Abstract
The apricot variety in Japan is “Prunus mume Sieb. et Zucc” (Ume). The Japanese have been growing Ume tree for more than 2000 years because of its health enhancing effects, and through cultivation, they have improved the Ume tree to produce healthier fruits. The purpose of this study was to investigate presence of anti-cancer substances in Ume. One kg Ume was squeezed to separate the soft fruit from the seed capsules. The soft Ume fraction was boiled at 90°C to 100°C to obtain 20 g of semi-solid Ume (misatol). The condensed fruit was dissolved in a water-diethylether. The fraction in diethylether was dried and further fractionated for experiments. The anti-cancer effects of the Ume were investigated on 2 established cancer cells—the Kato-III stomach cancer and the HL-60 promyelocytic leukaemia cell lines. Kato-III and HL-60 cells were grown in RPMI-1640 medium containing 10% foetal calf serum. The Ume extract in dimethyl sulphoxide was added to the cancer cell cultures at 1–10 μL/mL test (1 μL contained 20 μg extract). Without Ume, the cancer cells grew and formed colonies. When the Ume extract was added, cancer cells were dose dependently eliminated and at < 5 μL/mL, no cancer cell survived. Similarly, the condensed Ume showed strong anti-tumour effects on human pancreatic cancer and dog fibrosarcoma. The Ume preparation showed no toxic effect on normal human blood cells. In conclusion, this is the first study showing unequivocally presence of an anti-cancer agent in Ume fruit with both suppressive effect on the growth of cancer cells and lethal action on the already formed cancer cell colonies. As an abundant source of natural anti-cancer substance, Ume should have therapeutic benefit in tumour-bearing patients. Further, regular intake of Ume juice should suppress cancer initiation in healthy individuals.
INTRODUCTION
Many fruits contain nutrient substances that can prevent, cure or suppress various diseases, including cancer. They are nature's true medicines, and diets rich in fruits are consistently associated with a decreased risk of cancer and other chronic diseases. Apricot “Prunus armeniaca” is the fruit of a rosaceous tree (rosaceae), which is produced in most parts of the world.[Citation1] The name Prunus armeniaca is thought to be a misnomer based upon the long-held view that apricots initially originated in Armenia. It is now known that apricots originated in the Far East, most likely in the Himalayas, the Northern and Western regions of China from where they spread to Armenia and Russia.[Citation2,Citation3] The wild population in the hills of the South West Asia (including Armenia, from where Western botanists named the species) is regarded as a secondary center of diversity.[Citation1] Apricot is found semi-wild and wild in the northern hills of China and in a broad belt across the hills, mountains, and plateaus of Central Asia as far as the Caucasus Mountains. The first record of the domestication of apricots is an account of their cultivation in China, attributed to Emperor Yu, about 4000 years ago. It is likely that the tribe people of Central Asia established traditional rights to harvest their parts of the apricot forests for millennia before this time.
The apricot variety found in Japan is “Prunus mume Sieb. et Zucc” widely known as Ume. The Japanese started to grow Ume trees more than 2000 years ago, most likely imported from China. Soon they discovered the health enhancing effects of apricot fruits, and over many centuries, through cultivation, they have improved their apricot tree variety to produce healthier fruits. Accordingly, there is a long-standing view that the Japanese apricot juice can suppress cancer in tumour bearing hosts. This study was undertaken to investigate presence of anti-cancer substances in the Japanese apricot juice.
MATERIALS AND METHODS
Preparation of Condensed Ume Fruit
One kg Ume; about 30 Japanese apricot fruits (), harvested from Kanzawa farm in Gunma Prefecture, Japan, was squeezed to separate the soft fruit from the seed capsules. Then, the soft fruit was boiled at 90°C to 100°C for 7 days to yield about 20 g of semi-solid product (Misatol, Japanese apricot). This condensed Ume fruit was dissolved in a mixture of water and diethylether for extracting various investigational ingredients described below.
Preparation of Ume Extract
A low viscosity gel-like preparation was obtained by dissolving 3 g of condensed Ume fruit in 10 mL distilled water. This 10 mL gel-like preparation was added to 20 mL diethylether (Wako, Japan) and was mixed in an airtight container. This solution was gently agitated for 1 hour and after agitation, 2 separate layers had formed—the upper diethylether and the lower aqueous medium. To improve the separation, the container was centrifuged at 1500 rpm for 60 minutes. The diethylether fraction was filtrated through a filter paper (Toyoroshi, No. 2) and applied to a rotary evaporator (Tokyorika Kikai Inc., Japan) to obtain a dried Ume fruit powder. A 100 mg sample of the dried Ume was dissolved in 5mL dimethyl sulphoxide (DMSO) to obtain a 20 mg/mL stock Ume solution in DMSO for experiments. We investigated the anti-cancer activity of Ume by using the DMSO solution and also after further purification and fractionation to identify the active components on cancer cells.
Studies on Cancer Cells
The anti-cancer effects of the Ume extract was investigated on two human cancer cell lines, the HL-60 and the Kato-III. The former is an established human promyelocytic leukaemia cell line,[Citation4] while the latter is an established human gastric carcinoma cell line.[Citation5] For investigating the anti-cancer activities of the Ume fruit extract on tumour cell lines (HL-60 and Kato-III), the cells were grown in a culture medium containing RPMI–1640 (Sigma) with 10% foetal calf serum (FCS). Samples of human blood cells from a young volunteer were treated in the same manner to serve as control for any likely cell toxicity. Test medium complement was inactivated by heating at 56°C for 30 minutes.[Citation6] Each cell line was investigated at a final concentration of 1 × 105 cells/mL, seeded onto a 24-well culture plate (Sumitomo Bakelite Inc., Japan), at a volume of 1mL/well. The Ume solution in DMSO was added at 1 to 10 μL/mL to the wells. The same volume of DMSO solution was added to separate tests to serve as control. Similarly, the RPMI–1640 medium containing cells without Ume was used as positive control. The effects on cancer cells were investigated at 1, 2, and 3 days after incubation or as indicated otherwise. Cell morphology was observed by light microscopy. The cells were fixed and stained by Diff Quick solution (Kokusaisiyaku Inc., Japan) as described previously.[Citation7] The results are presented in and and and .
Table 1 Total inhibition of HL-60 promyelocytic leukaemia cell growth by Prunus mume Sieb. et Zucc (Ume) extract. The medium (RPMI-1640) with cells, but without Ume extract was used as positive control, while DMSO solution was added as control for the Ume. Data are presented as the mean ± SD values for n = 3 separate observations
Table 2 Total inhibition of Kato-III cell growth by Prunus mume Sieb. et Zucc (Ume) extract. The medium (RPMI-1640) with cells, but without Ume extract was used as positive control, while DMSO was added as control for the Ume extract. Data are presented as the mean ± SD values for n = 3 separate observations
Figure 2 Suppressive effects of the Japanese apricot, Ume (prunus mume Siev. et Zucc) on the growth of cancer cells. HL-60 promyelocytic leukaemia cell lines and Kato-III stomach cancer cell lines were grown in culture in the presence and absence of the Ume extract at 2 μL/mL. A), growth of HL-60 cells in the presence of the vehicle; B), HL-60 cells grown in the presence of Ume extract, no viable cell was found in the test sample; C), growth of Kato-III cells in the absence of the Ume extract; D), Kato-III cells grown in the presence of Ume extract. No viable cell was found in this test sample. E), human blood cells incubated in the presence of vehicle for 24 hr; and, F) human blood cells incubated in the presence of 10 μL/mL Ume extract. There was no loss of viability or cell damage in E and F.
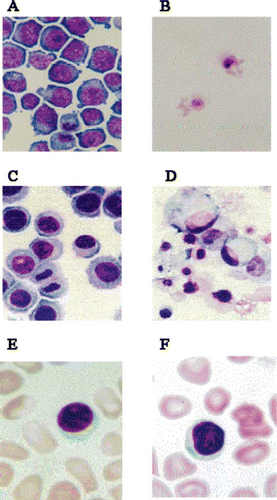
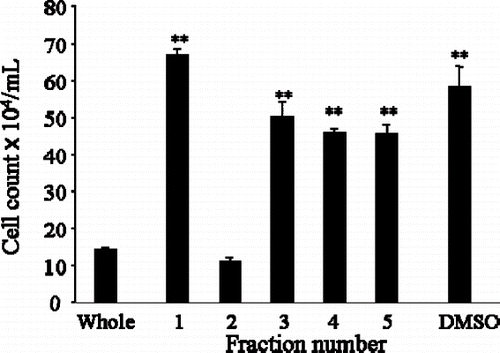
Figure 3 Silica gel chromatography column fractionated Japanese apricot juice product, Ume (Prunus mume Sieb. et Zucc). The extract derived from the condensed Ume juice killed human cancer cell lines, HL-60 and Kato-III cells shown in . This solution was subjected to Silica gel column and 5 fractions (Fra1 – Fra5) were collected, and after drying, they were dissolved in DMSO and individual fractions were tested on HL-60 promyelocytic cancer cell line. Only Fra2 showed inhibitory effects, other fractions were devoid of growth inhibition. ∗∗P < 0.01.
Determination of the Anti-Cancer Fraction of Ume
Starting from the hydrophobic extract described above, we proceeded to further fractionation to identify the anti-cancer substance(s) in the Ume fruit. The fractionation was done by applying a silica gel cromatography column (Wako Gel C-300) and following the instrument's operation manual. The mixed solvent for column chromatography was chloroform and methanol (CHCl3:CH3OH, 20:1; vol/vol). The dried Ume extract was dissolved in the mixed solvent (CHCl3:CH3OH) and applied to the silica gel column. Five fractions were obtained (Fra1 to Fra5). In each fraction, a dried powder was obtained after evaporating the organic solvents, and the resulting powder was dissolved in DMSO to a final concentration of 20 mg/mL DMSO. The anti-cancer activities of Fra1-Fra5 on HL-60 promyelocytic leukaemia cells were evaluated by investigating inhibition of cancer cell growth in the presence of the extract. HL-60 cells (3 × 106/mL) were cultured in RPMI–1640 as described above in the presence of 5 μL/well Ume extracts (Fra1-Fra5). A 5 μL of DMSO was added to serve as control, while 5μL of the culture medium was added to serve as positive control. To investigate the mechanism of anti-cancer effect, the morphology of the target cells was examined in a light microscope by using a published method.[Citation7]
RESULTS
Effects on Kato-III and HL-60 Cancer Cells
The hydrophobic fraction of the Ume fruit product that was collected in diethylether (after drying and dissolution in DMSO) was used to investigate the suppressive effects of the Ume fruit juice on the growth of HL-60 promyelocytic leukaemia cells and the Kato-III stomach cancer cells. The results are presented in and , and show complete inhibition of cancer cell growth. The lower fraction that separated in water (hydrophilic) during diethylether-water separation procedure was totally devoid of anti-cancer action (results not presented). As control vs Ume extract, tests were also run with the vehicle (DMSO), while another set of tests were run with the cells in the culture medium without Ume or DMSO. The inhibitory effects against HL-60 cells were observed just 1 day after incubation, and virtually no viable cell could be found at any concentration. In contrast, the inhibitory effect against Kato-III cells depended on the concentration of the Ume extract. However, after 3 days and at 5 μL/mL test, no viable Kato-III cell was detected. At a 2 μL/mL, Kato-III cells did not show proliferation, but the cell number did not change for 3 days. At a concentration of 1 μL/mL, the extract did not affect Kato-III cell proliferation. Further, the preparation at any concentration had no toxic effects on human blood cells (). To investigate the mechanism of anti-cancer effect, we observed the morphology of the target cells in a light microscope. When HL-60 and Kato-III were cultured in the presence of the Ume extract, the cells developed vacuoles and subsequently died at concentrations < 5μL/mL Ume extract. In , typical morphological changes in HL-60 and Kato-III cells are presented after being cultured for 3 days in the presence of 2μL/mL Ume extract or the vehicle. No viable cell could be found in the presence of the Ume extract.
Outcome of Fractionation
shows inhibition of HL-60 cell growth by fractionated Ume juice (“Prunus mume Sieb. et Zucc”). Five fractions (Fra1–Fra5) were collected from the silica gel column. Each fraction was separately tested against the growth of HL-60 promyelocytic leukaemia cell line. In , the first bar shows the inhibitory effects of unfractionated Ume extract and shows strong growth inhibitory effects. Bars 1 to 5 show the effects of Fra1-Fra5. Only Fra2 had the inhibitory effects seen with the unfractionated Ume extract; other fractions were devoid of HL-60 cell growth inhibition.
Anti-Tumour Effect on Fibrosarcoma
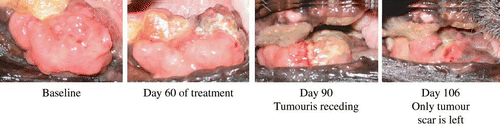
shows the effect of the unfractionated Ume extract on fibrosarcoma in a dog. Following the intriguing observations on Kato-III and HL-60 cancer cell lines, we attempted to investigate the effects of the Ume juice product on a spontaneously appearing and rapidly growing tumour in a 14-yr old German Shepherd dog. The tumour was in the gum. Tumour size was 50 mm (thickness) by 30 mm (width). We prepared a 5 mL solution of the extract that is equal to 1 g of the condensed Ume fruit described in the first section of Materials and Methods. This 5 mL preparation was added to 100 mL milk and was provided to the dog, which the dog slowly drank. Two days after taking the Ume, a 2 × 5 mm piece of the tumour fell off from the body of the tumour. Immediately, we fixed this specimen in formaldehyde solution followed by staining with haemotoxyline-eosin (HE), anti-bimentin antibody, anti-actin antibody for pathological examinations.[Citation8] The tumour was identified to be fibrosarcoma (staining was HE+, bimentin+ and actin−). In the dog, the tumour began to shrink soon after taking the Ume juice. We continued feeding the dog with the Ume juice once a day until the animal was virtually tumour free. The inhibition of growth of the tumour is thought to proceed in the following stages. First stage, inhibition of angiogenesis[Citation9] followed by apoptosis of tumour cells.[Citation10] Dying of the tumour tissue is shown in . After 106 days, the dog was tumour free and could continue a normal life.
Figure 4 Suppressive effect of the Japanese apricot “Prunus mume Sieb. et Zucc” (Ume) juice product on a rapidly growing oral malignant tumour (fibrosarcoma) in a German Shepherd dog. Prior to the treatment, the animal was unable to close its mouth. The dog was given 1 g of the condensed Ume fruit juice (described in the first section of Materials and Methods) in 100 mL milk every morning during the times shown and the status of the tumour was monitored and photographed by a veterinarian doctor. At the last observation, only tumour scar was seen, and the animal was able to feed normally.
Clinical Investigations on Human Pancreatic Cancer
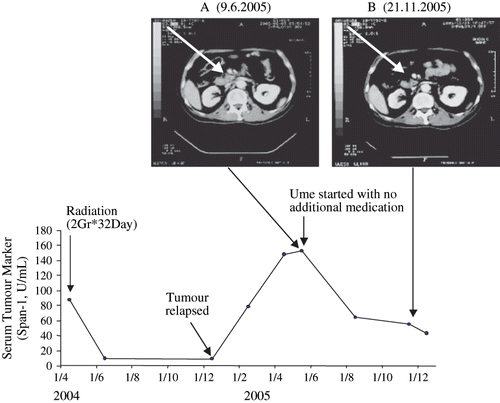
The prevalence of pancreatic cancer is reported to be as high as 15% in patients over 40 years old who have unexplained upper abdominal pain or weight loss and in whom upper gastro-intestinal endoscopy is negative.[Citation11] Pancreatic cancers have a particular propensity to secrete mucin into the blood either because of mechanical blockage of the pancreatic duct, loss of polarity of pancreatic cells or early angiogenesis.[Citation9,Citation11] Therefore, in these patients, a combination of an imaging test such as CT scanning together with a serological mucin assay such as SPan-1 has been shown to be very reliable for monitoring tumour progression and treatment efficacy.[Citation11] We used this method to investigate the anti-tumour effect of the Ume fruit extract on a rapidly growing pancreatic tumour in vivo. A 60-yr old man had been diagnosed to have cancer of the pancreace. His tumour had responded to radiation in April 2004, but had relapsed in December 2004 (). In June 2005, the patient started taking Ume at 2 g condensed Ume juice (in 50 mL water) per day without receiving radiation or any other medication. Within a few weeks, the tumour began to shrink together with a fall in serum tumour marker (Span-1). The last observation prior to this writing was on December 1, 2005. The patient was in remission. The essential details are presented in .
Figure 5 Part A, a CT Scan reveals the presence of a growing pancreatic tumour (at the arrow head). The CT Scan shows the tumour after a relapse following remission induced by radiation. The arrow head in part B shows the tumour has shranken to tumour scar during intake of condensed Ume fruit by the patient, 2 g/day for the entire time period shown. The lower part of the figure shows the fall of tumour marker (Span-1) during remission induced by radiation which then rises again when tumour has relapsed. The tumour marker fell again during the Ume intake and remains within the non-malignant assay range during the observation time.
DISCUSSION
The Japanese started growing “Prunus mume Sieb. et Zucc” (Ume) trees about 2000 years ago and through cultivation, with a major focus on growing trees that could produce high quality healthier fruits, have improved their apricot fruits variety. This signals that the Japanese discovered the health enhancing effects in the Ume fruit many years ago. In line with this thinking, Ume fruit has been known to be rich in minerals like iron (important for blood building), silicon (necessary for beautiful skin and healthy hair), beta-carotene (an excellent antioxidant), which potentially should prevent cancer. Although, not known as a citrus fruit, Ume juice contains surprisingly large amounts of fruit acids like lemons, but with a more pleasant taste. It helps to eliminate extra lactic acid found in the body. However, presence of a substance with such striking and unequivocal anti-cancer effect, which we found in the Japanese apricot is a novel observation.
To investigate the anti-cancer effects of the Ume fruit, we decided to have two well-established cancer cell lines: the Kato-III cell line, which is a known representative of the human gastric cancer cell[Citation5] and grows rapidly in culture; and, the HL-60 cell line that is a representative of the human myelocytic leukaemia cell,[Citation4] which also grows well under culture conditions. Therefore, the culture medium can be used to investigate both suppression of growth and elimination of already formed cancer cells. The suppressive effects of the Ume extract on the growth of Kato-III and the HL-60 cells were very striking because we had not expected to see an straight forward inhibitory effects on the growth of these cancer cell lines. At the concentrations we used, both the growth of the cells, as well as the existing cells, were affected, and at the final observation time point, no viable cell could be found in the test system. The concentrations of the Ume extract in the test medium can be achieved in humans by taking an equivalent of 1–2 grams of the condensed Ume fruit, and because it is a natural edible substance, there should be no safety concerns. In particular, we observed a full inhibitory effect against HL-60 cancer cells just 1 day after incubation, when virtually no viable cell could be found in the culture medium. In contrast, the effect against Kato-III cells depended on the concentration of Ume extract, and after 3 days, all Kato-III cells died at ≤5 μL/mL Ume extract. To investigate the mechanism of anti-cancer effect, we observed the morphology of the target cells in a light microscope. When HL-60 or Kato-III cells were cultured in the presence of the Ume extract, the cells developed vacuoles and subsequently died.
Having been intrigued by observations with the Ume extract on Kato-III and HL-60 cancer cells, we focused our endeavours on the identification of the active substance(s) in the Ume juice that had a lethal effect on cancer cells. Five distinct fractions, Fra1-Fra5 were separated with a silica column. These fractions were separately added to the culture medium containing HL-60 cells. Fra2 showed the HL-60 inhibitory effects seen with the unfractionated Ume extract; other fractions were devoid of anti-cancer effects.
Both inhibition of angiogenesis and apoptosis of tumour cells are essential for tumour elimination.[Citation9,Citation10] Our observations on the spontaneously appearing and rapidly growing fibrosarcoma and pancreatic tumour were most bewildering. The 1 g of condensed Ume fruit which was fed to the dog are within the nutritional range for humans. After taking the first few doses of the condensed Ume fruit, the tumour began to recede. This encouraged us to continue feeding the dog with the Ume juice once a day until the animal was virtually tumour free. We then proceeded to clinical investigations in collaboration with hospital physicians involved in the treatment of cancer. The cancer for our first investigation was a pancreatic tumour. The prevalence of pancreatic cancer is found to be as high as 15% in patients over 40 years old who have unexplained upper abdominal pain or weight loss and in whom upper gastro-intestinal endoscopy is negative.[Citation11] Pancreatic cancers have a particular propensity to secrete mucin into the blood either because of mechanical blockage of the pancreatic duct, loss of polarity of pancreatic cells, or early blood vessel invasion.[Citation9,Citation11] Therefore, in these patients, a combination of two methods like an imaging test such as CT scanning together with a serological mucin assay such as SPan-1 should be both logical and highly practical. This approach has been shown to enhance diagnostic accuracy with a valuable role in monitoring tumour response to therapy.[Citation11] Accordingly, we selected this method as one of the most reliable for our first investigation.
In conclusion, this is the first study showing unequivocally the presence of a substance in the Japanese apricot (Ume fruit) with both suppressive effect on the growth of cancer cells and lethal action on the already formed cancer cells. As the source of this anti-cancer agent is an edible fruit, the Japanese apricot, which may be considered as a natural source of anti-cancer medicine. Accordingly, the Japanese apricot is likely to serve as a novel treatment for tumour bearing hosts. Further, regular intake of the Japanese apricot juice should suppress cancer initiation in healthy individuals, a natural guard against cancer. To stop growth and proliferation of tumour cells, an intended medication should be able to stop blood flow into the tumour and induce dying of cancer cells. These can be achieved via destruction of the vascular system in the tumour (negative angiogenesis) and induction of apoptosis or necrosis in the cancer cells. Kato-III cells are known to die via necrosis, while HL-60 cells die via apoptosis. In this study, we found vacuolization of cancer cells in the presence of the Ume extract. To say that the precise mechanism(s) of anti-cancer effects of the Ume extract, and the chemical structure of its anti-cancer agent awaits to be revealed.
ACKNOWLEDGMENTS
The authors wish to thank the following organization for their generous financial support towards research on Ume. Gunma Industry Support Organization, the Nobuharu Kijima, and the Katsutoshi Tomiyama.
REFERENCES
- Gur , A. 1985 . “ Rosaceae — Deciduous Fruit Trees ” . In Handbook of Flowering , Edited by: Halevy , A.H. Vol. 1 , 355 – 389 . Boca Raton, FL : CRC Press .
- Gu , M. 1979 . Apricot Cultivars in China . Acta Horticulturae , 209 : 63 – 67 .
- Gulcan , R. 1988 . Apricot Cultivars in Near East . Acta Horticulturae , 209 : 49 – 54 .
- Gallagher , R. , Collins , S. , Trujillo , J. , McCredie , K. , Ahearn , M. , Tsai , S. , Metzgar , R. , Aulakh , G. , Ting , R. , Ruscetti , F. and Gallo , R. 1979 . Characterization of the Continuous, Differentiating Myeloid Cell Line (HL-60) from a Patient with Acute Promyelocytic Leukemia . Blood , 54 : 713 – 733 .
- Sekiguchi , M. , Sakakibara , K. and Fujii , G. 1978 . Establishment of Cultured Cell Lines Derived from a Human Gastric Carcinoma . Jpn. J. Exp. Med. , 48 : 61 – 68 .
- Hiraishi , K. , Takeda , Y. , Shiobara , N. , Shibusawa , H. , Jimma , F. , Kashiwagi , N. , Saniabadi , A.R. and Adachi , M. 2003 . Studies on the Mechanisms of Leukocyte Adhesion to Cellulose Acetate Beads: An In Vitro Model to Assess the Efficacy of Cellulose Acetate Carrier-Based Granulocyte and Monocyte Adsorptive Apheresis . Ther. Apher. , 7 : 334 – 340 .
- Cregan , P. , Yamamoto , A. , Lum , A. , VanDerHeide , T. , MacDonald , M. and Pulliam , L. 1990 . Comparison of Four Methods for Rapid Detection of Pneumocystis Carinii in Respiratory Specimens . J. Clin. Microbiol. , 28 : 2432 – 2436 .
- Erlandson , R.A. and Antonescu , C.R. 2004 . The Rise and Fall of Malignant Fibrous Histiocytoma . Ultrastruct. Pathol. , 28 : 283 – 289 .
- Folkman , J. 1971 . Tumor Angiogenesis: Therapeutic Implications . N. Engl. J. Med. , 285 : 1182 – 1186 .
- Kerr , J. , Wyllie , A. and Currie , A. 1972 . Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics . Br. J. Cancer , 26 : 239 – 257 .
- Rhodes , J.M. 1999 . Usefulness of Novel Tumour Markers . Ann. Oncol. , 10 ( Suppl. 4 ) : 118 – 121 .
