Abstract
This study was conducted to isolate and identify the antioxidative compound from fruit extracts of Mengkudu (Morinda citrifolia L.). The extract was fractionated on a Sephadex LH-20 column chromatography using ethanol as eluate. Six major fractions were isolated according to UV absorption intensity of phenolic contents at 725 nm. Antioxidative activity of these fractions was evaluated in a ferric thiocyanate method (FTC) and thiobarbituric acid test (TBA). The antioxidative activities were then compared to that of α-tocopherol (natural antioxidant) and butylated hydroxytoulene or BHT (synthetic antioxidant). Results showed that all isolated fractions demonstrated high antioxidative activity compared to either BHT or α-tocopherol. Further separation of these fractions on a high-performance liquid chromatography (HPLC) procedure based on water-methanol gradient with trifluoreacetic acid (TFA) for simultaneous analysis of flavonoids, indicated that they contain several active compounds. The major flavonoids that have been detected in M. citrifolia are catechin and epicatechin.
INTRODUCTION
Traditional cultures have been using the fruit, bark, leaves and roots of noni (Morinda citrifolia L.) for a very long time. It is said that Polynesian Islanders first cultivated and domesticated the noni tree over 2000 years ago for food and medical purposes. The plant has been reported to have broad effects including antimicrobial,[Citation1] anti-cancer activity,[Citation2] antioxidant properties,[Citation3] anti-inflammatory activity,[Citation4] analgesic activity,[Citation5] and cardiovascular activity.[Citation6]
About 160 phytochemical compounds have been identified in the noni plant, and the major micronutrients are phenolic compounds, organic acids, and alkaloids.[Citation7] The phenolic compounds were reported to be the major groups of functional micronutrient in noni. They include damnacanthal, scopoletin, morindone, alizarin, aucubin, nordamnacanthal, rubiadin, rubiadin-1-methyl ether, and other anthraquinone glycosides.[Citation8–10]
The presence of phenolic compounds in noni plant may have significant effect on their total antioxidant activity. Recent report by Su et al.[Citation10] revealed that neolignan and americanin A[Citation3] from n-BuOH-soluble partition part of MeOH extract of noni fruits was found to have significant antioxidant activity in two antioxidant bioassays. Similarly, Mohd et al.,[Citation3] reported that antioxidant properties of noni fruit extract exhibited strong inhibition of lipid oxidation. In other report by Wang and Su,[Citation7] found that the superoxide anion radicals (SAR) scavenging activity of noni juice was shown to be 2.8 times higher than that of vitamin C, 1.4 times of that pycnogenol (PYC), and almost of the same order as that of grape seed powder.
The Morinda citrifolia plant especially its fruit has been the object of many claims concerning its nutritional and functional properties. These beneficial effects may result from some compounds, in particular, scopoletin, nitric oxide, alkaloids, flavonoids, and sterols that attributed to the antioxidant potential of noni.[Citation11] As a result, consumption of this fruit is currently high. In Malaysia, the commercial interest in noni's fruit is tremendous and some local companies has started commercializing it as healthy juice as well as dietary supplements.
In this article, we describe the isolation and identification of antioxidative compounds from fruit of Mengkudu (Morinda citrifolia L.), as these may provide considerable contribution to ascertain the individual potency of the compounds, which have the potential of being incorporated into foods as functional ingredients.
MATERIALS AND METHODS
Sample Preparation
Plant materials used in this study includes fresh Mengkudu (M. citrifolia) fruit (seedless without core) obtained from Traditional Medicine Plant Plot, Universiti Putra Malaysia, Serdang, Selangor, Malaysia. The samples were washed with running tap water before being chopped into pieces. They were then oven dried at 45°C for 2 days and ground to powder.
Isolation and Identification of the Antioxidative Compound
M. citrifolia fruit was extracted according to the modified method of Chang, et al.[Citation12] The crude fruit extract was fractionated using Sephadex LH-20 column 1.5 cm diameter and 83 cm height, particle size 25–100 μm (Pharmacia, Uppsala, Sweden). Eluates collected (5 ml/tube) were measured at 725 nm after colour development for phenols,[Citation13] were then pooled into 6 fractions and solvent removed with rotary evaporator. Content of total phenolic compounds in each fraction was estimated using Folin-Ciocalteu reagent. TPCs (total phenolic compounds) were determined according to the method of Shahidi and Naczk.[Citation14]
Determination of Antioxidative Activity
The resulting fruit fractions after Sephadex LH-20 column chromatography were quantified by RP-HPLC on a Symmetry C18 column 150 × 3.9 mm, 5 μm, (Waters Milford, MA, USA). Pure flavonoids standards (flavanols i.e., (+)-catechin, (−)- epicatechin; flavonols i.e., quercetin, myricetin, kaempferol and rutin; flavones i.e., apigenin and luteolin; flavanones i.e., naringin), Na DEDTC and trifluoroacetic acids were obtained from Sigma Chemical Co. (St. Louis, MO, USA), methanol (HPLC grade) were purchased from Merck (Germany), hydrochloric acid was purchased from Malinckrodt Baker, Inc. (Kentucky, USA). Determination of antioxidative activity of the fractions was determined using FTC method adapted from Osawa and Namiki.[Citation13] The Thiobarbituric acid test (TBA) was conducted according to the method of Ottolenghi[Citation15] and Kikuzaki and Nakatani.[Citation16]
Statistical Analysis
All experiments were conducted in triplicates and statistical analysis was done according to the SAS[Citation17] User's Guide. Analysis of Variance was performed by ANOVA procedure. Duncan's multiple range tests were used to determine significant differences between the means.
RESULTS AND DISCUSSION
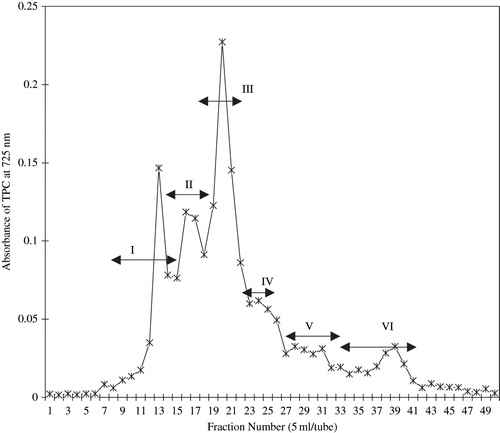
In this study, six peaks were clearly defined based on UV absorption intensity of phenolic compounds (absorbance at 725 nm) of fruit extract on Sephadex LH-20 column chromatography. Based on this data, samples were pooled into six major groups (I, II, III, IV, V, and VI) as presented in . Amarowicz et al.,[Citation18] and Wanasundara et al.,[Citation19] have reported that the Sephadex LH-20 provides an efficient medium for fractionation of plant phenolic especially in canola extract.
Phenolic substances that are known to possess high antioxidative activity are common phytochemicals in fruits and leafy vegetables. Most of them were classified in the two principal groups of phenol carboxylic acids and flavonoids, the latter being the most significant,[Citation20] and derivatives of flavan (2-phenyl-benzodihydropyran). The main subgroups were the colourless catechins, the red to blue-coloured anthocyanidins, the light-yellow flavonols and flavones, and the colourless proanthocyanidins.[Citation21] In this study, the content of phenolics, as (+)-catechin equivalents, in each of six fractions of fruit extract are given in . Result shows that the level of phenolic compounds in different fractions of fruit extract was not significantly (p < 0.05) different from each other except in Fraction IV. According to Pratt and Hudson,[Citation22] phenolic compounds were found abundantly in all parts of the plant, such as wood, bark, stems, leaves, fruit, root, flowers, pollen, and seeds.
Table 1 Total phenolic content of fruit extractFootnote a of Morinda citrifolia.
The oxidative deterioration of lipid-containing foods is responsible for the rancid odour and flavour during processing and storage, consequently decreasing the nutritional quality and safety of foods, due to the formation of secondary, potentially toxic compounds. The addition of antioxidant is a method of increasing the shelf life of foods. Antioxidative activity of phenolic compounds is based on their ability to donate hydrogen atom to free radicals. Many phenolic compounds, particularly flavonoids, exhibited a wide range of biological effect, including antibacterial, antiviral, and anti-inflammatory, anti-allergic, anti-thrombotic, and vasodilatory actions.[Citation23] Studies have also shown that some of these compounds were known as potent scavengers of free radicals and as such are potentially useful in the prevention of arteriosclerosis, cancer, diabetes, neurodegenerative diseases, arthritis, and others. Protective effects of diets high in fruits and vegetables have been attributed to the presence of these compounds.
and show the antioxidative activities of the six isolated fractions from fruit extract of M. citrifolia as measured by FTC and TBA method, respectively. The activity in decreasing order was BHT, I, α-tocopherol, V, IV, VI, III, and II. Fraction I displayed the strongest antioxidative activity, with activity that was not significantly (p < 0.05) different to that of BHT or α-tocopherol. This was true for both assay tested. Wang et al.,[Citation24] and Hertog et al.,[Citation25] reported that the antioxidant property of some vegetables and fruits were partly due to low molecular weight phenolic compounds, particularly the flavonoids, which were known to be potent antioxidant. Two known glycosides (rutin and asperulosidic acid) and a novel trisaccharide fatty acid ester [2,6-di-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose] were isolated from the n-butanol-soluble fraction obtained from the ethanol extracts of the fruit of M. citrifolia.[Citation24]
Figure 2 Antioxidative activity of Sephadex LH 20 column chromatographic fractions obtained from fruit extracts of M. citrifolia as measured by FTC method. Absorbance values represent triplicates of different samples analysed. Values with same letter (abc) are not significantly different (p < 0.05), between samples.
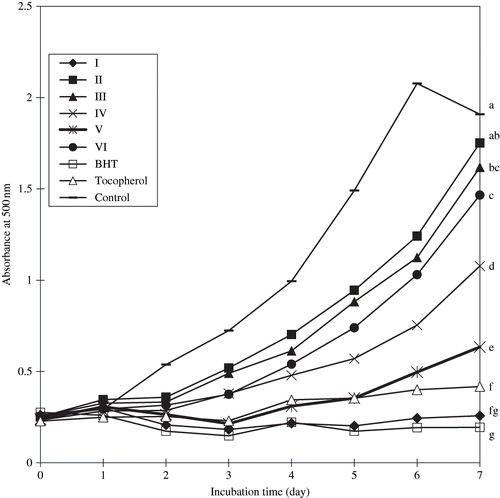
Figure 3 Antioxidative activity of Sephadex LH 20 column chromatographic fractions obtained from fruit extracts of M. citrifolia as measured by TBA method. Absorbance values represent triplicates of different samples analysed. Values with same letter (abc) are not significantly different (p < 0.05), between samples.
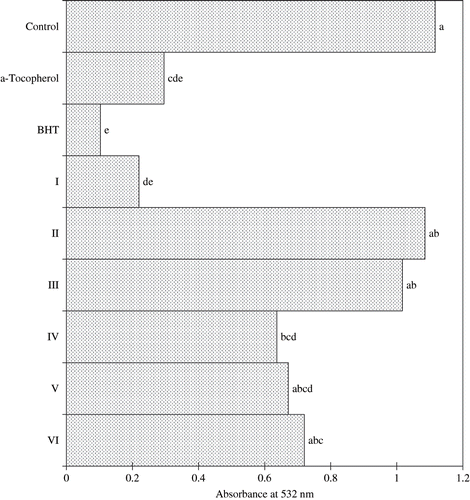
Interestingly, Fraction I, exhibited the highest antioxidative activity, although it contained only 2.21 mg/100 g of phenolic compounds () which was comparatively lower than that found in either fraction V or VI. This is probably due to the more potent antioxidant present in fraction I compared to that of fraction V or VI. The result also showed a lack of correlation between antioxidative activity and their content of phenolic compounds. According to Shahidi and Naczk,[Citation14] Folin-Ciocalteu method measured other constituents than phenolic, and its specificity is poor. The Folin-Ciocalteu reagent detected all phenolic groups found in the extract, including those found in the extractable proteins. These results suggested that factors other than total phenolic compounds may be playing a role in the antioxidant activity in the fruit fraction of M. citrifolia, and that their molecular structures play an important role in their antioxidant activity by developing antagonistic or synergistic effects in combination with themselves or with other constituents of the extracts.[Citation26–29] M. citrifolia fruits were also a good source of potassium and vitamin C. The high levels of potassium indicated the usefulness of the fruit as a survival food.[Citation26–29]
The presence of terpenoids in the fruits has also been reported suggesting that it may account for the biological activity of the fruit.[Citation26–29] The chemical composition of the fruit varied depending upon its stage of development. The green fruits contained high amounts of the medium sized fatty acids, palmitic and octadecanoic acids, while the ripe ones contained larger quantities of the short chain fatty acids, octanoic and hexanoic acids, and the rotten fruits contained greater levels of esterified octanoic and hexanoic acids, possibly from ethanolic fermentation.[Citation30–31]
Gardner et al.,[Citation32] suggested that phenolic compounds are the major contributor to the antioxidative activity of apple, pineapple, and vegetable juice. According to Rice-Evans et al.,[Citation33] phenolic compounds are found to be an effective hydrogen donor, which makes them good antioxidants. Even though the phenolic compound was reported to be highly responsible for the antioxidative activity in herbs, possible synergism of phenolic compounds with one another or with other components present in the extract may be responsible for observation in this study. On the basis of these results, all fractions were used for the identification of the antioxidants compounds.
The chromatograms of six fractions of fruit of M. citrifolia measured at 370 nm are given in . The flavonoids content of fraction I, II, III, IV, V, and VI of fruit of M. citrifolia is shown in . It is interesting to note that total catechin was the major flavonoid found in all the fractions collected. According to Balentine et al.,[Citation34] catechins were structurally, primarily, flavonols, and form 20–30% of dry weight of green tea. Major catechins found in fresh tea and green tea are (−) gallate (EGCG), (−) epigallocatechin (EGC), (−) epicatechin gallate (ECG), and (−) epicatechin (EC). In this study, results show that Fraction VI was found to contain the highest amount of total catechin (1111.2 mg/100 g), which was significantly (p < 0.05) higher than any other fractions. This result correlated well with the highest content of total phenolic found in Fraction VI of fruit extract of M. citrifolia ().
Figure 4 Chromatogram of fraction I, II, III, IV, V, VI obtained from fruit extracts of M. citrifola monitored at 370 nm. The chromatographic conditions are described in text.
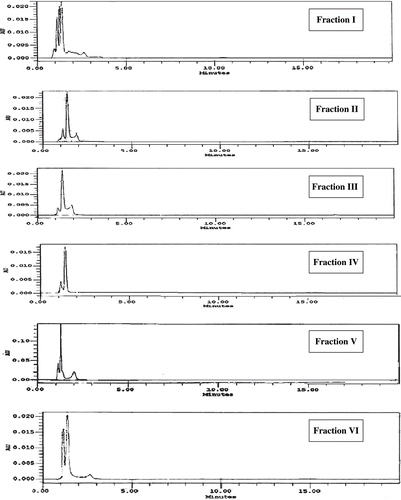
Figure 5 Flavonoid content of Sephadex LH 20 column chromatographic fractions obtained from fruit extracts of M. citrifolia. Values with same letter (ABC) are not significantly different at (p < 0.05).
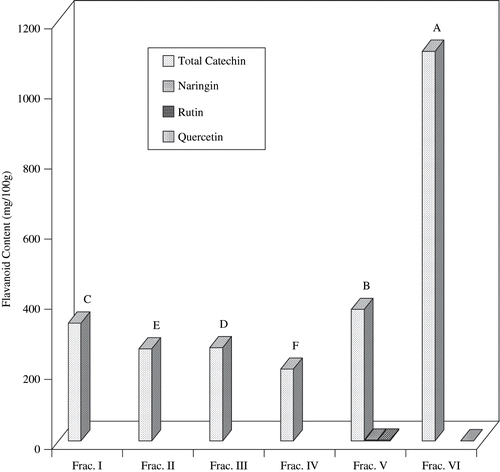
It is intriguing that Fraction VI exhibited lower antioxidative activities compared to other fractions tested, even though it contained the highest amount of catechin. Interestingly, Fraction I, exhibited the highest antioxidative activity, although it contained lower amount of catechin, as compared to that found in fraction VI. This is probably due to the more potent antioxidant present in fraction I compared to that of fraction VI. On the other hand, other flavonoids (naringin, rutin, and quercetin) are found only in trace amounts. Balentine et al.[Citation34] also reported that all green tea catechin were not equally active. Galloyl esters of catechins were more active than non-galloylated catechins because they had lower redox potentials. Some catechins, for example epicatechin, were found to be less effective against particular cancer cell lines, although they did show synergistic effects with other catechins and caffeine.[Citation35]
In vitro and in vivo experiments have shown that catechins were potentially beneficial to human health: they were potent antioxidants, anticarcinogens, antiinflammatory agents, and inhibitors of platelet aggregation.[Citation35] Scott et al.,[Citation36] stated that (+)-catechin of green tea leaves contributed to its antioxidative activity. According to Meyer et al.,[Citation37] catechins showed high activity in inhibiting copper-catalyzed oxidation of human LDL in vitro. Thus, catechins may be responsible for the therapeutic activities, such as antibacterial, anti-aging, antiarthritic, antituberculotic and antitumor in M. citrifolia.
CONCLUSIONS
It is encouraging to see that all the fractions studied demonstrated high antioxidative activity compared to either BHT or α-tocopherol. The potency of some of these compounds could provide scientific basis for the health benefits claims regarding M. citrifolia fruits in folk medicine and warrant further studies to assess their potential as effective natural remedies. The major flavonoids that have been detected in M. citrifolia fruits were catechin and epicatechin and were expressed as total catechin. Total catechins occurred at different concentrations in different fractions of the plant's fruit. Thus, fruit of M. citrifolia has great potential as an excellent source of catechin and should be studied more thoroughly to distinguish the exact catechins that were present. The information can be used as a guide to formulate a product from these species and also to serve as a reference point for future research. The present study also suggested that consumption of fruit of M. citrifolia might have potential health effects. In conclusion, these results further support the use of this fruit by the indigenous population as a traditional medicine. However, further investigation on toxicity needs to be carried out in future before it can be recommended as a natural source of antioxidants.
ACKNOWLEDGMENT
The authors would like to thank The Ministry of Science, Technology and Innovation of Malaysia for financing the project and the laboratory facilities for Universiti Putra Malaysia.
REFERENCES
- Saludes , J.P. , Garson , M.J. , Franzblau , S.G. and Aguinaldo , A.M. 2002 . Antitubercular Constituents from the Hexane Fraction of Morinda Citrifolia L. (Rubiaceae) . Phytotherapic Research , 16 : 683 – 685 .
- Hirazumi , A. , Furusawa , E. , Chou , S.C. and Hokama , Y. 1996 . Immunomodulation Contributes to the Anticancer Activity of Morinda Citrifolia (Noni) Fruit Juice . Proc. West. Pharmacol. Soc. , 39 : 7 – 9 .
- Mohd , Z. , Abdul-Hamid , A. and Osman , A. 2001 . Antioxidative Activity Extracts from Mengkudu (Morinda citrifolia L.) Root, Fruit and Leaf . Food Chem. , 78 : 227 – 231 .
- McKoy , M.L.G. , Thomas , E.A. and Simon , O.R. 2002 . Preliminary Investigation of the Anti-inflammatory Properties of An Aqueous Extract from Morinda Citrifolia (Noni) . Pharmacological Society , 45 : 76 – 78 .
- Wang , M.Y. , West , B. , Jensen , C.J. , Nowicki , D. , Su , C. , Palu , A.K. and Anderson , G. 2002 . Morinda Citrifolia (Noni): A Literature Review and Recent Advances in Noni Research . Acta Pharmacol. Sin. , 23 : 1127 – 1141 .
- Kamiya , K. , Tanaka , Y. , Endang , H. , Umar , M. and Satake , T. 2004 . Chemical Constituents of Morinda Citrifolia Fruits Inhibit Copperinduced Low-Density Lipoprotein Oxidation . J. Agric. Food Chem. , 52 : 5843 – 5848 .
- Wang , M.Y. and Su , C. 2001 . Cancer Preventive Effect of Morinda Citrifolia (Noni) . Ann. N. Y. Acad. Sci. , 952 : 161 – 168 .
- Morton , J.F. 1992 . The Ocean-going Noni, or Indian Mulberry (Morinda Citrifolia, Rubiaceae) and Some of Its “Colourful” Relatives . Ecological Botony , 46 : 241 – 256 .
- Dixon , A.R. , McMillen , H. and Etkin , N.L. 1999 . Ferment This: The Transformation of Noni, a Traditional Polynesian Medicine (Morinda Citrifolia, Rubiaceae) . Ecological Botony , 53 : 51 – 68 .
- Su , B. , Pawlus , A.D. , Jung , H. , Keller , W.J. , McLaughlin , J.L. and Kinghorn , A.D. 2005 . Chemical Constituents of the Fruits of Morinda Citrifolia(Noni) and Their Antioxidant Activity . J. Nat. Prod. , 68 : 592 – 595 .
- Chan-Blanco , Y. , Vaillan , F. , Perez , A.M. , Reynes , M. , Brillouet , J. and Brat , P. 2006 . The Noni Fruit (Morinda citrifolia L.): A Review of Agricultural Research, Nutritional and Therapeutic Properties . J. Food Comp. Anal. , 19 : 645 – 654 .
- Chang , S.S. , Ostric-Matijaseric , B. , Hsieh , O.A.L. and Huang , C.L. 1977 . Natural Antioxidant from Rosemary and Sage . J. Food Sci. , 42 : 1102 – 1106 .
- Osawa , T. and Namiki , M.A. 1981 . Novel Type of Antioxidant Isolated from Leaf Wax of Eucalyptus Leaves . J. Agric. Food Chem. , 45 : 735 – 739 .
- Shahidi , F. and Naczk , M. 1995 . Food Phenolics Sources, Chemistry, Effects, and Applications , Lancaster, Basel : Technomic Publishing .
- Ottolenghi , A. 1959 . Interaction of Ascorbic Acid and Mitochondrial Lipids . Arch. Biochem. Biophys. , 79 : 355 – 358 .
- Kikuzaki , H. and Nakatani , N. 1993 . Antioxidant Effects of Some Ginger Constituents . J. Food Sci. , 58 : 1407 – 1410 .
- SAS Institute . 1990 . SAS/STAT User's Guide , 6 , New York : The Institute .
- Amarowicz , R. , Kozlowska , H. , Shimoyamada , M. and Okubo , K. 1992 . Chomatographic Analysis of Rapeseed Glucoside Fractions . Polish Journal of Food and Nutrition Sciences , 42 : 89 – 93 .
- Wanasundara , U.N. , Amarowicz , R. and Shahidi , F. 1996 . Partial Characterization of Natural Antioxidants in Canola Meal . Food Res. Int. , 28 : 525 – 530 .
- Bitsch , R. 1996 . Pflanzenphenole und Ihre Gesundheitliche Wirkung . Naturw, Rdsch , 49 : 47 – 51 .
- Herrmann , K. 1994 . In Pflanzlichen Lebensmitteln Vorkommende Flavonoide als Antioxidantien . Gordian , 93 : 108 – 111 .
- Pratt , D.E. and Hudson , B.J.F. 1992 . “ Natural Antioxidants Not Exploited Commercially ” . In Food Antioxidants , Edited by: Hudson , B.J.F. 171 – 192 . London : Elsevier Applied Science .
- Cook , N.C. and Samman , S. 1996 . Flavonoids—Chemistry, Metabolism, Cardioprotective Effect and Dietary Sources . J. Nutr. Biochem. , 7 : 66 – 76 .
- Wang , H. , Cao , G. and Prior , R.L. 1996 . Total Antioxidant Capacity of Fruits . J. Agric. Food Chem. , 44 : 701 – 705 .
- Hertog , M.G.L. , Hollman , P.C.H. and Vennema , D.P. 1992 . Optimization of the Quantitative HPLC Determination of Potentially Anticarcinogenic Flavonoidsin Vegetables and Fruits . J. Agric. Food Chem. , 40 : 1591 – 1598 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Stucture-antioxidant Relationships of Flavonoids and Phenolic Acids . Free Radic. Biol. Med. , 20 : 933 – 956 .
- Moran , J.F. , Klucas , R.V. , Grayer , R.J. , Abian , J. and Becana , M. 1997 . Complexes of Iron with Phenolic Compounds from Soybean Nodules and Other Legume Tissues: Prooxidant and Antioxidant Properties . Free Radic. Biol. Med. , 22 : 861 – 870 .
- Lien , E.J. , Ren , S. , Bui , H.H. and Wang , R. 1999 . Quantitative Structure-activity Relationship Analysis of Phenolic Antioxidants . Free Radic. Biol. Med. , 26 : 285 – 294 .
- Peerzada , N. , Renaud , S. and Ryan , P. 1990 . Vitamin C and Elemental Composition of Some Bushfruits . J. Plant Nutr. , 13 : 787 – 793 .
- Legal , L. , David , J.R. and Jallon , J.M. 1994 . Molecular Basis of Morinda Citrifolia (L): Toxicity on Drosophila . J. Chem. Ecol. , 20 : 1931 – 1943 .
- Farine , J.P. , Legal , L. , Moreteau , B. and Le Quere , J.L. 1996 . Volatile Components of Ripe Fruits of Morinda Citrifolia and Their Effects on Drosphila . Phytochemistry , 41 : 433 – 438 .
- Gardner , P.T. , White , T.A.C. , McPhail , D.B. and Duthie , G.G. 2000 . The Relative Contributions of Vitamin C, Carotenoids and Phenolics to the Antioxidant Potential of Fruit Juices . Food Chem. , 68 : 471 – 474 .
- Rice-Evans , C.A. , Miller , N.J. , Bolwell , P.G. , Bramley , P.M. and Pridham , J.B. 1995 . The Relative Antioxidant Activities of Plant-derived Polyphenolic Flavonoids . Free Radical Res , 22 : 375 – 383 .
- Balentine , D.A. , Wiseman , S.A. and Bouwens , L.C.M. 1997 . The Chemistry of Tea Flavonoids . Crit. Rev. Food Sci. Nutr. , 37 : 693 – 740 .
- Kohlmeier , L. , Waterings , K.G.C. , Steck , S. and Kok , F.J. 1997 . Tea and Cancer Prevention: an Evaluation of the Epidemiologic Literature . Nutr. Cancer , 27 : 1 – 13 .
- Scott , B.C. , Butler , J. , Halliwell , B. and Aruoma , O.I. 1993 . Evaluation of the Antioxidant Actions of Ferulic Acid and Catechins . Free Radic. Biol. Med. , 19 : 241 – 253 .
- Meyer , A.S. , Heinonen , M. and Frankel , E.N. 1998 . Antioxidant Interactions of Catechin, Cyaniding, Caffeic Acid, Quercetin and Ellagic Acid on Human LDL Oxidation . Food Chem , 67 : 71 – 75 .