Abstract
Traditionally, the medical properties of garlic were recognized as early as 3000 BC. The functional benefits of garlic are its antimicrobial activity, anticancer activity, antioxidant activity, ability to reduce cardiovascular diseases, improving immune functions, and anti-diabetic activity. Recent studies identify the active functional components providing the medicinal benefits, as well as their mechanisms of action including the best possible ways to consume garlic. Allicin (diallyl-thiosulfinate) is one of the major organosulfur compounds in garlic considered to be biologically active. In this article, I review the chemistry of allicin and its stability during processing and storage, in-vivo and in-vitro functionality of allicin, and other functional components. In addition, I explore other potential alternative approaches of making its derivatives and their use for health benefits.
INTRODUCTION
Garlic (Allium sativum L.), like other plants, has an exquisite defense system, composed of as many different components as the human immune system. In order to protect itself from insects and fungi, garlic produces allicin by enzymatic reaction when it is injured. Thus, allicin is mother-nature's insecticide. Since ancient times, garlic has been used worldwide, not only as a food, but also as a medicine. As early as 3000 B.C., in ancient civilizations, including Egyptian, Phoenicians, Greek, Indian, Roman, Babylonian, Viking, and Chinese, garlic was used for treatment of heart conditions, arthritis, pulmonary complaints, abdominal growths (particularly uterine), respiratory infections, skin disease, symptoms of aging, diarrhea, headache, bites, worms, wounds, ulcers, and tumors.[Citation1,Citation2,Citation3] The ancient Chinese consumed garlic to achieve longevity. In the days of the Pharaohns, during the building of Great Pyramid of Cheops at Gizeh, when the supplies of garlic ran out the work force withdrew their labor. They knew well that garlic gave them strength and stamina. In the first century AD, Dioscorides, the chief physician of Roman army, prescribed garlic to his warriors and wrote: garlic cleans the arteries.[Citation4] The use of garlic to treat wounds surfaced repeatedly through the middle ages into World War II, when garlic was used to treat the wounds of soldiers.[Citation5]
Recently, other medical effects of garlic and certain constituents of garlic or other transformed products have been studied. The biological responses include reduction of risk factors for cardiovascular diseases and cancer, a stimulation of immune function, enhanced foreign compound detoxification, radio protection, restoration of physical strength, cholesterol-lowering, resistance to various stresses and potential anti-aging effects.[Citation6] Garlic consumption is correlated to reduced cancer risk, and its extracts and components effectively blocked experimentally induced tumors in a variety of sites including skin, breast, uterine cervix and colon, suggesting a general mechanism of action.[Citation7,Citation8,Citation9] Commercially available garlic supplements fall into 4 categories: dehydrated garlic powder, garlic oil, garlic oil macerate, and aged garlic extract.[Citation6] Aqueous garlic extract might exert its chemo-preventive effect by inducing apoptosis,[Citation10] cytotoxic to HeLa, a human cervical cancer cell line.[Citation11]
There is no standard available for intake of garlic.[Citation6] The 1988 German Kommission E monograph[Citation6] proposed that daily intake of ∼1–2 cloves garlic or ∼4 g of intact garlic may have health benefits. However, this recommendation was not substantiated with a scientific reference. Amagase, Petesch, Matsuura, Kasuga, and Itakura[Citation6] mentioned that in many recent clinical studies, the daily dose of dehydrated garlic powder has been ∼900 mg. Aged garlic extract intakes ranging from 1 to 7.2 g/day have been used with success. Studies showing immune enhancement in human showed as little as 1.8 g to as much as 10 g/day of age to be effective.[Citation12,Citation13,Citation14] Interestingly, no severe toxic side effects were reported in these clinical studies, even at high dosages.
Many organosulfur compounds, the major active principles in garlic, inhibited the proliferation of cancer cells, and some of them induced apoptosis in tumor cells of different tissue origin.[Citation15,Citation16,Citation17,Citation18,Citation19] Allicin [S-(2-propenyl) 2-propene-1-sulfinothioate] is one of the most widely known organosulfur compounds derived from garlic. The objectives of this review is to present the present knowledge on the chemistry of allicin and other organosulfer compounds and their stability during processing and storage, in-vivo and in-vitro functionality, and other potential alternative approaches of making its derivatives.
CHEMISTRY OF ALLICIN AND ITS STABILITY
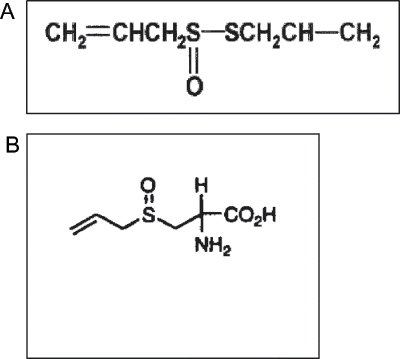
The pioneering studies of Semmler[Citation20] established the importance of diallyl disulfide and diallyl trisulfide in the flavor of garlic distillates. Allicin (diallyl-thiosulfinate) is the most biologically active compound of garlic. Allicin was discovered in 1944 by Cavallito and Bailey[Citation21] and then Cavallito et al.[Citation22] first noted its potent antimicrobial activity. The structure of allicin is shown in . Allicin is not present in raw garlic, but it is rapidly produced by the action of CS-lyase (allinase) on alliin. The structure of alliin is shown in . Allinase is activated by crushing or cutting the garlic cloves.[Citation1,Citation21,Citation22,Citation23] Allicin represents about 70% of the overall thiosulfinates present in the cloves upon mechanical crushing.[Citation24,Citation25,Citation26] Mechanistic and pharmacokinetic studies of allicin and its derivatives raise the need for a labeled compound. Labeling of this volatile and unstable liquid requires delicate handling. Miron et al.[Citation28] described a simple method for the preparation of 3H-labeled allicin.
The main sulfur compound in both raw garlic and garlic powder is alliin. On average, garlic cloves contain ∼8 g/kg alliin. A pure dehydration process, with no loss of ingredients, would result in 20–25 mg/g alliin content in the powder. However, garlic powders contain only 10 g/kg alliin at most, indicating that most of alliin is lost during dehydration. Crushed raw garlic is high in allicin, containing ∼37 mg/g.[Citation28] Diallyl thiosulfinate (allicin) was found to be a major constituent of solvent extracted garlic. Diallyl thiosulfinate undergoes dehydration, leading to the formation of 2 isomeric disulfides by a transformed rearrangement. After 24 hours, sulfur dioxide, diallyl mono-, di- and trisulfides were the major products of this reaction.[Citation29] Allyl and methyl sulfides are main components of commercial garlic oils. Allicin is more or less rapidly degraded into diallyl disulfide, vinyldithiins, and ajoenes, depending of the conditions: concentration, temperature and pH.[Citation30] Allicin, when exposed in heated aqueous solution, forms a lipid-soluble oligosulfide known as diallyl disulfide.[Citation31] Allyl mercaptan is an odorous compound that is the main component of garlic breath after eating garlic cloves. Allyl mercaptan is quantitatively formed from allicin or diallyl disulfide with cysteine via the intermediate compound S-allylmercaptocysteine when it comes in contact with whole blood.[Citation32] Allyl mercaptan, or a further metabolite of it may be the major means of the pharmacological (nontopical, nonenteral) action of allicin or diallyl disulfide.[Citation32]
Twenty-four botanical traits of Iranian garlic ecotypes were studied for its allicin content measured by HPLC method.[Citation33] Allicin content ranged from 0.16–13.0 mg/g. There was no significant correlation between the ecological condition and the allicin content. Cluster analysis on morphological and phytomedical characters arranged the ecotypes in six main groups. The ecotype of group A showed the highest botanical and pharmaceutical potential which could be considered in future breeding programs. Baghalian et al.[Citation33] pointed that probably the genetic factors could have more influence than ecology. Commercial allicin and allicin potential (alliin and alliinase activity) are being considered as indices for evaluation of the medicinal value of garlic preparations.
Lawson and Hughes[Citation34] studied the effects of pH, neutralization after acidification, time, and temperature on the yield of thiosulfinates release from garlic powder and garlic cloves. All dipropenyl thiosulfinates including allicin were formed at an optimum pH of 4.5–5.0. Below pH 3.6 no thiosulfinates were formed. Neutralization of the pH (previously at 3 or below) failed to restore thiosulfinate generation, thus allinase is completely and irreversibly inhibited by the acidic conditions found in the stomach. Yu and Wu[Citation35] studied the effects of pH on the formation of flavor compounds from allicin. They found two isomeric cyclic compounds 3-vinyl-1,2-dithiin and 2-vinyl-1,3-dithiin reached their highest levels around pH 6.5, whereas the formation of diallyl trisulphide, diallyl disulphide, methyl allyl disulphide, and diallyl sulphide was favored around pH 9.0. The effect of temperature (2–37°C) was found negligible on the rate of thiosulfinates formation. Allicin is formed from alliin by the action of allinase and gets metabolized rapidly into diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, S-allylmercaptocysteine, S-allyl cysteine, and vinyl dithiines.[Citation15,Citation36,Citation19,Citation37,Citation38]
The maximum rate of formation of allicin from garlic was observed at incubated temperature of 35°C, retarding effect of acetic acid (5–30%) or rectified spirit (5–30%). An increased rate of formation was evident with an increase in the pH (4.0–6.0) of the macerating medium.[Citation39] Similarly the rate of decomposition of allicin formed was found to be a maximum at incubation temperature of 35°C. The rate decreased with an increase in the strength of acetic acid, whereas rectified spirit/ethanol (5–30%) showed little effect. An increased pH (4.0–6.0) resulted in an increased rate of decomposition of allicin.[Citation39]
Raw garlic, when cut and placed on the tongue or lips, elicits painful burning and prickling sensations. Allicin and diallyl disulfide excite an allyl isothio-cyanate sensitive subpopulation of sensory neurons and induce vasodilation by activating capsaicin sensitive perivascular sensory nerve endings.[Citation40] Macpherson et al.[Citation41] showed that raw but not baked garlic activates TRPA1[Citation40] and TRPV1, two temperature-activated ion channels that belong to the transient receptor potential (TRP) family. These thermo-TRPs are present in the pain-sensing neurons that innervate the mouth. It was showed that allicin, an unstable component of fresh garlic, is the chemical responsible for TRP1 and TRPV1 activation and likely to cause garlic's pungency.
The loss of allicin in organic solvents during storage at −16 and 6°C was very low compared to storage at room temperature.[Citation2] At room temperature, it reduced to 10% within 3 days when stored in ethyl acetate, while in methanol it took around 13 days. This showed greater stability of allicin in methanol than in ethyl acetate. Iberl et al.[Citation42] reported that allicin was more stable in solvents capable of hydrogen bonding. Lawson and Gardner[Citation43] studied the composition (14 sulfur and 2 non-sulfur compounds) of fresh and commercial tablets, stability of potential active compounds, and availability of allyl thiosulfinates (mainly allicin) under both simulated gastrointestinal conditions and in vivo. The allyl thiosulfinates of blended fresh garlic were stable for at least 2 years when stored at −80°C. The dissolution release of thiosulfinates from the enteric-coated garlic tablets was found to be >95%. The bioavailability of allyl thiosulfinates from these tablets, measured as breath allyl methyl sulfide, was found to be complete and equivalent to that of crushed fresh garlic. S-allylcystein was stable for 12 months at ambient temperature. Stability of thiosulfinates of crushed garlic at 4°C in the absence of selected condiment revealed no significant decrease in any of the thiosulfinates at 12 days.
Drying of garlic at 60°C had no effect on alliin since there was only a 4% loss in yield for allicin, allyl methyl thiosulfinates and dimethyl thiosulfinates. However there was about 75% loss in yield for each of the 1-propenyl thiosulfinates, indicating that the drying process had destroyed much of the isoalliin.[Citation34]
FUNCTIONALITY OF ALLICIN AND OTHER ACTIVE COMPONENTS
Antimicrobial Activity
It was reported that allicin showed antibiotic activity.[Citation1,Citation21,Citation44,Citation45] Subramanyan et al.[Citation46] studied in vitro the effect of some of the more commonly used spices on intestinal bacteria in health and disease and reported that garlic was most potent in inhibiting the growth of some bacteria. Incorporation of garlic in the diet on caecal microflora of rats showed that garlic could considerably reduce the microflora in synthetic and stock diet fed animals.[Citation46] A significant decrease in caecal flora of rats was observed in rats fed poor rice diet supplemented with red gram dhal or butter milk along with garlic for 5 days. Nakagawa et al.[Citation47] reported the death and/or retarded growth in rats fed 5 ml of raw garlic extract per kg body weight. After feeding raw garlic extract for a period of 4 weeks to albino rats there was a decrease in total Streptococci, Coliforms, Lactobacilli, aerobes, and anaerobes. The effect on aerobes and anaerobes was found equally pronounced. None of the above changes were observed after feeding boiled garlic extract, instead stimulated the growth of certain intestinal bacteria, such as Streptococci and Coliforms.[Citation48] When garlic extract containing 8 μM of allicin was administered intragastrically to albino rats, a maximum of 0.4 μM in the intestine and 2.4 μM in caecum was detected after a period of 4 and 6 hr, respectively.[Citation49] About 50–60% reduction in microflora was observed in intestine after 4 hours of administration, but no such change was observed in caecum even after 6 hours. However, a 5-fold decrease in microflora was seen in caecum only at the end of 8 hours. Shashikanth et al.[Citation49] pointed that the gradual decrease in allicin content while passing through the gut is perhaps due to reducing agents in the gut, natural instability of allicin, antagonism by food materials and absorption in the intestine. Generally, the aerobes were more susceptible to allicin concentration in the gut than the anaerobes.
Sharma et al.[Citation50] demonstrated in vivo antibacterial property of Allium sativum water extracts on gram negative and gram positive flora of the gastro-intestinal tract of chicks indicating its effectiveness in inhibiting both types of flora. Garlic extract also inhibited some bacterial flora, which were resistant to some of the antibiotics used. Kumar and Sharma[Citation51] further indicated the inhibitory effect of garlic on enterotoxigenic Escherichia coli. The anti-microbial activity of a range of garlic products including dried garlic powder produced by different methods, commercial garlic products, and garlic oil was determined against a range of selected bacteria (Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Bacillus cereus, and a mixed lactic culture consisting of Lactobacillus delbrueckii subsp. Bulgaricus and Streptococcus thermophilus).[Citation52] Generally fresh garlic produced the greatest inhibition followed by freeze-dried powder. The results showed that both drying temperature and time had major effects on retaining the active components responsible for the inhibition of microbial growth. Allicin showed antibacterial effect against Helicobacter pylori.[Citation53] Helicobacter pylori is considered to be one of the main reasons of gastric and duodenal ulcers as well as gastric cancer. In vitro and in vivo research proved that Helicobacter pylori, resistant to many antibiotics, was sensitive to garlic extract in relatively low concentrations. Sivam et al.[Citation54] investigated the antimicrobial activity of aqueous extract of garlic against H. pylori and found that minimum inhibitory concentration of the extract was 40 μg allicin/ml. Cellini et al.[Citation55] tested 16 clinical isolates of H. pylori and showed the concentration of garlic extract required to inhibit the bacterial growth was between 2 and 5 mg/ml. The inhibitory concentration of garlic reported in the above studies is achievable in the stomach by consuming a medium size clove of garlic. Interestingly, H. Pylori can survive and grow in the acidic environment of stomach by producing abundant amounts of enzyme urease, which hydrolyzes urea present in gastric juice. The ammonia, generated from this reaction, produced a local alkaline microenvironment, thereby protecting the bacterium against the hostile acidic conditions.[Citation56] Juszkiewicz et al.[Citation57] found allicin or garlic extract inhibited the urease attributed to the reaction of allicin with SH-group. Thiol reagents (L-cysteine, 2-mercaptoethanol, glutathione, dithiothreithol) strongly protect the enzyme from the loss of enzyme activity, while urea and boric acid showed weaker protection.
Bakri and Douglas[Citation58] studied the inhibitory effect of garlic extract with allicin on oral bacteria including 13 gram-positive and 6 gram-negative types of bacteria, and one type of fungi. The garlic extract 57.1% (w/v) containing 220 μg/ml allicin inhibited the growth and killed most of the organisms tested. In general, the minimal inhibitory concentrations for Gram-negative strains varied from 0.4–6.87 μg/ml which were lower than those for Gram-positive strains (13.8–55.0 μg/ml) tested. Time-kill curves for Streptococcus mutans and Porphyromonas gingivalis showed that killing of the later started almost immediately, whereas there was a delay before S. mutans was killed. The garlic extract also inhibited the trypsin-like and total protease activity of P. gingivalis by 92.7% and 94.88%, respectively.
Garlic extracts showed to be antifungal[Citation59] and antiviral[Citation11] and antiprotozoal[Citation60] activity. Cu2+ showed a dose dependent fungicidal activity against Saccharomyces cerevisiae cells, and its lethal effect was extremely enhanced in the presence of allicin.[Citation61] Allicin influenced the mode of cell surface localization or the related function of AHP1 as a defense against phospholipids peroxidation by the external action of Cu2+.
There are several physiological processes in microorganisms which were affected by allicin, such as lipid bisynthesis[Citation62,Citation63] RNA synthesis,[Citation64] lowering of lipids in mammals,[Citation65,Citation66,Citation67,Citation68,Citation69,Citation70,Citation71] and aggregation of platelets.[Citation72,Citation73] Allicin was shown to be a specific inhibitor of acetyl-CoA synthetases from plants, yeast, and mammals. The bacterial acetyl-CoA-forming system, consisting of acetate kinase and phosphotransacetylase was inhibited. It was found to be specific to the enzymes of the fatty acid synthesis sequence.[Citation74] Allicin reacts very rapidly with free thiol groups, via thiol-disulphide exchange, therefore it is thought that its main mechanism of antimicrobial action is through interaction with thiol-containing enzymes, including cysteine proteases and alcohol dehydrogenases.[Citation75,Citation76]
Anticancer Activity
Alliin itself did not show any inhibition of tumor cell growth indicating that the antiproliferative effects of garlic was due to breakdown products of alliin.[Citation77] Allicin plays a major role in the antiproliferative effect of water-soluble garlic preparations and this effect may be attributed to the ability of allicin to transiently deplete the intracellular gluthathione (GSH) level. The extent of the decrease in GSH levels correlated well with the growth inhibitory activity of allicin.[Citation36] The antiproliferative effect of garlic is also clear. Allyl sulphur compounds are important antitumorigenic agents[Citation78] and diallyl disulfide reduced the size and the number of preneoplastic foci in rats liver induced by AFB1.[Citation79]
The protection of garlic against cancer aroused from several mechanisms including the blockage of nitrosamines formation and bioactivation.[Citation80] These substances are much suspected to be carcinogens and influence the cancer risk in humans. Dion et al.[Citation81] reported that their formations were retarded by S-allylcysteine. Garlic also decreased the bioactivation of carcinogens. Cytochrome P4502E1 is a hepatic phase I enzyme implicated in the metabolism of nitrosamine, and others carcinogens, and its activity is modulated by organo-sulfur compounds[Citation82] such as diallyl disulfide.[Citation83,Citation84] Ograno-sulfur from garlic blocked the bioactivation and carcinogenicity of non-nitrosamines such as aflatoxin B1 (AFB1), a cancer agent for the liver. Phase I enzymes CYP1A1, 1A2, 2B1, and 3A4 involved in carcinogen bioactivation showed a modified activities after diets supplementation in rats with garlic or several sulfur compound such as diallyl disulfite.[Citation85] The induction of phase II enzyme detoxication such as glutathione-S-transferase (GST), quinone reductase (QR), and uridinediphosphoglucuronate glucuronosyltransferase (UGT) by OSC, such as diallyl disulfite[Citation86,Citation87] was demonstrated and similar effects were observed with garlic consumption.[Citation88,Citation89]
Garlic intake can modify the risk of colon cancer to women because diallyl disulfide is an effective inhibitor of the growth of neoplastic CMT-13 cells and of N-acetytransferase activity in human colon adenocarcinoma cell line.[Citation90,Citation37] Furthermore, diallyl disulfide showed to be an effective inhibitor for the promotion phase of 9,10-dimethyl-1,2-benzanthracene induced skin tumors in the mouse.[Citation91]
Apoptosis, or programmed cell death, is a genetically controlled process, wherby the cell actively participates in its own destruction in response to environmental or developmental cues. Apoptosis is morphologically characterized by membrane blebbing, cytoplasmic, nuclear and chromatin condensation, and DNA fragmentation.[Citation92] Allicin showed effects on DNA processing, RNA synthesis,[Citation93] signal transduction and apoptosis.[Citation94] Certain effects of allicin are mediated through nitric oxide formation.[Citation78] Oommen et al[Citation95] studied the chemo-preventive action of allicin on the growth of cancer cells of murine and human origin by cell viability assay. They found that allicin inhibited the proliferation of cancer cells and induced apoptosis with typical features such as apoptotic bodies, DNA fragmentation, activation of caspases and poly (ADP-ribose) polymerase cleavage, thus these effects of allicin account partly for the anticarcinogenic properties of garlic. Park et al.[Citation96] showed that allicin induced apoptosis of the cells through caspase-independent apoptosis pathway, which was accompanied by the mitochondrial release of AIF and protein kinase A (PKA) appeared to play an important role in the caspase-independent apoptosis.
Shalinsky et al.[Citation97] studied the effects of allicin on other terminal prostaglandin biosynthetic enzymes, such as isomerase for PGE2 and on cyclooxygenase activity. They found that allicin selectively inhibited the GSH dependent PGH2 to PGE2 isomerase in the adenocarcinoma cell line. Macrophages play an important role in host defenses against tumors by killing them and produce secretory products, which resulted in the protection against bacterial, virus infection and malignant cell growth. Allicin is an efficient immunomodulator of macrophage secretory and cellular activities; it showed a differential effect on production of cytokines and cytotoxic molecules.[Citation98] It altered cellular functions of macrophages to kill tumor cells and produce various molecules, such as NO, H2O2, TNF-α, IL-1 (interleukin-1) and IL-6 after treated with various doses (1, 10, 100 ng/ml) for 20 hours.
Allicin and its corresponding sulfite inhibited the proliferation and induced apoptosis of several human non-leukaemia malignant cells including breast, bladder, colorectal, hepatic, prostate cancer, lymphoma, and skin tumor cell lines.[Citation99] Ajoene (which has greater chemical stability) showed to inhibit proliferation and induced apoptosis of human leukaemia CD34-negative cells including HL-60, U937, HEL and OCIM-I.[Citation100,Citation15,Citation101,Citation102] More significantly, ajoene showed to induce 30% apoptosis in myeloblasts from a chronic myeloid leukaemia patient in blastic crisis.[Citation100,Citation15] Acute myeloid leukaemia, is a heterogeneous malignant disease, has a major impact on resistance to chemotherapy and relapse.[Citation103,Citation104] Ajoene significantly enhanced the inhibitory effect of the two chemotherapeutic drugs, cytarabine and fludarabine on bcl-2-expression in KG1 cells.[Citation100] The two key antileukaemia biological actions of ajoene were the inhibition of proliferation and the induction of apoptosis. Studies showed the anti-proliferation activity of ajoene to be associated with a block in the G2/M phase of cell cycle in human myeloid leukaemia cells. The apoptosis inducing activity of ajoene is via the mitochondria dependent caspase cascade through a significant reduction of the anti-apoptotic bcl-2 that results in release of cytochrome c and the activation of caspase-3. Since acute myeloid leukaemia (AML) is a heterogeneous malignant disease in which disease progression at the level of CD34-positive cells has major impact on resistance to chemotherapy and release and the inability to undergo apoptosis is a crucial mechanism of multi-drug resistance in AML patients.[Citation99]
Antioxidant Activity
It was reported that soybean oil increased the stability of garlic flavor while cooking garlic with an oil-water mixture[Citation105] and garlic flavor acted as an inhibitor of soybean oil oxidation.[Citation106] Kim et al.[Citation107] studied the antioxidant activity of garlic homogenates blended with distilled water and different amounts of oil. Antioxidant activity of garlic aroma concentrate in the oil layer was found strong. Diallyl disulfide was found to be most stable compared to other two sulfur compounds disulfide and diallyl trisulfide when analyzed by HPLC. It was found that the effectiveness and strength of diallyl disulfide as an antioxidant could be increased by addition of α-tocopherol and L-ascorbyl palmitate in a lard system.[Citation107]
In vitro allicin acts by reacting with free thiol containing enzymes, serving as an efficient antioxidant by trapping of radicals.[Citation108,Citation76] Allicin was found to scavenge hydroxyl radicals[Citation109,Citation110] and to inhibit superoxide production by phorbol ester-activated human granulocytes.[Citation77] Allicin showed the modification of SH-dependent activities, an additional therapeutic property,[Citation109,Citation76,Citation110] and inhibitory effect on NO formation.[Citation78] Ajoene also showed the inhibitory effect of NO formation.[Citation78] The inflammatory environment in human atherosclerotic lesions resulted in an expression of the inducible form of nitric oxide synthase and subsequently in the formation of peroxynitrite. Peroxynitrite is a potent oxidant, which is formed when the synthesis of large amounts of NO coincides with superoxide production.[Citation111] Peroxynitrite is able to initiate LDL oxidation and to promote platelet aggregation[Citation112] and thus aggravates the atherogenic process. Peroxynitrite formation, however, occurred only if NO is produced in high enough concentrations to compete endogenous superoxide dismutase for superoxide. In D-galactosamine/lipopolysaccharide induced hepatitis rats, a significant increase of lipid peroxidation and decreased liver antioxidant enzyme levels were observed. Pretreatment with allicin prevented these alterations, thus provided hepatoprotective effect.[Citation113] Allicin showed antigenotoxic effect against methyl methanesulphonate induced genotoxic damage.[Citation114]
Reducing Cardiovascular Diseases
Cardiovascular diseases include a great number of factors such as high cholesterol, hypertension and increased platelet aggregation. The role of garlic in the reduction of cardiovascular diseases was historically proved but contradictory clinical studies emerged from different methodologies.[Citation30] The hypotensive effect is one of the earliest established properties of garlic preparations and was later confirmed in animals[Citation115] and in humans.[Citation116] The health benefit observed varies with the garlic products tested, i.e., garlic powder, aged garlic extract, garlic oil, and garlic oil macerate,[Citation6] with the state of cooked-, raw-, fried-, or boiled-garlic[Citation117,Citation118] and with the percentage of active components.[Citation119] The contradictory results may be due to several factors and the lack of knowledge about the substances present is certainly one of them.[Citation30] Garlic reduced blood pressure,[Citation67,Citation120] antioxidant,[Citation121] inhibited platelet aggregation,[Citation27,Citation70] and reduced blood glucose.[Citation67]
Allicin altered the lipid profile in hyperlipidemic rabbits.[Citation122] Synthetic preparation of allicin was found to lower blood pressure, insulin, and triglycerides levels in fructose-fed rats.[Citation123] Similar beneficial effect of allicin and enalapril (hypertensive drug) on blood pressure, insulin, and triglycerides were observed in fructose-induced hyperinsulinemic, hyperlipidemic and hypertensive rats, thus it reinforces the trend toward combining the nonpharmacologic approach with drug therapy.[Citation124] It also reduced the gain in weight of the groups fed fructose with allicin.[Citation125]
Atherosclerosis is one of the major risk factor in the development of hypertension and cardiovascular diseases. For centuries, garlic has been used for treating high blood pressure in China and Japan. Furthermore, garlic has been found to act as an antihypertensive agent[Citation126,Citation127] and has been officially recognized for the treatment of hypertension by the Japanese Food and Drug Administration.[Citation128] In hypercholesterolemic rabbits garlic elicited a significant reduction of the hypercholesterolemia in a way comparable to gemfibrozil while serum triglycerides values remain unchanged after garlic treatment.[Citation129] Ali et al.[Citation4] studied the effect of garlic powder (0.6% allicin) on the serum cholesterol, triglyceride, glucose, protein, and systolic blood pressure in rats fed with a high cholesterol diet. They found significant reduction in serum cholesterol, triglycerides, and blood pressure level, thus providing that garlic has beneficial functions. Effect on two risk factors for atherosclerosis: hyperlipidemia and hypertension. However, no changes were observed in the serum glucose and protein in all of the rats. Allicin showed significant vasodilator activity in the pulmonary vascular bed of rat when tone is increased experimentally. However, diallyl disulfide and allyl mercaptan, metabolites of allicin, did not possess this vasodilator action.[Citation31] Although the mechanism by which allicin induces vasosidilatation is uncertain, the results of the investigation of Kaye et al.[Citation31] suggest that this garlic derivative may be useful in the treatment of pulmonary hypertensive disorders, including primary pulmonary hypertension and chronic obstructive pulmonary disease. Allicin also showed vasodilator activity in the pulmonary vascular bed of cat and rat.[Citation130] When baseline tone in the pulmonary vascular bed of cat was raised, intralobar injections of allicin produced dose-related decrease in pulmonary arterial pressure without changing left arterial pressure. Allicin also decreased systemic arterial pressure in a dose-related manner.[Citation130] The pulmonary vasodilator responses to allicin were independent of the synthesis of endothelial derived relaxing factor or the activation of soluble guanylate cyclase. Other allicin analogs should be developed for clinical use because of allicin's vasodilator efficacy and apparent lack of toxicity.[Citation31] A modest dose (14 mg of allicin) was shown to cause decrease in diastolic blood pressure in severely hypertensive patients.[Citation131]
Inflammatory diseases such as atherosclerosis lead to coronary thrombosis. The platelet aggregation contributes to minimize the atherosclerosis, and it was reduced by allicin and two others thiosulphinates from onion.[Citation132] The antiplatelet activity in onion was significantly positively correlated with high sulfur level.[Citation133] Garlic powder also inhibited the platelet aggregation.[Citation134] Lawson et al.[Citation27] reported the variation of the antiplatelet activity of different garlic preparation, cloves or commercial products. These variations are due to the different nature of organo-sulfur compound present. Extracts of garlic was found to inhibit human platelet aggregation in vitro.[Citation135] The inhibition of human platelet aggregation did not involve an effect on cyclooxygenase thromboxane synthase activity, or on adenosine 3′, 5′-cyclic monophosphate levels.[Citation73]
Miron et al.[Citation136] synthesized allylmercaptocaptopril (CPSSA) by reacting captopril (antihypertensive drug, antiotension converting enzyme inhibitor) with pure allicin and studied their effects on fructose induced hypertensive groups of rats. They found that CPSSA decreased blood pressure and reduced triglycerides, whereas it found no effect on the insulin serum level. This new stable compound combines the beneficial properties of captopril and allicin and is a potential candidate for antihypertensive drug therapy. In rats, treatment with low dose of CPSSA (5 mg/kg day) lowered SBP but did not improve any further measured parameter, while treatment with a higher dose (50 mg/kg day) significantly decreased blood pressure, triglycerides, and homocysteine concentrations.[Citation137]
Improving Immune Function
Garlic decreases certain diseases caused by immune dysfunction. Thus, aged garlic extract presents an immunomodulation effect.[Citation138] The immunomodulatory effect of garlic or garlic constituents shows a modulation of cytokine production as mediator of inflammation. The nuclear factor-KB (NF-KB) is a central transcription factor and has a central role in the expression of genes that control immune response. NF-KB is strongly involved in the activation and regulation of key molecules associated with inflammatory diseases and cancer.[Citation139] It increases the expression of the genes of some cytokines. The inhibition of NF-KB by garlic products was indirectly controlled by a modulation of pro- and anti-inflammatory cytokines.[Citation140] Epithelial cells have an important role in intestinal inflammation and allicin showed anti-inflammatory properties.[Citation141] Allicin inhibited spontaneous and TNF-α induced secretion of pro-inflammatory cytokines and chemokines from intestinal epithelial cells.[Citation141] Josling[Citation142] found that an allicin containing supplement can prevent attack by common cold virus. One hundred forty six volunteers were randomized to receive a placebo or an allicin-containing garlic supplement, one capsule daily, over a 12‐week period. The active treatment group had significantly fewer colds than the placebo group. The placebo group, in contrast, recorded significantly more days challenged virally and a significantly longer duration of symptoms.
Effect on Protein and Fat Profile
Augusti and Mathew[Citation66] showed decreased serum protein levels in rats administered raw garlic extract. Raw garlic extract showed decreased total serum proteins as well as serum globulin when fed albino rats for 4 weeks, whereas boiled garlic extracts did not show any effects.[Citation48] The long term feeding (4 weeks) of raw and boiled garlic extract to albino rats resulted weight loss.[Citation48] Extracts of garlic were also reported to possess hypoglycemic[Citation121,Citation143] and hypocholesteremic properties.[Citation66] Allicin also reduced serum cholesterol and triglyceride levels, as well as atherosclerotic plaque formation, prevented platelet aggregation and decreased blood pressure.[Citation144,Citation62,Citation24,Citation145]
BIOAVAILABILITY OF ALLICIN
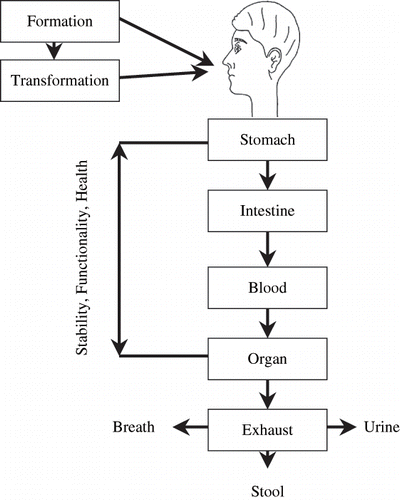
shows different pathways of active components in the body. Before one can evaluate the effectiveness of allicin in the body, it must be determined whether or not it can actually reach the target organs.[Citation58] If allicin or an allicin containing substance is ingested, it would be exposed to the acidic conditions of the stomach and then to the neutral conditions of the intestines. For these reasons, Freeman and Kodera[Citation2] studied the relative stability of allicin in blood, different solvents [ethyl acetate, methanol, water (pH 1.2 and 7.5)], simulated gastric fluid (SGF, pH 1.2), and simulated intestinal fluid (SIF, pH 7.5). At 37°C, allicin decomposed rapidly in methanol and ethyl acetate, however, it was more stable in protic polar methanol than in aprotic polar ethyl acetate. Approximately 90% of allicin remained after incubation at 37°C for 5 hours in water at pH 1.2 and 7.5. After 1 day at pH 1.2, about 80% of allicin remained, and at pH 7.5, about 62% of allicin remained. Interestingly, allicin did not appear to generate its normal transformation products at these pH values since an increase in the concentration of diallyl disulfide, ajoene, and dithiin was not observed. Therefore, gastric or intestinal pH may not be a significant factor affecting allicin availability or decomposition in the body during the digestive period. There was likely to inactivation of allinase, the enzyme that catalyzes the conversion of alliin to allicin at ≤ pH 3.[Citation34]
Food substances are normally present in the gastrointestinal tract, thus interaction of allicin with cow's milk was also examined by Freeman and Kodera.[Citation2] After exposure of milk for 1 hour, almost all allicin was recovered. Only traces of allicin could be detected after it was incubated in blood for 5 minutes. Allicin was more reactive to the blood cell fraction than to the plasma fraction. No allicin was detected after 3 minutes when it was incubated in blood cell fraction, while in the plasma fraction, the concentration of allicin decreased gradually and the half-life of allicin was estimated to be about 50 minutes. A rapid change in color of red blood cells to a dark color was observed after addition of allicin, which may be due to the rapid oxidization of iron in hemoglobin. Concurrent with the disappearance of allicin, the presence of diallyl disulfide was observed in the mixture of allicin and blood. Diallyl disulfide showed stability in blood. The concentration of diallyl disulfide remained unchanged after 1 hour.
Similar results were also observed in case of liver cell.[Citation146] After incubation for 3 minutes in liver homogenate, a decrease of 90% initial allicin was observed and the decrease of allicin was 99% after 6 minutes. On the other hand, it was observed that the metabolism of diallyl disulfide in mice reached a maximal concentration in the liver after 90 minutes of jp injection.[Citation147] Although allicin disappeared rapidly in the body after absorption, diallyl disulfide may be absorbed and delivered to organs.[Citation146,Citation147] Egen-Schwind et al.[Citation146] investigated pharmacokinetics of vinyldithiins (transformation products of allicin) after oral administration of 27 mg 1,3-vinyldithiin and 9 mg of 1,2-vinyldithiin to rats. In serum, kidney, and fat tissue, both vinyldithiins were detected over a period of 24 hours, whereas in liver only 1,3-vinyldithiin was found. 1,3-vinyldithiin seems to be less lipophilic and was rapidly eliminated from serum, kidney, and fat tissue, whereas 1,2‐vinyldithiin is more lipophilic and showed a tendency to accumulate in fat tissue. Allicin, the precursor of vinyldithiins, is metabolized more rapidly in liver homogenate than the vinyldithiins. Allicin is assumed to be the active component of garlic in vivo, but not in vitro. Allicin cannot be detected in the blood or urine after the ingestion of raw garlic or pure allicin within 1 to 24 hours after ingestion of 25 g raw garlic (∼90 mg allicin).[Citation27]
It was reported that allicin can be produced by human liver microsomes from diallyl disulfide. These suggested that despite its rapid disappearance from the bloodstream, allicin can be reformed in the process of interconversion of its metabolites and as a result, act intracellularly.[Citation148] Agarwal[Citation65] suggested that metabolites of allicin, rather than the agent itself, are responsible for this wide range of beneficial health effects. Several such metabolites are S-allylmercaptocysteine, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and S-allylcysteine. The pharmacokinetic studies of S-allyl cysteine demonstrated rapid absorption and almost 100% bioavailability after oral administration. In addition, since both safety and effectiveness of S-allyl cysteine have been reported, this compound appeared to play an important role in garlic's medicinal effects.[Citation149,Citation150]
Freeman and Kodera[Citation2] also studied the allicin content and allicin-producing potential of commercial garlic preparations. No allicin was observed in all commercial products. Although no product on the market contains a detectable amount of allicin (< 1 ppm) some garlic powder products claim to be able to generate certain amount of allicin, so-called “allicin potential” or “allicin yield.” Variable allicin potentials from 0.64–4.64 g/kg were observed in different brands of products, as well as among different lots of the same brand. After 1 hour at 37°C under SIF conditions, about 62% (2.86 g/kg) of the amount of allicin produced with water was observed, and after 1 hour under SGF condition, only 4% (0.19 g/kg) could be found. This low level of allicin production under SGF condition may be due to hydrolysis of allinase by acid and enzyme pepsin. Under simulated digestive conditions (sequential combination of SGF and SIF), only about 1% of allicin was observed. The small amount of allicin observed is not due to its decomposition during the incubation period because it was shown to be stable at gastric and intestinal pH for 2 to 3 hours. At pH 6.8 commercial products released very fast highly soluble compounds, amino acids, and dipeptides.[Citation30] Within less than 5 minutes nearly 100% of these compounds were released and allicin was formed. Alliin was very fast released from the powder and immediately metabolized to allicin (100%) within the pH range of 3.5–8.4. At pH 2, alliin was completely released within a minutes without further degradation to allicin. Higher pH values seem to stabilize the allicin, whereas lower pH values accelerated its degradation. However, sulfides and dithiines could not be detected in the release media within 30 minutes.[Citation30]
Arnault et al.[Citation30] analyzed alliin, allicin, and storage stability of 11 European products and found the majority of the products present a very different composition from what was claimed by the companies. Only one product possessed the legal status of a drug. For that product, values were found as claimed on the package. It was known that alliin and allicin content decreased in correlation to their storage time. This can be explained by the residual activity of the allinase. Their results clearly showed the instability of products when stored under ambient conditions for 18 months. The best products remained activity of 70–90%, whereas most of the products showed below 50% value. The BET monolayer value of freeze-dried garlic, which is considered as most stability of dried products during storage, was found 5.7 g/100 g sample at 20°C.[Citation151] However, Arnault et al.[Citation30] recommended residual water of less than 3 g/100 g sample (i.e., 3%), and a complete water vapor tight packaging material is needed for keeping their functionality. In addition, high packing of the tablet and organic film coating could also improve the stability up to 24 months.
The certitude that garlic provides beneficial effects on health lead the industry to market some garlic market products for human consumption in the last decades. There are 2 major types of market garlic products on the European pharmaceutical and food supplement market and a third one on the American market: (a) the oil macerates of fresh garlic, formulated commonly in soft gelatin capsules; (b) the dry powder products of fresh garlic formulated either as sugar or film coated tablets; and (c) dry powder products of aged garlic formulated either as sugar or film coated tablets.[Citation145] It is the task of the pharmaceutical science to formulate each active principle individually in such a way that the active principle is fully available under the physiological condition of the application. The appearances and characteristics of medicinal products are significantly influenced and controlled by the formulation technology (galenic) and the analytical technology. Both determine the ability to manufacture quality/stability, efficacy, and consumer acceptance of the final medicinal product.
Although not all of the active ingredients are known, ample research suggested that several biological components likely contribute to the observed beneficial effects of garlic.[Citation6] Other active organosulfur compounds found in garlic derived from allicin are diallyl disulfide, methyl allyl trisulfide, and ajoene. Allylic and allenic thiosulfinates are of biological interest due to their similarity to allicin. Braverman et al.[Citation152] developed a convenient preparation method of mixed allyl allenic and bis-allylic thiosulfinates.
Being a chemically reactive compound and hence rather unstable, allicin rapidly transforms into a number of derivatives found in aged garlic and various crushed garlic preparations. For garlic extract, whole or sliced garlic cloves are soaked in an extracting solution (e.g., purified water and diluted alcohol) for varying amount of time. After separation of the solution the extract is generally concentrated and used.[Citation6] Powdered forms of the extract are also available. The extract, especially aged, contains mainly the water soluble constituents in garlic and a small amount of oil-soluble compounds.[Citation153] The extract is characterized by water soluble sulfur containing compounds, including S-allyl cysteine (SAC) and S-allyl mercaptocysteine.[Citation154] S-allyl cysteine can be used for standardization because it is bioavailable and can be detected in the plasma, liver and kidney after oral intake. The bioavailability of SAC was 103% in mice, 98.2% in rats, and 87.2% in dogs.[Citation155] S-acetyl SAC was identified as a metabolite of SAC in the urine of dogs and humans. Other metabolites of garlic constituents, such as N-acetyl-S-(2-carboxypropyl)-cysteine, N-acetyl-cystein and hexahydrohippuric acid, were detected in human urine after ingestion of garlic.[Citation156] This suggested that SAC could be transformed by N-acetyltransferase. SAC and its metabolite(s) are possible compliance markers for clinical studies involving garlic.[Citation157]
Allyl mercaptan and diallyl disulfide were the first compounds identified as components that produce the strong odor detectable after ingestion of garlic.[Citation158,Citation159] Allicin, perfused into isolated rat livers, showed a remarkable first-pass effect and was metabolized as diallyl disulfide and allyl mercaptan, whereas ajoenes and vinyl-dithines were recovered in the effluent.[Citation146] The inhibition ability of platelet aggregation on the basis of ajoene and dithiin content may be inadequate because other compounds may act synergistically or independently to bring the effect.[Citation6] For example, aged garlic, which contains neither ajoene nor dithiin, significantly reduced platelet aggregation and adhesion in two double-blind, placebo-controlled clinical studies.[Citation160,Citation161] As a result, Amagase et al.[Citation6] pointed that the concentration of various compounds and their effects in vitro may not determine effectiveness. Preclinical or preferably clinical studies are required to confirm or refute the effectiveness of a product in question, whatever its chemical composition.
Aged garlic extract is aged for up to 20 months. During the aging process, the odorous, harsh, and irritating compounds of garlic are converted naturally into stable and safe sulfur compounds. Further the safety of aged garlic has been confirmed by various toxicological studies.[Citation162,Citation47,Citation163–166]
Allicin rapidly disappears from the circulation after intravenous injection suggesting that it is transformed into secondary products.[Citation2,Citation167] A variety of biological effects of allicin are attributed both to its SH-modifying and its antioxidant activity,[Citation75,Citation25,Citation110,Citation109] which also found in model systems.[Citation168,Citation76] Allicin can easily permeate cell membranes of phospholipids bilayers, carry out its activity intracellularly and interacted with SH groups.[Citation108] Fast diffusion and permeation of allicin across human red blood cell membranes was also demonstrated. Allicin did not induce leakage, fusion or aggregation of membrane. The high permeability of allicin through membranes may greatly enhance the intracellular interaction with thiols.[Citation108] It is important to find which by-product of allicin is the active species that modulates extra- and intra-cellularly processes.
It was found S-allylmercaptocysteine (CSSA) as the product of the reaction of cysteine with allicin.[Citation44] It is one of the active ingredients of aged garlic extract. The antiproliferative activity of CSSA on different cell lines was demonstrated, whereas S-allylcysteine showed no such effect.[Citation169 Citation–170,Citation19,Citation101] CSSA also revealed antioxidant activity[Citation154] and decreasing ocular pressure activities.[Citation171] Allicin elevated intracellular Ca2+ concentration in rabbit nonpigmented ciliary epithelial cells by allicin.[Citation171] The reduced glutathione most abundant non-protein thiol in mammalian systems has potential to interact with allicin. Rabinkov et al.[Citation172] studied the formation of S-allylmercaptoglutathione (GSSA) upon interaction of allicin with glutathione. The potential of GSSA to serve as vehicles for the prolonged action of allicin was demonstrated by its SH-modifying properties and high antioxidant activity. It showed reduction of OH radical reactions, and lowered the production of lipid peroxides. Thus it has a role in the biological activity of allicin and its derivatives. Though individual compounds, such as S-allyl cysteine showed activity, however Koch[Citation173] emphasized that the activity of various sulfur compounds could not alone be responsible for the benefits of garlic and fixation on a single group of components can lead to mistakes and wrong conclusions. Overall, the active principles in garlic was not fully characterized and it is assumed that the bioavailability of these sulfur containing compounds will play an important role in determining the biological response to various garlic preparations.[Citation6]
SAFETY AND TOXICITY
The effectiveness of garlic may be prevention rather than therapy, thus it may need long-term supplementation.[Citation6] Long-term use of supplements raises issues about toxicity. Although garlic has been used safely in cooking as a popular condiment or flavoring and used traditionally for medicinal purposes, it is commonly known that excessive consumption of garlic can cause problems. Recently Amagase et al.[Citation6] reviewed the safety of garlic preparation. Garlic produces odor on breath and skin[Citation174] and occasional allergic reactions.[Citation175] Other adverse effects associated with garlic are: stomach disorders and diarrhea,[Citation22,Citation176,Citation47] decrease of serum protein and calcium,[Citation177,Citation48] anemia,[Citation178 Citation–179,Citation35] bronchial asthma[Citation180 Citation–181] contact dermatitis,[Citation182–187] inhibition of supermatogenesis[Citation188 Citation–189] and damage of intestinal lining and the stomach.[Citation149 The following results were reported by Imada:[Citation190] (i) allicin is one of the major irritants in raw garlic; (ii) oil soluble sulfur compounds are more toxic than water soluble compounds; and (iii) when garlic is extracted in water for a certain period, its toxicity is greatly reduced. A number of toxicological and clinical studies of aged garlic showed no adverse effects. The safety of aged garlic was established in the following studies: acute and substance toxicity tests,[Citation163,Citation164] chronic toxicity test,[Citation165] mutagenicity tests,[Citation166] general toxicity tests,[Citation162,Citation47] teratogenicity tests, toxicity test conducted by the FDA, and clinical studies conducted on > 1000 subjects.[Citation191–193]
CONCLUSION
In 1892, the importance of diallyl disulfide and diallyl trisulfide in the flavor of garlic distillates was established. Allicin (diallyl-thiosulfinate), the most biologically active compound of garlic was discovered in 1944, and it is noted for its potent antimicrobial activity. Traditionally, many cultures used garlic for its different biological and medicinal effects. Based on this belief, trails of whole and garlic extracts started and continued in human and animal models. Recently attempts were being made to identify the functional components of garlic and to explore their mechanisms of action in human and animal models. In addition, other derived compounds from active garlic components are being developed to be used, which could provide more safety and effectiveness. The dose levels of effective functions and long-term toxicological safety need to be explored in more detail.
REFERENCES
- Block , E. 1985 . The Chemistry of Garlic and Onions . Sci. Am. , 252 : 114 – 119 .
- Freeman , F. and Kodera , Y. 1995 . Garlic Chemistry: Stability of S-(2-Propenyl) 2-Propene-1-Sulfinothioate (Allicin) in Blood, Solvents, and Simulated Physiological Fluids . Journal of Agricultural Food Chemistry , 43 : 2332 – 2338 .
- Rivlin , R.S. 2001 . Historical Perspective on the Use of Garlic . J. Nutr. , 131 : 951S – 954S .
- Ali , M. , Al-Qattan , K.K. , Al-Enezi , F. , Khanafer , R.M.A. and Mustafa , T. 2000 . Effect of Allicin from Garlic Powder on Serum Lipids and Blood Pressure in Rats Fed with a High Cholesterol Diet . Prostaglandins, Leukotrienes, and Essential Fatty Acids , 62 ( 4 ) : 253 – 259 .
- Essman , E.J. 1984 . The Medical Uses of Herbs . Fitoterapia , 55 : 279 – 289 .
- Amagase , H. , Petesch , B.L. , Matsuura , H. , Kasuga , S. and Itakura , Y. 2001 . Intake of Garlic and Its Components . J. Nutr. , 131 : 955S – 962S .
- Hussain , S.P. , Jannu , L.N. and Rao , A.R. 1990 . Chemopreventive Action of Garlic on Methylcholanthrene-Induced Carcinogenis in the Uterine Cervix of Mice . Cancer Letter. , 49 : 175 – 180 .
- Milner , J.A. 1996 . Garlic: Its Anticarcinogenic and Antitumorigenic Properties . Nutr. Rev. , 54 : A82 – S86 .
- Milner , J.A. 2001 . A Historical Perspective on Garlic and Cancer . J. Nutr. , 131 : 1027S – 1031S .
- Balasenthil , S. , Rao , K.S. and Nagini , S. 2002 . Garlic Induces Apoptosis during 7,12-Dimethylbenz[a]anthracene-Induced Hamster Buccal Pouch Carcinogenesis . Oral. Oncol. , 38 : 431 – 436 .
- Weber , N.D. , Andersen , D.O. , North , J.A. , Murray , B.K. , Lawson , L.D. and Hughes , B.G. 1992 . In Vitro Virucidal Effects of Allium Sativum(Garlic) Extract and Compounds . Planta Med. , 58 : 417 – 423 .
- Abdullah , T.H. , Kirkpatrick , D.V. and Carter , J. 1989 . Enhancement of Natural Killer Cell Activity in AIDS with Garlic . J. Oncol. , 21 : 52 – 53 .
- Kandil , O.M. , Abdellah , T.H. and Elkadi , A. 1987 . Garlic and the Immune System in Humans: Its Effects on Natural Killer Cells . Fed. Proc. , 46 : 441
- Kandil , O.M. , Abdullah , T.H. , Tabuni , A.M. and Elkadi , A. 1988 . Potential Role of Allium Sativumin Natural Cytotoxicity . Arch. AIDS Res. , 1 : 230 – 231 .
- Dirsch , V.M. , Gerbes , A.L. and Vollmar , A.M. 1998 . Ajoene, a Compound of Garlic, Induces Apoptosis in Human Promyeloleukemic Cells, Accompanied by Generation of Reactive Oxygen Species and Activation of Nuclear Factor Kappa B. . Mol. Pharmacol. , 53 : 402 – 407 .
- Kwon , K.B. , Yoo , S.J. , Ryu , D.G. , Yang , J.Y. , Rho , H.W. , Kim , J.S. , Park , J.W. , Kim , H.R. and Park , B.H. 2002 . Induction of Apoptosis by Diallyl Disulfide through Activation of Caspase-3 in Human Leukemia HL-60 Cells . Biochem. Pharmacol. , 63 : 41 – 47 .
- Pinto , J.T. , Lapsia , S. , Shah , A. , Santiago , H. and Kim , G. 2001 . Antipoliferative Effects of Garlic-Derived and Other Allium Related Compounds . Adv. Med. Biol. , 492 : 83 – 106 .
- Shirin , H. , Pinto , J.T. , Kawabata , Y. , Soh , J.W. , Delohery , T. , Moss , S.F. , Murty , V. , Rivlin , R.S. , Holt , P.R. and Weinstein , I.B. 2001 . Antiproliferative Effects of S-Allylmercaptocysteine on Colon Cancer Cells when Tested Alone or in Combination with Sulinac Sulfide . Cancer Res. , 61 : 725 – 731 .
- Sigounas , G. , Hooker , J. , Anagnostou , A. and Steiner , M. 1997 . S-Allylmer Captocysteine Inhibits Cell Proliferation and Reduces the Viability of Erythroleukemia, Breast and Prostate Cancer Cell Lines . Nutr. Cancer , 27 : 186 – 191 .
- Semmler , F.W. 1892 . Uber das atherische knoblauchs (Allium sativum) Arch . Pharm. , 230 : 434
- Cavallito , C.J. and Bailey , J.H. 1944 . Allicin, the Antibacterial Principle of Allium Sativum. I. Isolation, Physical Properties and Antibacterial Action . J. Am. Chem. Soc. , 66 : 1950 – 1951 .
- Caporaso , N. , Smith , S.M. and Eng , R.H.K. 1983 . Antifungal Activity in Human Urine and Serum After Ingestion of Garlic (Allium Sativum). . Antimicrob. Agents Chemother. , 23 : 700 – 702 .
- Stoll , A. and Seebeck , E. 1949 . AlliumCompounds. II. Enzymic Degradation of Alliins and the Properties of Allinase . Helv. Chim. Acta , 32 : 197 – 205 .
- Block , E. 1992 . The Organosulfur Chemistry of the Genus Allium-Implications for the Organic Chemistry of Sulfur . Angew. Chem. Int. Ed. Engl. , 31 : 1135 – 1178 .
- Han , J. , Lawson , L. , Han , G. and Han , P. 1995 . A Spectrophotometric Method for Quantitative Determination of Allicin and Total Garlic Thiosulfinates . Anal. Biochem. , 225 : 157 – 160 .
- Lawson , L.D. 1998 . “ Garlic: A Review of Its Medicinal Effects and Indicated Active Compounds. ” . In Phytomedicines of Europe: Their Chemistry and Biological Activity , Edited by: Lawson , L.D. and Bauer , R. 176 – 209 . Washington, DC : American Chemical Society .
- Lawson , L.D. , Ransom , D.K. and Hughes , B.G. 1992 . Inhibition of Whole Blood Platelet-Aggregation by Compounds in Garlic Clove Extracts and Commercial Garlic Products . Thromb. Res. , 65 : 141 – 156 .
- Miron , T. , Bercovici , T. , Rabinkov , A. , Wilchek , M. and Mirelman , D. 2004 . [3H]Allicin: Preparation and Applications . Analytical Biochemistry , 331 : 364 – 369 .
- Brodnitz , M.H. , Pascale , J.V. and Derslice , L.V. 1971 . Flavor Components of Garlic Extract . Journal of Agricultural Food Chemistry , 19 ( 2 ) : 273 – 275 .
- Arnault , I. , Haffner , T. , Siess , M.H. , Vollmar , A. , Kahane , R. and Auger , J. 2005 . Analytical Method for Appreciation of Garlic Therapeutic Potential and For Validation of a New Formulation . Journal of Pharmaceutical and Biomedical Analysis , 37 : 963 – 970 .
- Kaye , A.D. , De Witt , B.J. , Anwar , M. , Smith , D.E. , Feng , C.J. , Kadowitz , P.J. and Nossaman , B.D. 2000 . Analysis of Responses of Garlic Derivatives in the Pulmonary Vascular Bed of the Rat . J. Appl. Physiol. , 89 : 353 – 358 .
- Pierson , S. 1994 . Garlic Product Organosulfur Chemistry, Pharmacology and Toxicology: An Overview for Pharmacists . Pharmalert , 2 ( 5 )
- Baghalian , K. , Ziai , S.A. , Naghavi , M.R. , Badi , H.N. and Khalighi , A. 2005 . Evaluation of Allicin Content and Botanical Traits on Iranian Garlic (Allium sativumL.) Ecotypes . Scientia Horticulturae , 103 : 155 – 166 .
- Lawson , L.D. and Hughes , B.G. 1992 . Characterization of the Formation of Allicin and Other Thiosulfinates from Garlic . Planta Med. , 58 : 345 – 350 .
- Yu , T. and Wu , C. 1989 . Effects of pH on the Formation of Flavour Compounds of Disrupted Garlic . Journal of Chromatography , 462 : 137 – 145 .
- Hirsch , K. , Danilenko , M. , Giat , J. , Miron , T. , Rabinkov , A. , Wilchek , M. , Mirelman , D. , Levy , J. and Sharoni , Y. 2000 . Effect of Purified Allicin, the Major Ingredient of Freshly Crushed Garlic, on Cancer Cell Proliferation . Nutrition and Cancer , 38 ( 2 ) : 245 – 254 .
- Sundaram , S.G. and Milner , J.A. 1993 . Impact of Organosulfur Compounds in Garlic on Canine Mammary Tumor Cells in Culture . Cancer Lett. , 74 : 85 – 90 .
- Welch , C. , Wuarin , L. and Sidell , N. 1992 . Antiproliferative Effect of the Garlic Compound S-Allyl Cysteine on Human Neuroblastoma Cells in Vitro . Cancer Lett. , 63 : 211 – 219 .
- Mishra , R. , Upadhyay , S.K. and Maheshwari , P.N. 2001 . Stability of Allicin—A Kinetic Study . Indian Journal of Chemical Technology , 8 : 195 – 199 .
- Bautista , D.M. , Movahed , P. , Hinman , A. , Axelsson , H.E. , Sterner , O. , Hogestatt , E.D. , Julius , D. , Jordt , S. and Zygmunt , P.M. 2005 . Pungent Products from Garlic Activate the Sensory Ion Channel TRPA1 . PNAS , 102 ( 34 ) : 12248 – 12252 .
- Macpherson , L.J. , Geierstanger , B.H. , Viswanath , V. , Bandell , M. , Eid , S.R. , Hwang , S. and Patapoutian , A. 2005 . The Pungency of Garlic: Activation of TRPA1 and TRPV1 in Response to Allicin . Current Biology , 15 : 929 – 934 .
- Iberl , B. , Whinkler , G. and Knobloch , K. 1990 . Products of Allicin Transformation: Ajoenes and Dithiins Characterization and Their Determination by HPLC . Planta Med. , 56 : 202 – 211 .
- Lawson , L.D. and Gardner , C.D. 2005 . Composition, Stability and Bioavailability of Garlic Products Used in a Clinical Trial . Journal of Agricultural Food Chemistry , 53 : 6254 – 6261 .
- Cavallito , C.J. , Buck , J.S. and Suter , C.M. 1944 . Allicin, the Antibacterial Principle of Allium Sativum. II. Determination of the Chemical Structure . J. Am. Chem. Soc. , 66 : 1952 – 1954 .
- Hanley , A.B. and Fenwick , G.R. 1985 . Cultivated Alliums. . J. Plant Foods , 6 : 211 – 238 .
- Subramanyan , V. , Murthy , S.V. , Krishnamurthy , K. and Swaminanthan , M. 1958 . The Effect of Garlic on Certain Intestinal Bacteria . Food Science , 7 ( 8 ) : 223
- Nakagawa , S. , Masamoto , K. , Sumiyoshi , H. , Kunihiro , K. and Fuwa , T. 1980 . Effect of Raw Garlic Juice and Aged Garlic Extract on Growth of Young Rats and Their Organs After Peroral Administration . J. Toxicol. Sci. , 5 : 91 – 112 .
- Shashikanth , K.N. , Basappa , S.C. and Murthy , V.S. 1986 . Effect of Feeding Raw and Boiled Garlic (A. Sativum L.) Extracts on the Growth, Caecal Microflora and Serum Proteins of Albino Rats . Nutritional Reports International , 33 ( 2 ) : 313 – 319 .
- Shashikanth , K.N. , Basappa , S.C. and Murthy , V.S. 1985 . Allicin Concentration in the Gut of Rats and Its Influence on the Microfloa . Journal of Food Science and Technology , 22 : 440 – 444 .
- Sharma , V.D. , Sethi , M.S. , Kumar , A. and Rarota , J.R. 1977 . Antibacterial Property of Allium SativumLinn., in Vivoand in VitroStudies . Indian J. Exp. Biol. , 15 : 446
- Kumar , A. and Sharma , V.D. 1982 . Inhibitory Effect of Garlic (Allium SativumLinn.) on Enterotoxigenic Escherichia coli. . Indian J. Med. Res. Suppl. , 76 ( 2 ) : 66
- Rahman , M.S. , Al-Sheibani , H.I. , Al-Riziqi , M.H. , Mothershaw , A. , Guizani , N. and Bengtsson , G. 2006 . Assessment of the Anti-Microbial Activity of Dried Garlic Powders Produced by Different Methods of Drying . International Journal of Food Properties , 9 ( 3 ) : 503 – 513 .
- Jonkers , D. , van der Broek , E. , van Dooren , I. , Thijs , E. , Dorant , E. , Hageman , G. , Stobberingh , E. and Antimicrob , J. 1999 . Chemother. , 43 : 873 – 839 .
- Sivam , G.P. , Lampe , J.W. , Ulness , B. , Swanzy , S.R. and Potter , J.D. 1997 . Helicobacter Pylori—In Vitro Susceptibility to Garlic (Allium Sativum). . Extract Nutrition and Cancer , 27 ( 2 ) : 118 – 121 .
- Cellini , L. , Di Campli , E. , Masulli , M. , Di Bartolomeo , S. and Allocat , N. 1996 . Inhibition of Helicobacter Pyloriby Garlic Extract (Allium Sativum). . FEMS Immunology and Medical Microbiology , 13 : 273 – 277 .
- Ha , N.C. , Oh , S.T. , Sung , J.Y. , Cha , K.A. , Lee , M.H. and Oh , B.H. 2001 . Supermolecular Assembly and Acid Resistance of Helicobacter PyloriUrease . Nature-Structural Biology , 8 : 505 – 509 .
- Juszkiewicz , A. , Zaborska , A. , Laptas , A. and Olech , Z. 2004 . A Study of the Inhibition of Jack Bean Urease by Garlic Extract . Food Chemistry , 85 : 553 – 558 .
- Bakri , I.M. and Douglas , C.W.I. 2005 . Inhibitory Effect of Garlic Extract on Oral Bacteria . Archives of Oral Biology , 50 : 645 – 651 .
- Yoshida , S. , Kasuga , S. , Hayashi , N. , Ushiroguchi , T. , Matsuura , H. and Nakagawa , S. 1987 . Antigungal Activity of Ajoene Derived from Garlic . Appl. Environ. Microbiol. , 53 : 615 – 617 .
- Mirelman , D. , Monheit , D. and Varon , S. 1987 . Inhibition of Growth of Entamoeba Histolytica by Allicin, the Active Principle of Garlic Extract (Allium Sativum). . J. Infec. Dis. , 156 : 243 – 244 .
- Ogital , A. , Hirooka , K. , Yamamoto , Y. , Tsutsui , N. , Fujita , K. , Taniguchi , M. and Tanaka , T. 2005 . Synergistic Fungicidal Activity of Cu2+and Allicin, An Allyl Sulfur Compound from Garlic, and Its Relation to the Role of Alkyl Hydroperoxide Reductase 1 as a Cell Surface Defense in Saccharomyces Cerevisiae. . Toxicology , 215 : 205 – 213 .
- Adetumbi , M. , Javor , G.T. and Lau , B.H. 1986 . Antimicrob . Agents Chemother. , 30 : 499 – 501 .
- Ghannoum , M.A. 1988 . Studies on the Anticandidal Mode of Action of Allium Sativum (Garlic). . Journal of General Microbiology , 134 : 2917 – 2924 .
- Neuwirth , Z. , Sundstrom , D.C. and Thompson , N.H. 1988 . Antimicrob . Agents Chemother , 32 : 1763 – 1768 .
- Agarwal , K.C. 1996 . Therapeutic Actions of Garlic Constituents . Med. Res. Rev. , 16 : 111 – 124 .
- Augusti , K.T. and Mathew , P.T. 1973 . Effect of Long Term Feeding of the Aqueous Extracts of Onion (Allium Oepa Linn.) and Garlic (Allium Sativum Linn.) on Normal Rats . Indian J. Exptl. Biol. , 11 ( 3 ) : 239
- Ernst , E. 1987 . Cardiovascular Effects of Garlic (Allium Sativim): A Review . Pharmatherapeutics , 5 : 83 – 89 .
- Harenberg , J. , Giese , C. and Zimmermann , R. 1988 . Effect of Dried Garlic on Blood Coagulation, Fibrinolysis, Platelet-Aggregation and Serum Cholesterol Levels in Patients with Hyperlipoproteinemia . Atherosclerosis , 74 : 247 – 249 .
- Neil , H.A.W. and Silagy , C. 1994 . Garlic—Its Cardioprotective Properties . Curr. Opin. Lipidol. , 5 : 6 – 10 .
- Tayashree , J.A. , Gadkari , V. and Joshi , V.D. 1991 . Effect of Ingestion of Raw Garlic on Serum Cholesterol Levels, Clotting Time and Fibrinolytic Activity in Normal Subjects . J. Postgrad Med. , 37 : 128 – 131 .
- Warshafsky , S. , Kamer , R.S. and Sivak , S.L. 1993 . Effect of Garlic on Total Serum Cholesterol . Ann. Intern. Med. , 119 : 599 – 605 .
- Bordia , A. 1978 . Effect of Garlic on Human Platelet Aggregation in Vitro . Atherosclerosis , 30 : 355
- Mayeux , P.R. , Agrawal , K.C. , Tou , J.S.H. , King , B.T. , Lippton , H.L. , Hyman , A.L. , Kadowitz , P.J. and McNamara , D.B. 1988 . Agents and Actions , 25 : 182 – 190 .
- Focke , M. , Feld , A. and Lichtenthaler , H.K. 1990 . Allicin, A Naturally Occurring Antibiotic from Garlic, Specifically Inhibits Acertl-CoA Synthetase . Federation of European Biochemical Societies Litters , 261 ( 1 ) : 106 – 108 .
- Ankri , S. , Miron , T. , Rabinkov , A. , Wilechek , M. and Mirelman , D. 1997 . Allicin from Garlic Strongly Inhibits Cystein Proteinases and Cytophathic Effects of Entamoeba histolytica. . Antimicrob. Agents Chemother. , 41 : 2286 – 2288 .
- Rabinkov , A. , Miron , T. , Konstantinovski , L. , Wichek , M. , Mirelman , D. and Weiner , L. 1998 . The Mode of Action of Allic: Trapping of Radicals and Interaction with Thiol-Containing Proteins . Biochimica et Biophysica Acta , 1379 : 233 – 234 .
- Siegers , C.P. , Steffen , B. , Robke , A. and Pentz , R. 1999 . The Effects of Garlic Preparations Against Human Tumor Cell Proliferation . Phytomedicine , 6 : 7 – 11 .
- Dirsch , V.M. , Kiemer , A.K. , Wagner , H. and Vollmar , A.M. 1998 . Effect of Allicin and Ajoene, 2 Compounds of Garlic, on Inducible Nitric-Oxide Synthase . Atherosclerosis , 139 : 333 – 339 .
- Haber , D. , Suschetet , M. , Berges , R. , Astorg , P. and Siess , M.H. 1996 . Inhibition of Aflatoxin B1- and N‐nitrosodiethylamine-Induced Liver Preneoplastic Foci in Rats Fed Naturally Occurring Allyl sulfides . Nutr. Cancer , 25 : 61 – 70 .
- Atanasova-Goranova , V.K. , Dimova , V.K. and Pevicharova , G.T.J. 1997 . Nutr. , 78 : 335 – 345 .
- Dion , M.E. , Agler , M. and Milner , J.A. 1997 . Nutr. Cancer , 28 : 1 – 6 .
- Kwak , M.K. , Kim , S.G. , Kwak , J.Y. , Novak , R.F. and Kim , N.D. 1994 . Inhibition of Cytochrome P4502E1 Expression by Organosulfur Compounds Allylsulfide, Allylmercaptan and Allylmethylsulfide in Rats . Biochem. Pharmacol. , : 531 – 539 .
- Chen , L. , Lee , M. , Hong , J.Y. , Haug , W. , Wang , E. and Yang , C.S. 1994 . Biochem. Pharmacol. , 48 : 2199 – 2205 .
- Haber , D. , Siess , M.H. , Canivenc-Lavier , M. , Lebon , A.M. and Suschetet , M.J. 1995 . Toxicol. Environ. Health , 44 : 423 – 434 .
- Manson , M.M. , Ball , H.W. , Barrett , M.C. , Clark , H.L. , Judah , D.J. , Williamson , G. and Neal , G.E. 1997 . Carcinogenesis , 18 : 1728 – 1729 .
- Guyonnet , D. , Siess , M.H. , Le Bon , A.M. and Suschetet , M. 1999 . Modulation of Phase II Enzymes by Organosulfur Compounds from Allium Vegetables in Rat Tissues . Toxicol. Appl. Pharmacol. , 154 : 50 – 58 .
- Haber , D. , Siess , M.H. , Waziers , I. , Beau , P. and Suschetet , M. 1994 . Modification of Hepatic Drug-Metabolizing in Rat Fed Naturally Occurring Allyl Sulphides . Xenobiotica , 24 : 169 – 182 .
- Hatano , S. , Jimenez , A. and Wargovitch , M.J. 1996 . Chemopreventive Effect of S-allylcysteine and its Relationship to the Detoxification Enzyme Glutathione S-transferase . Carcinogenesis , 17 : 1041 – 1044 .
- Singh , A. and Singh , S.P. 1997 . Modulatory Potential of Smokeless Tobacco on the Garlic, Mace or Black Mustard-Altered Hepatic Detoxication System Enzymes, Sulfhydryl Content and Lipid Peroxidation in Murine System . Cancer Lett. , 118 : 109 – 114 .
- Chen , G.W. , Chung , J.G. , Hsieh , C.L. and Lin , J.G. 1998 . Effects of the Garlic Components Diallyl Sulfide and Diallyl Disulfide on Arylamine N-Acetyltransferase Activity in Human Colon Tumour Cells . Food Chem. Toxicol. , 36 : 761 – 770 .
- Belman , S. , Solomon , J. , Segal , A. , Block , E. and Barany , G. 1989 . Inhibition of Soybean Lipoxygenase and Mouse Skin Tumor Promotion by Onion and Garlic Components . J. Biochem. Toxicol. , 4 : 151 – 160 .
- Papini , E. , Satin , B. , Bucci , C. , De Bernard , M. , Telford , J.L. and Manetti , R. 1997 . The Small GTP Binding Protein Rab7 is Essential for Cellular Vacuolation Induced by Helicobacter PyloriCytotoxin . Eur. Mol. Biol. Org. J. , 16 : 15 – 24 .
- Feldberg , R.S. , Chang , S.C. , Kotik , A.N. , Nadler , M. , Neuwirth , Z. , Sundstrom , D.C. and Thompson , N.H. 1988 . In Vitro Mechanism of Inhibition of Bacterial Cell Growth by Allicin . Antimicrob. Agents Chemother. , 32 : 1763 – 1768 .
- Zheng , S. , Yang , H. , Zhang , S. , Wang , X. , Yu , L. , Lu , J. and Li , J. 1997 . Initial Study on Naturally Occurring Products from Traditional Chinese Herbs and Vegetables for Chemoprevention . J. Cell Biochem. , 27 : 106 – 112 .
- Oommen , S. , Anto , R.J. , Srinivas , G. and Karunagaran , D. 2004 . Allicin (From Garlic) Induces Caspase-Mediated Apoptosis in Cancer Cells . European Journal of Pharmacology , 485 : 97 – 103 .
- Park , S. , Cho , S. , Kwon , H. , Lee , K. , Rhee , D. and Pyo , S. 2005 . Caspase-Independent Cell Death by Allicin in Human Epithelial Carcinoma Cells: Involvement of PKA . Cancer Letters , 224 : 123 – 132 .
- Shalinsky , D.R. , McNamara , D.B. and Agrawal , K.C. 1989 . Inhibition of GSH-Dependent PGH2 Isomerase in Ammary Adenocarcinoma Cells by Allicin . Prostaglandins , 37 : 135 – 148 .
- Kang , N.S. , Moon , E.Y. , Cho , C.G. and Pyo , S. 2001 . Immunomodulating Effect of Garlic Component, Allicin, on Murine Peritoneal Macrophages . Nutrition Research , 21 : 617 – 626 .
- Hassan , H.T. 2004 . Ajoene (Natural Garlic Compound): A New Anti-Leukaemia Agent for AML Therapy . Leukemia Research , 28 : 667 – 671 .
- Ahmed , N. , Laverick , L. , Sammons , J. , Zhang , H. , Maslin , D.J. and Hassan , H.T. 2001 . Ajoene, a Garlic-Derived Natural Compound, Enhances Chemotherapy-Induced Apoptosis in Human Myeloid Leukaemia CD34-Positive Resistant Cells . Anticancer Research , 21 : 3519 – 3524 .
- Sigounas , G. , Hooker , J.L. , Li , W. , Anagnostou , A. and Steiner , M. 1997 . S-Allylmercaptocysteine, A Stable Thioallyl Compound, Induces Apoptosis in Erthroleukemia Cell Lines . Nutr. Cancer , 28 : 153 – 159 .
- Thatte , U. , Bagadey , S. and Dahanukar , S. 2000 . Modulation of Programmed Cell Death by Medicinal Plants . Cell Molecular Biology , 46 : 199 – 214 .
- Dalal , B.I. , Wu , V. and Barnett , M.J. 1997 . Induction Failure in de novoAML is Associated with Expression of High Levels of CD34 Antigen by the Leukemic Blasts . Leukemia Lymphoma , 26 : 299 – 306 .
- Hassan , H.T. and Zander , A.R. 1997 . Haematopoietic Cytokines in the Biology and Treatment of Acute Myeloid Leukaemia . Oncology Rep. , 4 : 1141 – 1149 .
- Kim , S.M. , Wu , C.M. , Kubota , K. and Kobayashi , A. 1995 . Effect of soybean oil on garlic volatile compounds isolated by distillation . J. Agric. Food Chem. , 43 : 449 – 452 .
- Kim , S.M. , Kobayashi , A. and Kubota , K. 1994 . Abstract paper . Annual Meeting of Nihon Koshinryo Kenkyukai . November 1994 , Kanazawa. pp. 13
- Kim , S.M. , Kubota , K. and Kobayashi , A. 1997 . Antioxidative Activity of Sulfur-Containing Flavor Compounds in Garlic . Biosci. Biotech. Biochem. , 61 ( 9 ) : 1482 – 1485 .
- Miron , T. , Rabinkov , A. , Mirelman , D. , Wilchek , M. and Weiner , L. 2000 . The Mode of Action of Allicin: Its Ready Permeability through Phospholipid Membranes May Contribute to Its Biological Activity . Biochimica et Biophysica Acta , 1463 : 20 – 30 .
- Prasad , K. , Laxdal , V.A. , Yu , M. and Raney , B.L. 1995 . Antioxidant Activity of Allicin, An Active Principle in Garlic . Mol. Cell. Biochem. , 148 : 183 – 189 .
- Wills , E.D. 1956 . Enzyme Inhibition by Allicin, the Active Principle of Garlic . Biochem. J. , 63 : 514 – 520 .
- Beckman , J.S. and Koppenol , W.H. 1996 . Nitric Oxide, Superoxide and Peroxynitrite: The Good, The Bad, and The Ugly . Am. J. Physiol. , 271 : C1424 – C1437 .
- Radomski , M.W. and Salas , E. 1995 . Nitric Oxide-Biological Mediator, Modulator and Factor of Injury: Its Role in the Pathogesis of Atherosclerosis . Atherosclerosis , 118 : S69 – S80 .
- Vimal , V. and Devaki , T. 2004 . Hepatoprotective Effect of Allicin on Tissue Defense System in Galactosamine/Endotoxin Challenged Rats . Journal of Ethnopharmacology , 90 : 151 – 154 .
- Siddique , Y.H. and Afzal , M. 2005 . Antigenotoxic Effect of Allicin Against Methyl Methanesulphonate Induced Genotoxic Damage . Journal of Environmental Biology , 26 ( 3 ) : 547 – 550 .
- Banerjee , A. 1979 . Effect of Aqueous Extract of Garlic on Arterial Blood Pressure of Normotensive and Hypertensive Rats . Artery , 2 : 369 – 373 .
- Auer , W. , Eiber , A. , Hertkorn , E. , Hoehfeld , E. , Kowhrle , U. , Lorenz , A. , Mader , F. , Merx , W. , Otto , G. , Schmid-Otto , B. and Taubenheim , H. 1990 . Hypertension and Hyperlipidemia: Garlic Helps in Mild Cases . Br. J. Clin. Pract. , 69 : 3 – 6 .
- Ali , M. and Thomson , M. 1995 . Consumption of a Garlic Clove a Day Could Be Beneficial in Preventing Thrombosis . Prostaglandins Leukotrienes Essential Fatty Acids. , 53 ( 3 ) : 211 – 212 .
- Chutani , S.K. and Bordia , A. 1981 . The Effect of Fried Versus Raw Garlic on Fibrinolytic Activity in Man . Atherosclerosis , 38 : 417 – 421 .
- Newall , C.A. , Anderson , L.A. and Phillipson , J.D. 1996 . Herbal Medicines—A Guide for Health Care Professionals , 129 – 133 . Cambridge, , UK : University Press .
- Foushee , D.B. , Ruffin , J. and Baanerjee , U. 1982 . Garlic as a Natural Agent for the Treatment of Hypertension: A Preliminary Report . Cystobios , 34 : 145 – 152 .
- Berthold , H.K. , Sudhop , T. and von Bergmann , K. 1998 . Effect of a Garlic Oil Preparation on Serum Lipoproteins and Cholesterol Metabolism: Randomized Controlled Trial . JAMA , 279 : 1900 – 1902 .
- Eilat , S. , Oestraicher , Y. , Rabinkov , A. , Ohad , D. , Mirelman , D. , Battler , A. , Eldar , M. and Vered , Z. 1995 . Alternation of Lipid Profile in Hyperlipidemic Rabbits by Allicin, an Active Constituent of Garlic . Coronary Artery Dis. , 6 : 985 – 990 .
- Elkayam , A. , Mirelman , D. , Peleg , E. , Wilchek , M. , Miron , T. , Rabinkov , A. , Sadetzki , S. and Rosenthal , T. 2000 . Comparison of the Effects of Allicinand Enalapril on Blood Pressure, Insulin and Triglycerides Levels in Fructose-Induced Hyperinsulinemic-Hyperlipidemic Hypertensive Rats . American Journal of Hypertension , 14 : 377 – 381 .
- Elkayam , A. , Mirelman , D. , Peleg , E. , Wilchek , M. , Miron , T. , Rabinkov , A. , Sadetzki , S. and Rosenthal , T. 2001 . The Effects of Allicin and Enalapril in Fructose-Induced Hyperinsulinemic Hyperlipidemic Hypertensive Rats . American Journal of Hypertension , 14 : 377 – 381 .
- Elkayam , A. , Mirelman , D. , Peleg , E. , Wilchek , M. , Miron , T. , Rabinkov , A. , Oron-Herman , M. and Rosenthal , T. 2003 . The Effects of Allicin on Weight in Fructose-Induced Hyperinsulinemic, Hyperlipidemic, Hypertensive Rats . American Journal of Hypertension , 16 : 1053 – 1056 .
- Al-Qattan , K.K. , Alnaqeeb , M.A. and Ali , M. 1999 . The Antihypertensive Effect of Garlic (Allium Sativum) in the Rat Two-Kidney, One-Clip Goldblatt Model . J. Ethanopharmacology , 66 : 217 – 222 .
- Ruffin , J. and Hunter , S.A. 1983 . An Evaluation of the Side Effects of Garlic as an Antihypertensive Agent . Cytobios , 37 : 85 – 89 .
- Bolton , S. , Null , G. and Troetel , W.M. 1982 . The Medical Uses of Garlic: Fact and Fiction . Am. Pharmacy , 22 : 448 – 451 .
- Ismail , M.F. , Gad , M.Z. and Hamdy , M.A. 1999 . A Study of the Hypolipidemic Properties of Pectin, Garlic and Ginseng in Hyperchloesterlemic Rabbits . Pharmacol Res. , 39 ( 2 ) : 157 – 166 .
- Kaye , A.D. , Nossaman , B.D. , Ibrahim , I.N. , Feng , C.J. , McNamara , D.B. , Agrawal , K.C. and Kadowitz , P.J. 1995 . Analysis of Responses of Allicin, a Compound from Garlic, in the Pulmonary Vascular Bed of the Cat and in the Rat . European Journal of Pharmacology , 276 : 21 – 26 .
- MacMahon , F.G. and Vargas , R. 1993 . Can Garlic Lower Blood Pressure? A Pilot Study . Pharmacotherapy , 13 ( 4 ) : 406
- Rendu , F. , Brohard-Bohn , B. , Pain , S. , Bachelot-Loza , C. and Auger , J. 2001 . Thiosulfinates inhibit platelet aggregation and microparticle shedding at a calpain-dependent step . Thromb. Haemost. , 86 : 1284 – 1291 .
- Goldman , I.L. , Kopelberg , M. , Debaene , J.E. and Schwart , B.S. 1996 . Thromb. Haemost. , 76 : 450 – 452 .
- Legnani , C. , Frascaro , M. , Guazzaloco , G. , Ludovici , S. , Cesarano , G. and Coccheri , S. 1993 . Effects of a dried garlic preparation on fibrinolysis and platelet aggregation in healthy subjects. , 43 : 119 – 122 .
- Apitz-Castro , R. , Cabrera , S. , Cruz , M.R. , Ledezma , E. and Jain , M.K. 1983 . Effects of Garlic Extract and of Three Pure Components Isolated from It on Human Platelet Aggregation, Arachidonate Metabolism, Release Reaction and Platelet Ultrastructure . Thromb Res. , 32 : 155 – 169 .
- Miron , T. , Rabinkov , A. , Peleg , E. , Rosenthal , T. , Mirelman , D. and Wilchek , M. 2004 . Allylmercaptocaptopril: A New Antihypertensive Drug . American Journal of Hypertension , 17 : 71 – 73 .
- Oron-Herman , M. , Rosenthal , T. , Mirelman , D. , Miron , T. , Rabinkov , A. , Wilchek , M. and Sela , B. 2005 . The Effects of S-allylmercaptocaptopril, the Synthetic Product of Allicin and Captopril, on Cardiovascular Risk Associated with the Metabolic Syndrome . Atherosclerosis , 183 : 238 – 243 .
- Kyo , E. , Uda , N. , Kasuga , S. and Itakura , Y.J. 2001 . Nutr. , 131 : 1075S – 1079S .
- Li , Q. and Verma , I.M. 2002 . Nat. Rev. Immunol. , 2 : 725 – 734 .
- Keiss , H.P. , Dirsch , V.M. , Hartung , T. , Haffner , T. , Trueman , L. , Auger , J. , Kahane , R. and Vollmar , A.J. 2003 . Nutr. , 133 : 2171 – 2175 .
- Lang , A. , Lahav , M. , Sakhnini , E. , Barshack , I. , Fidder , H.H. , Avidan , B. , Bardan , E. , Hershkoviz , R. , Bar-Meir , S. and Chowers , Y. 2004 . Allicin Inhibits Spontaneous and TNF-α Induced Secretion of Proinflammatory Cytokines and Chemokines from Intestinal Epithelial Cells . Clinical Nutrition , 23 : 1199 – 1208 .
- Josling , P. 2001 . Preventing the Common Cold with a Garlic Supplement: A Double-Blind, Placebo-Controlled Survey . Advances in Therapy , 18 ( 4 ) : 189 – 193 .
- Bhushan , S. , Sharma , S.P. , Singh , S.P. , Agarwal , S. , Indrayan , A. and Sethi , P. 1979 . Effect of Garlic on Normal Blood Cholesterol Level . Indian J. Physiol. Pharmacol. , 23 : 211
- Abramovitz , D. , Gavri , S. , Harats , D. , Levkovitz , H. , Mirelman , D. , Miron , T. , Eilat-Adar , S. , Rabinkov , A. , Wilchek , M. , Eldar , M. and Vered , Z. 1999 . Allicin-Induced Decrease in Formation of Fatty Steaks (Atherosclerosis) in Mice Fed on a Cholesterol-Rich Diet . Coron. Artery Dis. , 10 : 515 – 519 .
- Koch , H.P. and Lawson , L.D. 1996 . Garlic: The Science and Therapeutic Application of Allium sativum l. and Related Species , 2nd , Baltimore, MD : Williams and Wilkins .
- Egen-Schwind , C. , Eckard , R. , Jekat , F.W. and Winterhoff , H. 1992 . Pharmacokinetics of Vinyldithiins Transformation Products of Allicin . Planta Med. , 58 : 8 – 13 .
- Pushpendran , C.K. , Devasagayam , T.P.A. , Chintalwar , G.J. , Benerji , A. and Eapen , J. 1980 . The Metabolic Fate of[35S]-Diallyl Disulphide in Mince . Experientia , 36 : 1000 – 1001 .
- Teyssier , C. , Guenot , L. , Suschetet , M. and Siess , M.H. 1999 . Metabolism of Diallyl Disulfide by Human Liver Microsomal Cytochromes P-450 and Flavin-Containing Monooxygenases . Drug Metab. Dispos. , 27 : 835 – 841 .
- Kodera , Y. 1997 . “ Dietary Tolerance/Absorption/Metabolism of Phytochemicals in Garlic. ” . In Nutraceuticals-Designer Foods III, Garlic, Soy and Licorice; Lanchance, P.; Ed , 95 – 105 . Trumbell, CT : Food and Nutrition Press .
- Rosen , R. 2000 . Determination of Allicin and S-allyl Cysteine in Human Plasma and Urine After Consumption of Garlic and Garlic Products . Phytomed. , 7 ( 2 ) : 51
- Rahman , M.S. and Al-Belushi , R.H. 2006 . Dynamic Isopiestic Method (DIM): Measuring Moisture Sorption Isotherm of Freeze-Dried Garlic Powder and Other Potential Uses of DIM . International Journal of Food Properties , 9 ( 3 ) : 421 – 437 .
- Braverman , S. , Pechenick , T. , Gottlieb , H.E. and Sprecher , M.A . 2004 . Convenient Preparation of Mixed Allylic and Allenic Thiosulfinates . Tetrahedron Letters , 45 : 8235 – 8238 .
- Weinberg , D.S. , Manir , M.L. , Richardson , M.D. and Haibach , F.G. 1993 . Identification and Quantification of Organosulfur Compliance Markers in Garlic Extract . Journal of Agricultural Food Chemistry , 41 : 37 – 41 .
- Imai , J. , Ide , N. , Nagae , S. , Moriguchi , T. , Matsuura , H. and Itakura , Y. 1994 . Antioxidant and Radical Scavenging Effects of Aged Garlic Extract and its Constituents . Plants Med. , 60 : 417 – 420 .
- Nagae , S. , Ushijima , M. , Hatono , S. , Imai , J. , Kasuga , S. , Matsuura , H. , Itakura , Y. and Higashi , Y. 1994 . Pharmacokinetics of Garlic Compound S-Allyl Cysteine . Planta Med. , 60 : 214 – 217 .
- Jandke , J. and Spiteller , G. 1987 . Unusual Conjugates in Biological Profiles Originating from Consumption of Onions and Garlic . J. Chromatogr. , 421 : 1 – 8 .
- Steiner , M. and Li , W. 2001 . Aged Garlic Extract, a Modulator for Cardiovascular Risk Factors . J. Nutr. , 131 : 980S – 984S .
- Laakso , I. , Seppanen-Laakso , T. , Hiltunen , R. , Muller , B. , Jansen , H. and Knobloch , K. 1989 . Volatile Garlic Odor Components: Gas Phases and Adsorbed Exhaled Air Analyzed by Headspace Gas Chromatography-Mass Spectrometry . Planta Med. , 55 : 257 – 261 .
- Minami , T. , Boku , T. , Inada , K. , Morita , M. and Okazaki , Y. 1989 . Odor Components of Human Breath After the Ingestion of Grated Raw Garlic . J. Food Sci. , 54 : 763 – 765 .
- Rahman , K. and Billington , D. 2000 . Dietary Supplementation with Aged Garlic Extract Inhibits ADP-Induced Platelet Aggregation in Humans . J. Nutr. , 130 : 2662 – 2665 .
- Steiner , M. and Lin , R.I. 1998 . Changes in Platelet Function and Susceptibility Lipoproteins to Oxidation Associated with Administration of Aged Garlic Tract . J. Cardiovasc. Pharmacol. , 31 : 904 – 908 .
- Kanezawa , A. , Nakagawa , S. , Sumiyoshi , H. , Masamoto , K. , Harada , H. , Nakagami , S. , Date , S. , Yokota , A. , Nishikawa , M. and Fuwa , T. 1984 . General Toxicity Tests of Garlic Extract Preparation (Kyoleopin) Containing Vitamins . Oyo Yakuri , 27 : 909 – 929 .
- Nakagawa , S. , Masamoto , K. , Sumiyoshi , H. and Harada , H. 1984 . Acute Toxicity of Garlic Extract . J. Toxicol. Sci. , 9 : 57 – 60 .
- Nakagawa , S. , Sumiyoshi , H. , Masamoto , K. , Kanezawa , A. , Harada , H. , Nakagami , S. , Date , S. , Yokota , A. , Nishikawa , M. and Fuwa , T. 1984 . Acute and Subacute Toxicity Tests of a Ginseng and Garlic Preparation Containing Vitamin B1(Leopin-five). . Oyo Yakuri. , 27 : 1133 – 1150 .
- Sumiyoshi , H. , Kanezawa , A. , Masamoto , K. , Harada , H. , Nakagami , S. , Yoga , A. , Nishikawea , M. and Nakagawa , S. 1984 . Chronic Toxiocity Test of Extract in Rats . J. Toxicol. Sci. , 9 : 61 – 75 .
- Yoshida , S. , Hirao , Y. and Nakagawa , S. 1984 . Mutagenicity and Cytotoxic Tests of Garlic . J. Toxicol. Sci. , 9 : 77 – 86 .
- Lawson , L.D. and Wang , Z.J. 1993 . Prehepatic Fate of the Organosulfur Compounds Derived from Garlic (Allium Sativum). . Planta Med. , 59 : A688 – A689 .
- Miron , T. , Rabinkov , A. , Mirelman , D. , Weiner , L. and Wilchek , M. 1998 . A Spectrophotometric Assay for Allicin and Allinase (Alliin Lyase) Activity: Reaction of 2-Nitro-5-Thiobenzoate with Thiosulfinates . Anal Biochem. , 265 : 317 – 325 .
- Lee , E.S. , Steiner , M. and Lin , R. 1994 . Thioallyl Compounds: Potent Inhibitors of Cell Proliferation . Biochem. Biophys. Acta , 1221 : 73 – 77 .
- Pinto , J.T. , Qiao , C. , Xing , J. , Rivlin , R.S. , Protomastro , M.L. , Weissler , Y. , Tao , H. , Thaler , H. and Heston , W.D. 1997 . Effects of Garlic Thioallyl Derivatives on Growth, Glutathione Concentration, and Polyamine Formation of Human Prostate Carcinoma Cells in Culture . Am. J. Clin. Nutr. , 66 : 398 – 405 .
- Chu , T. , Burch , J.L. , De Paula Brotto , M.A. , Creazzo , T.L. , Han , J. , Han , G.Y. and Potter , D.E. 1996 . Elevation of Intracellular Ca2+ Concentration in Rabbit Nonpigmented Ciliary Epithelial Cells by Allicin . Comp. Biochem. Physiol. , 115C ( 1 ) : 89 – 94 .
- Rabinkov , A. , Miron , T. , Mirelman , D. , Wilchek , M. , Glozman , S. , Yavin , E. and Weiner , L. 2000 . S‐Allylmercaptoglutathione: The Reaction Product of Allicin with Glutathione Possesses SH‐Modifying and Antioxidant Properties . Biochimica Biophysica Acta , 1499 : 144 – 153 .
- Koch , H.P. 1993 . Saponine in Knoblauch und Kchenzwiebel . Deutsch Apotheker Zeitung 133 Jahrg Nr 41 , 14 ( 10 ) : 63 – 75 .
- Mader , F.H. 1990 . Treatment of Hyperlipidaemia with Garlic Powder Tablets . Arzneim. Forsch. , 40 : 3 – 8 .
- Siegers , C.P. 1992 . “ Allium Sativum ” . In Adverse Effects of Herbal Drug , Edited by: Smet , P.A. , Keller , G.M. , Hansel , K.R. and Chandler , R.F. 73 Berlin, , Germany : Springer-Verlag .
- Desai , H.G. , Kairo , R.H. and Choksi , A.P. 1990 . Effect of Ginger and Garlic on DNA Content of Gastric Aspirate . Indian J. Med. Res. , 92 : 139 – 141 .
- Miyamoto , T. 1938 . Effects of Garlic Water-Soluble but Alcohol-Insoluble Component and Garlic Volatile Oil on Blood Serum Protein and Residual Nitrogen . J. Manchurian Med. , 28 : 285 – 296 .
- Katsunuma , S. 1932 . On Effect of Garlic on Anemia . Exp. Med. , 18 : 442 – 444 .
- Kuzutani , S. 1934 . On Effects of Garlic (Allium Scorodoprasum L.) on Anemia . Clin. Pathol. Hematol. , 3 : 1175 – 1233 .
- Lybarger , J.A. , Gallagher , J.S. , Pulver , D.W. , Litwin , A. , Brooks , S. and Bernstein , I.L. 1982 . Occupational Asthma Induced by Inhalation and Ingestion of Garlic . J. Allergy Clin. Immunol. , 69 : 448 – 454 .
- von Kirsten , D. and Meister , W. 1985 . Berufsbedingte Knoblauchallergie . Ale. Gologie Jahrgang , 8 : 511 – 512 .
- Burden , E. , Ahmed , S. , Jain , M.K. , Crecely , R.W. , Apitz-Castro , R. and Cruz , M.R. 1984 . Ajoene: A Potent Antithrombic Agent from Garlic . J. Am. Chem. Soc. , 106 : 8295 – 8296 .
- Garty , B.Z. 1993 . Burns Garlic . Pediatrics , 91 : 658 – 659 .
- Lembo , G. , Balato , N. , Patruno , C. , Auricchio , L. and Ayala , F. 1991 . Allergic Contact Dermatitis Due to Garlic (Allium Sativum). . Contact Dermatitis , 25 : 330 – 331 .
- McFadden , J.P. , White , I.R. and Rycroft , R.J. 1992 . Allergic Contact Dermatitis from Garlic . Contact Dermatitis , 27 : 333 – 334 .
- Mitchell , J.C. 1980 . Contact Sensitivity to Garlic (Allium). . Contact Dermatol. , 6 : 356 – 357 .
- Parish , R.A. , Mcintrite , S. and Heimbach , D.M. 1987 . Garlic Burns: A Naturopathic Remedy Gone Awry . Pediatr. Emerg. Care , 3 : 258 – 260 .
- Dixit , V.P. and Joshi , S. 1982 . Effects of Chronic Administration of Garlic (Allium Sativum) on Testicular Function . Indian J. Exp. Biol. , 20 : 534 – 536 .
- Qian , Y.X. , Shen , P.J. , Xu , R.Y. , Liu , G.M. , Yang , H.Q. , Lu , Y.S. , Sun , P. , Zhang , R.W. , Qi , L.M. and Lu , Q.H. 1986 . Spermicidal Effect in Vitro by the Active Principle of Garlic . Contraception , 34 : 295 – 302 .
- Imada , O. 1990 . “ In Toxicity Aspects of Garlic, First World Congress on the Health Signifance of Garlic and Garlic Constituents ” . In Nutrition International 47 Irvine, CA
- Hasegawa , Y. , Kikuchi , N. , Kawashima , Y. , Shimizu , K. and Nishiyama , M. 1983 . Clinical Effects of Kyoleopin Against Various Indefinite Complaints in the Field of Internal Medicine . Shinyaku To Rinsho , 32 : 365 – 376 .
- Kawashima , Y. , Ochiai , Y. and Fujisaki , I. 1989 . Clinical Study of Kyoleopin for Patients with Hyperlipidemia . Shinryou To Shinyaku , 26 : 377 – 388 .
- Steiner , M. , Kham , A.H. , Holbert , D. and Lin , R.I.S. 1996 . A Double Blind Crossover Study of Aged Garlic Extract and Placebo Administration on Blood Lipids . Am. Clin. Nutr. , 64 : 866 – 870 .