Abstract
Seven tomato cultivars were studied for compositional changes during ripening at green, breaker, turner, and ripe stages. Result indicated changes in total soluble solids (4.15 to 6.62 g/100 g), acidity (0.36 to 0.54 g/100 g), reducing sugars (0.76 to 4.04 g/100 g), total sugars (1.67 to 5.52 g/100 g), lycopene in skin (0.07 to 14.28 mg/100 g), and in pulp (0.04 to 6.73 mg/100 g) during ripening from green to red ripe stage. Cultivar UC-828 was found superior with respect to total soluble solids (6.62 g/100 g), reducing sugars (4.04 g/100 g), and total sugars (5.52 g/100 g) where as cultivar 8–2–1–2–5 was found superior with respect to lycopene in skin (14.28 mg/100 g) and in pulp (6.73 mg/100 g). Hunter color values showed a change from negative value of ‘a’ (greenness) to positive values (redness) where as ‘b’ decreased. The (b/a) and tan−1 (b/a) showed a similar pattern of first increased and then decreased. The puncture resistance was decreased from 10.5 to 2.3 N indicating softening during ripening. Correlation studies showed that lycopene content best correlated with Hunter ‘a’ values during ripening (R2 = 0.84 to 0.93). Intercultivar variation in physicochemical parameters at all stages of ripening revealed that Castle Rock was the best cultivar.
INTRODUCTION
Tomato (Lycopersicon esculentum) constitutes one of the major vegetable crops around the world. Its quality depends upon the many factors such as cultivars, growing conditions, and on- or off-vine ripening. Tomatoes ripened on vine have better flavour where as ripened off vine reduces time to harvest, increases turnover, allows longer time for transportation and distribution, and increases shelf life.[Citation1]
The conversion of tomato fruit from the mature green to fully ripe state involves dramatic changes in color, flavour, and texture. The soluble solids depend on starch accumulation during the rapid growth phase of development.[Citation2,Citation3] Sugars contribute 65 to 70% of the total soluble solids in a tomato fruit.[Citation4] The organic acids in tomatoes consist mainly of citric and malic acids. The concentration of former increases to a maximum at the mature green stage and then remains constant during ripening, where as the concentration of later one decreases. The ratio of malic to citric acid falls from 1.3 to 0.6 during ripening.[Citation5] Sugars, acids, and their interactions are important to sweetness, sourness, and overall flavor intensity in tomatoes.[Citation6]
The major groups of pigments found in tomato are the chlorophyll and carotenoids. In green fruits the predominant pigment comprises a mixture of chlorophyll a and chlorophyll b.[Citation7] The lycopene biosynthesis increases dramatically during the ripening process as chloroplasts undergo transformation to chromoplasts.[Citation8] The lycopene content increased from 0.025 mg/100 g to over ripe tomatoes 7.050 mg /100 g, respectively.[Citation9] Color changes are attributed by the ripening and represent a key attribute, along with texture, for the determination of eating quality. The present work was under taken to study the newly developed and existing tomato cultivars for physicochemical changes during ripening.
MATERIALS AND METHODS
Raw Materials
Tomato cultivars (8-2-1-2-5, Castle Rock, IPA-3,UC-828, Pb Chhuhra, WIR-4285, and WIR-4329) of tomato harvested at mature green stages were obtained from Department of Vegetable, Punjab Agricultural University, Ludhiana, India. Fruits were sorted for uniformity of size, color, and freedom from blemishes. About one hundred tomatoes of each cultivar were placed in a chamber maintained at 20 ± 2 °C and 90–92 % RH. Random samples of 10 to 15 tomatoes were taken at each stage for analysis.
Physicochemical Analysis
Whole tomatoes were homogenized in a blender. Total soluble solids were quantified by using Abbe Mat Refractometer (Milton Roy Co., USA) at 21°C. Titrable acidity (as anhydrous citric acid) was determined using a pH meter (Elico, India). The homogenized samples were diluted with distilled demonized water (1:1 v/v), and the mixture was titrated to pH 8.1 with 0.1 N NaOH.[Citation10] Reducing and total sugars were determined according to Lane and Eyon method.[Citation11] Invert sugar reduces the copper in Fehling's solution to red, insoluble cuprous oxide. The sugar content in a food sample is estimated by determining the volume of the unknown sugar solution required to completely reduce a measured volume of Fehling's solution.
Lycopene
Skin or pulp (2 g) was extracted with 50 ml solvent (hexane: acetone: ethanol, 2:1:1) containing 0.05 g/100 ml butylated hydroxy toluene (BHT) till it become colorless. The extract was filtered and diluted with water and non-polar phase was collected. The absorbance of the lycopene hexane solution was measured at 503 nm. Lycopene concentration was computed on specific extinction coefficient 17.2 × 104 mol cm−1.[Citation12]
Color
The Hunter Color lab (Hunter Associates Laboratory, Reston, VA, USA, 45°/0° geometry, 10° observer) was calibrated and then color of pulp was measured. The ‘a’ (green-red) and ‘b’ (yellow-blue) values were used to calculate the hue angle [tan−1 (b/a)].
Puncture Test
Puncture strength at different ripening stages was measured with a texture analyzer (TAXT2, Stable Micro System, UK). Samples were placed on a heavy-duty platform and a cylindrical probe with 2 mm diameter at 1 mm/s test speed was allowed to penetrate up to a distance of 10 mm around the equatorial area. Peak force was measured around the equatorial region of tomatoes of different ripening stages, and the average of three values was reported.[Citation13] The data analysis was performed using the texture analysis software (TEE32, Stable Microsystems, UK).
Statistical Analysis
Analysis of variance (ANOVA) was performed to study the variation among the tomato cultivars and the ripening parameters. Duncan's multiple range test was used for pair wise comparison to classify the cultivars using statistical software SPSS 7.5 version. Evaluation was based on a 5% significance level.
RESULTS AND DISCUSSION
Total Soluble Solids (TSS)
Tomato homogenates showed a considerable increase in TSS from 4.15 to 6.62 g/100 g as ripening progressed from green to ripe stage (). The cultivar UC-828 showed the highest soluble solids at green (4.85 g/100 g), breaker (5.41 g/100 g), turner (5.87 g/100 g), and ripe (6.62 g/100 g) stages. ANOVA showed a significant (p ≤ 0.05) variation in TSS at different stages of ripening as well as among the cultivars. Duncan Multiple range test showed that cultivars Pb Chhuhra and WIR-4285 could be classified into group a, WIR-4329 and IPA-3 in group b, Castle Rock and 8-2-1-2-5 in group c, and UC-828 in group d. There were no significant differences within the group but a different group showed significant change in TSS at ripe stage. These values of TSS were quite similar to those reported in previous studies.[Citation14,Citation15] Increase in total soluble solids during fruit ripening was due to conversion of starch to sugars.[Citation16,Citation17] Different cultivars showed different TSS due to inherent characteristics.
Table 1 Changes in and total soluble solid and percent citric acid at green, breaker, turner, and ripe stages of fifteen tomato cultivars (n = 3).
Acidity
In general, a slight increase in acidity was observed in all cultivars. IPA-3 showed the highest value of acidity (0.43–0.54 g/100 g) while WIR-4329 showed lowest values (0.36–0.43 g/100 g). ANOVA did not show any significant change in acidity from green to ripe stages for the seven cultivars (). Thus, the results of the current study are in compliance with earlier studies, which reported acidity in the range of 0.4–0.7 g/100 g.[Citation14,Citation18]
Reducing and Total Sugars
Reducing and total sugars of tomato homogenate increased as ripening proceeded from mature green to ripe stage (). UC-828 showed highest value of reducing (1.33–4.04 g/100 g) and total sugars (1.52–5.52 g/100 g) from green to ripe stages, whereas WIR-4285 showed lowest values of reducing (0.76–2.20 g/100 g) and total (1.07–3.69 g/100 g) sugars. ANOVA showed a significant increase in both reducing and total sugars (p < 0.05). Duncan multiple range test showed that in case of the reducing sugars, Pb Chhuhra and WIR-4285, they can be classified into group a, WIR-4329 and IPA-3 in group b, Castle rock, 8-2-1-2-5, and UC-828 in group c at ripe stage. Similarly, in the case of total sugars, the Pb Chhuhra and WIR-4285 can be classified into group a, WIR-4329 and IPA-3 in group b, Castle rock in group c, and 8-2-1-2-5 and UC-828 in group d. Cultivars within the same group showed insignificant change in reducing and total sugars, but a different group had significantly different sugar content. Previous studies reported that climacteric fruits, in particular, may show considerable changes in sugar content during ripening.[Citation19–22,Citation15] Our results are in agreement with previous reported increase in reducing and total sugars from green to ripe stages.
Table 2 Changes in percent reducing and total sugars at green, breaker, turner and ripe stages of fifteen tomato cultivars (n = 3).
Lycopene
All the tomato cultivars showed increase in lycopene content both in skin and pulp during ripening (). The Cultivar 8-2-1-2-5 showed a highest increase from 0.09 to 14.28 mg/100 g in skin whereas 0.08 to 6.73 mg/100 g in pulp during the ripening process. The minimum increase was observed in WIR-4285 from 0.08 to 7.97 mg/100 g in skin and 0.06 to 3.12 mg/100 g in pulp. At green stage there was no variation in lycopene content among the cultivars, but at subsequent stages variation was evident depending on their ability to synthesize lycopene.
Table 3 Changes in lycopene from green, breaker, turner, and ripe stages of ripening (n = 3).
ANOVA showed that a significant increase in lycopene content during ripening in all cultivars. Duncan multiple range test revealed that lycopene content in skin at ripe stage classified WIR-4285 into group a, UC-828 into group b, IPA-3 into group c, WIR-4329 into group d, Pb Chhuhra into group e, Castle Rock into f, while 8-2-1-2-5 into group g. Whereas lycopene content in pulp at ripe stage classified WIR-4285 into group a, UC-828 and IPA-3 into group b, IPA-3 and WIR- 4329 into group c, WIR-4329 and Pb Chhuhra into group d, Castle Rock into group e, while 8-2-1-2-5 into group f. The results reveal that on an average tomato peels had 2.5 times the lycopene content found in pulp. The lycopene content, vary in tomatoes due to maturity, cultivars, and heat treatment.[Citation23,Citation24,Citation17] Drastic breakdown of chlorophyll was observed as ripening progressed. The destruction of chlorophyll from green to ripe stages may be due to the extensive accumulation of the carotenoids such as β-carotene and lycopene at turner and ripe stages.[Citation25,Citation26] As tomatoes developed from mature green to ripe, the increase in carotenoids content was related to the increase in lycopene content.[Citation9,Citation18] The lycopene content can said to be a good index to the level of maturation. Other studies also revealed a similar trend in lycopene content at different stages of maturity, ripening and cultivars.[Citation27–29] Thus present results supports the earlier studies that lycopene content in tomato varies due to maturity and cultivars.
Color
Hunter L a, b values were recorded at four stages of ripening. The Hunter ‘a’ values were negative (−9.31 to −5.92) at green stage indicating green color and then become positive during rest of the ripening stages (). The redness increased as the ripening progressed and attained the values in the range of 15.24 to 20.58. ANOVA revealed a significant change in the ‘a’ value (p < 0.05). Duncan multiple range test showed that WIR 4285 was classified in group a, IPA-3 in group b, UC-828 in group c, Pb Chhuhra, Castle Rock, and WIR-4329 in group d, while WIR-4329 and 8-2-1-2-5 in group e. These results supported the lycopene content that gave similar trends.
Table 4 Changes in hunter color ‘a’ and ‘b’ values of tomato homogenate at green, breaker, turner, and ripe stages (n = 3).
The Hunter ‘b’ values decreased as the ripening progressed indicating decreases in blueness (). The initial values of Hunter ‘b’ were 17.14–19.62 that decreased to 10.67–13.52 at ripe stage for seven cultivars. ANOVA showed a significant decline in Hunter ‘b’ value for seven cultivars. Duncan multiple range test revealed that 8–2–1–2–5 and Castle Rock were together in group a, Pb Chhuhra in group b, WIR-4329 in group c, IPA-3 and UC-828 in group d, while WIR-4285 in group e. Hunter L values did not vary significantly during the ripening (Data Not Shown).
The hue values or (b/a) values were negative at green stage and became positive at breaker stage. The positive values decreased at turner and ripe stages (). The hue value at mature green stage of all cultivars showed negative values (− 63.11 to − 72.69) indicating green color. As the color changed the hue value became positive 52.83 to 74.46 at breaker, 35.48 to 41.28 at turning, and 23.33 to 31.05 at ripe stage. Lower positive values indicated more redness. The (b/a) values followed the similar pattern as in the case of hue values. ANOVA indicated significant change in hue values and (b/a) (p < 0.05). Duncan multiple range test indicated that 8–2–1–2–5 and Castle Rock were in group a, Pb Chhuhra and WIR 4329 in group b, IPA-3 and UC-828 in group c, and WIR- 4285 in group d.
Table 5 Changes in hunter color ‘b/a’ values and homogenate hue of tomato homogenate at green breaker, turner, and ripe stages (n = 3).
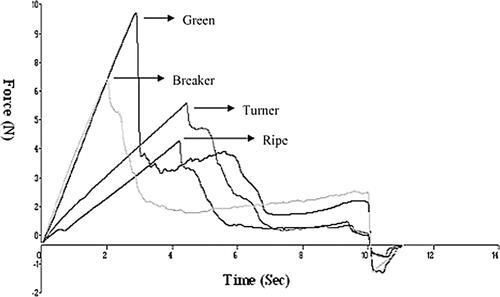
Puncture Test
The puncture strength measured as peak force required to puncture the tomato during ripening. Decline in firmness/puncture strength with the increase in ripening stages is shown in . Puncture strength of the tomato at green stage was in the range of 8.83–10.52 N that was decreased to 2.28–4.81 N at ripe stage for the seven cultivars under investigation (). Maximum firmness was reported for WIR-4285 where as least in case of WIR-4329. Firmness of tomato is an important criterion for marketing of fresh fruits. ANOVA revealed that there was a significant decline in firmness as the ripening proceeded (p < 0.05). Duncan multiple range test showed lot of overlapping of groups of the cultivars. WIR 4329, 8-2-1-2-5, UC-828 and IPA-3 were classified into group a; 8-2-1-2-5, UC-828, IPA-3, and Castle Rock into group b; UC-828, IPA-3, Castle Rock, and Pb Chhuhra into group c; and Castle Rock, Pb Chhuhra, and WIR-4285 into group d. The inter cultivar differences may be attributed to the variation in genetic makeup of the cultivars, which affect the physiological changes after the harvesting of the tomatoes. The difference at various ripening stages may be the activity of pectinases, which affect the middle lamellae of the cells and consequently lower the firmness.[Citation30]
Table 6 Changes in peak strength measured as peak force (N) during ripening (n = 3).
Correlation of Lycopene with Other Parameters
The ripening of the tomatoes of seven cultivars was studied using different parameters and these parameters were correlated with lycopene content of skin and pulp. Results revealed that lycopene content showed good correlation with Hunter ‘a’ followed by Hunter ‘b’, puncture strength and total sugars (). This study indicated that lycopene content could be predicted from Hunter ‘a’ values which are easier to measure as an index of ripening. The hue value tan−1 (b/a) or (b/a) did not correlate well with the lycopene synthesis in the tomato.
Table 7 Correlation coefficients of parameters with lycopene content of pulp and skin.
Classification of Cultivars
Duncan multiple range test was used to classify the cultivars into different groups and the same was summarized in the . There are three major criteria for selection of a tomato cultivar viz higher solids, deep red color, and firm texture. On the basis of total soluble solids and sugar contents, the most acceptable cultivar was UC-828 followed by 8-2-1-2-5 and Castle Rock. Based on color at the ripe stage, 8-2-1-2-5 was most desirable followed by WIR-4329 and Castle Rock. The firmness of tomatoes was observed in descending order of WIR-4285, Pb Chhuhra, and Castle Rock. Considering all the parameters it can be concluded that Castle Rock is better variety among the cultivars under investigation.
Table 8 Classification of the tomato cultivars employing Duncan Multiple range test.
ACKNOWLEDGMENTS
The University Grants Commission, New Delhi, supported this study. Dr M.S. Dhaliwal, Department of Vegetables, Punjab Agricultural University, Ludhiana is acknowledged for the supply of tomato cultivars.
REFERENCES
- Arias , R. , Lee , T.C. , Specca , D. and Janes , H. 2000 . Quality Comparison of Hydroponics Tomatoes (Lycopersicon esculentum) Ripened On and Off Vine . J. Food Sci , 65 ( 3 ) : 545 – 548 .
- Dinar , M. and Stevens , M.A. 1981 . The Relationship Between Starch Accumulation and Soluble Solids Content of Tomato Fruits . J. Am. Soc. Hort. Sci , 106 : 415 – 418 .
- Ho , L.C. and Hewitt , J.D. 1986 . “ Fruit Development ” . In The Tomato Crop , Edited by: Atherton , J.G. and Rudich , J. 201 – 239 . London : Chapman and Hall .
- Hobson , G.E. and Kilby , P. 1985 . “ Methods for Tomato Fruit Analysis as Indicators of Consumer Acceptability ” . In Annual Report–1984 , 129 – 136 . Littlehampton, , England : Glasshouse Crops Research Institute .
- Davies , J.N. and Hobson , G.E. 1981 . The Constituents of Tomato Fruit—the Influence of Environment, Nutrition and Genotype . CRC Crit. Rev. Food Sci. Nut , 15 : 205 – 280 .
- DeBruyn , J.W. , Garretsen , F. and Kooistra , E. 1971 . Variation in Taste and Chemical Composition of the Tomato (Lycopersicon esculentum Mill.) . Euphytica , 20 : 214 – 227 .
- Edwards , R.A. and Reuters , F.N. 1967 . Pigment Change During the Maturation of Fruit . J. Food Technol , 19 : 352 – 357 .
- Kirk , J.T.O. and Tilney-Basset , R.A.E. 1978 . The Plastids, their Chemistry, Structure, Growth and Inheritance , Amesterdam : Elsevier .
- Fraser , P.D. , Truesdale , M.R. , Bird , C.R. , Schuch , W. and Bramley , P.M. 1994 . Carotenoid Biosynthesis During Tomato Fruit Development . Plant Physiol , 105 : 405 – 413 .
- Thakur , B. , Singh , R. and Nelson , P. 1996 . Quality Attributes of Processed Tomato Products- a Review . Food Rev. Int , 12 ( 3 ) : 375 – 401 .
- Ranganna , S. 1986 . Hand Book of Analysis and Quality Control for Fruit and Vegetable Products , 2nd , New Delhi : Tata McGraw Hill .
- Sadler , G. , Davis , J. and Dezman , D. 1990 . Rapid Extraction of Lycopene and β- Carotene from Reconstituted Tomato Paste . J. Food Sci , 55 : 1460 – 1461 .
- Al-Hooti , S.N. , Sidhu , J.S. , Al-Saqer , J.M. , Al-Amiri , H.A. , Al-Othman , A. , Mansour , I.B. and Johari , M. 2004 . Developing Functional Foods Using Red Palm Olein: Objective Color and Instrumental Texture . Int. J. Food Prop , 7 ( 1 ) : 15 – 25 .
- Saini , S.P.S. and Singh , S. 1994 . Juice Composition of Some New Tomato Hybrids . J. Food Sci. Technol , 31 ( 3 ) : 231 – 232 .
- Ereifej , K.I. , Shibli , R.A. , Ajlouni , M.M. and Hussain , A. 1997 . Physico-chemical Characteristics and Processing Quality of Newly Introduced Seven Tomato Cultivars into Jordan in Comparison with Local Variety . J. Food Sci. Technol , 34 ( 2 ) : 171 – 174 .
- Biale , J.B. 1960 . The Post-harvest Biochemistry of Tropical and Subtropical Fruits . Adv. Food Res , 10 : 293 – 354 .
- Seymour , G.B. , Taylor , J.E. and Tucker , G.A. 1993 . Biochemistry Of Fruit Ripening , London : Chapman and Hall .
- Thompson , K.A. , Marshall , M.R. , Sims , C.A. , Wei , C.I. , Sargent , S.A. and Scott , J.W. 2000 . Cultivar, Maturity and Heat Treatment on Lycopene Content in Tomatoes . J. Food Sci , 65 ( 5 ) : 791 – 795 .
- Janes , B.E. 1941 . Some Chemical Differences Between Artificially Produced Parthenocarpic Fruits and Normal Seeded Fruits of Tomato . Am. J. Bot , 28 : 639 – 646 .
- Marre , S. and Murneek , A.E. 1953 . Carbohydrate Metabolism in the Tomato Fruit as Affected by Pollination, Fertilization and Application of Growth Regulators . Plant Physiol , 28 : 255 – 266 .
- Winsor , G.W. , Davies , J.N. and Massey , D.M. 1962 . Composition of Tomato Fruit. III. Juices from Whole Fruit and Locules at Different Stages of Ripeness . J Sci. Food Agic , 13 : 108
- Winsor , G.W. 1966 . Some Factors Affecting the Composition, Flavour and Firmness of Tomatoes . Sci. Hort , 18 : 27
- Stahl , W. and Sies , H. 1992 . Uptake of Lycopene and its Geometrical Isomers is Greater from Heat-processed Tomato Juice in Humans . J. Nut , 20 : 667 – 671 .
- Sharma , S.K. and Le , Maguer. M. 1996 . Lycopene in Tomatoes and Tomato Pulp Fractions . Ital. J. Food Sci , 2 : 107 – 113 .
- Rabinowitch , H.D. , Budowski , P. and Kedar , N. 1975 . Carotenoids and Epoxide Cycles in Mature-Green Tomatoes . Planta , 122 : 91
- Grierson , D. and Kader , A.A. 1986 . “ Fruit ripening and quality ” . In The Tomato Crop , Edited by: Atherton , J.G. and Rudich , J. 241 – 278 . London : Chapman and Hall .
- Al-Wandawi , H. , Rahman , M.A. and Al-Shaikhly , K. 1985 . Tomato Processing Waste as Essential Raw Materials Source . J. Agric. Food Chem , 33 : 804 – 807 .
- Abushita , A.A. , Daood , H.G. and Biacs , P.A. 2000 . Changes in Carotenoids and Antioxidant Vitamin in Tomato as a Function of Varietal and Technological Factors . J. Agric. Food Chem , 48 : 2075 – 2081 .
- Akanbi , C.T. and Oludemi , F.O. 2004 . Effect of Processing and Packaging on the Lycopene Content of Tomato Products . Int. J. Food Prop , 7 ( 1 ) : 139 – 152 .
- Phan , C.T. 1987 . “ Biochemical and Physiological Changes During the Harvest Period ” . In Post harvest Physiology of Vegetables , Edited by: Weichmannt , J. 9 – 22 . New York : Marcel Dekker Inc .