Abstract
The effect of degree of gelatinisation on the rheology and rehydration kinetics of instant rice produced by freeze-drying was studied. A Rapid Visco Analyser (RVA) was used to analyse the pasting properties of mixtures of ungelatinized and completely gelatinized dried rice starch at varying ratios in order to develop a standard curve between degree of gelatinization and peak viscosity. The degree of gelatinization of instant rice starch prepared by precooking (gelatinization) for varying times was determined using the calibration curve. It was found that 30 minutes or more of precooking produced completely gelatinized rice. The (RVA) pasting profiles for instant rice precooked for varying times showed that the higher the degree of gelatinization, the lower the viscosity peak, and their decrease was proportional to the degree of gelatinisation. The degree of gelatinisation increased with increase in precooking time. A study of rehydration kinetics showed than an increase in pre-gelatinization time increased the rehydration speed. The kinetics data was analysed using the Peleg's model.
INTRODUCTION
As a staple food, cereals provide more than 70% of energy intake in many parts of rural Africa and Asia.[Citation1] The cereal grains are seeds that contain the embryo of the future plant and food reserve materials for germination purposes. Cereal products include wheat, rice (Oryza sativa), maize (Zea Mays), rye (Secale cereale), barley (Hordeum spp.), oats (Avena sativa), sorghum (Sorghum vulgare) and millet. Rice, as the second major cereal in the human diet after wheat, is consumed in Eastern countries by about 2.7 billion people and some Western.[Citation2] The rice plant originated in Asia and was established in the Chinese diet by 2800 BC, and soon after in India.[Citation1] In Western countries, rice is widely used to manufacture products such as puddings, infant foods, puffed grains and breakfast cereals.[Citation3]
Because of changes in social life style, demographical changes and a trend towards increased convenience, there is an increasing need for quick and easy to prepare foods. Many food and beverage products are now processed beforehand, helping consumers to save time with less preparation. Instant rice, which is easy and quick to prepare, is in increased demanded since it takes a shorter time to cook them conventional rice. As rice serves as a common food type in many parts of the world, instant rice products with similar texture and taste to conventional cooked rice are needed to satisfy the consumer expectations. Instant rice is rice that can be rehydrated in a relatively short period of time, either by the addition of hot water or by the addition of water and microwave heating, after which the rice is ready for consumption.[Citation4] The rehydration characteristics of instant rice produced by freeze drying were studied by Seputro.[Citation5] Although there are many different techniques to make instant rice, the methods generally include first a soaking step, then steaming or boiling in hot water, followed by a second soaking and finally drying. The first soaking step involves soaking of the raw rice in water at ambient temperature in order to allow water absorption prior to the steaming process, so that gelatinisation of the starch can be facilitated.[Citation4] According to Ramesh and Rao,[Citation6] the optimum time for soaking raw rice was 30 minutes, giving a moisture content of 43–45% (dry basis). It was found that soaking for more than 30 minutes does not result in a significant increase in the rice moisture content. In the steaming or boiling stage, the rice starch is completely gelatinized and the moisture content increases to about 300% (dry basis).[Citation4,,Citation6] After this stage, rice is soaked in water for a second time, in order to arrest further gelatinization, and to remove starch on the rice surface, which prevents the stickiness.[Citation6] Dang and Copeland[Citation7] produced images of uncooked and cooked structure of rice using Atomic Force Microscopy. It was shown that a highly disorganized grain structure existed in cooked rice although the ordered layers were evident in uncooked rice. Pitting was observed in images of instant rice, due to starch partial hydrolysis by residual grain enzymes during the heat treatment, and/or the escape of trapped air bubbling through the surface. Some of the amylose and amylopectin leach out of the rice kernels during cooking and this relates to the stickiness of cooked rice.[Citation8,,Citation9] The proportions of total amylose and amylopectin in the starch leached into the cooking water or solubilised in the kernel are both reduced markedly as the steaming pressure is increased.[Citation10] Different varieties of cooked rice have different textures because of the different structure of the long linear chains, as free amylose or bound to the amylopectin in the starch molecule.[Citation11] All of these chains participate in intermolecular interaction, which then provide the structure and rigidity of the starch granules, and hence affect the texture of the cooked rice indirectly.[Citation11]
Drying methods and drying conditions play important roles in achieving the desired quality. Foods produced by freeze drying are characterized by high-quality characteristics, such as low bulk density, high porosity, superior taste and aroma retention, and better rehydration properties compared with products of alternative drying processes such as air, vacuum, microwave and osmotic drying.[Citation12] Freeze drying or lyophilisation is a two-step dehydration process, in which the solvent and/or the medium of suspension is first crystallized at low temperatures and then sublimated from the solid state directly to the vapour phase. The main advantages of freeze drying is preservation of most of the initial properties of the raw material such as shape, taste, colour, flavour, biological, or pharmaceutical activity.[Citation13] Low processing temperature, the absence of liquid water and rapid transition from a fully hydrated to nearly completely dehydrated state minimize the deterioration reactions that occur normally in drying processes, such as non-enzymatic browning, protein denaturation, and enzymatic reactions.[Citation14] Freeze drying also gives porous, non-shrunken dried products as a result of the structural rigidity afforded by frozen water where sublimation occurs; hence it gives a high rehydration capacity to freeze dried products.[Citation13,,Citation14]
The process of gelatinisation is unique to starches and important in many products and is responsible for their desired characteristics. Starch gelatinisation is a two-step process consisting of the initial swelling and its eventual dissolution even though the distinction between these two steps is limited.[Citation15] The swelling of starch granules is known to begin in the bulk, relatively mobile, amorphous fraction, and in the more restrained amorphous regions immediately adjacent to the crystalline zones.[Citation16]
An RVA plots time against viscosity as a sample starch paste is taken through a preset temperature profile. Starch which has been pregelatinised absorbs water more readily, causing the grains to swell and increasing the paste viscosity at low temperatures. A rapid increase in viscosity is observed at the gelatinisation temperature as amylose diffuses from the granules resulting in formation of a gel.[Citation17] This viscosity increase continued up to a peak viscosity, after which the viscosity of the ungelatinised starch decreases due to breakdown of the rice starch granules[Citation18] and of the gel structure, with the system transforming into a mixture of leached amylose molecules, melted amylopectin sites and granule fragments. In addition, there will be some effect on viscosity from melting of the crystalline region remaining in the swollen granule, disentanglement of the amylopectin molecules in the swollen particles, and loss of interaction between particles and the network.
Research on instant rice has not so far been extensive; so it is important to further analyse the properties of instant rice in order to produce a better product. The objective of this work was to study the effect of degree of gelatinization on rheological and rehydration kinetics of instant rice produced by freeze-drying. A Rapid Visco Analyser (RVA) was used to study the effect of degree of gelatinisation on the rheological properties. The rehydration kinetics was performed by soaking instant rice in water in order to determine the relationship between degree of gelatinisation and rehydration performance.
MATERIALS AND METHODS
Rice and Rice Flour
Long grain rough rice was provided by Ricegrowers Cooperative Ltd. (Leeton). White rice was prepared by removing the bran layer from long grain paddy using a Satake test rice miller, and then whole grain was separated from broken rice using a rice separation cylinder. As a control, rice flour was produced by grinding 100 g of white rice using a coffee grinder. The resulting rice flour was passed through a sieve of pore size 250 μm, then placed in a plastic bag, vacuum sealed, and stored in a a dessicator.
Dehydration of Completely Gelatinised Rice
To generate completely gelatinised rice, 200 g of white rice was soaked in one litre of water at room temperature (20°C) for 30 minutes then separated by straining. The soaked rice was cooked in 3.5 litre of water preheated to 100°C for 1 h in a conventional rice cooker, preset at 100°C, and stirred occasionally. The rice was cooled by dipping in 1.7 litre of water at room temperature for 5 min, then separated by strainer and placed into a freeze dryer at -20°C for 5 h.
Freeze drying was conducted for 24 h at a pressure of 60–80 Pa and a temperature of −20°C. Dehydrated gelatinised rice was placed into a plastic bag, vacuum sealed, and stored in a dessicator (silica gel) until required for experiments. Gelatinised rice flour was prepared by grinding the freeze-dried rice using a coffee grinder. The flour was placed in a plastic bag, vacuum sealed, and placed in a dessicator.
Instant Rice Preparation
The procedure for making fully-gelatinised rice was repeated, using 300 g of white rice and taking rice samples at 5 min intervals during cooking.
Moisture Content
The moisture content of all rice sample was determined by oven drying at 105°C for 20 h (AOAC method).
Rapid Visco Analyzer (RVA) Analysis
A Rapid Visco Analyzer (RVA) from Newport Scientific, Sydney was used to measure the viscosity of rice flour. A standard pasting curve was developed for both un-gelatinized and completely gelatinized dried rice flour. The RVA machine was prewarmed for 30 min before use, and samples were checked for homogeneity before starting each test.
Rehydration Kinetics
Instant rice (15 g) was placed into boiling water. Samples of 3–5 g were taken at 30 s intervals over a period of 15 min for determination of moisture content.
RESULTS AND DISCUSSION
To determine the effect of temperature on the viscosity of the rehydrated rice, three temperatures were measured:
-
the onset temperature, at which the viscosity first started to materially increase (Tonset);
-
the peak temperature, corresponding to the maximum rate of change in viscosity (Tpeak); and
-
the final or end temperature at which all of the granules have been completely gelatinized (Tend).
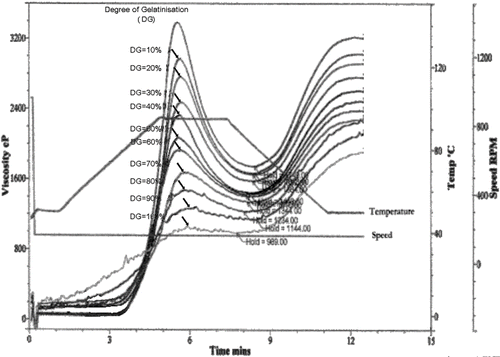
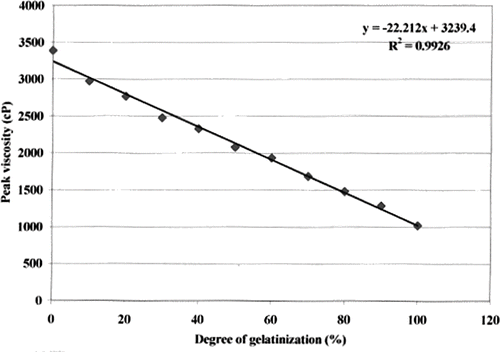
shows a sequence of curves of increasing gelatinisation on a plot of viscosity against time, as generated by the RVA. The peak viscosity was found to be linearly related to the fraction of gelatinisation G (). A higher fraction of gelatinization resulted in a lower peak viscosity. The resulting equation had a correlation coefficient of 0.996:
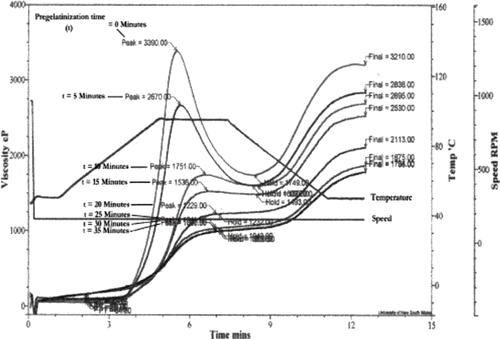
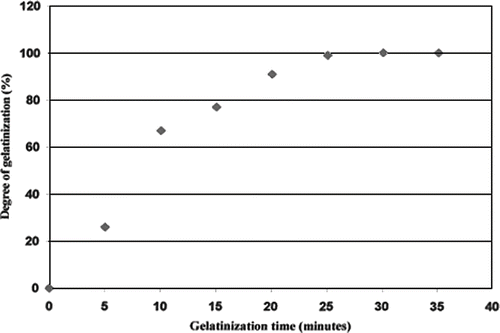
where Vp is the peak viscosity, in Pa.s. shows RVA plots for varying cooking times for the instant cooked rice. A longer precooking time resulted in a lower peak viscosity. Using the standard curve (), the fraction of gelatinisation was calculated, and found to increase exponentially with cooking time up to a maximum at 30 min (), after which longer cooking times had little effect. This finding agrees with the observation by Hsu et al.[Citation19] Similarly Bayram[Citation20] found that 40 min was required for 100% gelatinisation of wheat. Thus it can be concluded that 100% gelatinization is achieved if rice is precooked for 30 min or more prior to freeze drying.
This finding also supports the assumption used for cooking raw rice for 60 min to provide fully gelatinized rice starch, as used to develop the standard curve.
The rate of moisture uptake for instant rice for different degrees of precooking are shown in . Similar curves were observed for fresh rice. The rate of rehydration can be seen to increase with degree of gelatinization and the final moisture content after soaking. This suggests the precooked starch granules swell faster than the ungelatinized rice. This is similar to the phenomenon found in the paste viscosities for varying degress of gelatinisation (), where an increase in precooked rice viscosity was observed before the gelatinisation temperature, showing that unlike ungelatinised starch, precooked starch does not require heat to absorb water. This is in agreement with Lewicki,[Citation21] who observed that amorphous (i.e., gelatinised) regions of the solid part were the most accessible to the rehydration medium. However, they did not present their experimental data.
Figure 5 Effect of degree of precooking on rate of moisture uptake and comparison with Peleg's Model.
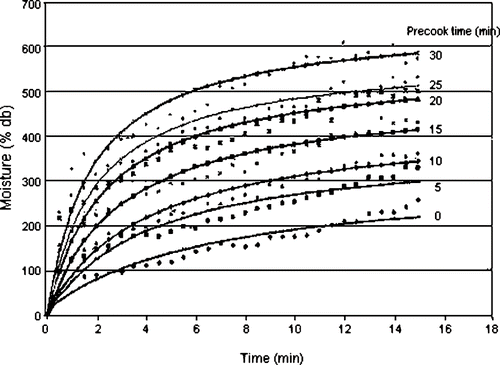
A second reason for faster moisture uptake in rehydration may be due to increased rice swelling caused by a longer precooking time; this swelling being preserved by the freeze-drying process, resulting in a high structural porosity.
The moisture uptake kinetics data were fitted to Peleg's model for rehydration:
where Mo and M are the moisture contents at the initial and subsequent times (dry basis), t is the soaking time (min), and k1 and k2 are model parameters. The equilibrium moisture content is defined as the final moisture at time t=∞:
The equation 5 can be linearised by an algebraic transform:
The model parameters were estimated from the slope and intercept of the linear plot of [t/(M−Mo)] against t and are presented in . The difference between the experimental data and the fitted Peleg model was not significantly different at p = 0.05. This result conforms with the work of Sopade et al.[Citation22] who adequately fitted the rehydration kinetics of amaranth grain using the Peleg model.
Table 1 Peleg's model parameters for the rehydration kinetics of instant rice
CONCLUSION
The degree of gelatinisation of instant rice can be determined using an RVA, based on a calibration curve obtained by proportional mixing of raw and fully gelatinised starch. The best indicator was found to be peak viscosity which decreased with increase in degree of gelatinisation. A 30 min precook of raw rice was sufficient to produce completely gelatinised instant rice. Precooked rice hydrated more quickly than raw rice, the rehydration kinetics being well characterised by Peleg's model.
ACKNOWLEDGMENT
The authors wish to acknowledge the provision of a Rapid Visco Analyser by Newport Scientific Pty Ltd.
REFERENCES
- Southgate , D.A.T. 2000 . “ Cereals and Cereal Products ” . In Human Nutrition and Dietetics , 10th , Edited by: Garrow , J.S. , James , W.P.T. and Ralph , A. London : Churchill Livingstone .
- Wu , D. , Shu , Q. , Wang , Z. and Xia , Y. 2002 . Effect of Gamma Irradiation on Starch Viscosity and Physicochemical Properties of Different Rice . Radiation Physics and Chemistry , 65 : 79 – 86 .
- Wang , H.H. , Sun , D.W. , Zeng , Q. and Lu , Y. 2000 . Effect of pH, Corn Starch and Phosphates on the Pasting Properties of Rice Flour . Journal of Food Engineering , 46 : 133 – 138 .
- Lee , E. and Wissgott , U. Instant Soakable Rice . United States Patent No US2001006696 . Filed January 4, 2001 and issued July 5, 2001.
- Seputro , S.P.B. 2003 . Rehydration and Properties of Instant Rice Produced by Freeze Drying , Sydney : Master Thesis. University of New South Wales .
- Ramesh , M.N. and Rao , S. 1996 . Drying Studies of Cooked Rice in a Vibrofluidised Bed Drier . Journal of Food Engineering , 27 : 389 – 396 .
- Dang , J.M.C. and Copeland , L. 2003 . Imaging Rice Grain Using Atomic Force Microscopy . Journal of Cereal Science , 37 : 165 – 170 .
- Priestley , R.J. 1977 . Studies on Parboiled Rice—Part 1: Comparison of the Characteristics of Raw and Parboiled Rice . Food Chemistry , 1 : 5 – 14 .
- Priestley , R. J. 1977 . Studies on Parboiled Rice—Part 2: Solubilization of Starch Fractions in Rice Varieties during Cooking. Developments in Food Carbohydrates . Food Chemistry , 1 : 141 – 151 .
- Priestley , R.J. 1977 . Studies on Parboiled Rice—Part 3: Characteristic of Parboiled Rice on Recooking . Food Chemistry , 2 : 43 – 50 .
- Ramesh , M. , Ali , S.Z. and Bhattacharya , K.R. 1999 . Structure of Rice and Its Relation to Cooked Rice Texture . Carbohydrate Polymers , 38 : 337 – 347 .
- Krokida , M.K. , Karathanos , V.T. and Maroulis , Z.B. 1998 . Effect of Freeze Drying Conditions on Shrinkage and Porosity of Dehydrated Agricultural Products . Journal of Food Engineering , 35 : 369 – 380 .
- Genin , N. , Rene , F. and Corrieu , G. 1996 . A Method for On-line Determination of Residual Water Content and Sublimation End-point during Freeze Drying . Chemical Engineering and Processing , 35 : 255 – 263 .
- Liapis , A.I. and Bruttini , R. 1995 . “ Freeze drying ” . In Handbook of Industrial Drying , Edited by: Mujumdar , A.S. New York : Marcel Dekker .
- Kokini , J.L. , Lai , L.S. and Chadid , L.L. 1992 . Effect of Starch Structure on Starch Rheological Properties . Food Technology , 46 : 124 – 139 .
- Hoover , R. and Vasanthan , T. 1994 . Effect of Heat-moisture Treatment on the Structure and Physicochemical Properties of Cereal, Legume, and Tuber Starches . Carbohydrate Research , 252 : 33 – 53 .
- Sopade , P.A. , Ajisegiri , E.S. and Badau , M.H. 1992 . The Use of Peleg Equation to Model Water Absorption in Some Cereal Grains during Soaking . Journal of Food Engineering , 15 : 269 – 283 .
- Han , X.Z. and Hamaker , B.R. 2001 . Amylopectin Fine Structure and Rice Starches Paste Breakdown . Journal of Cereal Science , 34 : 279 – 384 .
- Hsu , K.H. 1983 . A diffusion model with concentration dependent diffusion coefficient for describing water movement in legumes during soaking . Journal of Food Science , 48 : 618 – 622 .
- Bayram , M. , Öner , M.D. and Eren , S. 2004 . Effect of the Cooking Time and Temperature on the Dimensions and Crease of the Wheat Kernel during the Bulgur Production . Journal of Food Engineering , 64 : 43 – 51 .
- Lewicki , P.P. 1998 . Effect of Predrying Treatment, Drying and Rehydration in Plant Tissue—A Review . International Journal of Food Properties , 1 : 1 – 22 .
- Sopade , P.A. , Halley , P.J. and Junming , L.L. 2004 . Gelatinization of Starch in Mixtures of Sugars. I. Dynamic Rheological Properties and Behaviours of Starch-honey Systems . Journal of Food Engineering , 61 : 439 – 448 .