Abstract
The extraction of valerenic acids from valerian root by supercritical fluid extraction using CO2 under different operating conditions was compared to extraction by percolation using 70% ethanol. The yield of total valerenic acids under pressures of 10–20 MPa and temperatures of 40–50°C was about 85% of that achieved by percolation, while the addition of 5% ethanol or methanol as a modifier to the CO2 resulted in the same yield as percolation. Moreover, maximal extraction was achieved in the faster time of 20 minutes and resulted in a more highly concentrated extract than obtained by percolation which would aid the manufacturing of liquid or dried products. Further studies on a larger scale would clarify the use of this technique commercially.
INTRODUCTION
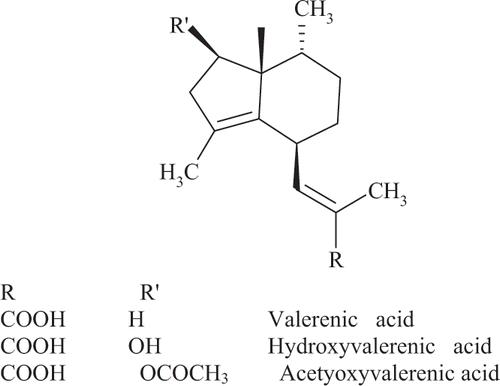
The roots and rhizomes of the medicinal herb valerian (Valeriana officinalis L. s.l.) are widely used as a sedative for nervous tension, insomnia, anxiety and stress.[Citation1] While the constituents responsible for these effects have not been fully elucidated, the valerenic acids have been shown to exhibit sedative activity and are often used as an indicator of medicinal quality.[Citation2–4] In addition, the valerenic acids are unique to V. officinalis and several closely related species and can be used to ensure plant species integrity.[Citation2–4] The valerenic acids are sesquiterpenes based on the dual ring valerane structure () with the major constituents being valerenic acid, acetoxyvalerenic acid and hydroxyvalerenic acid.[Citation3] In addition to use as a dried root powder, commercial products include infusions obtained from dried valerian roots by soaking in hot or cold water, and tinctures and liquid extracts obtained by maceration or percolation with 60–70% ethanol on a 1:5 herb:solvent ratio.[Citation5] Extracts are generally dried to ease incorporation before being manufactured into products such as tablets and capsules.[Citation6]
Supercritical fluid extraction (SFE) using carbon dioxide (CO2) has been demonstrated to offer advantages in the extraction of minor components from many biological materials. In general, supercritical CO2 behaves like a lipophilic solvent but unlike liquid solvents, its solvating power and selectively can be adjusted by manipulating the operating pressure and temperature.[Citation7,Citation8] The solvent power and selectivity can also be modified by incorporating small amounts of various liquid solvents to increase the affinity of the solvent mixture for the more polar compounds.[Citation8] In addition, CO2 is inert, pure, non-toxic, inexpensive and is gaseous under ambient conditions.[Citation7] Thus, solute separation, concentration and drying processes are made easier and the generation of liquid solvent waste and exposure of operators to toxic solvents can be minimized.[Citation7]
SFE technology has found some commercial application in the decaffeination of coffee and tea[Citation9] and extraction of flavor and oleoresin from spices.[Citation10] SFE on valerian using CO2 and trifluoromethane has been demonstrated for the extraction of valepotriates, a group of iridoidal sesquiterpenes[Citation11] with relatively mild conditions of 40°C, 9.6–15.0 MPa for 30 minutes giving substantial extraction efficiency and maximal extraction in less than 1 hour.[Citation12,Citation13] Although Morvai-Vitányi and Games[Citation13] did show that acetoxyvalerenic acid was supercritically extracted, they did not show to what extent nor did they optimise the conditions for extraction of valerenic acids. This study was conducted to determine the extent of extraction of valerenic acids from dried, powdered valerian root by SFE using CO2 under different pressures and temperatures and with the addition of ethanol and methanol as solvent modifiers.
MATERIALS AND METHODS
The SFE apparatus (Isco, Lincoln NE) consisted of two 260D syringe pumps, an SFX 2–10 supercritical fluid extractor, a restrictor temperature controller and a 260 series pump controller. Accurately weighed samples of about 0.5 g root powder were placed in the extractor chamber and the extracting solvent passed through the chamber under controlled temperature and pressure conditions. The effluent material was collected in methanol at 10, 20, and 30 minutes. In some trials, ethanol and methanol were added to the CO2 at 5% using the second syringe pump. There were three replicates for each extraction condition and the extractor was cleaned with 5 ml methanol between extractions. Extracts were adjusted to 10 ml in a volumetric flask with methanol.
In order to compare the efficiency of SFE extraction with current commercial practice, three samples (10 g) of the same valerian root powder were extracted with 70% ethanol by percolation. The valerian powder was placed in a separatory funnel with 70% ethanol (50 ml, valerian to solvent ratio 1:5) and allowed to macerate for 1 hour before passing by gravity through the bed of root material. The effluent was collected into a 100 ml volumetric flask and made up to volume with 70% ethanol before assaying by HPLC.
The total valerenic acids (valerenic, acetoxyvalerenic, and hydroxyvalerenic acids) were determined by HPLC on two aliquots (20 μl) of an extract. The HPLC method was that described by Shohet et al.,[Citation14] which was a modification of that reported by Bos et al.[Citation3] The method involved separation on a reversed phase column with gradient elution of a mobile phase of acetonitrile and aqueous phosphoric acid and peak detection at 225 nm. Quantification was achieved using an external standard of biphenyl (99.5%, Aldrich, Milwaukee WIS), which had been calibrated to valerenic acid and hydroxyvalerenic acid (Indofine, Belle Mead NJ). The limit of detection for the three valerenic acids in solution was determined as 0.01 μg/20 μl which equated to 0.01 mg/g of dried root. The moisture content of the root powder was determined from a fresh sample (1 g) that was dried in a vacuum oven (Thermoline, Sydney) at 60°C and −70 kPa until constant weight was achieved. All analytical data were corrected for moisture content and expressed on a dry weight basis.
RESULTS AND DISCUSSION
The extraction levels of valerenic acids from dried valerian root powder by the traditional method of solvent percolation and by SFE with CO2 over a 30 minute period at different pressure, temperature, and in the presence of solvent modifier are presented in . For all extractions, including percolation, the ratio of acetoxyvalerenic acid to valerenic acid was similar (average ratio was 0.87–0.93:1) and hydroxyvalerenic acid was never detected. Hence, only the data for total valerenic acids are presented.
Table 1 Extraction of total valerenic acids by SFE using CO2 at different times, pressures, temperatures and solvent modifiers.
The extraction of valerenic acids by percolation with 70% ethanol at a 5:1 solvent: valerian ratio was found to be 2.4 mg/g. This can be considered a benchmark since this is a common commercial process for dried valerian. Extraction by SFE with CO2 was found to be maximal after 30 minutes under all the operating conditions evaluated. The yield of total valerenic acids under pressures of 10–20 MPa and temperatures of 40–50°C was 2.0–2.1 mg/g, about 85% of that achieved by percolation. The addition of 5% ethanol or methanol as a modifier to the CO2 increased the yield of valerenic acids to 2.4–2.5 mg/g, the same yield as for percolation. Valerenic and acetoxyvalerenic acid contain a carboxylic acid group () that would increase their polarity, even though they are lipids found in essential oil of the plant. Thus, addition of a polar solvent to CO2, an essentially lipophilic solvent, led to an increase in extraction of these compounds.
The extraction of valerenic acids by SFE was, however, not uniform over the 30 minute extraction period. For all extractions at 15 and 20 MPa, > 90% of the total was obtained in the first 10 minutes and 97% by 20 minutes. At 10 MPa, there was significantly less extraction in the first 20 minutes but after 30 minutes, the total was not significantly different than that at the other pressures. Increasing the temperature of the extractor had no effect on the rate of extraction of valerenic acids while the addition of modifiers at 5% significantly increased the extraction in the first 10 minutes and over 30 minutes.
The findings indicate that use of SFE on valerian root powder with CO2 at the relatively mild conditions of 15 MPa and 40°C and addition of ethanol or methanol at 5% would seem to offer advantages over the current technology of percolation in that a similar yield of valerenic acids was obtained but required a shorter extraction time of 20 minutes compared to 90 minutes. In addition, the extract obtained was more highly concentrated which would assist manufacturers to achieve a wider range of desired concentrations in liquid end products by dilution or allow more cost effective total drying of the extract for tablet or capsule manufacturing. However, further studies on a larger scale are required to determine its potential for commercial application with valerian.
ACKNOWLEDGMENTS
We wish to than the Rural Industries Research and Development Corporation and Mediherb for funding support.
Notes
4. Bos R.; Analytical and Phytochemical Studies on Valerian and Valerian Based Preparations. Ph.D. Thesis. University of Groningen, the Netherlands 1997
REFERENCES
- Houghton , P.J. 1988 . The biological activity of valerian and related plants . J. Ethnopharmacol , 22 : 121 – 142 . [INFOTRIEVE] [CSA]
- Hänsel , R. and Schulz , J. 1982 . Valerensäuren und Valerenal als Leitstoffe des offizinellen Baldrians . Bestimmung mittels HPLC-Technik. Z. Phytother , 3 : 333 – 340 . [CSA]
- Bos , R. , Woerdenbag , H.J. , Hendriks , H. , Zwaving , J.H. , De Smet , P.A.G.M. , Tittel , G. , shy;Wikström , H.V. and Scheffer , J.J.C. 1996 . Analytical aspects of phytotherapeutic valerian preparations . Phytochem. Anal. , 7 : 143 – 151 . [CSA] [CROSSREF]
- 4. Bos R.; Analytical and Phytochemical Studies on Valerian and Valerian Based Preparations. Ph.D. Thesis. University of Groningen, the Netherlands 1997
- Upton , R. 1999 . “ Commercial sources and handling ” . In Valerian Root, Valeriana officinalis, Analytical, Quality Control and Therapeutic Monograph , Edited by: Upton , R. 6 – 7 . Santa Cruz, CA : American Herbal Pharmacopoeia .
- Foss , R. and Houghton , P.J. 1997 . “ Valeriana products ” . In Valerian. The Genus Valeriana , Edited by: Houghton , P.J. 129 – 137 . Amsterdam : Hardwood Academic Publishers .
- Saito , M. , Yamauchi , Y. and Okuyama , T. 1994 . “ Introduction ” . In Fractionation by Packed-Column SFC and SFE , Edited by: Saito , M. , Yamauchi , Y. and Okuyama , T. 15 New York : VCH Publishers .
- Reverchon , E. 1997 . Supercritical fluid extraction and fractionation of essential oils and related products . J. Supercrit. Fluids , 10 : 1 – 37 . [CSA] [CROSSREF]
- Lee , S. 1988 . Supercritical decaffeination . Tea and Coffee Trade Journal , 160 : 3 – 5 . [CSA]
- King , M.B. and Bott , T.R. 1993 . Extraction of Natural Products using Near-Critical Solvents , 325 Glasgow : Blackie Academic & Professional .
- Stahl , E. and Schütz , E. 1980 . Extraktion labiler Naturstoffe mit überkritischen Gasen. 4. Mitt.: Extraktion der Valepotriate aus Valeriana wallichii . Planta Med. , 40 : 262 – 270 . [CSA]
- Unger , K.K. and Roumeliotis , P. 1983 . On line high-pressure extraction-high-performance liquid chromatography. 1. Equipment design and operation variables . J. Chromatogr. , 282 : 519 – 526 . [CSA] [CROSSREF]
- Morvai-Vitányi , M. and Games , D.E. 1993 . Analysis of supercritical fluid extracts of valerian roots by combined chromatography/mass spectrometry . Acta Horticult , 344 : 386 – 403 . [CSA]
- Shohet , D. , Wills , R.B.H. and Stuart , D.L. 2001 . Valepotriates and valerenic acids in commercial preparations of valerian available in Australia . Pharmazie , 56 : 860 – 863 . [INFOTRIEVE] [CSA]