Abstract
The composition of egg yolk lipids differs greatly from most other food lipids, as it combines high contents of triglycerides and phospholipids, as well as a high proportion of cholesterol. The lipids are organized in lipoproteins and are dispersed in aqueous phase. Classical extraction methods using chloroform and methanol have not been optimized for such a matrix, therefore quantitative extraction yields of egg lipids can be inaccurate. In this study, the original method of Bligh and Dyer is evaluated and adapted for egg lipids. Furthermore, a direct extraction/derivatization method is presented. Solvent extraction resulted in a lipid yield of 31.8 ± 0.89% and total fatty acid yield was 257 ± 2 mg/g yolk whereas the direct method yielded 250 ± 7 mg/g yolk. Gas chromatographic analyses confirmed that lipid recovery was complete for triglycerides and phospholipids as well.
INTRODUCTION
The fatty acid composition of egg yolk lipids and its variation in response to feed composition has attracted the interest of nutritionists, since egg lipids contain appreciable amounts of long-chain polyunsaturated omega-3 fatty acids (LCn3PUFA). Among LCn3PUFA, docosahexaenoic acid (DHA, C22:6n3) is the most abundant in egg lipids and its concentration is highly dependent on feed composition. DHA is mainly located in the phospholipid (PL) fraction of egg lipids and is highly sensitive to oxidation. Accurate quantification of PUFA such as DHA requires complete recovery of PL and highest possible protection against autooxidation.
Compared to most other food matrices, egg yolk is rather unique as it combines high amounts of total fat and high proportions of membrane components (PL, cholesterol). Egg lipids are organized in lipoproteins and dispersed in aqueous phase. Quantitative lipid extraction of such a matrix can be a challenge, and strictly obeying the procedures of classical methods such as the Folch et al.[Citation1] or the Bligh and Dyer[Citation2] methods can be insufficient. As there are no definite procedures established for the extraction of egg lipids, many variations of the classical procedures are found in literature. While some authors follow the original protocols,[Citation3–5] others apply some modifications, mainly by using higher solvent/sample ratios.[Citation6]
Prerequisite for extraction of unsaturated fats and oils is consistent protection against lipid oxidation. Adding synthetic antioxidants, such as butylated hydroxyl toluene (BHT)[Citation7] or ethoxyquin,[Citation8] to the extraction solvent can help. For the extraction of egg lipids the use of chloroform and methanol as solvents is probably the best option. Nevertheless, attempts have been made to perform lipid extraction from biological matrices by using less toxic solvents,[Citation9] or by more sophisticated techniques such as supercritical fluid extraction.[Citation10,Citation11] Chloroform and methanol in combination exhibit strong dissolving power for the entire range of polarity found in lipids, as well as the ability to break up membranes and denaturate (lipo-) proteins. The method of Bligh and Dyer has been developed for cod muscle, which is a very lean matrix with less than 1% lipid content, but with a high proportion of water (ca 80%).[Citation2] This method has become very popular in food analysis, especially for sample with high water content. Therefore, it is also the preferred method for the extraction of lipids from (liquid) egg yolk. The authors of that study gave detailed instructions on how to adapt their method for other matrices than cod muscle.[Citation2] However, most food samples have either a high content of fat, which is mostly TAG, or they are lean and have a high proportion of PL and other membrane components. Bligh and Dyer suggested to keep the proportions of solvents as described in the original procedure, and to adapt the amount of solvent to the sample size (not the expected amount of lipid). For total recoveries in fatty samples they recommended a second wash with chloroform alone and to combine the lipid phases. Other authors recommend using solvents in at least the15-fold excess in order to obtain complete extraction for fatty matrices such as milk products.[Citation12]
In this article, the original Bligh and Dyer (OBD) procedure[Citation2] was tested versus the adaptation of this method using the 15-fold excess of solvent (modified Bligh and Dyer—MBD). We tested whether the additional surplus of solvent improves lipid yield, measured as weigh-out of extract and of GC analysis of FA. The second part of this article was the evaluation of a direct extraction/transmethylation method (DM), in which the homogenized egg yolk was subjected to direct derivatization. Such methods have been developed successfully for biological and food matrices such as human milk, adipose tissue,[Citation13] plasma, feces, liver,[Citation14] processed food[Citation15] and fresh fish.[Citation16] For adaptation to egg lipids it is necessary to evaluate whether full recoveries are obtained for the entire range of polarity, e.g., that FA from TAG and PL are equally and quantitatively recovered.
MATERIALS AND METHODS
Eggs were purchased at a local supermarket. All chemicals were of analytical grade (p.a.) and were obtained from Roth chemicals (Lactan, Graz, Austria), unless otherwise stated. Toluene and chloroform were treated with 100 mg/l BHT. All determinations were performed in quadruplicates and statistical evaluation was performed using SigmaStat software (SigmaStat Inc., Chicago, IL.). ANOVA was carried out on a 95% confidence level (P < 0.05) and differences among groups were determined by the Tukey test.
Egg Preparation
Egg yolks were separated from the white and weighed. For comparison of the different methods, 4 yolks were pooled and homogenized on an IKA Ultra Turrax T18 (IKA-Werke GmbH & Co. KG., Staufen, Germany). From this representative pool, ca. 1 g was weighed to nearest 0.1 mg into the appropriate glassware (20 ml reaction tubes with screw caps for the OBD method, 200 ml glass bottles with screw caps for the MBD method. The rest of the yolk homogenate was weighed into Petri-dishes (made of glass) in amounts of ca. 10 g each (to nearest 0.01 g) and the dishes were frozen at −70°C.
Solvent Extraction (OBD)
This method resembled the original Bligh and Dyer protocol,[Citation2] including the adaptation to other food matrices recommended by the authors of this study. As a first step, 1 ml of distilled water was added to the reaction vial containing 1 g of accurately weighed yolk. This was done in order to keep the proportion of chloroform, methanol and water at 1:2:0.8, as it is described in the original procedure (1 g of yolk contains ca. 0.5 g of water, the addition of 1 g water increases the total amount of water to ca. 1.5 g in 2 g total). Yolk and water were dispersed by gentle shaking by hand and 2 ml of chloroform and 4 ml of methanol were added. After shaking for 90 s (Ms 2 Minishaker, IKA-Werke GmbH & Co. KG., Staufen, Germany), another 2 ml of chloroform were added. The mixture was shaken again for 30 seconds and 2 ml of water were added followed by a short shaking (5 s). The tubes were centrifuged at 1100 rpm for 10 minutes and the lower phase was recovered by using a double pipette technique. In brief, one Pasteur pipette was shortened about 5 cm with a glass cutter and introduced into the lower phase by applying a little overpressure on the rubber ball. After penetrating the protein disc carefully, the rubber ball was removed that the clean lipid phase could enter the pipette without contamination by the upper phase. Then the lipid phase was recovered by a second pipette, which was inserted through the first pipette. After recovering most of the lower phase the pipettes were removed and the short pipette was cleaned with a small amount of fresh chloroform (ca. 1 ml), which was added to the isolated lipid extract. The remaining upper phase was washed with 4 ml chloroform by shaking again for 30 seconds. After centrifuging for another 10 minutes, the lower phase was recovered again with the aforementioned procedure using the same pipettes in order to recover any possible remnants from the inside glass wall. This washing step was repeated one more time in order to achieve complete recovery. The combined lower phases were collected in pre-weighed 100 ml round bottom flasks.
Solvent Extraction (MBD)
The yolk was weighed into a 200 ml Pyrex glass bottle with screw cap. According to the OBD method, water was added first, but in an amount of 25 ml in order to match the required ratio of solvents described by Bligh and Dyer.[Citation2] Then, 35 ml methanol and 70 ml chloroform were added and the sample was stirred on a magnetic stirrer for 90 seconds. After addition of 35 ml chloroform, 30 seconds stirring and addition of 35 ml water, the mixture was transferred into a 250 ml separatory funnel, and the headspace was flushed with nitrogen. After phase separation, the lower phase was recovered and the upper phase washed again with 35 ml chloroform. The lipid phases were combined in pre-weighed 250 ml round bottom flasks.
Treatment of the Lipid Extracts
The solvents (chloroform, methanol and traces of water) were removed with a rotary evaporator (Büchi R220, Büchi Labortechnik AG, Flawil, Switzerland). In order to avoid foaming of the egg lipids, the vacuum should not be beyond 330 mbar until the solvent has disappeared. The temperature of the water bath was set at 40°C. For removing solvent traces the vacuum was reduced to ca. 190 mbar, after all visible parts of the solvents were evaporated. The lipids were re-dissolved in ca. 5 ml of acetone and dried again under vacuum. The clean lipids were kept in an evacuated desiccator over night and the lipid content was determined gravimetrically. The extracts were dissolved in exactly 25 ml toluene. From this mixture 1 ml was pipetted into a 20 ml reaction vial and 1 ml of toluene containing 1 mg internal standard (metyl-heptadecanoate, Larodan, Malmö, Sweden) was added.
Direct Extraction/Transmethylation
The yolk that had been frozen in the Petri dishes was freeze-dried over night using an Edwards Modulyo 4K freeze drier (Edwards, Kniese & Co., Marburg, Germany). Dry matter was determined by back-weighing the lyophilisate. The dried yolk was powdered in a stone mortar by hand and ca. 50 mg of the powder was weighed to nearest 0.1 mg into a 20 ml reaction vial. To the reaction vial 1 ml of toluene and 1 ml of internal standard (4 ml metyl-heptadecanoate in toluene) were added.
Transmethylation
Transmethylation was performed with 5% methanolic HCl according to Ulberth and Henninger.[Citation15] HCl/methanol was freshly prepared each day by following the procedure described by Christie.[Citation7] Three ml of methanolic HCl were added to the reaction vials containing the lipid extracts or lyophylisates described before. The headspace was flushed with nitrogen and the vials were sealed tightly with Teflon-lined screw caps. The reaction was performed in a 70°C water bath, and the vials were shaken once after they reached the reaction temperature (ca. 1 min after they were put in the water). After 2 hours, the vials were cooled in cold water and 5 ml of 6 % aqueous K2CO3 was added. The vials were shaken and centrifuged at 1100 rpm for 10 minutes. The upper phase was recovered and dried over ca. 1 g of Na2SO4 and transferred into auto-sampler vials.
Gas Chromatography
GLC analyses were performed on a Carlo Erba 5160 system. A RTX 225 capillary column (25 m length, 0.25mm ID) was operated with hydrogen as carrier gas at a flow rate of 3 ml/min (head pressure 90 kPa). The temperature program was 170°C for 2 minutes followed by a 5°C/min ramp to 220°C and a final hold time of 10 minutes. Injector and FID temperatures were 250°C. The injector was operated in split mode (split rate 1/50) using an empty glass liner (4 mm ID). The injection was performed by using the hot needle (thermospray) technique[Citation17] with additional solvent flush by injecting 1 μl sample ahead of 0.8 μl solvent (toluene). The FAME were identified tentatively by comparison with authentic standards. No response factors were used to correct for unequal detector response.[Citation15]
RESULTS AND DISCUSSION
Solvent Extraction
When the Bligh and Dyer lipid extraction was performed using a 15-fold excess of solvent (MBD), the weigh-out of lipids was higher (P < 0.01) than when following the OBD procedure ().[Citation2] Both values were close to the lipid contents described in the literature of 31.0 to 33.0%.[Citation18] Possible errors in gravimetric measurement of lipids can be incomplete extraction of specific lipids such as TAG or PL, unspecific losses by manipulation of the sample or, when results are too high, co-extraction of non lipid material. Therefore, the lipid yield was evaluated by GLC as well. shows the FA profiles, expressed as mg FA/g yolk for both extraction methods plus the DM. In this experiment, none of the values measured (single FA and total FA) differed between the two extraction methods (P > 0.05). This indicated that the higher weigh-out found in the MBD method was due to co-extraction of non-lipid material or incomplete removal of solvents.
Table 1 Lipid content of egg lipids obtained from 2 different extraction methods (% lipid/yolk).
Table 2 Fatty acid content of egg yolk lipids after applying different extraction methods (mg/g yolk, means ± standard deviation)Footnote a .
Among the main lipid classes of egg yolk, TAG are the least polar, whereas PC are the most polar. Investigations of the positional distribution of FA between TAG and PL showed, that around 90% of C16:0 and C18:1n9 are located in TAG, whereas around 90% of C20:4n6 and C22:6n3 are located in PL.[Citation3] Therefore, these FA can be used as indicators for extraction efficiency of TAG and PL respectively. As the increased solvent/sample ratio of the MBD method did not increase the yield of total and individual FA versus the OBD method (P > 0.05), it was concluded that extraction was complete for the entire range of lipids. Complete extraction of cholesterol, which is more polar than TAG but less polar than PL, can be assumed.
These results clearly show that the recommendations of Bligh and Dyer[Citation2] regarding adaptation of their method to other samples as cod muscle are applicable for extracting egg yolk lipids and increased solvent/sample ratios might lead to co-extraction of non-lipid material. According to Smedes and Askland,[Citation19] the (lower) lipid phase of the Bligh and Dyer extract contains around 1% of the water and 10% of the chloroform, whereas the upper (aqueous) phase contains around 3% of the chloroform used in the assay. Increasing the solvent/sample ratio should, in theory, not alter the distribution coefficients of particular lipids between the phases, as long as the influence of the sample itself is not taken into account. Thus it is likely to assume that the higher concentration of PL in the lower phase can lower the co-extraction of non-lipids. Completeness of PL extraction can be assumed, as neither the MBD method, nor the direct method produced higher yields of the PL-indicator FA (C22:4n6, C22:6n3). However, quantitative extraction of neutral lipids required 2 additional washing steps with chloroform alone, a procedure suggested by Bligh and Dyer.[Citation2] Incomplete extraction of neutral lipids from egg yolk can be easily detected by visible traces of yellow pigment (lutein) in the lipid phase.
According to data in the literature, the lipid class profile of egg yolk from the laying hen is rather constant and consists of ca. 65% TAG and 28.3% PL, of which 73% is PC and 15.5% is PE, and 5.2% (free) cholesterol.[Citation20] Calculating the average relative amount of FA in each of these lipid classes, which can be deduced from the average molecular weight fraction of FA in TAG, PC and PE, it can be estimated that the approximate FA content of egg yolk lipid is between 82–83%. With this assumption, the total FA content, measured by GLC, can be converted into lipid-equivalents and a recovery can be calculated versus the gravimetric results. The recovery, calculated from the data of and was 98.15 ± 0.77% for the OBD procedure and 89.99 ± 4.23% for the MBD method, indicating a high accuracy of the OBD method.
Direct Method (DM)
Also the yields of individual and total FA obtained from the DM could not be distinguished (P > 0.05) from those obtained by separate solvent extraction (). In order to test the robustness against traces of water in the reaction system, an additional experiment was performed, in which water was added to the assay. The amounts of water added were between 0 and 10 mg/mg dry yolk which corresponded to a water content of 0–16.7% in the HCl-methanol phase. In these experiments the internal standard was added after the reaction was completed and the sample had been neutralized by K2CO3. A duplicate sample was analyzed in the conventional way and used as a reference. Recoveries of total FA are presented in and for some individual FA in . The breakdown-point of the reaction is obviously in a region where the water content of the methanol is exceeding 8%. In this experimental set-up, this would approximately match a water content of 4 mg water per mg dry yolk. This would represent a water content of 80% in liquid yolk, which by far exceeds the natural water content of egg yolk. Therefore, we performed an additional experiment to see whether the DM would work on fresh yolk as well. The cross-mark on the graph in shows the mean recovery of this experiment (n = 4) at the point that represents the water content of fresh yolk. In , it can be observed that the addition of 10 mg water/mg yolk caused a severe breakdown of methyl ester recovery, but FA, which are mainly found in TAG were much more affected than FA found in PL. The PL-bound C20:4n6 and C22:6n3 were found at a level of 87.9 and 99.9% respectively, whereas the TAG-bound C18:1n9 was recovered at a level of 28.6% only. The recovery of C18:0 was 58.5%, which is in agreement with the previous observation, as C18:0 is equally distributed between PL and TAG in egg yolk lipids.[Citation3]
Figure 1 Recovery of fatty acids after addition of water to the tramsmethylation reaction a . a The crossmark represents the recovery of directly transmethylated fresh (liquid) yolk.
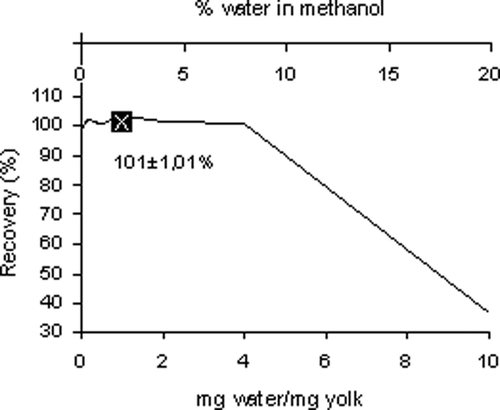
Table 3 Recovery of individual fatty acids after addition of water to the transesterification mixture
presents the FA profiles measured by direct transmethylation of fresh egg yolk. This experiment was conducted in order to estimate the precision of sampling liquid yolk (ca. 100 mg per assay), expressed by the standard deviation. In comparison with the data in , the precision of this method was comparable to the precision found in the OBD procedure (relative standard deviation of total FA around 1%) and superior to the other two methods (relative standard deviation around 7%).
Table 4 Fatty acid content of egg yolk after direct transmethylation of fresh egg yolk
CONCLUSION
Performing direct extraction/methylation procedure instead of lipid extraction followed by derivatization produces equally accurate results for the analysis of fatty acids from egg yolk. Moreover, this method is faster to perform and needs much less input of manual work and chemicals. Consequently, sources of error are reduced as well. If clean lipid extracts are required for whatever reason, one should follow the original Bligh and Dyer protocol and avoid all of the numerous modifications reported for this method.
ACKNOWLEDGMENT
The author is grateful to Mrs. N. Schamberger for providing skilled technical assistance.
REFERENCES
- Folch , J. , Lees , M. and Stanley , G.H.S. 1957 . A simple method for the isolation and purification of total lipides from animal tissues . J. Biol. Chem. , 226 : 497 – 509 . [INFOTRIEVE]
- Bligh , E.G. and Dyer , W.J. 1959 . A Rapid Method of Total Lipid Extraction and Purification . Canadian Journal of Biochemistry and Physiology , 37 : 911 – 917 . [INFOTRIEVE]
- Schreiner , M. , Hulan , H.W. , Razzazi-Fazeli , E. , Bohm , J. and Iben , C. 2004 . Feeding laying hens seal blubber oil: effects on egg yolk incorporation, stereospecific distribution of omega-3 fatty acids, and sensory aspects . Poult. Sci. , 83 ( 3 ) : 462 – 473 . [INFOTRIEVE]
- Nash , D.M. , Hamilton , R.M.G. and Hulan , H.W. 1995 . The effect of dietary herring meal on the omega-3 fatty acid content of plasma and egg yolk lipids of laying hens . Canadian Journal of Animal Science , 75 : 247 – 253 .
- Nash , D.M. , Hamilton , R.M.G. , Sanford , K.A. and Hulan , H.W. 1996 . The effect of dietary menhaden meal and storage on the omega-3 fatty acids and sensory attributes of egg yolk in laying hens . Canadian Journal of Animal Science , 76 : 377 – 383 .
- Pacetti , D. , Hulan , H.W. , Schreiner , M. , Boselli , E. and Frega , N.G. 2005 . Positional analysis of Egg Triacylglycerols and Phospholipids from Hens Fed Diets Enriched in Refined Seal Blubber Oil . J. Sci. Food. Agric. , 85 ( 10 ) : 1703 – 1714 . [CROSSREF]
- Christie , W.W. 2003 . Lipid analysis , Bridgewater, , England : The Oily Press .
- De Koning , A.J. 2002 . The antioxidant ethoxyquin and its analogues: A review . Int. J. Food Prop. , 5 ( 2 ) : 451 – 461 . [CROSSREF]
- Hara , A. and Radin , N.S. 1978 . Lipid extraction of tissues with a low-toxicity solvent . Anal. Biochem. , 90 ( 1 ) : 420 – 426 . [INFOTRIEVE] [CROSSREF]
- Boselli , E. and Caboni , M.F. 2000 . Supercritical carbon dioxide extraction of phospholipids from dried egg yolk without organic modifier . Journal of Supercritical Fluids , 19 : 45 – 50 . [CROSSREF]
- Yener , M.E. 2001 . Estimation of lipid properties related to supercritical fluid extraction . Int. J. Food Prop. , 4 ( 1 ) : 45 – 57 . [CROSSREF]
- Kramer , K.G. and Zhou , J. 2001 . Conjugated Linoleic Acid and Octadecanoic Acids: Extraction and isolation of lipids . European Journal of Lipid Science and Technology , 103 : 594 – 600 . [CROSSREF]
- Lepage , G. and Roy , C.C. 1984 . Improved recovery of fatty acid through direct transesterification without prior extraction or purification . J. Lipid Res. , 25 ( 12 ) : 1391 – 1396 . [INFOTRIEVE]
- Lepage , G. and Roy , C.C. 1986 . Direct transesterification of all classes of lipids in a one step reaction . J. Lipid Res. , 27 : 114 – 120 . [INFOTRIEVE]
- Ulberth , F. and Henninger , M. 1992 . One-step extraction/methylation method for determining the fatty acid composition of processed foods . J. Am. Oil Chem. Soc. , 69 ( 2 ) : 174 – 177 .
- Henninger , M. and Ulberth , F. 1997 . Fettsäurespektren von heimischen Fischen, Seefischen und Fischölen . Dtsch. Lebensm.-Rundsch. , 93 ( 6 ) : 178 – 183 .
- Grob , K. and Biedermann , M. 2000 . Video-taped sample evaporation in hot chambers simulating gas chromatography split/splitless injectors I. . Thermospray injection. J. Chromatogr. A. , 897 : 137 – 146 .
- Souci , S.W. , Fachmann , W. and Kraut , H. 2000 . Food composition and nutrition tables , New York : CRC Press .
- Smedes , F. and Askland , T.K. 1999 . Revisiting the Development of the Bligh and Dyer Total Lipid Determination Method . Mar. Pollut. Bull. , 38 ( 3 ) : 193 – 200 . [CROSSREF]
- Belitz , H.D. and Grosch , W. 1992 . Food Chemistry , New York : Springer Verlag .