Abstract
Moisture sorption (adsorption and desorption) isotherm of freeze-dried garlic powder was measured by static and dynamic isopiestic methods. Isotherms measured by static isopiestic method produced deviations from the dynamic method at the lower and higher water activity ranges. Dynamic method showed that the temperature had more effects on desorption isotherms compared to the adsorption isotherm, and the degree of isotherm hysteresis reduced with the increase of equilibration temperature. The isotherm was fitted with BET, GAB, and Norrish models. Critical evaluations on the physical meaning of the model parameters are presented. The dynamic method has the potential to be used in studying other structural and drying characteristics of foods.
INTRODUCTION
The concept of water activity in foods has been developed to provide a reliable assessment of the microbial growth, lipid oxidation, non-enzymatic and enzymatic reactions, vitamins losses, and textural characteristics.[Citation1] Moisture sorption isotherm shows in graphical form the variation in water activity with the change in moisture content of sample at a specified temperature. A number of methods have been reported in the literature to measure or estimate the water activity of foods. Water activity measurement methods include the following: manometric, isopiestic, and hygrometric methods. The selection of appropriate method or instrument depends on its portability, speed, cost, simplicity and types of foods to be measured.[Citation2] Static isopiestic method is one of the most popular and widely used techniques to measure the moisture sorption isotherms of food. In this method sample of known mass (around 2–3 gm) is stored in a closed chamber and allowed to reach equilibrium with an atmosphere of known equilibrium relative humidity (or equilibrate with a standard of known water activity). Static desiccator is most commonly used to generate different relative humidity atmospheres with supersaturated salt solutions. More details of this method are given by Sablani et al.,[Citation3] Rahman,[Citation2] and Spiess and Wolf.[Citation4] The main advantages of this method are its simplicity, low cost, ability to handle many samples at a time and requirement of low skills to operate. The main disadvantage of this method is the long equilibration time, which is usually from 3–6 weeks. In many instances it could take few months to equilibrate.[Citation5] For this reason it is questioned whether the microbial and physico-chemical stability remain valid in the sample during long experimental period, especially at higher water activity. Although it is recommended to place toluene or thymol in the chamber for slowing the microbial growth, there is no option available to ensure physical and chemical stability in the course of equilibration period. In addition, care should be taken to use these chemicals for their toxicity due to inhalation from the desiccator chamber during sample preparation and performing weighing process. The condition of equilibrium is determined by reweighing the sample at intervals until constant mass is reached. Lewicki and Pomaranska-Lazuka[Citation5] studied the effect of the individual operations such as opening the desiccator, and transferring samples to the balance for checking constant mass. It was shown that the most important was the disturbance of equilibrium caused by opening the desiccator, taking the sample and closing it again. The error depends on the water activity and number of openings. At lower water activity (aw < 0.6) a maximum of about 20% overestimation in sample mass was observed, while at high water activity (0.6 < aw < 0.8) an underestimation of 20% was observed. The process of equilibration can be enhanced in desiccators with or without vacuum. It was shown that equilibration of samples under vacuum or without vacuum yields different water contents.[Citation6] All materials showed clearly that water contents were higher at the high water activities (> 0.60) and lower at the low water activities (< 0.400) using vacuum desiccators because of a probable difference in humidity between the external atmosphere and inside the desiccators with or without vacuum. Equilibration water contents were achieved after 2–3 days of storage using vacuum desiccators, while equilibrium time was 2–3 weeks using desiccators without vacuum over the whole range of relative humidity for all 3 g samples of different materials tested. In addition it is always not possible to find salts, which could be used for each and every water activity ranging from 0 to 1. Using static isopiestic method it is also difficult to measure adsorption and desorption isotherm for the same sample.
The dynamic isopiestic method could overcome the disadvantages of the static isopiestic method. The main advantages of the dynamic method, such as Symmetrical Gravimetric Analyzer (SGA) are: (i) its ability for rapid or fast equilibration of the sample; (ii) it uses same sample for whole isotherm; (iii) there is no need to remove the sample from the controlled humidity chamber during the complete isotherm measurement; (iv) easy measurement of adsorption and desorption isotherms for the same sample; (v) micro-level sample in the order of mg could be used; (vi) and, it is possible to measure at any increasing or decreasing water activity steps and oscillation mode. It is difficult to find detailed studies on the potential of this method although recently limited papers are presented on the uses of dynamic isopiestic method in foods.[Citation7–9] The reasons why this process gives fast equilibration (main advantage) are not studied in the literature. These could be due to small sample size, continuous circulation of gas, surface area, and small chamber or cell volume. This dynamic isopiestic method is also defined as dynamic vapor sorption (DVS) method. The objective of this study was to generate complete adsorption and desorption isotherms of freeze-dried garlic powder using dynamic method and to compare with the widely used static isopiestic method. In addition, possibility of using dynamic isopiestic method for measuring other sorption and structural related properties are explored.
MATERIALS AND METHODS
Sample Preparation
The garlic cloves were sliced into 2 mm thick pieces and slices were frozen in a freezer at −40°C for at least 48 hours. Frozen samples were then placed in an automatically controlled freeze drier (SP Industries Company, New York). The plate temperature was set at −20°C with a vacuum of 108 Pa in the chamber, while the condensing plate temperature was set at −65°C. The frozen sample was dried for about 48 hours. The freeze-dried slices were ground to form a powder and subsequently stored in air tight sealed bottle at −18°C until used in the isotherm experiments.
Static Isopiestic Method
The data developed by Al-Waili et al.[Citation10] by isopiestic method are used for comparison. The procedures used for static isopiestic method by Al-Waili et al.[Citation10] were as follows. Freeze-dried garlic powder (around 2 g) was placed in open weighing bottles and stored in air-sealed glass jars while maintaining equilibrium relative humidity of the atmosphere with saturated salt solutions. A layer of solid salts was maintained in the slurries during the whole period of equilibration. This is to confirm the solutions always remain saturated. The salts were: LiCl, CH3 COOK, MgCl2, K2CO3, NaBr, SrCl2, and KCl. Relative humidity values for these solutions were obtained from compilation by Rahman.[Citation2] Another 5 ml beaker with thymol (around 0.5 g) was placed inside each glass jar to avoid microbial growth in the sample and solutions. The jars were placed in a room controlled at 20°C. Detailed of this method are given by Sablani et al.[Citation3] The samples were equilibrated for about 6 weeks until constant mass was achieved.
Dynamic Isopiestic Method Apparatus
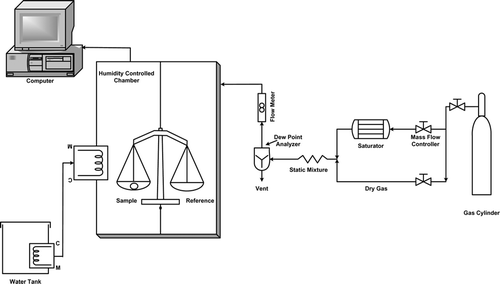
The Symmetrical Gravimetric Analyzer (SGA) Model 100 (SGA–100) from VTI Corporation, Florida was used to generate the sorption and drying data. It is an instrument designed for obtaining water sorption data on solid samples at relative humidity values between 2 and 98% and temperatures between 5 and 60°C. The drying of the samples could be done up to 80°C. A flow diagram of the set up is given in . This apparatus was designed in such a way that entire microbalance is inserted in a forced nitrogen circulation system, provided with a dynamic nitrogen humidification system, electronically controlled by an accurate mass flow controller. Identical conditions of temperature and humidity for sample and a reference were achieved by using a symmetrical two-chamber aluminum block. The nitrogen gas with a specific humidity was passed over the sample, as well as through the microbalance reference. The relative humidity was determined by relative humidity measuring probe. In order to ensure a constant atmospheric composition during the test and to optimize the nitrogen flux, the difference between the values read by two detectors was controlled. Sample mass changes were recorded using CI electronics microbalance sensitive to 0.1 µm and capacity of 5 gm. The entire apparatus was maintained at specific temperatures through circulating cold or warm water from a water bath (stability of 0.01°C) around the chamber. The equipment was tested and calibrated with sodium chloride and PVP (providone, N-vinyl pyrrolidone) within 10–80% relative humidity with drying cycle at 60°C. The microbalance was calibrated with 100 mg weight before each isotherm measurement.
Experimental Procedure
Around 20 mg of freeze-dried powder sample was placed in the sample balance and dried at 70°C with zero relative humidity for 2 hours. Water adsorption started at a specific temperature (for example 20°C) from 5% to 90% relative humidity with intervals of 5%. Desorption started from 90% to 5% with similar intervals of relative humidity of 5%. Sorption equilibrium conditions (mass stability) at certain humidity values were fixed as the condition to which the mass percentage variation was lower than 0.05% for 10 minutes. The flow rate of nitrogen gas was maintained at 4.8 × 10−3 m3/s over the sectional area of 3.1 cm × 3.8 cm. The acquisition system automatically recorded the mass of sample, mass change, sample temperature, and reference and sample chambers' relative humidity. The moisture content of the equilibrated sample was estimated by material balance from the change in mass and the moisture content of the sample before placing in the sample balance. The water content and total solids were measured gravimetrically by drying samples in air convection drier at 105°C for at least 20 hours. The statistical comparison of the data was done by one-way analysis of variance and by Tukey's paired test using SAS[Citation11] at 5% significance level.
Isotherm Models
The BET and GAB equations are widely used for modeling of the sorption isotherms of foods. The BET equation is:[Citation12]
Anderson[Citation14] modified BET equation for multi-layers for strengthening the physical meaning and the fitting ability of the model. Anderson's equation was later derived kinetically by DeBoer[Citation15] and statistically by Guggenheim,[Citation16] which was then named as GAB equation. The GAB is one of the most popular and widely used for foods. The equation is:
In EquationEqs. 3 and Equation4, T is the absolute temperature (K), R is the universal gas constant (8.314 kJ/mol K), and ΔHC and ΔHK are heat of sorption functions (kJ/mol). The GAB isotherm equation is an extension of the BET model taking into account the modified properties of the sorbate in the multilayer region and the bulk liquid properties through the introduction of a third constant K. The GAB parameters can be estimated by linear multiple regression after parabolic transformation of non-linear EquationEq. 2. Another option is to use non-linear optimization techniques.
The Norrish model for predicting the water activity of a solution has been derived from thermodynamic principles as follows:[Citation17]
RESULTS AND DISCUSSION
Moisture Sorption Isotherm
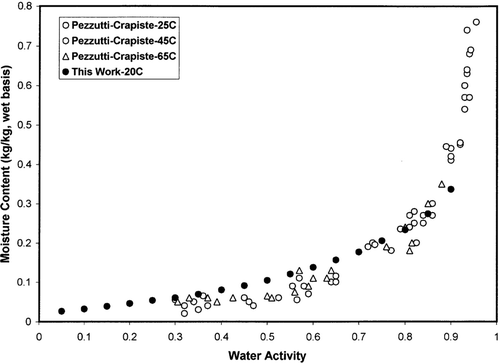
Initial experiments indicated that an equilibration (0.05% change for 10 minutes) time of 2 to 3 hours at each water activity should be enough for the garlic sample of around 30 mg. Based on this the maximum experimental equilibration time was set for 2 hours at each relative humidity. This showed that the equilibration time could be significantly reduced by dynamic method compared to the weeks or months required by the static method. shows the adsorption and desorption data of freeze-dried garlic powder as a function of equilibration temperature. In the case of adsorption, equilibrium water content increased with the increase of water activity, and opposite trends observed in the case of desorption. shows the adsorption isotherm of freeze-dried garlic powder measured by static and dynamic isopiestic methods. Both methods showed similar isotherm with in water activity values of 0.35–0.70. At the low water activity range (aw < 0.35), static isopiestic method showed overestimation of the moisture content, while at high water activity range (aw > 0.70) it showed underestimation of moisture content. Similar observation was also found by Lewicki and Pomaranska-Lazuka[Citation5] for static isopiestic method due to the opening of desiccator, and transferring samples to the balance for checking constant mass. One of the reasons could be due to the higher relative humidity of the room atmosphere, which caused condensation or adsorption of humidity resulting in overestimation at low water activity. In case of desorption, evaporation could occur for the high water activity sample. In addition, high water activity equilibration for long periods of time may also cause continuous physical and chemical deterioration of the sample. shows comparison of adsorption isotherm data from Pezzuti and Crapiste[Citation19] and this work. The little difference could be due to variation of the sample preparation and measurement method. Pezzuti and Crapiste[Citation19] prepared the dried garlic samples by vacuum drying at 50°C and stored in desiccators to allow equilibration of internal temperature and moisture content. The water activity of the samples was then measured by a Rotronic hygrometer. The hygrometers are more sensitive compared to the static and dynamic methods at water activity above 0.9.
Table 1 Adsorption moisture isotherm data of garlic by isopiestic and SGA methods.
Figure 2 Comparisons with data from Pezzutti and Crapiste.[Citation19]
![Figure 2 Comparisons with data from Pezzutti and Crapiste.[Citation19]](/cms/asset/f49a208d-4e74-4501-bd5b-3e73abb8edcc/ljfp_a_159596_o_f0002g.gif)
Figure 3 Moisture adsorption isotherms of freeze-dried garlic powder measured by static and dynamic isopiestic methods.
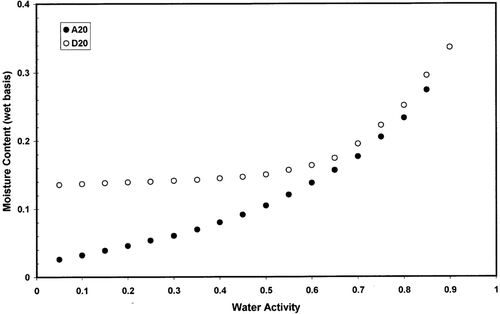
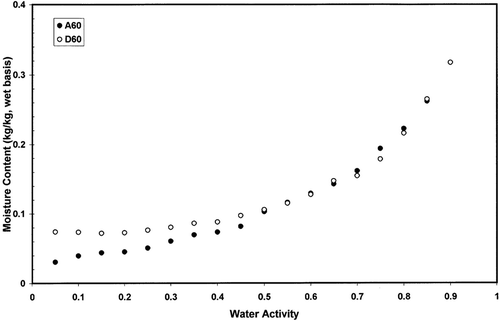
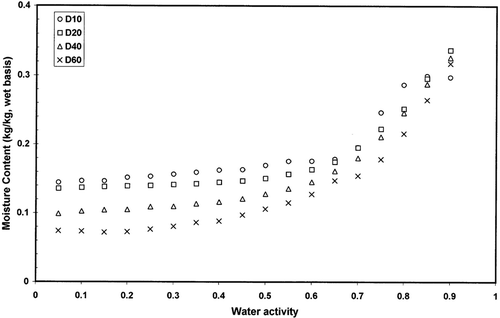
and shows adsorption and desorption isotherms of freeze-dried garlic powder as a function of equilibration temperature. Temperature showed significant effect on the adsorption and desorption isotherms (p < 0.05). Temperature showed higher effect in the case of desorption isotherm () compared to the adsorption isotherm (). and showed the hysteresis of the sorption isotherm at 60 and 20°C indicating higher temperature reduced the degree of hysterisis. At 20°C hysteresis extended up to water activity of 0.90, while at 60°C hysteresis started below water activity of 0.45. Iglesias and Chirife[Citation20] concluded that the total amount of hysteresis, its distribution relative to water activity, and the temperature dependency of the total hysteresis varied widely for different foods. For some foods, the effect of increasing temperature was to decrease or eliminate the total hysteresis (thyme, winter savory, sweet marjoram, trout cooked, chicken cooked, chicken raw, pork, apple, rice, bovin serum albumin, and tapioca), while for others the total hysteresis remained constant (ginger, nutmeg) or even increased (anis, cinnamon, chamomile, coriander).[Citation20–22] The desorption hysteresis loop usually ends at monolayer, but in some cases it extends down to an activity of zero.[Citation13] At 20°C hysteresis of gaziantep cheese extended up to water activity of 0.1.[Citation23] At 4.4°C, air-dried apple showed hysteresis from 0.6 to zero, freeze-dried cooked pork from 0.9 to zero, and freeze-dried rice from 0.95 to zero water activity.[Citation21] More data on hysteresis are presented in the literature for different foods.[Citation24–30] However, physico-chemical processes involved in the variation of hysteresis in foods have not been explored clearly in the literature. In addition, it is difficult to find desorption data of foods in the literature from dynamic method for comparison. Most of the desorption data generated in the literature were based on the isopiestic or hydrometric method, which may not be clearly compared with dynamic desorption.
Figure 4 Moisture adsorption isotherms of freeze dried garlic powder as a function of temperature measured by dynamic method.
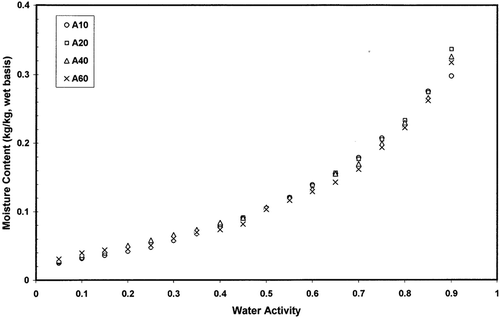
Figure 5 Moisture desorption isotherms of freeze dried garlic powder as a function of temperature measured by dynamic method.
Several theories have been proposed to explain hysteresis. From a capillary condensation mechanism, two theories exist.[Citation20] The hysteresis can be explained by a change in contact angle of sorbent between adsorption and desorption.[Citation31, Citation32] The ink-bottle-theory assumes capillaries to be composed of narrow necks with large bodies.[Citation33, Citation34] On adsorption, the capillary do not completely fill until the activity corresponding to the larger radius. During desorption, the smaller radius controls the emptying of the capillary so that the activity is lowered considerably according to the Kelvin equation.[Citation13] A capillary condensation mechanism alone is not able to explain the presence of hysteresis in foods. This is due to the fact that the hysteresis loop extends down to low water activities, in this region the capillary condensation mechanism is doubtful to operate.[Citation35] In addition, molecular structural changes may also cause hysteresis. In case of proteins, the hysteresis was caused by a deformation of the polypeptide chains within the protein molecule as the polar adsorbate settled into suitable position.[Citation36] Iglesias et al.[Citation37] found that collapse of the matrix shifted the isotherm up in the case of adsorption and move it down in case of desorption. This may be one of the processes that could happen in slow adsorption and desorption. The presence of phase transformations in sugar containing sorbates may produce hysteresis loops.[Citation38] Iglesias and Chirife[Citation20] concluded that it is not possible to give a single explanation of the hysteresis phenomena in foods; each food is a complex combination of various constituents which can sorb water independently, but can also interact among themselves giving increased or decreased water sorption capacities. Based on the authors' knowledge, no prediction procedure of hysteresis in foods has been proposed so far in the literature.
Isotherm Models
The isotherm data were fitted with the BET, GAB, and Norrish models. The model parameters are shown in . The BET monolayer values showed a decreasing trends with the increasing equilibration temperature, and BET-monolayer values estimated from desorption isotherms were much higher than the values estimated from adsorption isotherms (). Adsorption isotherms showed positive values for B, whereas desorption isotherms showed negative values. In the case of desorption isotherms, difficulty was observed to converge the iterations in non-linear regression. The convergence was strongly dependent on the initial value in case of desorption isotherms. The BET-monolayer is an effective method for estimating the amount of bound water to specific polar sites in dehydrated food systems. The BET equation provides an average value of heat of sorption while it changes with the water activity. The physical meaning of B in BET may not be valid in many cases. Iglesias and Chirife[Citation20] showed that there is limited validity of the relation of B with heat of sorption due to the number of assumptions involved in BET theory. The value of B constant cannot be taken as more than a very rough guide to the magnitude related to heat of sorption. They identified many instances when estimated heat of binding of water from temperature dependency of B was completely wrong although in many instances it was valid. In addition, it is more difficult to explain when monolayer decreases significantly with increasing temperature.[Citation39] Selected situations are presented when the values of B are very difficult to explain. Maskan and Gogus[Citation40] reported that negative values of B were impossibility and was not reported in the literature. Comino[Citation41] found negative values of B for Limonene-β-cyclodextrin powders. In case of all desorption data, the values of B were negative, whereas in case of adsorption only 9.6% oil content showed negative values. In case of Arabian sweet isotherm in the temperature range of 20–50°C, Ahmed et al.[Citation42] also found negative values of B for BET and C for GAB models. Similarly, Dincer and Esin[Citation43] also observed negative values of B for macaroni in the temperature range 30–70°C. Young[Citation44] also found infinity value for B in the case of peanuts. In case of hazelnut, the values of B was found in the order of 106 at 3°C, whereas at 10 and 30°C the values were in the order of 100 and 10.[Citation45] However, the lack of reliability of the energy parameter B, does not preclude the use of BET equation for the determination of monolayer value.[Citation39] It is well documented for its physical meaning and validity for its monolayer value.
Table 2 Model parameters for BET, GAB, and Norrish.
In selecting isotherm models two points need to be considered. One is the accuracy of the model and another one is physical meaning of the model's parameters. Accuracy indicates the mathematical representation of the experimental data, and physical meaning indicates more explanation of the physico-chemical process. The whole range of isotherms up to water activity of 0.9 could be predicted using GAB and Norrish models using the estimated parameters shown in . The GAB model was found most accurate up to water activity 0.9. It is now well accepted that GAB is one of the most accurate models for predicting isotherm of foods. In many cases, GAB was found accurate and residual was observed random.[Citation27] Chen and Jayas[Citation46] evaluated GAB equation for the isotherms of agricultural products. They found uniform scattered in residual plots of some high protein and high oil materials. Clear patterns in residual plots were found in high starch and some high protein products. The lack of fit for starchy foods was also observed by Bizot.[Citation47] A clear pattern of residual plots was also found for garlic slices,[Citation48] and sweet potato slices.[Citation49] The physical meaning of GAB and Norrish parameters may not be valid in all cases although there have been given emphasis in the literature on the physical meaning. These aspects are explained below from the reported literature. Gogus et al.[Citation25] found completely unreliable monolayer values (very high 40% to 138%) for Turkish delight estimated from GAB model although it gave the most accurate prediction. Theoretically the values of K should be less than unity.[Citation50] However in the literature huge number of papers presented the values of K higher than unity, which was unsound. Lewicki[Citation51] showed that the GAB model described well sigmoidal type isotherms when parameters are kept in the following regions: 0.24 < K < 1 and 5.67 ≤ C ≤ ∞. Outside these regions the isotherm is either no longer sigmoid or the monolayer capacity is estimated with the error larger than ± 15.5%. Keeping constants in the above regions fulfils the requirements of the BET model.
It is common to use an Arrhenius-type equation for three parameters of the GAB, which transforms six parameters temperature dependent GAB equation. Chen and Jayas[Citation46] identified that Arrhenius function did not show a strong relationship between GAB parameters and temperature. In this case the basis of C and K based on the heat of sorption for mono- and multi-layers adsorption could be questioned for its physical meaning. Different regression methods and optimization techniques could be used to estimate the parameters, which may result in different values or many local optimization regions. When it happens, the parameters could be varied significantly based on the initial values in the iteration process as well as the variation of location of optimum region. When computing the estimated parameters of the GAB equation for some sorption isotherms of rapeseed, it was very difficult to obtain convergence for the C value. Neither the Gauss-Newton nor the steepest descent method could obtain the convergence value of C. The value for C always approached infinity no matter what numerical technique was used.[Citation46] Isotherm data of microcrystalline cellulose (MCC) and cake provided negative values of C and positive value for starch when temperature was varied from 100 to 130°C.[Citation52, Citation53] When isotherm data of MCC cellulose and potato starch from 92 to 135°C was fitted with GAB model Bassal et al.[Citation54] found the values of C was positive. The expected values of C should be negative at temperatures above 100°C. Based on the points discussed above, GAB could lose its theoretical basis and transform into just a 3-parameter regression model.[Citation55] These are the generic problems when theoretical based model is extended to fit the experimental data and attempt to relate with physics.[Citation56] In this situation, we strongly believe that only BET model having two parameters (which could be estimated by graphical methods always giving same values from same set of data) could be related with the physics for only monolayer value. Computer software could be used to do regression or non-linear optimization for BET, but it could also be checked with the graphical solution or use the graphical values as the initial values for optimization.
Other Potential Uses of Dynamic Method
Dynamic method has high potential to be used for micro level drying studies. Micro level drying kinetics could be performed as a function of air composition (modified atmosphere drying with varied oxygen, carbon dioxide, and nitrogen levels), relative humidity, temperature, and varied sample geometry and structure. May et al.[Citation7] studied the isothermal drying kinetics of foods using a thermo-gravimetric analyzer (Perkin-Elmer 7 series), which was automated and can handle samples between 10–100 mg. It recorded mass automatically at set intervals with a precision of 0.1 mg. They used sample size of about 30 mg and recorded mass against time at a set temperature of 40°C with an accuracy of 0.1°C. The purge gas used was nitrogen at a constant velocity of 1.25 × 10−3 m/s. Drying curves of five different foods (apple, potato, carrot, asparagus, and garlic) were analyzed and found different drying characteristics with varied constant and falling rate periods. Lin and Chen[Citation57] discussed a prototype set-up for controlled air humidity and temperature chamber for isothermal drying kinetics and moisture sorption isotherm. Teoh et al.[Citation8] used dynamic vapor sorption (DVS-2000, Surface Measurement Systems, London) analysis to measure cornmeal snack moisture isotherm and compared with the values by equilibrating sample in proximity equilibration cell[Citation58] and measured the water activity using Aqualab CX-2 (Pullman, Washington). They identified that DVS analysis could produce rapid isotherms and to accurately condition the individual model systems to the same moisture content. With such rapid equilibration times, it was possible to obtain isotherm points at up to water activity of 0.95, without worry about microbial and other physico-chemical degradation, and increasing the accuracy of the isotherm modeling at higher water activities. Rahman et al.[Citation59] studied the quality of dried lamb meat produced by simulating modified atmosphere (nitrogen gas) drying using dynamic system SGA-100. The dried lamb meat was evaluated for their microbial and physico-chemical characteristics. Sannino et al.[Citation9] used DVS-1000 system to study drying process of lasagna pasta at controlled humidity and temperature with a sensing device to measure the electrical conductivity of pasta during the drying process. An anomalous diffusion mechanism has been observed, typical of the formation of a layered sample structure: a glassy shell on the surface of the pasta slice, which inhibits a fast diffusion from the humidity, rubbery internal portion. Internal stresses at the interface of the glassy-rubbery surfaces are responsible for crack formation and propagation and thus lasagna sample de-lamination and breakage.
The kinetics of adsorption and desorption isotherms could be used to explore other structural characteristics, such as glass transition. It is expected to observe a break in the mass transfer rate constant or moisture diffusivity at the glass-rubber transition. The mass transfer rate constant or diffusivity could be estimated from the adsorption or desorption kinetics of samples having same initial moisture content and be plotted as a function of temperature in order to identify a break in the plot at glass transition. This has not yet been tested. However, Garcia and Pilosof[Citation60] attempted to correlate water sorption kinetics with the glass transition temperature. They measure adsorption and desorption kinetics by placing samples in desiccator at 30°C with different relative humidity values maintained by saturated salts. Garcia and Pilosof[Citation60] plotted rate constant as a function of relative humidity and observed a change in slope at the relative humidity when sample was transformed to glassy state. In general, dynamic method in micro level has high potential to be used in foods. Del-Nobile et al.[Citation61] developed a new approach based on the use of oscillatory sorption tests to determine the water-transport properties of chitosan-based edible films. Oscillatory sorption tests as well as stepwise sorption tests were conducted at 25°C on chitosan films. Two different models were fitted to the experimental data to determine the relationship between the water-diffusion coefficient and the local water concentration. One of the two tested models accounts only for stochastic diffusion and the other accounts also for the superposition of polymer relaxation to stochastic diffusion. A comparison between experimental and predicted water permeability pointed out that stepwise sorption tests cannot be used to determine the dependence of water-diffusion coefficient on local water concentration when the diffusion process has characteristic time much smaller than that of polymer relaxation. In fact, in these cases, the diffusion process controls only the very early stage of sorption kinetics, whereas the remaining part of the transient is controlled by polymer relaxation.[Citation61] In the future, other potential applications of the dynamic methods could be found in the literature.
CONCLUSION
Moisture sorption isotherm of garlic powder measured by static isopiestic method produced deviations from the dynamic method at the lower and higher water activity ranges. The temperature had more effects on desorption isotherms compared to the adsorption isotherm, and the degree of isotherm hysteresis reduced with the increase of equilibration temperature. The BET-monolayer values were found lower when adsorption isotherms were used. The GAB model was found to be more accurate over the water activity range up to 0.9. The dynamic method has the potential to be used in studying micro level drying characteristics, conditioning sample at different water content and water activity, simulating modified atmosphere drying, and identifying other structural characteristics, such as glass-rubber transition, and surface cracking.
ACKNOWLEDGMENTS
This work was supported by the Sultan Qaboos University research grants IG/AGR/BIOR/05/01. The author would like to thank Dr. Bhesh Bhandari for his valuable comments on the manuscript.
Notes
41. Comino, P.R. The Sorption Isotherm Properties of Limonene-β-cyclodextrin complex powder. Master of Philosophy Thesis, The University of Queensland, 2005.
REFERENCES
- Rahman , M.S. and Labuza , T.P. 1999 . “ Water activity and food preservation ” . In Handbook of Food Preservation , Edited by: Rahman , M.S. 339 – 382 . New York : Marcel Dekker .
- Rahman , M.S. 1995 . Food Properties Handbook , Boca Raton, Florida : CRC Press .
- Sablani , S.S. , Rahman , M.S. and Labuza , T.P. 2001 . Current Protocols in Food Analytical Chemistry , Edited by: Wrolstad , R.E. A2.3.1 – A2.3.10 . New York : John Wiley & Sons .
- Spiess , W.E.L. and Wolf , W. 1987 . “ Water activity ” . In Theory and Applications to Food , Edited by: Rockland , L.B. and Beuchat , L.R. 215 – 233 . New York : Marcel Dekker .
- Lewicki , P.P. and Pomaranska-Lazuka , W. 2003 . Errors in static desiccator method of water sorption isotherms estimation . International Journal of Food Properties , 6 ( 3 ) : 557 – 563 .
- Laaksonen , T.J. , Roos , V.H. and Labuza , T.P. 2001 . Comparisons of the use of desiccators with or without vacuum for water sorption and glass transition studies . International Journal of Food Properties , 4 ( 3 ) : 545 – 563 . [CROSSREF]
- May , B.K. , Shanks , R.A. , Sinclair , A.J. , Halmos , A.L. and Tran , V.N. 1997 . A study of drying characteristics of foods using thermogravimetric analyzer . Food Australia , 49 ( 5 ) : 218 – 220 .
- Teoh , H.M. , Schmidt , S.J. , Day , G.A. and Faller , J.F. 2001 . Investigation of cornmeal components using dynamic vapor sorption and differential scanning calorimetry . Journal of Food Science , 66 ( 3 ) : 434 – 440 .
- Sannino , A. , Capone , S. , Siciliano , P. , Ficarella , A. , Vasanelli , L. and Maffezzolo , A. 2005 . Monitoring the drying process of lasagna pasta through a novel sensing device-based method . Journal of Food Engineering , 69 : 51 – 59 . [CROSSREF]
- Al-Waili , N.S. , Rahman , M.S. and Sablani , S.S. 2005 . Moisture adsorption isotherm of freeze-dried garlic powders . Proceedings of the International Conference on Postharvest Technology and Quality Management in Arid Tropics held in Sultan Qaboos University . 31 January–2 February 2005 , Muscat, Sultanate of Oman.
- SAS . 2001 . SAS users' guide: Statistics , NC : SAS Institute .
- Brunauer , S. , Emmet , P.H. and Teller , E. 1938 . Adsorption of gases in multimolecular layers . Journal of American Chemical Society , 60 ( 2 ) : 309 – 319 . [CROSSREF]
- Labuza , T.P. 1968 . Sorption phenomena in foods . Food Technology , 22 : 263 – 272 .
- Anderson , R.B. 1946 . Modification of the Brunauer, Emmett and Teller equation . Journal of the American Chemical Society , 68 : 686 – 691 . [CROSSREF]
- DeBoer , J.H. 1953 . The Dynamic Character of Adsorption , 61 – 81 . Oxford : Clarendon Press .
- Guggenheim , E.A. 1966 . Application of Statistical Mechanics , 186 – 206 . Oxford : Clarendon Press .
- Norrish , R.S. 1966 . An equation for the activity coefficients and equilibrium relative humidities of water in confectionery syrups . Journal of Food Technology , 1 : 25 – 39 .
- Rahman , M.S. , Perera , C.O. and Thebaud , C. 1998 . Desorption isotherm and heat pump drying kinetics of peas . Food Research International , 30 ( 7 ) : 485 – 491 . [CROSSREF]
- Pezzutti , A. and Crapiste , G.H. 1997 . Sorption equilibrium and drying characteristics of garlic . Journal of Food Engineering , 31 : 113 – 123 . [CROSSREF]
- Iglesias , H.A. and Chirife , J. 1976 . Isosteric heats of water sorption on dehydrated foods. Part II. Hysteresis and heat of sorption comparison with B.E.T theory . Food Science and Technology , 9 : 123 – 127 .
- Wolf , M. , Walker , J.E. and Kapsalis , J.G. 1972 . Water vapor sorption hysteresis in dehydrated food . Journal of Agricultural Food Chemistry , 20 : 1073 – 1077 . [CROSSREF]
- Seehof , J.M. , Keilin , B. and Benson , S.W. 1953 . The surface areas of proteins. V. The mechanism of water sorption . Journal of American Chemical Society , 75 : 2427 – 2430 . [CROSSREF]
- Kaya , S. and Oner , M.D. 1996 . Water activity and moisture sorption isotherms of gaziantep cheese . Journal of Food Quality , 19 : 121 – 132 .
- Yanniotis , S. and Zarmboutis , I. 1996 . Water sorption isotherms of pistachio nuts . Food Science and Technology , 29 ( 4 ) : 372 – 375 .
- Gogus , F. , Maskan , M. and Kaya , A. 1998 . Sorption isotherms of Turkish delight . Journal of Food Processing and Preservation , 22 : 345 – 357 .
- Iglesias , O. and Bueno , J.L. 1999 . Water agar-agar equilibrium: determination and correlation of sorption isotherms . International Journal of Food Science and Technology , 34 : 209 – 216 . [CROSSREF]
- Menkov , N.D. 2000 . Moisture sorption isotherms of chickpea seeds at several temperatures . Journal of Food Engineering , 45 : 189 – 194 . [CROSSREF]
- Menkov , N.D. 2000 . Moisture sorption isotherms of lentil seeds at several temperatures . Journal of Food Engineering , 44 : 205 – 211 . [CROSSREF]
- Cervantes , M.S.S. , Gonzalez , I.A. , Kubiak , K.N. and Alvarado , M.A.G. 1993 . Study on sorption isotherms of shrimp heads . Drying Technology , 11 ( 6 ) : 1459 – 1466 .
- Tolaba , M.P. and Suarez , C. 1990 . Desorption isotherms of shelled maize: whole, dehulled and hulls . International Journal of Food Science and Technology , 25 : 435 – 441 .
- Foster , A.G. 1932 . The sorption of condensable vapors by porous solids. Part I. The applicability of the capillary theory . Transactions of the Fraday Society , 28 : 645 – 657 . [CROSSREF]
- Cohan , L.H. 1938 . Sorption hysteresis and the vapor pressure concave surface . Journal of American Chemical Society , 60 : 433 [CROSSREF]
- Kraemer , E.D. 1931 . A Treatise on Physical Chemistry , Edited by: Taylor , H.S. 1661 London : Macmillan & Co .
- McBain , J.W. 1935 . An explanation of hysteresis in the hydration and dehydration of gels . Journal of American Chemical Society , 57 : 699 – 700 . [CROSSREF]
- Karel , M. 1973 . Recent research and development in the field of low-moisture and intermediate-moisture foods . CRC Critical Reviews in Food Technology , 3 : 329 – 373 .
- Benson , S.W. and Richardson , R.L. 1955 . A study of hysteresis in the sorption of polar gases by native and denatured proteins . Journal of American Chemical Society , 77 : 2585 – 2590 . [CROSSREF]
- Iglesias , H.A. , Schebor , C. , Buera , M.P. and Chirife , J. 2000 . Sorption isotherm and calorimetric behavior of amorphous/crystalline raffinose-water systems . Journal of Food Science , 65 ( 4 ) : 646 – 650 .
- Iglesias , H.A. , Chirife , J. and Lombardi , J.L. 1975 . Water sorption isotherms in sugar beet root . Food Technology , 10 : 299 – 308 .
- Iglesias , H.A. and Chirife , J. 1976 . Isosteric heats of water sorption on dehydrated foods. Part I. Analysis of differential heat curves . Food Science and Technology , 9 : 116 – 122 .
- Maskan , M. and Gogus , F. 1997 . The fitting of various models to water sorption isotherms of Pistachio nut paste . Journal of Food Engineering , 33 : 227 – 237 . [CROSSREF]
- 41. Comino, P.R. The Sorption Isotherm Properties of Limonene-β-cyclodextrin complex powder. Master of Philosophy Thesis, The University of Queensland, 2005.
- Ahmed , J. , Khan , A.R. and Hanan , A.S. 2004 . Moisture adsorption of an Arabian sweet (basbusa) at different temperatures . Journal of Food Engineering , 64 : 187 – 192 . [CROSSREF]
- Dincer , T.D. and Esin , A. 1996 . Sorption isotherms for macaroni . Journal of Food Engineering , 27 : 211 – 228 . [CROSSREF]
- Young , J.H. 1976 . Evaluation of models to describe sorption and desorption equilibrium moisture content isotherms of Virginia-type peanuts . Transactions of the ASAE , 19 : 146 – 155 .
- Lopez , A. , Pique , M.T. , Clop , M. , Tasias , J. , Romero , A. , Boatella , J. and Garcia , J. 1995 . The hygroscopic hehaviour of the hazelnut . Journal of Food Engineering , 25 : 197 – 208 . [CROSSREF]
- Chen , C. and Jayas , D.S. 1998 . Evaluation of the GAB equation for the isotherms of agricultural products . Transactions of the ASAE , 41 ( 6 ) : 1755 – 1760 .
- Bizot , H. 1984 . “ Using the G.A.B model to construct sorption isotherms ” . In Physical Properties of Foods , Edited by: Jowitt , J. 43 – 54 . New York : Elsevier Applied Sciences .
- Madamba , P.S. , Driscoll , R.H. and Buckle , K.A. 1994 . Predicting the sorption behavior of garlic slices . Drying Technology , 12 ( 3 ) : 669 – 683 .
- Chen , C. and Tsao , T. 1997 . Equilibrium relative humidity characteristics of sweet potato slices . Journal of Agricultural Machinery , 7 ( 1 ) : 99 – 113 .
- Chirife , J. , Timmermann , E.O. , Iglesias , H.A. and Boquet , R. 1992 . Some features of the parameter k of the GAB equation as applied to sorption isotherms of selected food materials . Journal of Food Engineering , 15 : 75 – 82 . [CROSSREF]
- Lewicki , P.P. 1997 . The applicability of the GAB model to food water sorption isotherms . International Journal of Food Science and Technology , 32 : 553 – 557 .
- Bassal , A. and Vasseur , J. 1992 . “ Measurement of water activity at high temperature ” . In Drying ‘92 , Edited by: Mujumdar , A.S. 312 – 321 . London : Elsevier Science Publishers .
- Bassal , A. , Vasseur , J. and Loncin , M. 1993 . Sorption isotherms of food materials above 100°C . Food Science and Technology , 26 : 505 – 511 .
- Bassal , A. , Vasseur , J. and Lebert , A. 1993 . Measurement of water activity above 100°C . Journal of Food Science , 58 ( 2 ) : 449 – 452 .
- Rahman , M.S. 2005 . Dried food properties: challenges ahead . Drying Technology , 23 ( 4 ) : 695 – 715 .
- Rahman , M.S. 2004 . State diagram of date flesh using differential scanning calorimetry (DSC) . International Journal of Food Properties , 7 ( 3 ) : 407 – 428 . [CROSSREF]
- Lin , S.X.Q. and Chen , X.D. 2005 . An effective laboratory air humidity generator for drying research . Journal of Food Engineering , 68 : 125 – 131 . [CROSSREF]
- Lang , K.W. , McCune , T.D. and Steinberg , M.P. 1981 . A proximity equilibrium cell for rapid determination of sorption isotherms . Journal of Food Science , 46 : 936 – 938 .
- Rahamn , M.S. , Salman , Z. , Kadim , I.T. , Mothershaw , A. , Al-Riziqi , M.H. , Guizani , N. , Mahgoub , O. and Ali , A. 2005 . Microbial and physico-chemical characteristics of dried meat processed by different methods . International Journal of Food Engineering , 1 ( 2 ) : 1 – 14 .
- Garcia , L.H. and Pilosof , A.M.R. 2000 . Kinetics of water sorption in okara and its relationship to the glass transition temperature . Drying Technology , 18 ( 9 ) : 2105 – 2116 .
- Del-Nobile , M.A. , Buonocore , G.G. and Conte , A. 2004 . Oscillatory sorption tests for determining the water-transport properties of chitosan-based edible films . Journal of Food Science , 69 ( 1 ) : 44 – 49 .