ABSTRACT
Five desi (PBG-1, PDG-4, PDG-3, GL-769, and GPF-2) and one kabuli type (L-550) chickpea cultivars were evaluated for their seed mass, volume, hydration capacity, swelling capacity, cooking time, and instrumental textural properties (hardness, cohesiveness, gumminess, and chewiness). Flour was prepared from these chickpea cultivars and various physicochemical and functional properties were determined. The pasting (pasting temperature, peak viscosity, breakdown, and final viscosity) and gelatinization (T o, T p, T c, and ΔH gel) properties of these flours were measured using Rapid Visco Analyzer (RVA) and Differential Scanning Calorimeter (DSC), respectively. Starch was also isolated from chickpea cultivars and evaluated for amylose content, swelling power, solubility, and syneresis values. Physicochemical, cooking, and instrumental textural properties of seeds of different chickpea cultivars were related to physicochemical, gelatinization, and pasting properties of their flours and physicochemical properties of their starches. Selected properties of chickpea seeds were significantly correlated with the properties of their starches and flours. Hardness value of soaked chickpea seeds was positively correlated to cooking time, seed mass, seed volume, hydration, and swelling capacity (p < 0.01). Water solubility index (WSI) of chickpea flours was positively correlated to seed mass, volume, hydration capacity, and hardness value (p < 0.05). Selected instrumental textural parameters of seeds had positive correlation with ΔH gel of flours (p < 0.01). Peak viscosity of flours showed positive correlation to breakdown, final viscosity, bulk density, and negative correlation to cohesiveness of soaked seeds (p < 0.01). Final viscosity showed negative correlation to bulk density and water absorption index (WAI) (p < 0.01) of flours.
INTRODUCTION
Legumes have been considered as the most significant food source for people of low incomes.[Citation1] Dry legumes are important ingredient of diet in many parts of the world. Among dry legumes, green peas, dry beans, chickpeas, and lentils are the most common. Starch is the major carbohydrate of legume seeds and is considered of commercial importance due to its high industrial demand.[Citation2] Chickpea (Cicer arietinum) is the world's third most important grain legume after beans and peas.[Citation3] Chickpea and other food legumes contribute significant amounts of protein, carbohydrate, vitamins, and minerals to the diets of people living in the Mediterranean region.[Citation4–5] The breeding programs are carried out in search for high yielding chickpea cultivars to meet the increasing demand for chickpea seeds.[Citation6] Genotypic variation in chemical composition of chickpea seeds have been reported.[Citation7]
Textural characteristics of legumes may be dependent upon seed microstructure, chemical, and physical changes occurring during processing. Cooking time is a highly significant aspect of cooking quality. One of the main drawbacks that limit the utilization of legumes is their long cooking time.[Citation8] Sefa Dedeh and Stanley[Citation9] reported that seed size and seed coat thickness play an important role in the water absorption characteristics of legume seeds. The varietal differences in cooking quality have been reported to exist even in the same legume.[Citation10] Starch, the principal carbohydrate constituent of a majority of plant materials, merits a detailed investigation to understand better its biochemical and functional characteristics as well as variations.[Citation11] Applications of starch in food systems are primarily governed by gelatinization, gelation, pasting, solubility, swelling, and digestibility properties.[Citation12]
Differential Scanning calorimeter has been used to study thermal properties associated with starch gelatinization. Gelatinization, the process by which the internal structure of the granule is broken down and the whole granule disintegrates releasing the polysaccharide into the surrounding medium, is accompanied by a variety of changes.[Citation13] In view of the increasing utilization of grain legumes in composite flours for various food formulations, their functional properties are assuming greater significance.[Citation14] The functional properties of legume flours are provided not only by proteins, but also by the complex carbohydrates and other components such as pectins and mucilages. There is a lack of information on seed-starch-flour property relationships among chickpea cultivars. Therefore, the present investigation was undertaken to establish relationships between physicochemical, cooking, and instrumental textural properties of seeds of different chickpea cultivars with the physicochemical, gelatinization, and pasting properties of flours and starches isolated from these cultivars.
MATERIALS AND METHODS
Materials
Representative samples of six improved commercial chickpea cultivars viz. PBG-1, PDG-4, PDG-3, GL-769, GPF-2, and L-550 from April, 2002, harvest were obtained from Punjab Agricultural University, Ludhiana, India. The cultivars PBG-1, PDG-4, PDG-3, GL-769, GPF-2 are of desi type, while L-550 cultivar is of kabuli type.
Physicochemical Properties of Seeds
Seed mass (mass of 100 seeds), seed volume (volume of 100 seeds), hydration capacity (increase in weight of seeds after soaking/total number of seeds), and swelling capacity (increase in volume of seeds after soaking/total number of seeds) were evaluated using the method of Williams et al.[Citation8]
Cooking Time
Cooking time was determined using the method of Williams et al.[Citation8] For the determination of cooking time, about 300 ml distilled water was brought to boiling point in a 500 ml beaker fitted with condenser to avoid evaporation losses during boiling, and then a 100-g seed sample was added. Boiling was continued, and boiled samples at intervals of 2 minutes were drawn and tested for their softness by pressing between the forefinger and thumb. The time taken to achieve the desirable softness was recorded as cooking time of the sample.
Instrumental Texture Profile Analysis (TPA)
Chickpea seeds (50 g) were soaked in 100 ml distilled water in beakers, which were kept covered at room temperature. After a soaking period of 10 hours, excess water was drained, and the soaked seed samples were kept for instrumental textural analysis. Instrumental TPA of soaked chickpea seeds was performed using a single grain of each cultivar, which was placed on the base plate of the Instron Universal Testing Machine (Model 4464, Instron, Buckinghamshire, England) and kept parallel to the plate for testing. The soaked seeds were subjected to 80% compression with a cylindrical probe (38 mm diameter) at a crosshead speed of 1 mm/s twice in two cycles using a 10 kg load cell. The instrumental textural parameters of hardness, cohesiveness, gumminess, and chewiness were determined as described by Szczesniak.[Citation15] Eight replicates were performed on each sample.
Starch Isolation
Starch was isolated from different chickpea cultivars using the method of Singh et al.[Citation12] Various chickpea cultivars (300 g) were steeped in water containing 0.16% sodium hydrogen sulphite for 12 hours at 50°C. The steep water was drained off, and grains were ground in a laboratory blender. The ground slurry was screened through nylon cloth (100 mesh). The material left over the nylon cloth was washed thoroughly with distilled water. The filtrate slurry was allowed to stand for 1 hour. The supernatant was removed by suction, and the settled starch layer was resuspended in distilled water and centrifuged in wide-mouthed cups at 3000 rpm for 5 minutes. The upper non-white layer was scrapped off. The white layer was resuspended in distilled water and recentrifuged for 3 to 4 times. The starch was then collected and dried in an oven at 40°C for 12 hours.
Physicochemical Properties of Starches
Amylose content of isolated chickpea starches was determined in triplicate using the method of Williams et al.[Citation16] Swelling power and solubility were determined in triplicate using method of Leach et al.[Citation17] For the measurement of syneresis, starch suspension (2 kg/100 L water) was heated at 85°C for 30 minutes in a temperature controlled water bath, followed by rapid cooling in an cold water bath to room temperature. The starch sample was stored for 24, 48, and 120 hours at 4°C. Syneresis was measured (in triplicate) as percent amount of water released after centrifugation at 3000 rpm for 15 minutes.
Preparation of Chickpea Flours
Seeds of different chickpea cultivars were ground to pass through the sieve no. 72 (British Sieve Standards) to obtain flour. The flour samples were defatted by solvent extraction process using n-hexane and then dried at temperature of 40°C in a hot air cabinet drier; and after cooling, they were packed in air tight containers.
Physicochemical and Functional Properties of Flours
For the measurement of bulk density, flour samples were gently filled in 10 ml graduated cylinder, previously tared. The bottom of the cylinder was gently tapped on a laboratory bench several times until there was no further diminution of the sample level after filling to the 10 ml mark. Bulk density was calculated as mass of sample per unit volume of sample (kg/m3). Measurements were made in triplicate. Water absorption index (WAI) and water solubility index (WSI) of chickpea flours were determined by slightly modifying the method of Anderson et al.[Citation18] Flour sample (2.5 g) was dispersed in 30 ml of distilled water, using a glass rod, and cooked at 90°C for 15 minutes in a water bath. The cooked paste was cooled to room temperature and transferred to tared centrifuge tubes, and then centrifuged at 3000 rpm for 10 minutes. The supernatant was decanted for determination of its solid content into a tared evaporating dish, and the sediment was weighed. The weight of dry solids was recovered by evaporating the supernatant overnight at 110°C. WSI and WAI were calculated by the equations:
Water absorption capacity of chickpea flours was measured by the centrifugation method of Sosulski.[Citation19] The sample (3.0 g) was dispersed in 25 ml of distilled water and placed in preweighed centrifuge tubes. The dispersions were stirred occasionally, held for 30 minutes, followed by centrifugation for 25 minutes at 3000 rpm. The supernatant was decanted, excess moisture was removed by draining for 25 minutes at 50°C, and sample was reweighed. For the determination of oil absorption the method of Lin et al.[Citation20] was used. Samples (0.5 g) were mixed with 6 ml of corn oil in preweighed centrifuge tubes. The contents were stirred for 1 minutes with a thin brass wire to disperse the sample in the oil. After a holding period of 30 minutes, the tubes were centrifuged for 25 minutes at 3000 rpm. The separated oil was then removed and the tubes were inverted for 25 minutes to drain the oil prior to reweighing. The water and oil absorption capacities were expressed as grams of water or oil bound per gram of the sample on a dry basis.
Gelatinization Properties of Flours
Gelatinization characteristics of chickpea flours were studied by using Differential Scanning Calorimeter—821e (Mettler Toledo, Switzerland) equipped with a thermal analysis data station. Flour (3.5 mg, dry weight) was loaded into a 40 μl capacity aluminium pan (Mettler, ME-27331), and distilled water was added with the help of a Hamilton microsyringe to achieve a flour-water suspension containing 70% water. Samples were hermetically sealed, allowed to stand for 1 hour at room temperature, and reweighed before heating in the DSC. The DSC analyzer was calibrated using indium, and an empty aluminium pan was used as reference. Sample pans were heated at a rate of 10°C/min from 20 to 100°C. Onset temperature (T o), peak temperature (T p), conclusion temperature (T c), and enthalpy of gelatinization (ΔH gel) were calculated automatically. Enthalpies were calculated on a dry flour basis.
Pasting Properties of Flours
Pasting properties of chickpea flours were studied by using Rapid Visco Analyzer (Newport Scientific Pty Ltd, Warriewood NSW 2102, Australia). Viscosity profiles of flours from different chickpea cultivars were recorded using flours suspensions (8%, w/w; 28 g total weight). The temperature-time conditions included a heating step from 50 to 95°C at 6°C/min (after an equilibration time of 1 minute at 50°C), a holding phase at 95°C for 5 minutes, a cooling step from 95 to 50°C at 6°C/min, and a holding phase at 50°C for 2 minutes. Each sample was analyzed in triplicate.
Statistical Analysis
The data reported in all the tables are an average of triplicate observations. The data were subjected to one-way analysis of variance (ANOVA) and correlation coefficients were calculated using Minitab Statistical Software (MINITAB 2002, Version 13, USA).
RESULTS AND DISCUSSION
Physicochemical characteristics of seeds of different chickpea cultivars varied significantly (). Seed mass and seed volume of different chickpea cultivars ranged between 12.46–21.94 g and 9.8–17.0 ml per 100 seeds, respectively. Highest seed mass and volume of 21.94 g and 17.0 ml, was observed for L-550 (kabuli type) against lowest seed weight (12.46 g) and seed volume (9.8 ml) for PBG-1 (desi type). Seeds of different chickpea cultivars showed hydration and swelling capacity per seed in the range of 0.12–0.20 and 0.11–0.23, respectively. Williams et al.[Citation8] reported mean swelling capacity and hydration capacity 0.361 and 0.346, respectively, for different chickpea cultivars. Hydration capacity was lowest for PBG-1 and GPF-2, both desi types. L-550 was different from all other chickpea cultivars with respect to its highest swelling and hydration capacity.
Table 1 Physicochemical, cooking, and textural properties of seeds of different chickpea cultivars.
The water absorbing capacity of seeds depends on cell wall structure, composition of seed, and compactness of the cells in the seed.[Citation21] A strong positive correlation of seed mass with hydration capacity () and swelling capacity (r = 0.952) was observed. Seed volume had a highly significant positive correlation with swelling capacity (r = 0.965, p < 0.01) and hydration capacity (r = 0.993). A positive correlation of swelling capacity with hydration capacity () was observed. Cooking time for different chickpea cultivars ranged between 62.4 to 95.0 minutes, lowest for PBG-1 and highest for L-550. The longer cooking time requirement for L-550 could be attributed to its larger seed mass, as seed size governs the distance to which water must penetrate in order to reach the innermost portion of seeds. Cooking time showed a significant positive correlation with seed mass (r = 0.978), seed volume (r = 0.978, p < 0.01), and swelling capacity (r = 0.956). Instrumental texture measurements of the soaked chickpea seeds from different cultivars are presented in . The pattern of textural changes during soaking reflects the hydration properties of the seeds. Hardness value of soaked chickpea seeds ranged between 106.9 to 209.7 N—the lowest for GL-769 and highest for L-550. PDG-4 had lowest cohesiveness (0.06), gumminess (7.83 N), and chewiness (23.8 Nmm) against highest gumminess (16.3 N) and chewiness (40.0 Jm) for L-550 cultivar. It was observed that cultivars with higher cooking time had higher hardness value for their soaked seeds. The interrelationships among the textural parameters showed a significant correlation of hardness with gumminess (r = 0.829, p < 0.05) and of gumminess with chewiness (r = 0.964). Hardness value for soaked chickpea seeds showed positive correlation to seed mass (r = 0.960), seed volume (r = 0.967), hydration capacity (), and swelling capacity (r = 0.996).
Figure 1 A. Relationship between seed mass and hydration capacity of seeds from different chickpea cultivars; B. relationship between swelling capacity and hydration capacity of seeds from different chickpea cultivars; C. relationship between hydration capacity and hardness value of seeds from different chickpea cultivars; D. relationship between amylose content and syneresis after 120h of storage of starches from different chickpea cultivars; E. relationship between T p (°C) of starches and chewiness of soaked seeds from different chickpea cultivars; F. relationship between pasting temperature and final viscosity of flours from different chickpea cultivars; G. relationship between peak viscosity of flours and cohesiveness of soaked seeds from different chickpea cultivars; H. relationship between final viscosity and water absorption index (WAI) of flours from different chickpea cultivars.
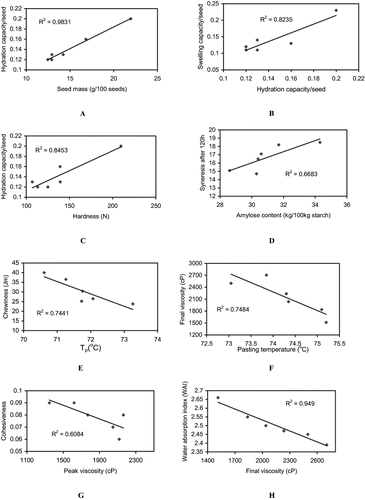
Significant differences in various physicochemical properties were observed among starches from different chickpea cultivars (). Chickpea starches had amylose content ranging between 28.6–34.3 (kg/100 kg starch)—the highest for GPF-2 and lowest for PBG-1 starch. Amylose content in the range of 30.4–32.2 and 28.3–33.5 kg/100 kg starch for chickpea starches has been reported earlier by El faki et al.[Citation11] and Lineback and Ke.[Citation22] Swelling power of chickpea starches varied from 11.4–13.6 g/g, whereas solubility values (amount of solids leached out in the solution during heating of starch suspension) were in the range of 13.2–14.9 (kg/100 kg starch). Starch swelling occurs concomitantly with loss of birefringence and precedes solubilization. Highest swelling power for PBG-1 and lowest for GL-769 starch was observed (). Highest solubility for GL-769 was observed, whereas PBG-1 starch had lowest value for the same. Schoch and Maywald[Citation23] observed a restricted swelling power in the range of 16 to 20 for chickpea and three other legume starches. Swelling power of chickpea starches showed significant negative correlation with solubility (r =−0.770). The syneresis of gels prepared from starches separated from different chickpea cultivars was measured as the amount of water released from gels during storage (up to 120 h) at 4°C (). We observed the highest syneresis rate for GPF-2 starch, and the lowest for GL-769 starch. The syneresis values progressively increased with the increase in storage of starch pastes from all chickpea cultivars. A significant positive correlation between amylose content and syneresis values () of starches was observed.
Table 2 Physicochemical properties and syneresis of starches from different chickpea cultivars.
The genotypes belonging to two distinct chickpea groups showed large differences in certain physicochemical and functional properties of their flours (). Bulk density for chickpea flours varied from 0.536–0.571 kg/m3, highest for L-550 flour and lowest for GPF-2 flour. The water absorption index (WAI) and water solubility index (WSI) for different chickpea flours ranged between 2.39–2.66, and 20.42–22.89 kg/100 kg flour, respectively. WAI was observed to be highest for GPF-2 flour and lowest for L-550 flour. L-550 flour showed significantly higher WSI (22.89) and lower WAI (2.39), as compared to flours from desi chickpea cultivars. WSI was positively correlated to seed mass, seed volume, hydration capacity and hardness value for soaked seeds (p < 0.05). WAI showed positive correlation to amylose content (r = 0.778) and negative correlation with bulk density (r = -0.991). The water absorption capacity (WAC) and oil absorption capacity (OAC) of different chickpea flours ranged between 1.33–1.47 g/g and 1.05–1.17g/g, respectively. Kabuli chickpea flour (L-550) showed significantly higher OAC (1.24 g/g) and lower WAC (1.33g/g), as compared to desi chickpea flours. This may be attributed to the presence of greater amounts of hydrophobic constituents in kabuli chickpea flour.
Table 3 Physicochemical, functional, thermal, and thermal properties of flours from different chickpea cultivars.
According to Hodge and Osman[Citation24] flours with high water absorption have more hydrophilic constituents like polysaccharides. In DSC analysis, the onset temperature (T o), peak temperature (T p), conclusion temperature (T c), and enthalpies of gelatinization (ΔH gel) for chickpea flours are shown in . T o, T p, T c and ΔH gel for chickpea flours ranged between 65.43–67.99, 70.61–73.26, 77.03–79.45°C, and 3.48–4.92J/g, respectively. PDG-4 (desi cultivar) flour had highest values of T o, T p, T c, and ΔH gel, whereas L-550 (kabuli type) flour showed lowest values for the same. The lowest transition temperatures (T o, T p, T c ) and gelatinization enthalpy values for L-550 flour indicates that lesser energy is needed to break the intermolecular bonds in starch granules of this flour to achieve gelatinization. A positive correlation of T o with T p (r = 0.941), T c(r = 0.858) and negative correlation with cohesiveness (r = −0.806) was observed. T p was positively correlated to T c (r = 0.937) and ΔH gel (r = 0.776) and negatively to gumminess (r = −0.841) and chewiness () of soaked seeds. Solubility of chickpea starches showed negative correlation to ΔH gel of chickpea flours (r = −0.798).
Significant differences were observed in pasting characteristics of flours from different chickpea cultivars. Pasting temperature of flours from different chickpea cultivars ranged between 73.05–75.20°C, with the highest for GPF-2 and lowest for PBG-1 flour were observed. The high pasting temperature of GPF-2 flour indicates the presence of starch in this flour that is highly resistance to swelling and rupturing. Peak viscosity, final and breakdown viscosity of different chickpea flours varied from 1348–2163, 1515–2704, and 71–269 cP, respectively. Peak viscosity was highest for L-550 and lowest for GPF-2 flour. Lowest breakdown was observed in GPF-2 flour, thereby indicating its paste stability. Flour from kabuli chickpea cultivar had a low pasting temperature (73.85°C), the highest peak viscosity (2163), and final viscosity (2704). Pasting temperature showed negative correlation with peak viscosity (r = −0.795), final viscosity (), and bulk density (r = −0.813) of chickpea flours. Peak viscosity of flours showed positive correlation to breakdown (r = 0.954), final viscosity (r = 0.946), bulk density (r = 0.961), and negative correlation to cohesiveness of soaked seeds (). Final viscosity showed positive correlation to bulk density (r = 0.989) and negative to WAI () of chickpea flours. Breakdown viscosity was positively correlated to final viscosity (r = 0.813), bulk density (r = 0.842), and negatively correlated to cohesiveness (r = −0.957) of soaked chickpea seeds.
CONCLUSION
Various physico-chemical, cooking and instrumental textural properties of chickpea seeds were observed to correlate with the functional, gelatinization, and pasting properties of their flours and physicochemical properties of their starches. A positive correlation of WSI of chickpea flours with seed mass, volume, hydration capacity, and hardness value of chickpea seeds was observed. Peak viscosity of flours showed positive correlation to breakdown, final viscosity, bulk density, and negative correlation to cohesiveness of soaked seeds. Final viscosity showed negative correlation to bulk density and WAI of flours. Therefore, selected properties of chickpea seeds might be useful in assessing the properties of their flours and starches.
REFERENCES
- Bressani , R. and Elias , L.G. 1979 . The problems of legume protein digestibility . J. Food Sci , 39 : 661 – 670 .
- Hoover , R. and Sosulski , F. 1991 . Composition, structure, functionality and chemical modification of legume starches: a review . Can. J. Physiol. Pharmacol , 69 : 79 – 92 .
- Food and Agriculture Organization . 1993 . Food and Agriculture Organization Yearbook , Vol. 46 , 105 – 115 . Rome, , Italy : FAO . Production 1992
- Bahl , P.N. 1990 . “ The role of food legumes in the diets of the populations of Mediterranean areas and associated nutritional factors ” . In Role of legumes in the farming systems of the Mediterranean Areas , Edited by: Osman , A.E. , Ibrahim , M.H. and Jones , M.A. 143 – 149 . Dordrecht, , The Netherlands : The Kluwer Academic Publishers .
- Singh , K.B. , Bejiga , G. and Malhotra , R.S. 1993 . Genotype-environment interactions for protein content in chickpea . J. Sci. Food Agric , 63 : 87 – 90 .
- Ereifej , K.I. , Al-Karaki , G.N. and Hammouri , M.K. 2001 . Seed chemical composition of improved chickpea cultivars grown under semiarid Mediterranean conditions . Int. J. Food Prop , 4 ( 2 ) : 239 – 246 .
- Gil , J. , Nadal , S. , Luna , D. , Moreno , M. and De Haro , A. 1996 . Variability of some physico-chemical characters in Desi and Kabuli chickpea types . J. Sci. Food Agric , 71 : 179 – 184 .
- Williams , P.C. , Nakoul , H. and Singh , K.B. 1983 . Relationship between cooking time and some physical characteristics in Chickpea (Cicer arietinum L) . J. Sci. Food Agric , 34 : 492 – 496 .
- Sefa-Dedah , S. and Stanley , D.W. 1979 . Textural implications of the microstructure of legumes . Food Technol , 33 : 77 – 83 .
- Narasimha , H.V. and Desikachar , H.S.R. 1978 . Objective methods for studying cookability of Tur pulse (Cajanus cajan) and factors affecting varietal differences in cooking . J. Food. Sci. Technol , 15 : 47 – 50 .
- El faki , H.A. , Desikachar , H.S.R. , Paramahans , S.V. and Thavanathan , R.N. 1983 . Physicochemical characteristics of starches from chickpea, cowpea and horse gram . Starch , 35 : 118 – 122 .
- Singh , N. , Sandhu , K.S. and Kaur , M. 2004 . Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars . J. Food Engng , 63 ( 4 ) : 441 – 449 .
- Sandhu , K.S. , Singh , N. and Kaur , M. 2004 . Characteristics of the different corn types and their grain fractions: physicochemical, thermal, morphological, and rheological properties of starches . J. Food Engng , 64 ( 1 ) : 119 – 127 .
- Singh , U. 2001 . Functional properties of grain legume flours . J. Food. Sci. Technol , 38 ( 3 ) : 191 – 199 .
- Szczesniak , A.S. 1975 . General foods texture profile revisited-ten years perspective . J. Texture. Studies , 6 : 5 – 8 .
- Williams , P.C. , Kuzina , F.D. and Hlynka , I. 1970 . A rapid colorimetric procedure for estimating the amylose content of starches and flours . Cereal Chem , 47 : 411 – 420 .
- Leach , H.W. , McCowen , L.D. and Schoch , T.J. 1959 . Structure of the starch granule. I. Swelling and solubility patterns of various starches . Cereal Chem , 36 : 535 – 545 .
- Anderson , R.A. , Conway , H.F. , Pfeifer , V.F. and Griffin , E. 1969 . Gelatinization of corn grits by roll and extrusion cooking . Cereal Sci. Today , 14 : 4–7 – 11–12 .
- Sosulski , F.W. 1962 . The centrifuge method for determining flour absorption in hard red spring wheats . Cereal Chem , 39 : 344 – 350 .
- Lin , M.J.Y. , Humbert , E.S. and Sosulski , F.W. 1974 . Certain functional properties of sunflower meal products . J. Food Sci , 39 : 368
- Muller , F.M. 1967 . Cooking quality of pulses . J. Sci. Food. Agric , 18 : 292 – 295 .
- Lineback , D.R. and Ke , C.H. 1975 . Starches and low molecular weight carbohydrates from chickpea and horse gram flours . Cereal Chem , 52 : 334 – 347 .
- Schoch , T.J. and Maywald , E.C. 1968 . Preparation and properties of various legume starches . Cereal Chem , 45 : 564 – 573 .
- Hodge , J.C. and Osman , E.M. 1976 . “ Carbohydrates ” . In Principles of Food Science, Part I, Food Chemistry , Edited by: Fennema , R.O. 97 – 200 . New York : Marcel Dekker Inc. .