Abstract
Tg′, a glass transition temperature under conditions of maximal freeze concentration, is important to the stability of frozen food. The procedures for using differential scanning calorimetry (DSC) to measure Tg′ have been reported under different experimental conditions. The aims of this study were to investigate the Tg′ of cooked rice stick noodles, and to determine the effect of the annealing temperature on its Tg′ value using DSC. Cooked rice stick noodles in aluminum DSC pans were scanned from −60°C to 25°C at 5°C/min. to locate the apparent Tg′, which was −5.3°C in the non-annealed state. When subjected to 4 different annealing temperatures of −2, −6, −8 and −10°C for 15 minutes, the Tg′ of cooked rice stick noodles was −5.3, −4.0, −4.2, and −4.9°C, respectively. The value of Tg′ was clearly observed in the annealed sample at −6°C. The annealing process allowed time for the maximum formation of ice. This study showed that annealing at a temperature slightly below Tg′ gave a higher and more accurate value.
INTRODUCTION
Glass transition is a nature of second-order phase transition that occurs over the temperature range at which amorphous solid materials (glassy materials) are transformed into the viscous, liquid state.[Citation1] A special glass transition temperature, denoted as Tg′ has been defined as the glass transition of the maximally freeze-concentrated system.[Citation2] Tg′ can be determined by observing a step change in heat capacity and changes in mechanical and dielectric properties. Differential Scanning Calorimetry (DSC) is one of the most common methods used in determining the glass transition temperature. DSC measures the change in heat capacity between the glassy and rubbery states and is indicated by a change in the baseline in a DSC thermogram.[Citation1,Citation3]
The glass transition behavior of food plays a key role in the quality, and the storage stability of frozen products because the rates of deteriorative changes in frozen food is closely related to the Tg′.[Citation1,Citation4,Citation5] The significance of such glass transitions to the stability of frozen food has been discussed extensively by Slade and Levine.[Citation6] Consequently, knowledge about the Tg′ of food is important. However, reported procedures for using DSC to measure Tg′ differ in regard to sample size, cooling rates, warming rates, holding times and temperatures, and annealing conditions (or lack thereof).[Citation7–14] It has been found that annealing affects the Tg′ of foods and solutions.[Citation12,Citation15–22] For example, the glass transition of a 30% fructose solution occurred at −58°C after quench freezing but this temperature increased to −48°C as a result of the slow freezing and annealing process.[Citation20] The Tg′ values of a gelatinized starch sample after annealing were higher than that of the non-annealed sample.[Citation12] Rahman[Citation21] showed that annealing date flesh at Tm′ −1 assisted in clearly identifying glass transition temperatures. Tm′ in his study was the end point of freezing under conditions where freeze concentration was at its maximum.
There is a scarcity of information on the Tg′ of real food systems partly due to their complexity. Recently, there has been an increasing demand for frozen ready-to-eat food made from cooked rice noodles. Cooked rice noodles could be a good example of a starch-based food in which the starch granules pass through the process of gelatinization, retrogradation, and melting of the retrograded starch. However, very limited information on the Tg′ of cooked Thai rice noodles is available in the literature. The objectives of this study were to investigate the Tg′ of cooked rice stick noodles and to determine the effect of the annealing temperatures on the Tg′.
MATERIAL AND METHODS
Material
Rice stick noodles in this study were made from Thai rice grain (Luang 11 grown in the Kalasin Province area); it contained 12.27% moisture, 5.18% protein, 0.28% fat, 0.21% ash, and 82.50% carbohydrate.[Citation23]
Rice Stick Noodle Processing
First, rice flour was made from rice grain by wet milling and drying at 45°C for 15 hours. Then, rice stick noodle processing began by mixing the rice flour with water (40% wt/wt rice flour) and allowing it to soak for 3 hours. The slurry was spread on a sheet of thin cloth and steamed for 3 minutes over boiling water, air cooled for 15 minutes, and aged at 2°C for 5 hours. Finally, the rice noodle sheets were cut into strips and dried at 40°C for 1 hour. The noodles when cooked for 2 minutes in boiling water and cooled for 1 minute in 10°C water, appeared white in color (L∗ = 96.00, a∗ = 0.83, b∗ = 3.56 using a Minolta spectrophotometer) and elastic in texture (tensile maximum force = 0.338 N, tensile distance = 24.125 mm using a Texture Analyzer TA-XT2 with a Spaghetti tensile grip and 5 kg load cell).
Sample Preparation
The dried rice stick noodles were cooked in boiling water for 2 minutes and measured for moisture content using a Moisture Balance MB 200 (Jebson & Jessen (Thailand) Ltd.). When the sample reached a water content of approximately 70% (on a dry weight basis), a 6 to 7 mg sample was then placed into a weighed volatile aluminum sample pan (no. 2190062), which was immediately weighed and hermetically sealed.
DSC Determination of Tg′ and the Annealing Study
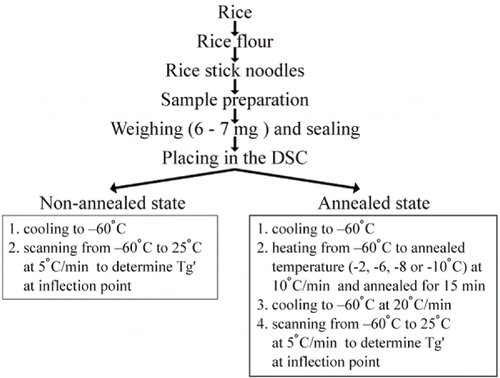
illustrates steps in sample preparation for determining glass transition temperatures. A Pyris1 DSC (Perkin Elmer, USA) was used for this study. The calorimeter (equipped with an Intracooler) was allowed to cool to −60°C before the samples were tested. Nitrogen gas was purged through the measuring cells continuously at a pressure of 20 psi to obtain a consistent temperature. To avoid condensation, the instrument cover lid was heated and the sample headspace was purged with a shield of dry nitrogen gas when loading the samples. The instrument was calibrated using indium (m.p. 156.6°C, ΔHm 28.45 J/g) and water (m.p. 0°C, ΔHm 334.5 J/g), and an empty aluminum pan was used as a reference. After immediate cooling, the sealed pans were heated from −60°C to 25°C at 5°C/min. to determine the glass transition temperature of the samples in its non-annealed state. The other sealed pans were annealed at four different temperatures (2, −6, −8, or −10°C) in the vicinity of the apparent Tg′ of the non-annealed sample. All samples were heated from −60°C to the annealing temperature at 10°C/min., and annealed at that temperature for 15 minutes in order to determine quasi equilibrium Tg′, a time that has been used by several researchers to determine the Tg′ for gelatinized starches.[Citation12,Citation14,Citation22,Citation24] Then the sample was cooled to −60°C at 20°C/min. and rescanned by heating from −60°C to 25°C at 5°C/min. to locate the Tg′. The Tg′ was indicated by an inflection point, which was determined by using the computer software program associated with the Perkin Elmer instrument. Each sample was analyzed in triplicate.
RESULTS AND DISCUSSION
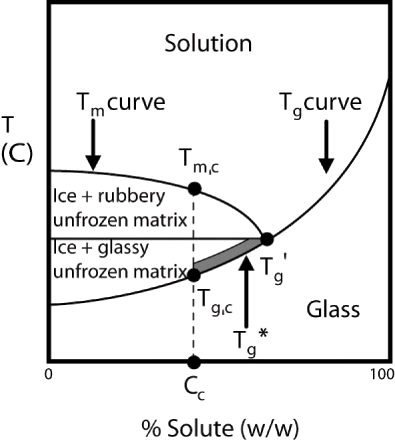
Many researchers have studies glass transition in various foods and model systems and defined and named several points along the Tg curve as part of their studies. As defined by Tananuwong and Reid,[Citation25] in a system that is allowed to form the maximum amount of ice, the glass transition of this maximally freeze concentrated phase occurs at Tg′ (), this is independent of the initial solute concentration. However, if the maximum amount of ice is not formed in the system, the resulting unfrozen matrix will be more dilute. According to Tananuwong and Reid,[Citation25] the glass transition temperature of this partially freeze concentrated phase, denoted as Tg∗, is lower than Tg′ and falls between Tgc (the glass transition temperature of a homogeneous amorphous matrix) and Tg′ along the Tg curve with the exact point depending on the concentration of the unfrozen phase. Several researchers have also defined another term Tg″ as the intersection of the freezing curve with the glass line by maintaining the similar curvature.[Citation26–28] Rahman et al.[Citation29] introduced Tg‴, the glass transition of the rubbery solids matrix in the frozen samples. Tg‴ can be located on a DSC scan of the sample being heated from −90°to 50°C.
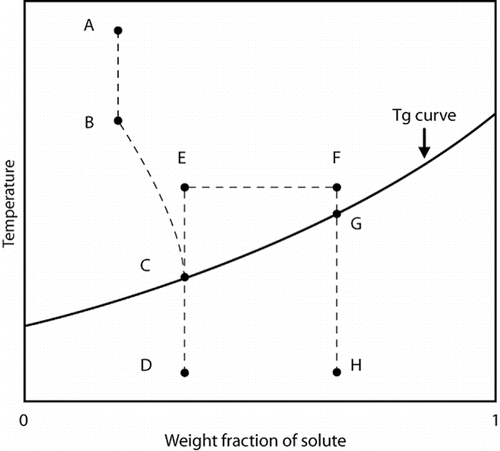
In addition Hsu et al.[Citation24] have pointed out that the annealing process affects the Tg′ of glass forming solutions. illustrates several changes which occur during annealing. When the temperature is decreased from point A to point B, this is the initial freezing temperature, but no ice crystals form. As the temperature is decreased below point B, ice crystals form and the concentration of unfrozen solute is initiated. As ice crystals continue to form, the solute concentration increases and the equilibrium freezing temperature is depressed for the unfrozen fraction (point B to point C). At point C (Tg), ice crystal formation ceases, and the physical state of the unfrozen fraction changes to an amorphous solid glass. When the temperature is decreased from point C to point D, the concentration of the unfrozen fraction does not change until the sample temperature is increased to the annealing temperature (point E). At this point and throughout the annealing period (point E to point F), additional water from the solution is converted into ice. When the concentrated solution is cooled (point H), the solution becomes supersaturated and forms the glass. Therefore, as the sample is warmed from point H onward, the glass transition peak is found at the point G (Tg′).
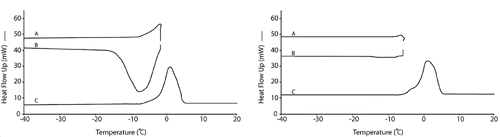
DSC thermograms of the cooked rice stick noodles isothermally annealed at different temperatures are shown in . The Tg′ of the cooked rice stick noodles was −5.3°C (A) in the non-annealed state. At the four different annealing temperatures of −2 (B), −6 (C), −8 (D), and −10°C (E), the Tg′ of cooked rice stick noodles was −5.3, −4.0, −4.2, and −4.9°C respectively.
Figure 4 DSC thermograms of the cooked rice stick noodles during annealing at different temperature for 15 minutes before the determination of Tg′: (A) non – annealing, (B) −2°C, (C) −6°C (D) −8°C and (E) −10°C.
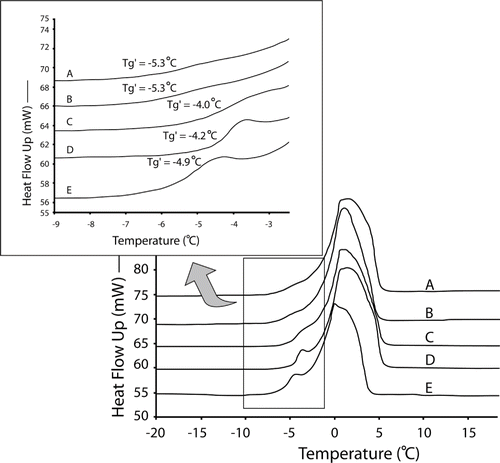
The Tg′ values and accuracy increased when the annealing temperature was increased except when the sample was annealed at −2°C where it had a Tg′ value, which was the same as the non-annealed sample. The annealing temperature of −6°C resulted in the highest Tg′ value among the four annealing temperatures. This might have occurred because this temperature, which was slightly below the Tg′ of the cooked rice stick noodles (−5.3°C), was high enough to have sufficient molecular mobility for ice formation and yet also low enough to maintain the amorphous glass matrix as discussed by Lim et al.[Citation12] Roos and Karel[Citation13] showed that with isothermal annealing for 15 minutes at −35°C, one degree below the proposed Tm′ (onset of ice melting) at −34°C, the Tg′ of the 20% sucrose solution was −46°C, which was higher than the non-annealed Tg′ (−50°C). A different study by Roos and Karel[Citation30] found that the annealing led to a constant Tg′ value because the annealing process allowed time for the maximum formation of ice. At the lowest annealing temperature of −10°C, the value of Tg′ increased less than the annealed Tg′ at −6 and −8°C. Annealing at 10°C gave a Tg′ of −4.9°C (a slight increase from −5.3°C with the non-annealed sample) while annealing at −6 and −8°C gave a higher Tg′ of −4.0 and −4.2°C, respectively. These results agreed with those of Lim et al.,[Citation12] where the Tg′ value of the gelatinized potato starch after annealing at −9°C increased less than the annealed Tg′ at −6°C. It is believed that at this temperature, the gelatinized potato system had a more rigid glass solid matrix and less molecular mobility was available for maximizing ice formation in the time allowed. In our study at the highest annealing temperature (−2°C), the value of Tg′ equaled that of the non-annealed Tg′, possibly because the dissolving water from the melting ice plasticized the glass matrix at a temperature close to the ice melting peak. The presence of dissolving water can be observed from the ice melting endotherm (line A) and from the ice forming exotherm (line B) in left, while with annealing at the other temperatures, ice melting peaks were not observed ( right). Therefore, our experiment showed that annealing at a temperature slightly below the Tg′ of the non-annealed state improves the accuracy of the Tg′ determination in a real food system, i.e., cooked rice stick noodles.
Figure 5 DSC thermograms of the cooked rice stick noodles during annealing at −2°C and −6°C (left and right, respectively) for 15 minutes before the determination of Tg′ : (A) heating from −60°C to annealing temperature at 10°C/min. and annealed for 15 minutes, (B) cooling to −60°C at 20°C/min., and (C) scanning from −60°C to 25°C at 5°C/min. to determine Tg′.
CONCLUSIONS
The results indicate that the Tg′ of cooked rice stick noodles is −5.3 ± 0.1°C in the non-annealed state. Annealing at −6°C, which was slightly below the Tg′ of the cooked rice stick noodles, gave the highest and most accurate Tg′ value (−4.0 ± 0.0°C) because the annealing process led to the maximum formation of ice. Annealing at a temperature above Tg′ of the annealed state caused ice melting while with annealing at lower temperatures, no ice melting peaks were observed.
ACKNOWLEDGMENTS
This research work was supported by the Kasetsart University Graduate School and the Ministry of University Affairs, Thailand. The authors also thank Ms. Pamela Tom, Mr. Adrian Hillman, and Ms. Angela Foin for reading the manuscript, and Mr. Supadej Israkarn for drawing the figures.
REFERENCES
- Roos , Y.H. 1995 . Phase Transition in Foods , New York : Academic Press .
- Levine , H. and Slade , L. 1988 . Principles of Cryostabilization Technology from Structure/Property Relationships of Carbohydrate/Water Systems: A Review . Cryo-lett , 9 : 21 – 63 .
- Labuza, T.; Nelson, K.; Coppersmith, C. Glass Transition Temperatures of Food Systems, 1992. www.fsci.umn.edu/Ted Labuza/PDF files/Isotherm Folder/Tg%20compilation.pdf
- Bhandari , B.R. and Howes , T. 2000 . Glass Transition in Processing and Stability of Food . Food Aust , 52 ( 12 ) : 579 – 585 .
- Roos , Y.H. 1995 . Glass Transition-Related Physicochemical Changes in Foods . Food Technol , 49 ( 10 ) : 97 – 102 .
- Slade , L. and Levine , H. 1995 . Glass Transitions and Water-Food Structure Interactions . Adv. Food Nutr. Res , 38 : 103 – 233 .
- Ferrero , C. and Zaritzky , N.E. 2000 . Effect of Freezing Rate and Frozen Storage on Starch-Sucrose-Hydrocolloid Systems . J. Sci. Food Agric , 80 : 2149 – 2158 .
- Huang , R.M. , Chang , W.H. , Chang , Y.H. and Lii , C.Y. 1994 . Phase Transitions of Rice Starch and Flour Gels . Cereal Chem , 71 ( 2 ) : 202 – 207 .
- Jang , J.K. , Lee , S.H. , Cho , S.C. and Pyun , Y.R. 2001 . Effect of Sucrose on Glass Transition, Glatinization, and Retrogradation of Wheat Starch . Cereal Chem , 78 ( 2 ) : 186 – 192 .
- Jouppila , K. and Roos , Y.H. 1997 . The Physical State of Amorphous Corn Starch and its Impact on Crystallization . Carbohydr. Polym , 32 : 95 – 104 .
- Laaksonen , T.J. and Roos , Y.H. 2001 . Thermal and Dynamic-Mechanical Properties of Frozen Wheat Doughs with Added Sucrose, NaCl, Ascorbic Acid, and Their Mixtures . Int. J. Food Prop , 4 ( 2 ) : 201 – 213 .
- Lim , M.H. , Wu , H. and Reid , D.S. 2000 . The Effect of Starch Gelatinization and Solute Concentrations on Tg′ of Starch Model System . J. Sci. Food Agric , 80 : 1757 – 1762 .
- Roos , Y. and Karel , M. 1991 . Amorphous State and Delayed Ice Formation in Sucrose Solutions . Int. J. Food Sci. and Technol , 26 : 553 – 566 .
- Wang , Y.J. and Jane , J. 1994 . Correlation Between Glass Transition Temperature and Starch Retrogradation in the Presence of Sugars and Maltodextrins . Cereal Chem , 71 ( 6 ) : 527 – 531 .
- Brake , N.C. and Fennema , O.R. 1999 . Glass transition values of muscle tissue . J. Food Sci , 64 ( 1 ) : 10 – 15 .
- Jensen , K.N. , Jorgensen , B.M. and Nielsen , J. 2003 . Low-Temperature Transitions in Cod and Tuna Determined by Differential Scanning Calorimetry . Lebensm. Wiss. U. Technol , 36 : 369 – 374 .
- Bai , Y. , Rahman , M.S. , Perera , C.O. , Smith , B. and Melton , L.D. 2001 . State Diagram of Apple Slices: Glass Transition and Freezing Curves . Food Res. Int , 34 : 89 – 95 .
- Martinez-Monzo , J. , Martinez-Navarrete , N. , Chiralt , A. and Fito , P. 1998 . Mechanical and Structural Changes in Apple (var. Granny Smith) Due to Vacuum Impregnation with Cryoprotectants . J. Food Sci , 63 ( 3 ) : 499 – 503 .
- Delgado , A.E. and Sun , D.W. 2002 . Desorption Isotherms and Glass Transition Temperature for Chicken Meat . J. Food Eng , 55 : 1 – 8 .
- Carrington , A.K. , Goff , H.D. and Stanley , D.W. 1996 . Structure and Stability of the Glassy State in Rapidly and Slowly Cooled Carbohydrate Solutions . Food Res. Int , 29 ( 2 ) : 207 – 213 .
- Rahman , M.S. 2004 . State Diagram of Date Flesh Using Differential Scanning Calorimetry (DSC) . Int. J. Food Prop , 7 ( 3 ) : 407 – 428 .
- Singh , K.J. and Roos , Y.H. 2005 . Frozen State Transitions of Sucrose-Protein-Cornstarch Mixtures . J. Food Sci , 70 ( 3 ) : 198 – 204 .
- American Association of Cereal Chemists . 2000 . Approved Methods of the American Association of Cereal Chemists , 10th , St. Paul, MN : AACC .
- Hsu , C.L. , Heldman , D.R. , Taylor , T.A. and Kramer , H.L. 2003 . Influence of Cooling Rate on Glass Transition Temperature of Sucrose Solutions and Rice Starch Gel . J. Food Sci , 68 ( 6 ) : 1970 – 1975 .
- Tananuwong , K. and Reid , D.S. 2004 . Differential Scanning Calorimetry Study of Glass Transition in Frozen Starch Gels . J. Agric. Food Chem , 52 : 4308 – 4317 .
- Sereno , A.M. , Sa , M.M. and Figueiredo , A.M. . Glass Transitions and State Diagrams for Freeze-Dried and Osmotically Dehydrated Apple . Proceedings of the 11th International Drying Symposium (IDS'98) . August 19–22 , Halkidiki, Greece.
- Kantor , Z. , Pitsi , G. and Thoen , J. 1999 . Glass Transition Temperature of Honey as a Function of Water Content as Determined by Differential Scanning Calorimetry . J. Agric. Food Chem , 47 : 2327 – 2330 .
- Kasapis , S. , Rahman , M.S. , Guizani , N. and Al-Aamri , M. 2000 . State Diagram of Temperature vs. Date Solids Obtained from Mature Fruit . J. Agric. Food Chem , 48 : 3779 – 3784 .
- Rahman , M.S. , Sablani , S.S. , Al-Habsi , N. , Al-Maskri , S. and Al-Belushi , R. 2005 . State Diagram of Freeze-Dried Garlic Powder by Differential Scanning Calorimetry and Cooling Curve Methods . J. Food Sci , 70 ( 2 ) : 135 – 141 .
- Roos , Y. and Karel , M. 1991 . Water and Molecular Weight Effects on Glass Transitions in Amorphous Carbohydrates and Carbohydrate Solutions . J. Food Sci , 56 ( 6 ) : 1676 – 1681 .