Abstract
Kinetics of air drying of fresh and osmotically pretreated pear slices was analyzed. Osmotic treatments were applied for 0.5, 3, and 48 hours in 50°Brix sucrose solutions at 30°C. Air drying was carried out at 45, 55, and 65°C, 2.2 m/s air rate. Drying curves were modelled through a Fickian equation, obtaining the effective diffusion coefficient in each case. This coefficient was markedly affected by the temperature and by the osmotic pretreatment, ranging between 6.5 × 10− 12 to 5.8 × 10 − 10 m2/s. A significant relationship between the De, and the inverse of the initial solute content of the samples was found. Activation energy of the drying process was similar for fresh and osmotically pretreated samples for 0.5 and 3 hours, but increased considerably when long osmotic treatment time was applied. Total drying time increased in osmodehydrated samples, but process yield, in terms of sample weight loss, increased. These effects were more marked when long osmotic pretreatment times were used.
INTRODUCTION
Osmotic dehydration (OD) of fruits and vegetables by immersion in liquids with a water activity lower than that of food has received considerable attention in recent years as an air-drying pretreatment. This dehydration method, in which the simultaneous transfer of water out and solutes into the material takes place, can be used before air drying in order to reduce the food water content by 30 to 70% of the original amount. Several studies applying osmotic dehydration before drying report that yield and quality of fully dehydrated or intermediate moisture products are improved.[Citation1] Among the advantages of OD pre-treatments which have been reported, can be found: a) protection afforded to the product against structural collapse and structural disorganization, b) better texture and lower shrinkage,[Citation2,Citation3,Citation4] c) smaller changes in color and flavor than those caused by air drying by thermal effects,[Citation3,Citation5] d) shortening of the second stage drying time by up to 60% with a minimum energy savings of 20%.[Citation6,Citation7]
Published results reveal that osmotic pre-treatment implies changes in mass transport properties of the product during air drying as a result of the structural changes induced by the pre-treatment, as it can be observed in previous works.[Citation8,Citation9] In general, a decrease in the water effective diffusion coefficient (De) has been observed in osmotically pretreated tissues; the greater the solute uptake in this step, the greater the De decrease.[Citation1,Citation3,Citation6,Citation7]
Very little data on air drying of pears has been found. Zogzas et al.,[Citation10] reported De values in drying of fresh pear at 65°C. The study of Park et al.,[Citation11] into drying of pear var. Bartlett at several temperatures and drying rates, concluded that De increased with increasing drying temperature and air velocity. Park et al.,[Citation12] analyzed the drying behavior of pear cubes (var. Anjou), and the influence of osmotic pretreatment at different temperatures and drying rates. These authors observed that water effective diffusivity (De) increases linearly with air temperature and air velocity in osmodehydrated samples, due to the reduction of superficial hardening when osmotic treatment was applied. The objective of this article was to analyze the kinetics of the air drying of pear slices (var. Blanquilla) with and without OD pre-treatment, as a function of the air temperature and time of OD pretreatment.
MATERIALS AND METHODS
Raw Material
Pear cv. Blanquilla purchased at the local market was used as a raw material. Non‐peeled pear slices taken from central parts of fruit of around 25 g, 80 mm diameter, and 10 mm thick were used in all the experiments.
Drying Process
For air drying process, a laboratory scale drier with temperature and air rate control described by Xue et al.,[Citation13] was used. Drying experiments were carried out using air flow through the sample at 2.2 m/s, assuming an internal control in mass transfer, as it was deduced from previous works.[Citation13] Drying temperatures were: 45°C (38–53% relative humidity), 55°C (43–53% relative humidity), and 65°C (39–53% relative humidity). Only one fruit slice was dried in each experiment, and they were made in triplicate.
Osmotic Pre-treatment
Experiments of osmotic dehydration were performed on pear slices prior to drying process, in a pilot plant at controlled temperature (30°C) and solution flow rate. Pear samples were osmotically dehydrated for 0.5, 3, and 48 hours in a 55° Brix sucrose solution containing 0.5% citric acid as antibrowning agent.[Citation14] Solution-fruit ratio was large enough (20:1) to avoid significant changes in the solution concentration throughout the process.
Analytical Determinations
Moisture content was analyzed by vacuum drying at 60°C and 100 mbar pressure until constant weight by the AOAC.[Citation15] Sample water activity was determined using a Decagon Aqualab (model CX-3) at 25°C. Soluble solids were determined by using a refractometer (ATAGO model 3T, Japan) at 20°C. All measurements were carried out in triplicate. Equations for determination of sugar gain and water loss were determined according to Chafer et al.[Citation16]
RESULTS AND DISCUSSION
shows the values of mass fractions of water and soluble solids and water activity of pear samples before and after osmotic treatments. Water loss (ΔMw) and sugar gain (ΔMs), which occurred in each OD treatment, is also shown in this table, referred by mass unit of initial product before drying, as the dried solid weight is not constant in the osmodehydrated samples. Solute gain and water loss increase in line with the time of processing. Samples osmotically dehydrated for 48 hours were practically equilibrated with the osmotic solution used in terms of water and solute contents and water activity. The high sugar gain (about 30% with respect to the initial sample weight) reached in this treatment is remarkable. So, notable structural changes will be expected in these samples, related to the high total solids retained in the cellular matrix and their interactions. In this sense, an enhancement of tissue firmness has been reported for osmotically pre-treated apple samples as the sugar gain increases.[Citation9]
Table 1 Mass fractions of water and solutes, water activity of fresh and OD treated pear slices, and water loss and sugar gain for the different treatments.
Drying Behavior
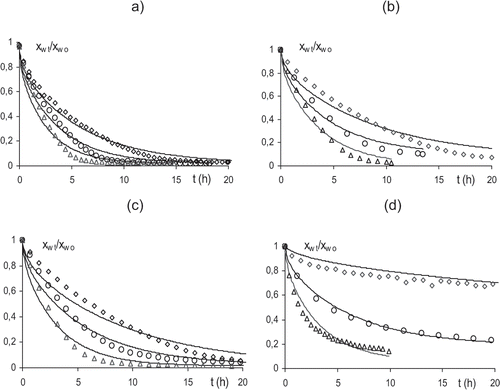
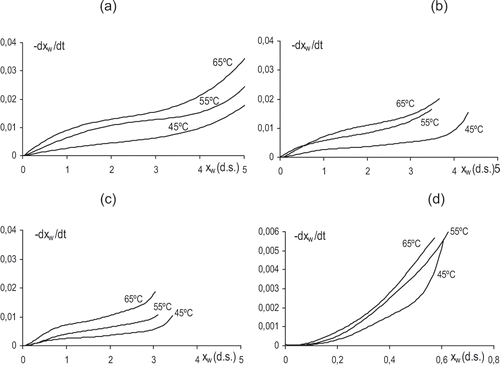
After OD treatment, samples were dried using hot air at 45, 55, 65°C. Although relative humidity was not exactly the same in all drying experiments, differences in its value did not imply a notable change in drying curves, as deduced from the non significant differences observed in the obtained replicates at the same temperature (small enough to consider an average drying curve at a determined temperature). Mean drying curves at the different air temperatures are plotted in . From these data, drying rate curves were obtained and are shown in . Drying rate curves show a sigmoid shape with two drying periods, similar to that described for other fresh fruits.[Citation17,Citation18] The lack of steadiness in the drying rate in the first period, points out that the diffusion process controls the water transport in the sample interface, where the driving force is progressively reduced as the water activity reduces in the external parts of the tissue. From a specific moisture content, when a great number of cell layers in the tissue have lost a considerable amount of water, dried cell layers offer a much greater resistance to water diffusion through the interface and the drying rate slows down rapidly. In pre-treated samples, where moisture range covered throughout the process was narrow, the two periods appear less marked in the curve. Drying rate decreased when OD pretreatments were applied, as has been reported by El‐Aouar et al.[Citation19] This can be explained by the presence of a surface layer of collapsed cells developed during osmotic treatment in the sample that will contribute to increasing mass transport resistance near the interface in air drying experiments. This resistance may also be affected by the sugar uptake which takes place during the osmotic pretreatment, mainly in the intercellular spaces.[Citation1,Citation18] The reduction of process driving force due to pretreatment will contribute to the decrease in water transport rate. All these effects were more notable when longer OD times were used. So, the OD treatment for 48 hours implied the greatest differences in air drying behavior. In this case, deeper dehydration and greater sugar uptake and certain sample shrinkage took place. All these effects give rise to a drying rate below 0.006 kg water/s kg d.s., much lower than in the other treatments.
Drying Kinetics
Effective water diffusion coefficients (De) were obtained for the different treatments by applying the integrated Fick second law for long times and a plane sheet geometry, using 10 terms of the series equation, where constant values of initial moisture () and equilibrium moisture of 0.008 w/w (d.b.) for non-treated and 0.5 hour osmotically pre-treated samples and 0.046, 0.082 w/w (d.b.) for 3 and 48 hour OD treatments were used, respectively. Equilibrium values were taken as the mean of experimental values in drying curves from the time when successive moisture content differ less than 0.001. shows the obtained values, which range from 6.5 × 10−12 to 5.8 × 10−10 m2/s, and also, the determination coefficients (R2) obtained from the fitting. De values increase as the temperature rises and decrease as the osmotic processing time gets longer. The empirical sense of the obtained De parameter is remarkable as the OD samples had an uneven initial water distribution at the beginning of the drying process, and therefore one of the Fick equation integration conditions is not fulfilled. Nevertheless, this parameter allows us to estimate the differences in water mass transport properties of pear samples due to osmotic pretreatments and the associated structural and compositional changes. The De values found were in the same order as values reported by Zogzas et al.,[Citation10] for fresh pear (9.6 × 10−10 m2/s at 65°C) and by Park et al.[Citation12] for osmodehydrated samples.
Table 2 Water effective diffusion coefficients obtained in the different drying treatments as a function of temperature.
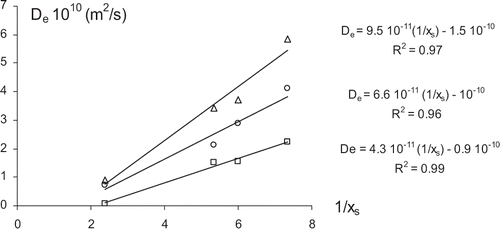
Linear relationships were found between the De values and the inverse of the soluble solid contents of the samples as shown in , for each temperature. De values for samples osmotically dehydrated for 0.5 and 3 hours were quite similar and decreased by around 30% with respect to fresh samples. This small increment in the process time seems not to imply structural differences in plant tissue relevant to its overall mass transport properties. However, samples osmodehydrated for long times (48 hours) showed much lower De values, which are reduced by around 82–97% with respect to non-pretreated samples. The profound changes in the cellular structure of the tissue which occur during the long term osmo-dehydration will be one of the main factors responsible for the abrupt change in the tissue mass transport properties. Another important contribution to the reduction of the mass transport rate is the great increase in the viscosity of the fruit liquid phase achieved during the osmotic pre-concentration of the sample. From a practical point of view, when samples are osmotically pretreated, higher temperature requirements are necessary to reach reasonably high drying rates, especially when long-term osmotic pretreatments were applied.
Temperature Influence
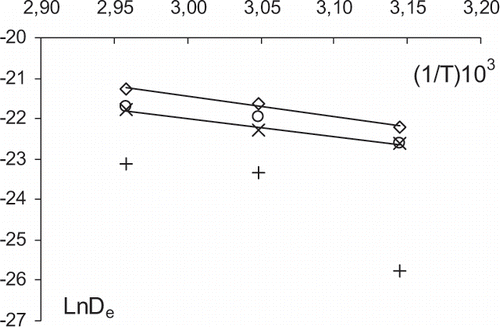
Temperature influence on De values was modeled by using Arrhenius equation. shows the fitted model for samples submitted to the different pretreatments. As it can be seen, the slope of the fitted line for OD0.5h and OD3h treatments were very similar, so the same activation energy (Ea) value has been obtained. These values were 43 and 38 kJ/mol for the non pretreated and short time pretreated samples, respectively (R2 values were 0.981 and 0.929). These values are similar to those found by other authors for non pretreated pear (cv. Bartlett, cv. Anjou) and for pre-osmosed apple and pear using short osmotic times.[Citation11,Citation18] In samples pretreated for 48 hours, the influence of the temperature on the De coefficients deviates from Arrhenius model. The slope is similar to the other treatments between 55 and 65°C, but the De value at 45°C decreased more sharply than expected. This could be related to a viscous effect in the concentrated fruit liquid phase, since viscosity of highly concentrated systems is much more sensitive to the temperature.[Citation20]
Figure 4 Arrhenius relationship between water effective diffusivity (De) and temperature (1/T, K−1) for untreated samples (⋄) and for osmotically pre-dehydrated samples for 0.5 h (○), 3 h (×) and 48 h (+).
A stepwise multiple regression analysis was carried out to find an empirical model to predict the effective water diffusion coefficient as a function of temperature and initial soluble solid contents of samples. The fitted model given by equation 1 explains 97.5% of the variability in De values. The accurate estimation of the data by the EquationEq. 1 can be useful for practical determinations.
Process Yield
Differences in the yield of drying process (air or combined) were estimated for samples with 40% (wet basis) moisture content. By applying the Fickian equation and the obtained De values (), air drying times have been calculated for each treatment. shows these values for each drying step (osmotic and air stages), together with the mass loss (ΔM) reached in the samples. The total drying time (OD + air drying) increases in combined treatments despite the fact that air drying time is reduced when the longest osmotic pretreatment was applied; the longer the osmotic treatment, the greater the total drying time. Nevertheless, osmotic pretreatments implied an increase in the process yield, since the total weight loss was lower in these cases. The effect is especially notable in the longest osmotic pretreatment because of the great sugar gain.
Table 3 Total drying time (h) and total mass variations (%) during the both OD and air drying processes for the different treatments.
CONCLUSIONS
Drying kinetics of osmotically pre-treated pear could be modelled by Fick equation, thus obtaining the values of the effective water-diffusion coefficients in the osmosed tissues. Osmotic pre-treatment implied lower air drying rates due to the increase of the internal mass transport resistance, and a greater temperature influence on drying kinetics. These effects were enhanced as the osmotic treatment time increases, since this suppose greater solid gains. In samples osmosed for a longer time i.e. such as those produced in fruit candying processes), higher air temperature are needed during drying to attain acceptable processing times.
NOMENCLATURE
| De | = |
Effective diffusion coefficient (m2/s), |
| xio | = |
Initial moisture content(i = w) or solute content (i = s) (kg water or solute per kg d.m) |
| xit | = |
Moisture content (i = w) or solute content (i = s) at the time t (kg water or solute per kg d.m) |
| ΔM | = |
Weight loss (kg sample/kg initial weight) |
| ΔMi | = |
Water loss (i = w) and sugar gain (i = s) (kg water or solids/kg initial weight) |
| OD0.5h | = |
Osmotic Dehydration for 0.5 hour |
| OD3h | = |
Osmotic Dehydration for 3 hours |
| OD48h | = |
Osmotic Dehydration for 48 hours |
| T | = |
Temperature (°C) |
REFERENCES
- Nieto , A. , Salvatori , D. , Castro , M.A. and Alzamora , S.M. 1998 . Air drying behaviour of apples as affected by blanching and glucose impregnation . Journal of Food Engineering , 36 : 63 – 79 .
- Lazarides , H.N. , Fito , P. , Chiralt , A. , Gekas , V. and Lenart , A. 1999 . “ Advances in osmotic dehydration ” . In Processing Foods. Quality optimization and Proccess assessment , Edited by: Oliveira , F.A.R. and Oliveira , J.C. 175 – 199 . Boca Raton, FL : CRC Press .
- Sankat , C.K. , Castaigne , F. and Maharaj , R. 1996 . The air drying behaviour of fresh and osmotically dehydrated banana slices . International Journal of Food Science and Technology , 31 ( 2 ) : 123 – 135 . [CROSSREF]
- Lewicki , P.P. and Sitkiewicz , I. 1999 . Effect of pre-drying treatment and storage on rheological properties of dried onion . International Journal of Food Properties , 2 ( 1 ) : 23 – 37 .
- Krokida , M.K. , Maroulis , Z.B. and Saravacos , G.D. 2001 . The effect of the method of drying on the colour of dehydrated products . International Journal of Food Science and Technology , 36 ( 1 ) : 53 – 59 . [CROSSREF]
- Lenart , A. 1994 . “ Osmotic dehydration of fruits before drying ” . In Minimal Processing of Foods and Process Optimization , Edited by: Singh , R.P. and Oliveira , F.A.R. 87 – 105 . London : CRC Press .
- Alvarez , C.A. , Aguerre , R. , Gomez , R. , Vidales , S. , Alzamora , S.M. and Gerschenson , L.N. 1995 . Air dehydration of strawberries: effects of blanching and osmotic pre treatments on the kinetics of moisture transport . Journal of Food Engineering , 25 : 167 – 178 . [CROSSREF]
- Lewicki , P.P. 1998 . Effect of pre-drying treatment, drying and rehydration on plant tissue properties . International Journal of Food Properties , 1 ( 1 ) : 1 – 22 .
- Mandala , I.G. , Anagnostaras , E.F. and Oikonomou , C.K. 2005 . Influence of osmotic dehydration condcitions on apple air-drying kinetics and their quality characteristics . Journal of Food Engineering , 69 : 307 – 316 . [CROSSREF]
- Zogzas , N.P. , Maroulis , Z.B. and Marinos-Kouris , D. 1996 . Moisture diffusivity data compilation in foodstuffs . Drying Technology , 14 ( 10 ) : 2225 – 2253 .
- Park , K.J. , Bin , A. and Brod , F.P.R. 2003 . Drying of pear d'Anjou with and without osmotic dehydration . Journal of Food Engineering , 56 ( 1 ) : 97 – 103 . [CROSSREF]
- Park , K.J. , Yado , M.K.M. and Brod , F.P.R. 2001 . Drying studies of sliced pear Bartlett (Pyrus sp.) . Ciencia e Tecnología de Alimentos , 21 ( 3 ) : 288 – 292 .
- Xue , K. , Cháfer , M. , González-Martínez , C. , Fito , P. and Chiralt , A. 2003 . “ Secado por aire caliente de pera var. Blanquilla ” . In Ingeniería de Alimentos: nuevas fronteras en el siglo XXI , Edited by: Fito , P. , Mulet , A. , Chiralt , A. and Andrés , A. Vol. 11 (11) , 235 – 240 . Valencia : Universidad Politécnica de Valencia .
- Pérez , L. , González-Martínez , C. , Cháfer , M. and Chiralt , A. 2002 . “ Cambios de color en pera mínimamente procesada (var. Blanquilla) ” . In Actas de II Congreso Español de Ingeniería de Alimentos , Lleida, , Spain : Universitat de Lleida . CD-ROM
- Association of Official Analytical Chemists . 1980 . Official Method of Analysis of the Association of Official Analytical Chemists , 13th , Washington, D.C. : AOAC .
- Chafer , M. , Perez , S. and Chiralt , A. 2003 . Kinetics of solute gain and water loss during osmotic dehydration of orange slices . Food Science and Technology International , 9 ( 6 ) : 389 – 396 . [CROSSREF]
- Lewicki , P. and Lenart , A. 1997 . “ Effect of osmotic pre-treatment on convection drying of selected fruits ” . In Osmotic treatments for the food industry , Edited by: Sereno , A. 29 – 40 . Porto, , Portugal : FEUP ediçoes .
- Simal , S. , Deyá , E. , Frau , M. and Rosselló , C. 1997 . Simple modelling of air curves of fresh and osmotically pre-dehydrated apple cubes . Journal of Food Engineering , 33 : 139 – 150 . [CROSSREF]
- El-Aouar , A.A. , Azoubel , P.M. and Murr , F.E.X. 2003 . Drying kinetics of fresh and osmotically pre‐treated papaya (Carica papaya L.) . Journal of Food Engineering , 59 : 85 – 91 . [CROSSREF]
- Muller , H.G. 1997 . Introducción a la reologia de los alimentos , 171 Zaragoza, , Spain : Acribia .