Abstract
The effects of three saline solutions, LiCl, NaCl, and KCl, on the gelatinization temperature of starch-salt systems were studied. The same but systems with the addition of NaOH were also studied. A rise of gelatinization temperature of all starch-salt-water systems studied was observed. The gelatinization range (ΔTgr) of starch-salt-water systems, was not affected by the nature of the cation; instead it was affected by its concentration; the rise of the gelatinization temperature (ΔTgel) and gelatinization enthalpy (ΔH) were not affected by the nature and concentration of the cation. The rise of the gelatinization temperature (ΔTgel) of starch-salt-NaOH-water systems was significantly affected by the nature and concentration of the cation, and by the concentration of NaOH. Li+ was responsible for a smaller rise of gelatinization temperature and K+ for a greater rise of gelatinization temperature. Increases in the gelatinization temperature of up to 48.0°C were measured. These results may indicate that the rise in gelatinization temperature of starch-salt-NaOH-water systems could be explained based on the assumption that starch behaves as a weak ion exchanger governed by the Donnan potential.
INTRODUCTION
The gelatinization temperature of a starch suspension is an important feature in the manufacture of starch derivatives with the condition that the granule structure must be preserved. If in these processes the temperature of the suspension rises beyond the gelatinization temperature, the starch will start to gelatinize and become soluble in water. Under this fact, the derivatization process cannot be continued. Consequently, the product is lost and there is a large amount of work involved in cleaning out the equipment.[Citation1] In addition, the derivatization produces a decrease in the gelatinization temperature of the starch. Therefore, only a limited degree of derivatization can be obtained. Some derivatization processes are highly exothermic and require complex temperature controls in order to avoid gelatinization and to maintain an adequate reaction rate. In these processes, it is usually necessary to ionize the hydroxyl groups of starch. This ionization is best accomplished by making the starch suspension highly alkaline. A pH of at least 9 is required, and one above 10 is preferred, taking care not to exceed a pH of 12 since the starch granules would swell and start to gelatinize. Sodium hydroxide is the most commonly alkali used for starch modification reaction. However, to prevent a pH rising above 12 in any part of the reaction vessel, the sodium hydroxide is usually diluted to a concentration less than a 4% by weight before injecting it into the starch slurry. This produces an increase of the reaction vessel capacity in order to hold the excess water introduced with the sodium hydroxide. Moreover, the dilution of starch slurry causes a loss in reaction efficiency and more reagents are needed to complete the reaction.[Citation2]
In practice, it has been found that the gelatinization temperature of starch can be raised by adding some salts like sodium sulphate, sodium chloride, etc.[Citation3,Citation4,Citation5,Citation6] The presence of salts also allows the pH rising up to 12.5 without causing swelling or gelatinization. Salts concentration can reach values as high as 55% by weight and even higher values, with a great reduction of the amount of water introduced into the slurry with the pH adjusting agent.[Citation2] Several authors have tried to explain how electrolytes affect the gelatinization temperature of starch, but they have not succeeded because they have assumed a mechanism that did not perform were in some simple systems.[Citation4,Citation7] Oosten[Citation1] has proposed a hypothesis to explain electrolyte effects on gelatinization temperature based on the assumption that starch behaves as a weak ion exchanger governed by the Donnan potential . However, there are not enough experiments to support his hypothesis. Ion exchangers, are insoluble solid materials which carry exchangeable cations or anions. They consist of a framework, which is held together by covalent bonds or lattice energy. This framework carries a positive or negative electric charge, which is compensated by ions of opposite charge, called counter ions . The counter ions are free to move within the framework and can be replaced by other ions of the same charge. The framework of a cation exchanger is usually a macromolecular or crystalline polyanion.[Citation8]
When a cation exchanger is placed in an electrolyte solution, both the electrolyte solution and the exchanger will exchange counter ions with each other until equilibrium is reached. Although the driving force for the counter ion exchange is a simple diffusion, the process disturbs the electroneutrality. An accumulation of negative charges will occur in the cation exchanger and an accumulation of positive charges will occur in the solution. Consequently, the concentration ratio of counter ions will not necessarily be the same in both phases. The electric potential difference generated between the two phases is called Donnan potential. In general, an ion exchanger prefers some species instead of other compounds because of interactions between the charged framework and counter ions. As a rule, a cation exchanger prefers the counter ion of highest valence, and of smallest solvated equivalent volume (solvated equivalent volume for Li+ > Na+ > K+ > Rb+ > Cs+), and the least associated with the co-ion.[Citation8]
Oosten[Citation9] observed a substantial rise of the gelatinization temperature by adding NaCl and NaOH, when compared with NaCl. Oosten used a Brabender amylograph to determine the gelatinization temperature of different salt systems. The limitation of Brabender amylograph to work above 100 °C did not let him detect substantial increases of Tgel in some salt systems. DSC (Differential Scanning Calorimetry) is particularly useful to investigate the phase transitions of starch-water[Citation10] systems because it allows: (1) the study of starch gelatinization over a wide range of starch/water ratios, (2) the determination of gelatinization temperatures above 100 °C, and (3) the estimation of transition enthalpies.[Citation11,Citation12,Citation13,Citation14,Citation15,Citation16,Citation17,Citation18]
The effects of three salt solutions, LiCl, NaCl, and KCl, at different concentrations on the gelatinization temperature of starch-salt systems were studied. The same systems in the presence of NaOH were also studied. Weak-acid cation exchangers have an increased selectivity for the cation of these salts (selectivity of K+ > Na+ > Li+). Based on the Donnan potential to explain the rise in the gelatinization temperature by adding salt and hydroxide, we hypothesize that the use of salts with the same anions and different univalent cations in starch-salt-NaOH-water systems should increase the gelatinization temperature in a proportion related to the selectivity of cations. The aim of this study was to try to explain the effect of the substantial rise of gelatinization temperature of starch by adding salts and sodium hydroxide.
MATERIALS AND METHODS
Common corn starch (CCS) was obtained from the National Starch and Chemical Company. CCS was washed with dionized water twice to remove possible traces of ions and was then filtered and dried overnight in a vacuum oven at 50°C. AACC standard method[Citation19] was used for moisture determination. All reagents used were analytical grade or better (Fisher Scientific Company).
Preparation of Salts-NaOH Solutions
Salt solutions of LiCl, NaCl, and KCl (2 and 4 molal) were prepared. Additional salt solutions were prepared by adding NaOH to a portion of the previous salt solutions to obtain 0.25 m and 0.5 m NaOH, respectively.
Differential Scanning Calorimetry
Thermal analysis was performed using a differential scanning calorimeter (DSC 7, PerkinElmer Corp., Norwalk, CT) equipped with a thermal analysis data station (PerkinElmer). Indium was used as a calibration standard. The reference cell contained a sealed, empty, stainless steel pan. Starch samples (∼ 15.0 mg, dwb) were weighed into preweighed stainless steel pans (Perkin-Elmer). Deionized water or salt solutions were added to obtain ∼ 30% (w/w) starch suspensions. The samples were stirred with a needle. Pans were sealed, the total weights were determined, and the suspensions were stored overnight at room temperature. All samples were heated at 5°C up to 180°C, at a heating rate of 10°C/min. Gelatinization enthalpy, onset, peak and end transition temperatures were calculated (7 Series Software, Perkin-Elmer). Analyses were performed in duplicate and mean values and standard deviation are reported.
Statistical Analysis
Statistical analysis was made using the program Statgraphics plus.[Citation20] To compare values of temperature and gelatinization energy, an ANOVA test was used. To compare individual means an a posteriori test (LSD) was used.
RESULTS
A rise of gelatinization temperature of all starch-salt-water systems studied was observed. Gelatinization range (ΔTgr) was affected by cation concentration (p < 0.026) but not by its nature. Rise of gelatinization temperature (ΔTgel) was affected by both variables, cation nature (p < 0.018) and concentration (p < 0.0001); but gelatinization enthalpy (ΔH) was not affected by these variables (see ). The addition of NaOH at different concentrations affected ΔTgr (p < 0.0001), ΔTgel (p < 0.0001) and ΔH (p < 0.0001). Moreover, this addition produced a significant influence of cation nature in ΔTgr and cation concentration in ΔH, not found in systems without NaOH, ΔTgel varied with both variables in the system without NaOH.
Table 1 Thermal analyses of CCS-water and CCS-water-salt systems.
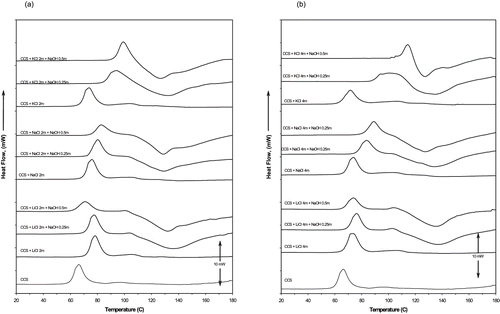
The CCS-LiCl 2 m systems reduced ΔTgel by adding NaOH. The effect was diminished in CCS-LiCl 4 m systems. The CCS-NaCl 2 m systems increased ΔTgel by adding NaOH. However, this effect was enhaced in CCS-NaCl 4m systems by adding NaOH. The CCS-KCl 2m systems increased ΔTgel by adding NaOH. This effect was enhanced in CCS-KCl 4m systems, as shown in . The CCS-LiCl 2 m and CCS-LiCl 4 m systems increased ΔTgr by adding NaOH 0.5m. The CCS-NaCl 2 m and CCS-NaCl 4 m systems increased ΔTgr by adding NaOH. The CCS-KCl 2 m systems increased ΔTgr by adding NaOH. This effect was enhanced in CCS-KCl 4 m systems by adding NaOH 0.5 m, as shown in . In all starch-salt-water systems the ΔH was reduced by adding NaOH, as shown in . The DSC thermograms of CCS, starch-salt systems (2 m and 4 m salt), starch-salt-NaOH systems (0.25 m and 0.5 m NaOH) are illustrated in . All starch-salt-NaOH-water systems showed an exotherm with a peak (Tp) located between 125 – 135°C.
Table 2 Thermal analyses of CCS-water-salt-NaOH systems.
DISCUSSION
The weak acid character of the starch chain in the dissociation of alcoholic groups present in the polymer is expressed according to the following equation:
When starch granules are in suspension they absorb water and dissociate shown in EquationEq. 1 and this dissociation has a pKa = 12.5. The concentration gradient of H+ will induce the H+ to migrate from the granules to the water, reducing the pH of the water from 7 to 6.25. This migration takes place because outside the granules the pH is influenced by the dissociation of the water molecules, which have a pK = 14. At this point, there will be a potential difference between the starch granules and the water phase, called Donnan potential.
When salts such as LiCl, NaCl, or KCl are added to starch suspensions, some alcoholic groups in the starch granule are converted to alcoholate, as shown in EquationEq. 1. The alcoholate form is better dissociated and some H+ are replaced by the cations into the granules, increasing therefore the Donnan potential. The released H+ produce a pH reduction and the absorbed cations cause the rise in the gelatinization temperature of the starch-salt-water systems. Because these salts are highly dissociated in the solution, they are not a good binding agent for the released H+, and consequently both the absorption of cations and the increase in the Donnan potential are rather limited. The Donnan potential excludes anions (Cl− and OH−) from the granules and so the rise in the gelatinization temperature is rather limited.
When the salt concentration is increased, H+ are no longer replaced by cations because the solution is still not a good binding agent for the H+ and it can be released from the granules. The Donnan potential decreases as a consequence of the increase of Cl− in the solution. In this way, the decrease of the Donnan potential reduces its ability to exclude anions from the granule, and consequently reduces the rise of the gelatinization temperature. The H-bonds formed among inter- and intramolecular chains of starch are the main forces responsible for maintaining the granule structure. Anion penetration into the granules disrupts H-bonds facilitating gelatinization.[Citation1] Limited absorption of cation and limited exchange of H+ for cations in starch-salt systems at low salt concentration is consistent with the current results of no significant change of ΔTgr and ΔH. When more salt is added, more anion penetration occurs and the granules are weaker. This also appears consistent with the smaller ΔTgel found in the present article.
After adding NaOH to starch-salt systems, the absorption of cations is very much enhanced due to the consumption of H+ by the OH−. The increased number of cations results in a higher Donnan potential, which more efficiently excludes the gelatinizing anions, and therefore increases the gelatinization temperature. The current results suggest that K+ rather than Na+, and Na+ rather than Li+ are absorbed by starch granules. This cation selectivity effect becomes more obvious when the rise of ΔTgel in starch-salt-NaOH systems at the same levels of concentration is compared. An evidence of the lowest Li+ selectivity presented by the starch granules can be inferred by observing the decrease of ΔTgel in starch-LiCl systems when the concentration of sodium hydroxide is increased. In addition, the highest selectivity of K+ is manifested by the substantial rise of ΔTgel in all starch-KCl systems when the concentration of NaOH is increased. The intermediate selectivity of Na+ by the starch granules becomes evident due to the rise of ΔTgel in starch-NaCl systems and this only occurs when sodium hydroxide is added at the highest concentration of NaCl (see ). The penetration of the cations into the granules in starch-salt-NaOH systems seems to explain the reduction of the ΔH since no significant variation of ΔH was observed in starch-water or starch-water-salt systems. All starch-salt-NaOH-water systems showed an exotherm with a peak (Tp) located between 125 – 135°C, as shown in . The presence of an exotherm peak located in the same range of temperatures was also detected in starch-NaOH-water systems. These results suggest that a degradation reaction occurring between starch and NaOH.
CONCLUSIONS
The assumption that starch behaves as a weak cation exchanger governed by the Donnan potential clearly explains the substantial rise of the gelatinization temperature of common corn starch by adding univalent salts and hydroxide. However, more studies are needed to determine the validity of the model in more complex systems and at a higher salt-hydroxide concentration.
REFERENCES
- Oosten , B.J. 1982 . Tentative Hypothesis to Explain How Electrolytes Affect the Gelatinization Temperature of Starches in Water . Starch/Stärke , 34 : 233
- Subbaratnam , A.V. and Powell , E.L. Alkaline Buffer for Granular Starch Modification Reaction . U.S. Patent 3,687,728 . 1972 . issued August 29
- Banks , W. and Greenwood , C.T. 1975 . Starch and Its Components , New York : Halsted Press, Wiley and Sons .
- Gough , B.M. and Pybus , J.N. 1973 . Effect of Metal Cations on the Swelling and Gelatinization Behavior of Large Wheat Starch Granules . Starch/Stärke , 25 : 123
- Evans , I.D. and Haisman , D.R. 1982 . The Effect of Solute on the Gelatinization Temperature Range of Potato Starch . Starch/Stärke , 34 : 224
- Takahashi , K. and Wada , K. 1992 . Reversibility of salt effects on the thermal stability of potato starch granules . Journal of Food Science , 57 ( 5 )
- Gerlsma , S.Y. 1970 . Gelatinization Temperature of Starch, as Influenced by Polyhydric and Monohydric Alcohols, Phenols, Carboxylic Acids and Some Other Additives . Starch/Stärke , 22 : 3
- Helfferich , F. 1962 . Ion Exchange , Vol. 6 , 82 – 134 . London : McGraw Hill, Inc. .
- Oosten , B.J. 1979 . Substantial Rise of Gelatinization Temperature of Starch by Adding Hydroxide . Starch/Stärke , 31 : 228
- Saif , S.M.H. , Lan , Y. and Sweat , V.E. 2003 . Gelatinization Properties of Rice Flour . International Journal of Food Properties , 6 ( 3 ) : 531 – 542 .
- Wooton , M. and Bamunuarachchi , A. 1979 . Application of Differential Scanning Calorimetry to Starch Gelatinization. I. Commercial Native and Modified Starches . Starch/Stärke , 31 : 201
- Wooton , M. and Bamunuarachchi , A. 1980 . Application of Differential Scanning Calorimetry to Starch Gelatinization. III. Effect of Sucrose and Sodium Chloride . Starch/Stärke , 32 : 126
- Biliarderis , C.G. , Maurice , T.J. and Vose , J.R. 1980 . Starch Gelatinization Phenomena Studied by Differential Scanning Calorimetry . J. Fd. Sc , 45 : 1669
- Cooke , D. and Gidley , M.J. 1992 . Loss of Crystalline and Molecular Order During Starch Gelatinization: Origin of Enthalpic Transition . Carbohydrate Research , 227 : 103 – 112 .
- Hwang , C.H. , Heldman , D.R. , Chao , R.R. and Taylor , T.A. 1999 . Changes in Specific Heat of Corn Starch Due to Gelatinization . Journal of Food Science , 64 ( 1 )
- Zhong , Z. and Susan Sun , X. 2004 . Thermal Characterization and Phase Behavior of Corn Starch Studied by Differential Scanning Calorimetry . Journal of Food Engineering , 69 ( 4 )
- Singh , N. , Singh Sandhu , K. and Kaur , M. 2004 . Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars . Journal of Food Engineering , 63 ( 4 )
- Jyothi , A.N. , Moorthy , S.N. and Vimala , B. 2003 . Physicochemical and Functional Properties of Starch from Two Species of Curcuma . International Journal of Food Properties , 6 ( 1 ) : 135 – 145 .
- American Association of Cereal Chemists . 1983 . “ Method 44–15A ” . In Approved Method of the AACC , St. Paul, MN : AACC .
- 1993 . “ Statgraphics, Plus. Version 7 for DOS ” . In Statgraphics User Manual , Rockville, MD : Manugistics, Inc .