Abstract
The effect of polyhydric alcohol, mannitol, on the properties of proteins from Sardinella longiceps has been investigated. Calcium ATPase activity of the proteins decreased during frozen storage. Ca2+ATPase activity of mannitol treated samples was higher up to 60 days of storage as compared to untreated samples. Emulsion capacity of proteins decreased as a function of storage. Thermal denaturation profile of proteins revealed no change in apparent thermal transition temperature (app.Tm) from a control value of 57.5°C, in presence of mannitol. Scanning electron micrographs showed an increase in porosity of muscle tissues in the presence of mannitol. These results indicate the stabilizing effect of mannitol at low temperature on the fish muscle protein by retaining structure and functional properties of the muscle during frozen storage.
INTRODUCTION
Freezing and frozen storage induces various changes to the proteins, and these are reflected in a decrease of functional property. Various additives are used to reduce the freeze denaturation of muscle proteins. During frozen storage emphasis is given to additives, which does not induce any alteration of the product profile. Additives like polyhydric alcohols are used for the commercial production of surimi promoting stabilization of proteins during frozen storage. Stabilization of proteins from fish during frozen storage helps to retain the functional and sensory characteristics, which is desirable in the product formulation. Normally, cosolvents are used as additives to bring in stabilization and macroscopic property changes to products, not only the sensorial parts of a product and its deterioration during storage, but also to bring in desired effect.
Cryoprotectants are compounds that protect or stabilize a product during freezing and frozen storage. Cryoprotectants are compounds that extend the shelf life and quality of frozen foods.[Citation1] The presence of cryoprotectants is important to ensure maximum functionality of frozen surimi because freezing induces protein denaturation and aggregation.[Citation2] Sugars, sorbitol, phosphates, and salts are commonly used as cryoprotectants in surimi industry. The amount of each added additive varies considerably between various producers. For example, approximately 4–5% sugars, 0–5% sorbitol, 0–3% salts, and 0–0.3% phosphates are used. During extended frozen storage, the protein undergoes conformational changes, where hydrophobic portion are exposed that leads to the aggregation and loss of solubility by hydrophobic interactions of the exposed residues.[Citation3]
Sardinella longiceps is a pelagic fish with high fat content. It is normally used for fishmeal and oil production especially during glut season. In order to utilize these resources for human consumption, it is needed to design strategies for improving the keeping quality of sardine either by processing or by introducing better methods of preservation. Production of surimi (water washed fish flesh added with cryoprotectants) is a good option for efficient utilization of oil sardine resources for human consumption. Because of the intrinsic nature of muscle like dark muscle, high fat content, and high quantity of endogenous enzymes necessitates special attention for stabilization of proteins during frozen storage. Mannitol perhaps is a better cryoprotectant as mannitol inhibits ATPase enzyme activity at lower concentrations, and also it will not affect the structure of actomyosin at this low concentration.[Citation4] The objective of this study was to investigate the effect of polyhydric alcohol, mannitol, on the stabilization of sardine proteins, to analyze the effect of different concentrations of mannitol on the properties of mince from sardine, and also to evaluate the structural, functional, and physicochemical properties proteins during frozen storage as a function of time.
MATERIALS AND METHODS
Materials
Fresh fishes (Sardinella longiceps), commonly known as Indian oil sardine, were procured from landing center and transported to the laboratory in iced condition (1:1 ice to fish) with in an hour of landing. The average length of fish used for the study was in the range of 12–15 cm, and the weight of fish was in the range of 30–40 g.
Chemicals
Mannitol, sodium dihydrogen phosphate, disodium hydrogen phosphate, ammonium molybdate, ferrous sulfate, tricholoro acetic acid, calcium chloride, glycine, sulfuric acid, sodium chloride, and sodium hydroxide were procured from E. Merck (India), Mumbai, India. Sodium carbonate, Folin & Ciocalteu's phenol reagent, and copper sulfate were procured from Sisco Research Laboratories Pvt. Ltd. Mumbi, India. Sepharose 6B gel, adenosine triphosphate disodium salt, tris(hydroxymethyl) aminomethane, acrylamide, N,N′-methylene bisacrylamide, sodium dodecyl sulfate, N,N,N′,N′-tetramethylene diamine, 2-mercaptoethanol, ammonium persulfate, bromo phenol blue, bovine serum albumin and standard molecular weight markers were procured from Sigma Chemical Co., St. Louis, Missouri, USA.
Methods
Processing of fish
Fishes were washed thoroughly with chilled water to remove slime and dirt. Head and viscera were removed manually. Eviscerated fishes were washed with chilled water and used for meat picking. Meat picking was done mechanically with the help of a reciprocating meat picker having a pore diameter of 5 mm. Picked meat was washed with three volumes of chilled water, and the washing procedure was repeated in triplicate. Excess water was removed by centrifuging in a basket centrifuge. Prepared meat was minced using a mincer and mixed with the selected cryoprotectant, mannitol, at 2%, 4%, and 6% (w/w) using a silent cutter, packed in polythene bags, and frozen for 90 minutes at −35°C by an air-blast freezer. Frozen samples were kept at −20°C for monitoring the storage stability. Quartz double distilled water was used throughout the study.
Ca2+ ATPase activity
Ca2+ ATPase activity of the extracted proteins were assessed by the method of Perry.[Citation5] ATPase activity of mince was carried out after extracting the proteins with glycine-NaOH buffer (pH 9.0, 0.2 M) containing 0.5 M KCl. The reaction mixture consists of 0.03ml of ATP (50 mM), 0.2ml of calcium chloride (0.1 M), 1.0 ml of buffer and 0.2 ml of muscle extract. Reaction was started by the addition of ATP solution to the mixture. The mixture was incubated at 25 ± 2°C for 15 minutes in a shaking water bath (Grant OLS 200, Grant Instruments (Cambridge) Ltd, England). The reaction was stopped with the addition of 1 ml of 15% TCA. The solution was centrifuged at 6000 × g for 20 minutes. Inorganic phosphorous content was estimated in the filtrate by the method of Taussky and Shorr.[Citation6] To 3 ml of sample containing 4–8 µg of phosphorous 2 ml of ferrous sulfate molybdate reagent (5 g of FeSO4.7H2O dissolved in 1% ammonium molybdate in 1 N sulfuric acid) was added and the intensity of colour read at 660 nm. The colour develops within 1 minute and is stable for nearly 2 hours. Standard graph is plotted against Pi content using potassium dihydrogen phosphate and straight line is obtained. ATPase activity was expressed as moles of Pi/mg protein/min.
Emulsion capacity
Emulsion capacity of proteins was determined by the method of Pearce and Kinsella.[Citation7] Proteins were extracted from the mince with phosphate buffer (0.05 M, pH 7.5) containing 1.0 M NaCl and used for emulsion capacity determination. Temperature of the mixture was maintained at 4°C during the process of extraction. The slurry was centrifuged at 8000 × g at 4°C for 20 minutes using a refrigerated centrifuge (Biofuge 15R, Heraeus, Germany). The supernatant was used for emulsion capacity determination. To 10 ml of protein extract, 5 ml of refined groundnut oil was added and mixed thoroughly at 6000 rpm for 10 seconds using an Ultra turrax homogenizer. Homogenization was continued at a speed of 11,000 rpm with addition of oil at a flow rate of 0.5–0.6 ml/sec, until phase inversion was recorded. Protein content of the extract was determined by the method of Lowry.[Citation8] Emulsion capacity was expressed as mL oil/ mg protein in the system.
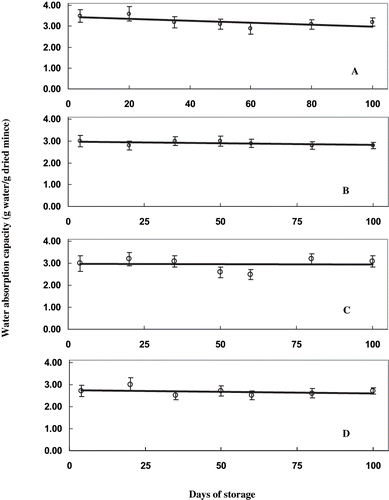
Water absorption capacity
Mince prepared from sardine was freeze-dried and 0.5 g of freeze dried mince mixed with 10ml of distilled water and kept for 30 minutes with occasional mixing. The mixture was centrifuged at 7000 × g for 15 minutes at room temperature using a centrifuge (Biofuge 15R, Hereus, Germany). Supernatant was discarded without the loss of solid materials. Tubes were kept at 50°C at an angle of 45° for 15 minutes to remove the water adhering along the sides of tubes. The difference between initial and final weight was taken for the calculation of water absorption capacity of material and expressed as g water per g of dried material.
Thermal denaturation curve
The proteins from the mince were extracted with phosphate buffer (0.05 M, pH 7.5) containing 1.0 M NaCl. A known protein concentration of this solution was equilibrated to 25°C in special thermal melting cuvettes and melting curve was followed at 287 nm in the temperature range of 25°– 90°C at 1°C interval using Gilford response II spectrophotometer (Cleveland, Ohio). Spectral data were stored and analyzed using specific software with the instrument. The apparent Tm was calculated either by first derivative plot of absorbance or Van't Hoff plot.[Citation9]
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Sodium dodecyl sulfate polyacrylamide gel electrophoretic pattern of proteins extracted with the buffer (0.125 M Tris HCl, 4% SDS, 20% glycerol, pH 7.2) were carried out in 10% polyacrylamide gel according to Laemmlli.[Citation10]
Scanning electron microscopy (SEM)
Scanning electron microscopic studies of the freeze dried fish mince were carried out using surface scanning electron microscope (LEO 435 VP, Cambridge, UK). Before loading the samples in to the system, the samples were coated with gold, using Poloron SEM coating system E-5000. Average coating time was 2–3 min. Thickness of the coating was 200–300 nm. The coated samples were loaded on the system and the image was viewed under 20 kV potential using 35 mm picoh camera (Leo 435 VP).
RESULTS AND DISCUSSION
ATPase enzyme activity of proteins from frozen mince was monitored during storage. The profile of ATPase enzyme activity during frozen storage of mince is shown in . ATPase activity was less in the case of untreated samples than the samples treated with mannitol immediately after freezing. ATPase activity from frozen mince reduced during frozen storage resulted sharp decrease in ATPase activity occurred up to 50 days of storage. Mannitol treated samples have shown higher activity up to 60 days of storage. After 75 days of storage, ATPase activity of mince attained a higher value. The reduction in activity was 80% after 50 days of storage.
Figure 1 Ca2+ ATPase activity of proteins extracted from sardine mince treated with different concentrations of mannitol during frozen storage. Ca2+ ATPase activity is expressed as moles of inorganic phosphate released per mg of protein per minute. A) control samples, without mannitol treatment; (B) samples treated with 2% mannitol; (C) samples treated with 4% mannitol; and, (D) samples treated with 6% mannitol.
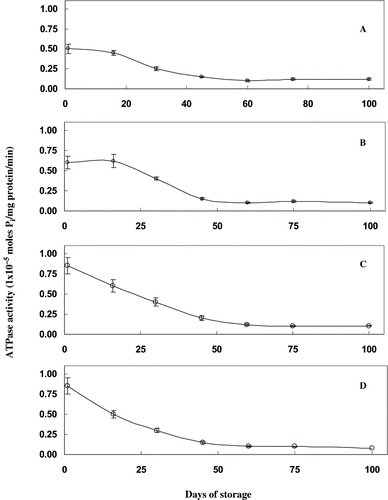
The reduction in the ATPase activity during frozen storage indicates that the integrity of actin and myosin molecules was lost during the process of freezing and frozen storage. The loss of ATPase activity is not necessarily be paralleled with frozen storage induced aggregation of myosin.[Citation11] The loss of ATPase activity does not necessarily imply that the myosin head has been unfolded; it only indicates that myosin head undergoes some conformational changes.[Citation12] The globular head of myosin is responsible for its enzymatic (ATPase) activity, which is sensitive to changes in the configuration of the molecule around the enzymatic site.[Citation13] The higher ATPase activity of extract from mannitol treated samples than control samples during freezing clearly showed that this polyhydric alcohol protected the myosin and actin from freeze denaturation.
The protein profile of the samples with and without mannitol were analysed by SDS-PAGE after extracting the proteins with sample buffer is shown in . The major bands were contributed by myosin and actin components of mince. There was no significant change in the protein pattern due to the treatment with mannitol at different concentrations. With increase in concentration up to 6% mannitol in the mince, the protein profile remained unaltered. During storage under low temperature, the intensity of bands corresponding to actin and myosin increased and resulted in the lower resolution of bands. During frozen storage there was aggregation of proteins, especially the high molecular weight myosin heavy chain. Actin molecules were the one that predominantly showed aggregation during storage. The rate of aggregation in presence of mannitol 4% and 6% were less than control samples.
Figure 2 SDS–PAGE pattern of proteins extracted in sample buffer from sardine mince mixed with different concentrations of mannitol and frozen stored. A) immediately after freezing; and, B) at 112 days of storage. (1) standard marker proteins; (2) mince without mannitol treatment; (3) mince treated with 2% mannitol; (4) mince treated with 4% mannitol; and, (5) mince treated with 6% mannitol. MHC–Myosin Heavy Chain, MLC-Myosin Light Chain.
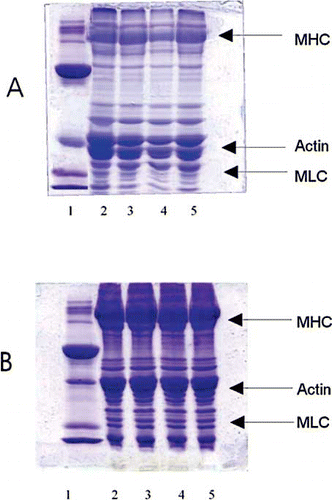
The protein profile by SDS-PAGE showed a difference in pattern of these extractable proteins due to the storage under low temperature. The presence of mannitol during storage did not alter the protein profile. During frozen storage the aggregation of proteins was observed, especially the high molecular weight myosin heavy chain. During freezing and frozen storage the protein – protein interaction favours the formation of protein dimers. From the literature, it is evident that during freezing, the MHC was polymerised mostly as dimers.[Citation12] The formation of aggregates could involve various kinds of interaction between protein molecules, and different kinds of linkages are possible between the proteins. The covalent cross-linking between the MHC molecules is predominantly formed by disulfide bonds.[Citation14] During frozen storage, high-molecular weight salt soluble aggregates formed in which the proteins are partially linked by non disulfide covalent bonds, but it does not exclude the possible involvement of other kinds of bonding.[Citation15] Since the low molecular weight protein bands did not show any change in the pattern, it could be concluded that the proteolytic activity was considerably low in the minced meat. Washing of meat with water must have significantly leached most of the sarcoplasmic protein fractions, which include proteolytic enzymes and myoglobin, thus resulted in low proteolytic activity of mince during frozen storage. The formation of aggregates is mainly contributed by the protein – protein interaction of myosin heavy chain. The presence of mannitol at and above 4% affected in decreasing the aggregation of proteins.
The composition of proteins in mince influences the behaviour of mince proteins during heating. During heating the proteins undergo denaturation and this can be monitored by the change in apparent Tm of proteins. This also helps to understand the behavior of proteins in presence of various additives and also during treatments with them. The thermal denaturation spectra of fish proteins showed in , indicated two transitions one major transition at 57°C and a smaller transition at 71°C. The major transition is taken as the apparent Tm of proteins during thermal denaturation. The proteins extracted from sardine mince showed an apparent Tm of 57 ± 1.5°C (). Apparent Tm of proteins increased by 1.9°C in presence of mannitol. All the samples treated with mannitol shown higher values in apparent Tm than control samples. Maximum increase was shown by the presence of 2% mannitol. The effect of mannitol in stabilization was seen by the Tm recorded especially at lower concentrations where a clear bimodal pattern at 2% was seen. Where in the control above 2% such bimodal transitions occur at (57°C and 71°C) during storage is not observed. This may be due to change in the surface properties of the proteins at these concentrations of mannitol. At higher concentrations of cosolvents, the bimodality in terms of Tm values as a function of storage was also observed. Apparent Tm as recorded during frozen storage decreased with storage time. Control samples showed a gradual decrease in the values of apparent Tm with an increase in storage time. In presence of 2% mannitol, the decrease was rapid and it reached values less than that of control samples. Higher concentration of mannitol in the samples has been shown apparent Tm values more than those of control samples during storage. Samples with 4% mannitol were able to keep the higher values with up to 60 days of storage. Thereafter, it decreased rapidly and reached values very near to that of control samples. The analysis of variance of apparent Tm values during storage in presence of mannitol indicated significant difference in apparent Tm values due to different concentrations of mannitol, as well as due to the effect of storage time.
Figure 3 Thermal denaturation profile of fish proteins during heating from 25 – 95°C at pH 7.5. A). The absorbance at 287 nm was monitored during heating in thermal cuvettes with a heating rate of 1°C per minute B) first derivative plot of absorbance vs temperature.
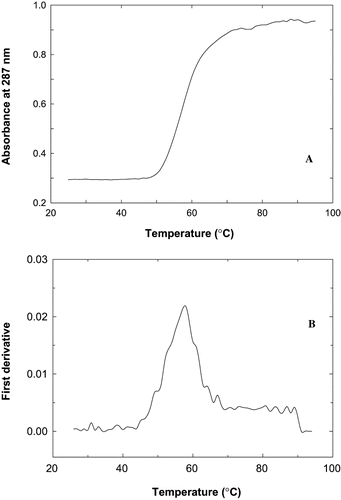
Table 1 Apparent Tm (°C) of proteins from sardine mince treated with different concentrations of mannitol during frozen storage
Since proteins are the major constituents of mince, thermal properties mainly depend on the properties of proteins present in mince. The changes in structure-function of proteins will have an indirect effect in the changes in the thermal properties of mince. Thermal transition of sardine mince during frozen storage may be influenced by the presence of additives also. The transition temperature, which is measured as apparent thermal transition temperature of extracted protein, was 57 ± 1.5°C. Thermal transition of extracted proteins from mince was mainly contributed by myosin molecules present in the mince. Apparent Tm of proteins from sardine mince increased by ∼2°C in presence of mannitol. Sugars and polyhydric alcohols increase the thermal denaturation temperature of globular proteins in aqueous solutions. They have also been shown to reduce the extent of unfolding of globular proteins by indirect solvent effect. DSC thermogram of muscle depleted of sarcoplasmic protein fractions, a clear endothermic peak (Tm = 60.1°C) was observed in case of shark muscle.[Citation16] The different transition regions are due to the involvement of different sub fragments of fish proteins.[Citation17] Sano et al.[Citation18] suggested that the transformation of myosin in the temperature range of 51°C – 80°C is due to the interaction among the head portion of myosin molecules.
The functional and physicochemical properties of proteins are influenced by changes in macro structure of mince. The changes in the structure of mince and its proteins can be monitored by scanning electron microscopy. Microstructure of mince in presence of various concentrations of mannitol and the effect of frozen storage was assessed with the help of scanning electron microscopy (). There were changes observed in the microstructure of mince in presence of various concentration of mannitol. Mince treated with 6% mannitol showed more porous structure during frozen storage. The structural integrity of mince remained almost similar during the period of storage. SEM is not able to give a clear picture of structural changes in the mince during frozen storage. The porosity of surface of muscle increased with increase in concentration of mannitol in the mince. During frozen storage, there was a reduction in the surface porosity of meat, which clearly showed the aggregation phenomenon of proteins during frozen storage. This loss of structure was prevalent in control samples.
Figure 4 Scanning electron micrographs of sardine mince during frozen storage in presence of mannitol. A) control samples, without mannitol treatment immediately after freezing; B) at 120 days of storage; C) sardine mince treated with 6% mannitol immediately after freezing; and, D) at 120 days of storage.
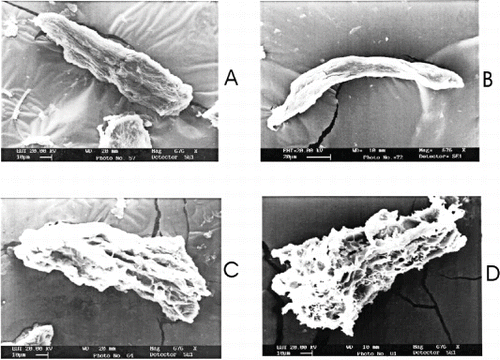
It is evident from the micrographs that higher concentration of mannitol was able to retain this structure, even after 120 days of storage. The structural changes in the actomyosin complex may directly affect different functional properties of the minced meat.
The surface properties changed due to the presence of mannitol in the mince. This might be either due to the change in water structure around the protein molecules or along with it by the solubilization of proteins by the presence of these additives. The retention of porosity of surface of muscle might have been due to the higher water content present in the microenvironment of proteins. This indicates the reduced aggregation of proteins during storage in presence of mannitol compared to control samples. The changes in structure bring changes in enzymatic properties of mince. The change in microstructure clearly indicated the change in the structure of myosin head, which is responsible for the ATPase activity. This also indicates the formation of aggregates during frozen storage. In the presence of mannitol as cryoprotectant the aggregation of protein was shown to be inhibited. The addition of different additives have been shown to alter in microstructure in thermally induced fish protein gels.[Citation19]
Emulsion capacity of extracted proteins from mince was studied upon storage at low temperature (). Emulsion capacity of samples treated with 6% mannitol was higher than that of the control samples even though statistically it may not be significant. Lower concentrations of mannitol induced decrease in emulsion capacity.
Table 2 Emulsion capacity of proteins from sardine mince treated with different concentrations of mannitol during frozen storage. Emulsion capacity expressed as ml oil/ mg protein
There was no significant difference in the emulsion capacity of proteins from sardine mince during frozen storage. But in the case of samples treated with 6% mannitol there was considerable decrease in emulsion capacity after 50 days of storage and reached values much less than the control samples. There was no significant difference in the emulsion capacity of proteins in presence of various concentrations of mannitol during storage as shown by statistical evaluation. The analysis of variance showed that there was no significant difference in the emulsion capacity due to the presence of different concentrations of mannitol during storage. This reduction in emulsion capacity values during frozen storage could be due to formation of aggregates.
Functionality of proteins from sardine mince decreased due to treatments and storage. Emulsion capacity showed a decreasing trend with increase in storage period. Three dimensional structures of proteins can be stabilized by both covalent and non covalent interactions. In general, protein flexibility is also a function of noncovalent interactions such as hydrogen bonding, van der Waals forces, electrostatic and hydropohobic interactions. The low temperature induced denaturation of proteins in mince resulted in the reduction of emulsion capacity of extract. Presence of different concentrations of polyhydric alcohol did not affect the emulsion capacity significantly. The reason for this might be that the exposure of residues is more important in the formation of stable emulsions than that of overall structure of proteins. The presence of polyhydric alcohols did not bring significant change in the exposure of side chains of actin and myosin molecules of mince.
The changes in the microenvironment of proteins can be well understood by overall monitoring the water absorption capacity of freeze-dried samples. The changes in the water absorption capacity of proteins in presence of varying concentrations of mannitol during frozen storage are shown in . The water absorption capacity of the samples treated with mannitol recorded lower values than the control samples indicating the presence of the additive reduced this functionality of mince. The reduction was more for the samples with higher concentration of mannitol. Water absorption capacity of untreated samples was higher than treated samples during the entire period of storage. The reduction in water absorption capacity was similar in the case of control samples and samples in presence of 2%, 4%, and 6% mannitol, respectively. Water absorption capacity is the other important functionality of proteins from mince. The retention of water between the protein molecules reduced during the storage. The water holding capacity depends on the nature of muscle structure and on the nature of additives present in between the protein molecules. The cohesion between water molecules is much larger than that between stabilizer molecules and is a dominant factor for the hydrophobic behavior of the non-polar solutes.[Citation20] Mannitol treated samples retained the ability to hold water better than untreated samples. Myofibrillar proteins such as myosin partially unfolds when the temperature falls below a particular value and the exposed nonpolar groups can then interact with similar groups in the neighboring protein leading to aggregation that lead to adverse change in the texture and water holding capacity of muscle.[Citation21,Citation22] The reduction in water absorption capacity during storage can be probably attributed to the formation of aggregates. The presence of mannitol inhibited the formation of aggregates such that better water retention during storage is observed. Also the changes were brought about by an alteration in the physical structure of muscle in the mince. This was also shown by the data of macro structure of the mince at high magnification by SEM analysis.
CONCLUSIONS
The effect of mannitol on the structure, function, and stability of proteins from fish mince both in the control and frozen stored samples suggests that both covalent and noncovalent interactions in the myosin heavy chain of the proteins play a crucial role. The results also indicate that to a certain extent mannitol prevents aggregation of proteins in fish mince during storage. It also stabilizes the proteins against denaturation through an indirect effect of altering the bulk solvent structure. These results substantiates that the cosolvents which are known to be preferentially excluded from the vicinity of the protein surface perhaps contribute to the stability of proteins through indirect thermodynamic booster effect.
ACKNOWLEDGMENTS
The financial support by Indian Council of Agricultural Research, New Delhi for this study and also Senior Research Fellowship to Sijo Mathew from Council of Scientific and Industrial Research, New Delhi is gratefully acknowledged.
REFERENCES
- Park , Y. , Kelleher , S.D. , McClements , D.J. and Decker , E.A. 2004 . Incorporation and Stabilization of Omega-3 Fatty Acids Insurimi Made from Cod (Gadus Morhua) . Journal of Agricultural and Food Chemistry , 52 : 597 – 601 .
- Mac Donald , G.A. , Lanier , T.C. and Carvajul , P.A. 2000 . “ Stabilization of Proteins in Surimi ” . In Surimi and Surimi Sea food , Edited by: Park , J.W. 91 – 125 . New York : Marcel Dekker .
- Benjakul , S. , Visessanguan , W. , Thongkaew , C. and Tanaka , M. 2003 . Comparative Study on Physicochemical Changes of Muscle Proteins from Some Tropical Fish During Frozen Storage . Food Research International , 36 : 787 – 795 .
- Mathew , S. and Prakash , V. 2005 . Polyhydric Alcohols Mediated Inhibition of Calcium Activated Adenosine Triphosphatase Activity of Fish Skeletal Muscle Actomyosin . International Journal of Food Properties , 8 ( 2 ) : 255 – 265 .
- Perry , S.V. 1995 . Myosin Adenosine Triphosphatase . Methods in Enzymology , 2 : 582 – 588 .
- Taussky , H.H. and Shorr , E.A. 1953 . Microcalorimetric Method for Determination of Inorganic Phosphorous . Journal of Biological Chemistry , 202 : 675 – 685 .
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying Properties of Proteins: Evaluation of Turbidimetric Techniques . Journal of Agricultural and Food Chemistry , 26 : 716 – 723 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.C. and Randall , R.J. 1951 . Protein Measurement with the Folin Phenol Reagent . Journal of Biological Chemistry , 193 : 265 – 275 .
- Pace , C.N. and Scholtz , J.M. 1997 . “ Measuring the Conformational Stability of a Protein ” . In Protein Structure a Practical Approach , Edited by: Creighton , T.E. 299 – 321 . New York : Oxford University Press .
- Laemmlli , U.K. 1970 . Cleavage of Structural Protein during Assembly of the Head Bacteriophage T4 . Nature , 227 : 680 – 685 .
- Ramirez , J.A. , Martin-Polo , M.O. and Bandman , E. 2000 . Fish Myosin Aggregation as Affected by Freezing and Initial Physical State . Journal of Food Science , 65 : 556 – 560 .
- Sultanbawa , Y. and Li-Chan , E.C.Y. 2001 . Structural Changes in Natural Actomyosin and Surimi from Ling Cod (Ophiodon elongatus) during Frozen Storage in the Absence or Presence of Cryoprotectants . Journal of Agricultural and Food Chemistry , 49 : 4716 – 4725 .
- Mackie , I.M. 1993 . The Effect of Freezing on Flesh Proteins . Food Reviews International , 9 : 575 – 610 .
- Lian , P.Z. , Lee , C.M. and Hufnagel , L. 2000 . Physicochemical Properties of Frozen Red Hake (Urophycis chuss) Mince as Affected by Cryoprotective Ingredients . Journal of Food Science , 65 : 1117 – 1123 .
- Mazo , M.L. , Torrejon , P. , Careche , M. and Tejada , M. 1999 . Characteristics of the Salt Soluble Fraction of Hake (Merluccius merluccius) Fillets Stored at −20°C and −30°C . Journal of Agricultural and Food Chemistry , 47 : 1372 – 1377 .
- Chen , H. 1995 . Thermal Stability and Gel Forming Ability of Shark Muscle as Related to Ionic Strength . Journal of Food Science , 60 : 1237 – 1240 .
- Zhang , J. , Farkas , B.E. and Hale , S.A. 2001 . Thermal Properties of Skipjack Tuna (Katsuwonus pelamis) . International Journal of Food Properties , 4 ( 1 ) : 81 – 90 .
- Sano , T. , Noguchi , S.F. , Matsumoto , J.J. and Tsuchiya , T. 1990 . Effect of Ionic Strength on Dynamic Viscoelastic Behavior of Myosin During Thermal Gelation . Journal of Food Science , 55 : 51 – 55 .
- Gao , J.C. , Piggot , G.M. and Reine , B. 1999 . Gelforming Additive Effects on Properties of Thermally Induced Minced Fish Gel . Journal of Food Science , 64 : 414 – 417 .
- Carrillo-Nava , E. , Dohnal , V. and Costas , M. 2004 . Modeling Gibbs Energies of Solution for a Non-Polar Solute in Aqueous Solutions of the Protein Stabilizers Glycerol and Ethylene Glycol . Biophysical Chemistry , 107 : 19 – 24 .
- McClements , D.J. 2002 . Modulation of Globular Protein Functionality by Weakly Interacting Cosolvents . Critical Reviews in Food Science and Nutrition , 42 : 417 – 471 .
- Jiang , S.T. , Hwang , B.O. and Tsao , C.T. 1987 . Effect of Adenosine Nucleotides and Their Derivatives on Denaturation of Myofibrillar Proteins in vitro During Frozen Storage at −20°C . Journal of Food Science , 52 : 117 – 123 .