recently, we adapted our end-point method to determine the antioxidant potential of lipophilic extracts,[Citation19] meaning that both hydrophilic and lipophilic antioxidant activities can be estimated using the same chromogen radical and similar conditions.[Citation20,Citation21] In this respect, many hydrophilic antioxidant methods are not suitable for or adaptable to lipophilic measurements and therefore for obtaining a real measurement of the total antioxidant capacity of foods. To obtain such reliable data, hydrophilic and lipophilic antioxidant measurements using the same chemical principle are necessary. The object of this study was to determine the possible gradient of hydrophilic and lipophilic antioxidant activity, and the total phenol content of the steam and different leaves of a number of lettuce varieties (Iceberg, Romaine and Baby head).
Materials and Methods
Reagents
2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as the crystallized diammonium salt and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma Chem. Co (Madrid, Spain). H2O2 (30%, v/v) was obtained from Aldrich Chem. Co. (Madrid, Spain). The concentrations of ABTS and hydrogen peroxide were determined by measuring their absorbance using ϵ340 nm = 36 mM−1 cm−1 for ABTS, ϵ240 nm = 43.6 M−1 cm−1 for H2O2. Horseradish peroxidase (HRP) type VI was obtained from Sigma (ϵ403 nm = 100 mM−1 cm−1).[Citation22]
Spectrophotometry
Spectrophotometric measurements were recorded with a Perkin-Elmer Lambda-2S UV-VIS spectrophotometer interfaced on-line with a PC-computer. The temperature was controlled at 25 ± 0.1° C using a Haake D1G circulating bath with a heater/cooler.[Citation22]
Plant Material
Lettuce head (Lactuca sativa L.) varieties Iceberg, Romaine and Baby head were obtained from the Sociedad Cooperativa Coáguilas (Águilas, Murcia, S.E. Spain). All the lettuce heads were sorted into outer, medium and inner leaves, and stem. Extractions were carried out using one gram of fresh lettuce material, homogenized in 10 mL of 50 mM Na phosphate (pH 7.5) in a Euroturrax T20 (IKA, Germany) for 1 min. The homogenate was centrifuged for 10 min at 4° C at 1000 × g in a Sorvall RC5B Plus (Dupont, USA), and the supernatant was transferred to a decantation funnel. The pellet was re-suspended in 10 mL of ethyl acetate, homogenized in a Euroturrax T20 and centrifuged for 10 min at 4° C at 1000 × g, after which the supernatant was transferred to the decantation funnel. This procedure was performed twice, until a colourless pellet was obtained. The different extractions were mixed and allowed to separate in the funnel and then the two phases (organic and aqueous) were collected. The aqueous phase was analyzed immediately for hydrophilic antioxidant activity and total aqueous phenols. The organic phase was evaporated under a stream of nitrogen, and stored at −20° C until analysis, when it was re‐dissolved in 4 mL of ethyl acetate. An aliquot of 1 mL was used to measure lipophilic antioxidant activity. The remaining 3 mL were dried under nitrogen and washed with 1 mL of methanol (70%) to measure the total phenolic compounds present in the organic phase. In all cases, three replicate analyses were performed.
Antioxidant Activity
The antioxidant activity was measured using the ABTS/HRP decolouration method,[Citation18,Citation19] which is based on the capacity of different components to scavenge the ABTS radical cation (ABTS•+) compared to a standard antioxidant (Trolox) in a dose-response curve. For the hydrophilic antioxidant activity (HAA), the reaction mixture contained 2 mM ABTS, 30 μM H2O2 and 0.25 μM HRP in 50 mM Na phosphate buffer (pH 7.5) in a total volume of 1 mL. The assay temperature was 25° C. The reaction was monitored at 730 nm until absorbance became stable. Then, 10 μL of the aqueous phase was added to the reaction medium and the decrease in absorbance, which is proportional to the ABTS•+ quenched, was determined after 5 min. For the lipophilic antioxidant activity (LAA), the reaction mixture contained 1 mM ABTS, 30 μM H2O2 and 6 μM HRP in acidified ethanol (phosphoric acid 0.7%, v/v), in a total volume of 1 mL. In this case, 10 μL of the organic phase were added to the reaction medium and the decrease in absorbance at 730 nm was determined after 5 min. The total time needed to carry out each assay was approximately 6 min. The absorbance decrease was determined from the difference between the A730 values before and after addition of sample. In both cases, the antioxidant activity was expressed as Trolox equivalents per fresh weight (mg·100 g−1 FW).
Total Phenolic Content
Folin-Ciocalteu's reagent (Panreac, Spain) was used to determine the total content of phenolic compounds. 500 μL of sample was placed in a glass test tube, and 1 mL of water (Milli-Q) and 2.5 mL of Folin-Ciocalteu's reagent (ten-fold diluted) were added. The reaction medium was allowed to react in the dark for 1 h, and the absorbance at 755 nm was measured. The results were expressed as mg of gallic acid equivalents (GAE) per 100 g FW.[Citation23]
Statistical Analysis
For statistical analysis of the results the SPSS program was used, applying the LSD multiple range test to establish significant differences between the parameters being evaluated.
Results
Variations in the hydrophilic antioxidant activity (HAA) are shown in , where it can be seen that Romaine was significantly different from Iceberg and Baby head (p < 0.05). Taking into account the leaf type, only in Romaine was the highest hydrophilic antioxidant capacity obtained in the external leaves, while in Iceberg and Baby head, the inner leaves showed the highest HAA levels, although the differences were very small in the case of Iceberg variety. Romaine leaves showed HAA values 10–25 times higher than the other varieties.
Table 1 Antioxidant activity (HAA and LAA) in the different leaf-types from the three lettuce varieties assayed.
As regards lipophilic antioxidant activity (LAA), Romaine lettuce also differed from Iceberg or Baby head (p < 0.05). In the three varieties, LAA increased from the stem to the outermost leaves (), with significant difference between each type of leaf analysed (p < 0.05). The steepest gradient was observed in Romaine, from 3.6 to 15.4 mg TE 100 g−1, which represented an increase of around 330%; in the Iceberg, the increase was less pronounced from 1.5 to 5.5 mg TE 100 g−1, representing about the 266%. In Baby head the increase was about 30%, from 2.4 to 3.1 mg TE 100 g−1. The LAA of the stem was practically the same in all three types assayed.
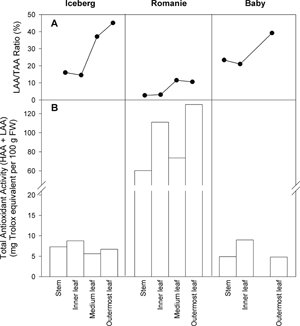
shows the values of total antioxidant activity (TAA) in the leaves and stem of each lettuce variety. TAA is obtained as the sum of HAA and LAA values because in both determinations the same principle was used to obtain the antioxidant activities. Romaine showed the highest values due, mainly, to the contribution of HAA. The TAA values of Iceberg and Baby head were very similar and about 10 times lower than in Romaine lettuce. An important aspect here was the contribution of LAA to TAA (). Thus, Iceberg and Baby head presented higher LAA/TAA ratios than Romaine because of its high HAA value. As can be seen in , all three types presented the highest LAA/TAA ratios in the most external leaves, pointing to a significant role of photosynthetic pigments in LAA.
Figure 1 Total antioxidant activity in the leaves and stem of Iceberg, Romaine and Baby head lettuce varieties. Panel A: LAA/TAA ratios in each leaf-type. Panel B: TAA as HAA + LAA in each leaf-type for the three different lettuce varieties assayed.
The phenolic content of the lettuce leaves is shown in . The highest phenolic content was obtained in the aqueous phase, Romaine especially, showing an aqueous phenolic content two-times higher than the other varieties. Slight changes were observed in the aqueous phenol levels between the different leaves, although the greatest increase was once again observed in Romaine lettuce (from 2.6 to 8.5 mg GAE 100 g−1 FW). In addition, Romaine leaves had a high phenolic content in all leaf types assayed.
No changes were observed in the organic phenol content of the leaves assayed in Iceberg and Baby lettuce, and only Romaine lettuce showed a significant increase in the content of organic phenolic compounds content from stem to outermost leaves (from 0.5 to 1.9 mg GAE 100 g−1 FW). The total phenolic content (organic plus aqueous phenolic content) was higher in Romaine lettuce than in the other two varieties assayed and, as regards the different leaves, the highest levels of phenolics were obtained in the most photosynthetic ones, the outermost leaves.
Table 2 Total phenolic content in the different leaf-types from the three lettuce varieties assayed.
Discussion
One of the greatest advantages in determining antioxidant activity is that individual compounds need not be measured. However, in the case of plant material, polyphenols are an important group of compounds, which contribute to the antioxidant activity. In our search for a relationship between the phenolic content and antioxidant activity, only in the Romaine lettuce a good correlation was observed between HAA and aqueous phenols (r2 = 0.90, p < 0.05), and between LAA and organic phenols (r2 = 0.97, p < 0.05). However, these correlations do not imply any quantitative correlation in each tissue analyzed. In all cases, the phenolic content was less than the corresponding HAA or LAA. For example, Romaine lettuce had about double the phenolic content of the other lettuces, but between 10–25 times more HAA than the others (Tables and ). These finding indicate that compounds other than phenolics contribute to the antioxidant activities. However, it is important to take into consideration that different phenolic compounds can contribute in different ways to HAA because their relative antioxidant activity differs widely.[Citation24,Citation25] In the same way, other families of compounds (for example: carotenoids, glucosinolates, organic acids and aminoacids) with different potentials may contribute to the total antioxidant activity. For this reason it is very important to obtain real TAA measurements by methods or tests that permit HAA and LAA to be summed. At the present time, only our methods and the Oxygen Radical Absorbance Capacity with fluorescein (ORACFL)Citation26,Citation27] method allow this.
From a nutritional point view, Romaine variety is more interesting than the other varieties since it presents the highest HAA, LAA and TAA values (). The three varieties studied present an increasing gradient of LAA from the stem to the outermost leaves, while HAA shows a non-specific distribution. However, Iceberg and Baby head varieties present a higher LAA/TAA ratio than Romaine (). The LAA/TAA ratio permits us to know the contribution of lipophilic antioxidants to the total antioxidant activity, which can be used to compare different plant/food varieties. This parameter is especially relevant in products rich in carotenoids or lipidic components, such as tomato, leafy vegetables, oily fruits, nuts, etc., and also for studying non-green (etiolated) materials.[Citation27] The LAA/TAA ratios point to a more relevant role for lipophilic antioxidants with respect to the total activity in the order: Iceberg > Baby > Romaine. The LAA gradient may exist because the outermost leaves present more developed photosynthetic tissues with a high lipophilic antioxidant content, possibly carotenoids, tocopherols and plastoquinones.[Citation28] This same behaviour is apparent during leaf ontogeny, where the youngest leaves present the lowest LAA, while a higher carotenoid content is related with a higher LAA and older-leaves.[Citation29] Lastly, knowledge of the different TAA values (and the LAA/TAA ratios) in the different types of leaf could be of interest for selecting suitable leaves to make salads or other ready-to-eat mixed vegetable dishes.
Acknowledgments
This work was supported by the projects: 502/PI/04 of Fundación Séneca de la Comunidad Autónoma de Murcia, and MCyT-AGL2003-00638 (co-financed by FEDER). Antonio Cano had a post-doctoral grant from the University of Murcia.
Notes
xValues are mg Trolox equivalent per 100 g of fresh weight.
yValues followed by different letter are significantly different at the 0.05 level.
xValues are mg Gallic acid equivalent per 100 g of fresh weight.
yValues followed by different letter are significantly different at the 0.05 level.
References
- Ferreres , F. , Gil , M.I. , Castaner , M. and Tomás-Barberán , F.A. 1997 . Phenolic metabolites in red pigmented lettuce. Changes with minimal processing and cold storage . J. Agric. Food Chem. , 45 : 4249 – 4254 .
- Serafini , M. , Bugianesi , R. , Salucci , M. , Azzini , E. , Rguzzini , A. and Maiani , G. 2002 . Effect of acute ingestion of fresh and stored lettuce (Lactuca sativa) on plasma total antioxidant capacity and antioxidant levels in human subjects . British J. Nutr. , 88 : 615 – 623 . [CROSSREF]
- Ames , B.N. , Shigenaga , M.K. and Hagen , T.M. 1993 . Oxidant, antioxidant, and the degenerative diseases of aging . Proc. Natl. Acad. Sci. USA , 90 : 7922 – 7915 .
- Hertog , M.G.L. , Feskens , E.J.M. , Hollman , P.C.H. , Katan , M.B. and Kromhout , D. 1993 . Dietary antioxidant flavonoids and the risk of coronary heart disease: the Zutphen Elverly Study . The Lancet , 342 : 1007 – 1011 . [CROSSREF]
- Halliwell , B. and Gutteridge , J.M.C. 1999 . Free Radicals in Biology and Medicine, , 3rd , New York : Oxford University Press .
- Cantuti-Castelvetri , I. , Shukitt-Hale , B. and Joseph , J.A. 2000 . Neurobehavioral aspects of antioxidants in aging . Intl. J. Dev. Neurosci , 18 : 367 – 381 . [CROSSREF]
- Temple , N.J. and Gladwin , K.K. 2003 . Fruit, vegetables, and the prevention of cancer. Research challenges . Nutrition , 19 : 467 – 470 . [PUBMED] [CROSSREF]
- Bub , A. , Watzl , B. , Blockhaus , M. , Briviba , K. , Liegibel , U. , Muller , H. , Pool-Zobel , B.L. and Rechkemmer , G. 2003 . Fruit juice consumption modulates antioxidative status, immune status and DNA damage . J. Nutr. Biochem. , 14 : 90 – 98 . [PUBMED] [CROSSREF]
- Franceschi , S. , Biddi , E. , Vecchia , C. , Talamini , R. , D'Avanzo , B. and Wegni , E. 1994 . Tomatoes and risk of digestive-tract cancers . Inter. J. Cancer , 59 : 181 – 184 .
- Mayne , S.T. , Janerich , D.T. , Greenwald , P. , Chorost , S. , Tucci , C. , Zaman , M.B. , Melamed , R. , Kiely , M. and McKneally , M.F. 1996 . Dietary β-carotene and lug cancer risk in U.S. nonsmokers . J. Nat. Cancer Inst. , 86 : 33 – 38 .
- Burton , G.W. and Ingold , K.U. 1984 . β-carotene: a usual type of lipid antioxidant . Science , 244 : 569 – 573 .
- Palozza , P. and Krinsky , N.I. 1991 . The inhibition of radical-initiated peroxidation of microsomal lipids by both α-tocopherol and β-carotene . Free Rad. Biol. Med. , 11 : 407 – 414 . [PUBMED] [CROSSREF]
- Furuya , M. 1993 . Phytochromes: their molecular species, gene families, and function. Annu. Rev. Plant Physiol . Plant Mol. Biol. , 44 : 617 – 645 . [CROSSREF]
- Short , T.W. and Briggs , W.R. 1994 . The transduction of blue light signal in higher plants. Annu. Rev. Plant Physiol . Plant Mol. Biol. , 45 : 143 – 171 . [CROSSREF]
- Dekker , M. , Verkerk , R. , Van der Sluis , A.A. , Khokhar , S. and Jongen , W.M.F. 1999 . Analysing the antioxidant activity of food products: Processing and matrix effects . Toxicol. in Vitro , 13 : 797 – 799 . [CROSSREF]
- Rice-Evans , C.A. and Miller , N.J. 1994 . Total antioxidant status in plasma and body fluids . Meth. Enzymol. , 234 : 279 – 293 . [PUBMED]
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antoixidant activity . Lebensm.-Wiss. u.-Technol , 25 : 28 – 30 .
- Cano , A. , Hernández-Ruiz , J. , García-Cánovas , F. , Acosta , M. and Arnao , M.B. 1998 . An end-point method for estimation of the total antioxidant activity in plant material . Phytochem. Anal. , 9 : 196 – 202 . [CROSSREF]
- Cano , A. , Acosta , M. and Arnao , M.B. A . 2000 . method to measure antioxidant activity in organic media: application to lipophilic vitamins . Redox Rep. , 5 : 365 – 370 . [PUBMED] [CROSSREF]
- Arnao , M.B. , Cano , A. and Acosta , M. 2001 . The hydrophilic and lipophilic contribution to total antioxidant activity . Food Chem. , 73 : 239 – 244 . [CROSSREF]
- Cano , A. , Acosta , M. and Arnao , M.B. 2003 . Hydrophilic and lipophilic antioxidant activity changes during on-vine ripening of tomatoes (Lycopersicon esculentum Mill.) . Postharvest Biol. Technol. , 28 : 59 – 65 . [CROSSREF]
- Hiner , A.N.P. , Hernández-Ruiz , J. , García-Cánovas , F. , Arnao , M.B. and Acosta , M. 1996 . A comparative study of the purity, enzyme activity and inactivation of commercially available horseradish peroxidase isoenzyme A and C . Biotechnol. Bioengin. , 50 : 655 – 662 . [CROSSREF]
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenols with phosphomolibdic-phosphotungstic acid reagents . Am. J. Enol. Vitic. , 16 : 144 – 158 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Rad. Biol. Med. , 20 : 933 – 956 . [PUBMED] [CROSSREF]
- Arnao , M.B. , Cano , A. and Acosta , M. 1999 . Methods to measure the antioxidant activity in plant material. A comparative discussion . Free Rad. Res. , 31 : S89 – S96 .
- Prior , R.L. , Hoan , H. , Gu , L. , Wu , X. , Bacchiocca , M. , Howard , L. , Hampsch-Woodill , M. , Huang , D. , Ou , B. and Jacob , R. 2003 . Assay for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological food samples . J. Agric. Food Chem. , 51 : 3273 – 3279 . [PUBMED] [CROSSREF]
- Wu , X. , Gu , L. , Holden , J. , Haytowitz , D.B. , Gebhardt , S.E. , Beecher , G. and Prior , R.L. 2004 . Development of a database for total antioxidant capacity in foods: a preliminary study . J. Food Compos. Anal. , 17 : 407 – 422 . [CROSSREF]
- Niyogi , K.K. 1999 . Protection revisited: genetic and molecular approaches . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 50 : 333 – 359 . [PUBMED] [CROSSREF]
- Arnao , M.B. , Cano , A. , Alcolea , J.F. and Acosta , M. 2001 . Estimation of free radical-quenching activity of leaf pigment extracts . Phytochem. Anal. , 12 : 138 – 143 . [PUBMED] [CROSSREF]