Abstract
Cassava starch suspension was homogenized at different pressures (0, 20, 40, 60, 80, and 100 MPa) with a high-pressure homogenizer. To investigate the effect of high-pressure homogenization on the structure of cassava starch, the samples were characterized using microscopy, laser scattering, and X-ray diffraction techniques, with native and heat gelatinized cassava starches as controlled samples. The temperature of starch suspension increased linearly with applied pressure at a rate of 0.187°C/MPa. Microscopy studies showed that cassava starch was partly gelatinized after high-pressure homogenization, and the degree of gelatinization increased with homogenizing pressure. Results of laser scattering measurements suggested a considerable increase in particle size after homogenization at 100 MPa as a result of granule swelling. The X-ray diffraction pattern showed that there was no evident change after homogenization suggesting that the crystalline structure of starch granules was resistant to high-pressure homogenization.
INTRODUCTION
The world's first homogenizer presented at the 1900 World Fair in Paris was invented by Auguste Gaulin. More recently, in the early 1990s, a new generation of homogenizers, referred to as high-pressure homogenizers, has been developed. This new technology has a different reaction chamber geometry that can attain a pressure up to 500 MPa. These high-pressure homogenizers have opened up new areas of application for industry.[Citation1] Chemical, pharmaceutical, specialty food, and biotechnology facilities all use homogenizers to emulsify, disperse, mix, and process their products.[Citation2] However, it was only after about 1970 that an understanding of the homogenization mechanism was obtained through fundamental studies.[Citation1] The mechanism has been thought to be the result of a combination of several different effects, including shear in the liquid and between the valve faces, impact against the side walls, velocity changes and turbulence, cavitation forces, turbulence itself, and more recently the velocity gradient.[Citation3]
Starch is a kind of macromolecular compound composed of high sugars. It is widely used in paper, textile, adhesive, sweetener, and food industries. Among the different starches used in industry, cassava starch is very important because of its easy extractability, high viscosity, and paste clarity.[Citation4,Citation5] When heated in water, starch granules undergo an order-disorder transition known as gelatinization. Starch gelatinization is the collapse of molecular orders within the starch granule manifested in irreversible changes in properties such as granule swelling, native crystallite melting, loss of birefringence, and starch solubilization.[Citation6] Research results also indicate that starches can be gelatinized by high pressure even at room temperature, and pressure induced gelatinization is significantly different from heat induced gelatinization.[Citation7,Citation8,Citation9,Citation10] It is well established that the gelatinization patterns of starches depend not only on their chemical structure but also on the level and kind of energy supplied.[Citation11] Heat treatment and pressure process provide necessary energy for starch gelatinization in different ways.
High-pressure homogenizations where high pressures are experienced over very short times are different from static high-pressure systems, because pressure induced phenomena of cavitation, shear, turbulence, and temperature rise are involved simultaneously.[Citation1] With the specific energy output, high-pressure homogenizers were reported to introduce novel changes to products. However, high-pressure homogenizers were rarely utilized in starch processing. There is limited information available on the effects of high-pressure homogenization treatment on starch structure and properties. It would be then very important to obtain a detailed understanding of the effects of such a process on the structure and properties of starches. In this study, the cassava starch suspension was homogenized at different pressures with a high-pressure homogenizer. The effect of high-pressure homogenization on the structure of cassava starch was investigated using microscopy, laser scattering, and X-ray diffraction techniques.
MATERIALS AND METHODS
Materials
Commercial cassava starch was purchased from Beijing Quanfeng Starch Company, China. The moisture content of the starch was determined by drying 3 replicated samples in an air-oven at 105°C to constant weight. The average moisture content of the cassava starch was determined as 9.3% (w/w). An analytical grade anhydrous alcohol (95%, w/w) was obtained from Beijing Beihua Chemical Company.
High-pressure Homogenization of Cassava Starch Suspension
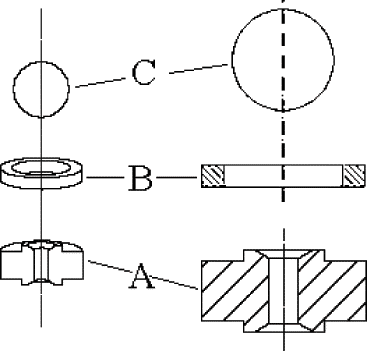
Five percent (w/w) starch suspension was prepared by adding air-oven dried cassava starch in deionized water at room temperature (27 ± 1°C). Well mixed suspension was homogenized in a high-pressure homogenizer (NS1001L-PANDA 2K, Niro Soavi S.p.A., Italy) at 0, 20, 40, 60, 80, and 100 MPa for one pass. Approximately 250 mL suspension was processed at each pressure level. PANDA 2K was a two-stage homogenizer, and only the first high-pressure valve, shown as , was used. The suspension temperatures were monitored just before and immediately after homogenization with an immersion thermometer. Controlled experiment was done as described above with deionized water (24.6°C).
Figure 1 PS type homogenizing valve used in experiment, where A is tungsten carbide valve seat; B is stainless steel impact ring; and C is ceramic ball.
Homogenized samples were compared to native and heat gelatinized cassava starches in order to provide an elucidation for the mechanism of high-pressure homogenization. Heat gelatinization was accomplished by heating starch-water suspension (5%, w/w) in water bath for 2 h at the temperature that 100 MPa homogenized sample achieved after the treatment.
Microscopy Study of Cassava Starch Granules
To investigate the structural changes of starch granules after high-pressure homogenization, starch samples treated under different conditions were immediately observed under optical microscope (CX31 Biological Microscope, Olympus Corporation, Japan) equipped with a CCD camera module. The detailed structure of 100 MPa homogenized sample was viewed and photographed with a scanning electron microscope (SEM) (KYKY-2800, KYKY Technology Development Ltd., China). In order to prepare starch samples for SEM observations, the following procedure was used.
After filtering out starch granules from the homogenized starch suspensions, they were dehydrated with anhydrous alcohol, and subsequently they were oven-dried at 80°C for 45 min in order to obtain dry starch samples. Dried starch samples were pulverized and then preserved in desiccator. 100 MPa homogenized sample was sprayed on a metal plate previously covered with double-sided adhesive and sputtered with a layer of gold using an ion coater (IB-3, Eiko, Japan) under vacuum. The samples were examined using SEM at 20 kV accelerating voltage.
Laser Scattering Measurement
Particle size distributions of 0 and 100 MPa homogenized samples were determined using the laser scattering method. The diffractometer used was a Mastersizer 2000 laser diffractometer (Malvern Instruments, UK) equipped with a He–Ne laser (wavelength 632.8 nm). The samples were measured within 3 h after high-pressure homogenization and dispersed by ultrasound in the diffractometer cell before measurements. The size distributions were calculated from the intensity of light diffracted at each angle using Mie theory. Refractive indices of water and starch we used were 1.33 and 1.53 at 27°C, respectively. The absorbance of starch granules was taken as 0.1.[Citation12]
X-ray Diffraction Analysis of Cassava Starch
Dried starch samples for X-ray diffraction were prepared as described above. A XD-2 X-ray diffractometer (Beijing Purkinje General Instrument Co., Ltd., China) was used. Starch samples were pulverized to pass 360 mesh using a specific carnelian mortar. X-ray powder diffraction analyses were performed at 36 kV and 20 mA with nickel-filtered Cu-K α (wavelength 1.5405 Å) radiation. The scattered intensities were measured with a scintillation counter. Powdered samples were scanned from 4° to 60° (2θ) with a scanning speed of 0.25°min−1 and sampling interval of 0.02°. Each sample was scanned at least in duplicate.
RESULTS AND DISCUSSION
Temperature Changes of Cassava Starch Suspension and Deionized Water during High-pressure Homogenization
The temperature changes of starch suspension and deionized water after homogenization at different pressures are shown in . Experimental data were regressively analyzed by the least squares method to model the pressure effect on the temperatures of starch suspensions and deionized water. Two linear regression equations between pressure and temperature were obtained from the experimental data ().
Figure 2 Effect of homogenizing pressure on the temperatures of 5% cassava starch suspension and deionized water. Initial temperatures of the starch suspension and deionized water were 27.2 and 24.6°C, respectively.
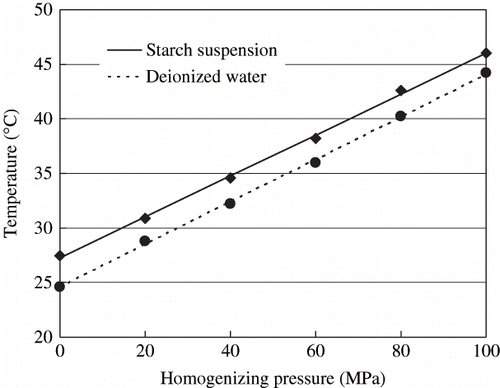
Table 1 Regression analysis between homogenizing pressure and temperature of starch suspension and deionized water (p < 0.05)
As it can be seen from , both equations have good correlation coefficients (R is very close to 1) and pass the statistical test for the effectiveness (Fcal > Fcri ) at a confidence level of 0.95. This suggests that the relationship between the temperature and homogenizing pressure can be adequately described by a simple linear equation. As can be seen from and , the temperatures of starch suspensions and deionized water increase with increasing homogenizing pressure. The slopes which indicate the dependence of temperature on homogenizing pressure of starch suspension and deionized water are 0.187°C/MPa and 0.194°C/MPa, respectively.
The linear increase in temperature has been widely reported by other researchers, though different values for the extent of temperature change with pressure were reported. Floury, Desrumaux, and Lardières reported an increase of 12.1°C per 100 MPa after homogenizing oil-in-water emulsion at pressures up to 350 MPa, and they stated that the strong warming up of the fluid was due to viscous stress caused by the high velocity of the fluid flow, which was then impinged on the ceramic valve.[Citation13] But they did not mention how the mechanical energy was dissipated as heat in the fluid. Sandra and Dalgleish reported a temperature rise of 14°C after homogenizing at 186 MPa for 6 passes.[Citation14] And they explained it with the theory of Hayes and Kelly, which attributed the temperature rise to high pressure, high shear, turbulence, and cavitation when sample passed through the homogenization valve.[Citation15] However, more detailed explanation was not included, either.
In our experiment, the temperature of starch suspension increased 18.7°C after homogenizing the starch suspension at a pressure of 100 MPa, which was in excellent agreement with the findings of Thiebaud et al.[Citation16] For deionized water the value was 0.194°C/MPa, which was slightly higher than that of starch suspension. In other words, the presence of starch granules slightly reduced the temperature rise. So it could be concluded that the heat was not generated from the impingement of starch granules on the ceramic ball or impact ring, nor was it resulted from the friction between different starch granules or starch granules and high-pressure valve. If the temperature rise is due to viscous stress, as described by Floury et al.,[Citation13] there would be a significant difference in temperature rise between starch suspension and deionized water because of their diverse viscosities caused by different composition, and the temperature of deionized water would rise more slowly than starch suspension. However, experimental evidence was contrary to this assumption. The temperature rise could not be the result of high pressure compression since starch granules and water can not be compressed easily.[Citation16,Citation17] So we assume that the temperature rise might be resulted from acute change of pressure in very short time. The acute change of pressure can induce the formation of cavities filled with gas or vapor as pressure decreased and their collapse as soon as the pressure increased again, which is commonly referred to as cavitation. The sudden collapse of vapor bubbles caused by rapid increase of pressure generate strong shock waves and temperatures up to 104 K.[Citation18] The addition of starch reduced temperature rise by restraining the formation of cavities.
Micrographs of Cassava Starch Granules
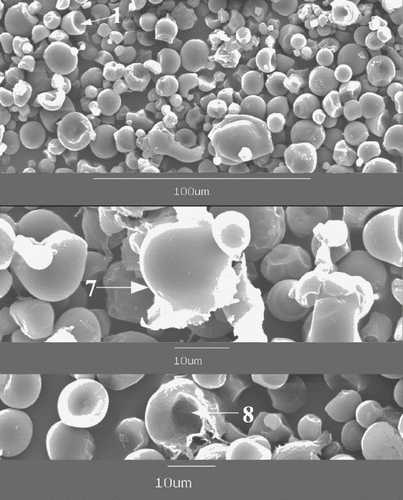
Micrographs of starch granules are shown in and . Native cassava starch granules (A) used in this study were considerably irregular in shape with oval, round and truncated (1) granules. Sriroth et al. observed similar types of cassava starch agglomerates cultivated in Thailand.[Citation19] Almost all of the truncated granules had concave spherical surface at the truncated end. This specific structure presented a black ring (2) in bright field micrographs. Some of the granules had a small pit (3) at the surface, which was generally considered to be the growing point of starch granules, while others had surface rupture (4) caused by preprocessing such as drying. Homogenization at 0, 20, and 40 MPa had no obvious effect on the structure of cassava starch granules, starch granules retained granule shape (B, C, & D). When the homogenizing pressure was increased to 60 MPa, few starch granules began to lose their native pattern (E-5 & 6). More starch granules were characterized by significant deformations when homogenizing pressure was increased to 80 and 100 MPa (F, G). And most of the deformed granules disintegrated into fragments (7). Furthermore, some of the fragments showed gel-like structures (8). Błaszczak et al. obtained similar gel structures by hydrostatically pressurizing potato starch.[Citation11] Some starch granules also deformed after heating them at 46°C for 2 h (H).
Figure 3 Micrographs of cassava starch granules treated under different conditions: (A) native, (B) homogenized at 0 MPa, (C) 20 MPa; (D) 40 MPa; (E) 60 MPa; (F) 80 MPa; (G) 100 MPa; and (H) heated at 46°C for 2 h.
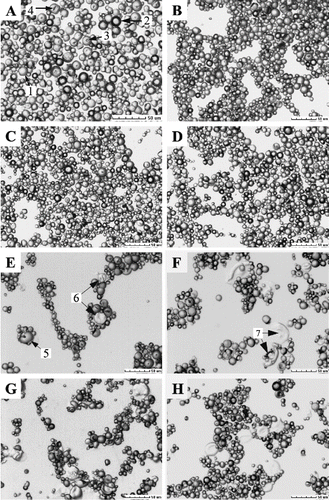
While being treated in high-pressure homogenizer, as described above, the starch suspensions underwent a temperature rise caused by dynamic high-pressure, which induced starch gelatinization manifested in deformation of starch granules. The degree of gelatinization increased with homogenizing pressure, because the temperature rose linearly with applied pressure. It was observed that the deformation of homogenized starch granules and heat treated ones was similar suggesting that the deformation of starch granules was mainly caused by temperature rise. However, some fragmented granules were observed only in the homogenized starch samples due to shear and/or impact forces generated during the homogenization process. It was noted that the starch fragments were big in size, which indicated that big granules were more sensitive to high-pressure homogenization.
Even though the temperature of the starch suspensions in our experiment (46°C) was much lower than the critical gelatinization temperature (64°C),[Citation4] the structural change of starch granules did happen. It implies that gelatinization course starts at the temperature lower than critical gelatinization temperature.
Particle Size Distributions of Cassava Starch Granules
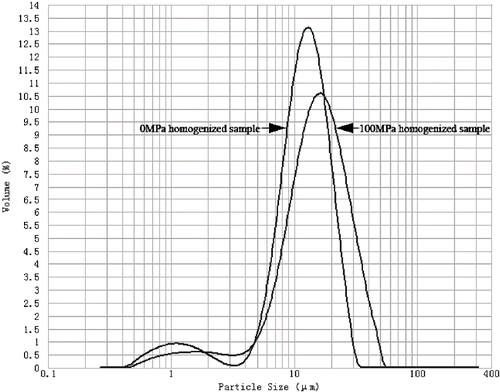
Results of particle size measurements using the laser scattering technique are expressed as size distributions () and mean diameters (). As shown in , the particle size distribution of 0 MPa homogenized sample was characterized by a main peak at about 13.3 μm and a small shoulder around 1.1 μm. d(0.1), d(0.5), and d(0.9) of the 0 MPa homogenized sample were 5.609, 12.080, and 20.686 μm, respectively. The particle size of the homogenized cassava starch obtained in this study was similar to that of native cassava starches obtained by previous researchers.[Citation19,Citation20] However, it should be emphasized that a direct comparison of the particle size distributions was not possible since the aforementioned researchers did not use the laser scattering technique to measure the particle size distribution. Homogenization at 100 MPa led to a shift of the size distribution curve to higher value and a slight decrease of the main peak. Corresponded to this change, the mean diameters of the starch granules homogenized at 100 MPa, listed in , increased considerably.
Table 2 Diameters of cassava starch granules homogenized at 0 and 100 MPa (μm)
It is known that starch granules absorb water and swell to some degree in warm water.[Citation4,Citation21] Stolt, Oinonen, and Autio found similar granule swelling induced by high hydrostatic pressure.[Citation22] While being homogenized, starch molecules combined with water molecules activated by heat and high-pressure. Starch granules that absorbed a large quantity of water swelled rapidly. The structure change which indicated starch gelatinization as described above caused size increase in turn. The pronounced size increase of 100 MPa homogenized sample in our experiment was the result of high pressure compression as well as the temperature rise induced by dynamic high-pressure. Stute et al. reported different swelling patterns of various starches in detail.[Citation23] They found that cassava starch showed a much higher and completely different swelling pattern in contrast to corn and arrow root starches.
X-ray Diffraction Analysis of Cassava Starch Treated under Different Pressures
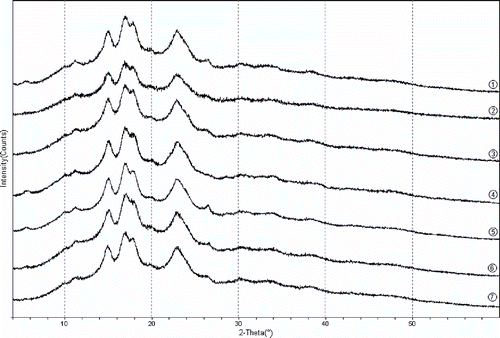
The X-ray diffraction patterns obtained are presented in . As can be seen from the XRD patterns, all samples showed typical A-type diffraction pattern with main peaks at about 15.0°, 17.0°, 17.9°, and 22.9° (2θ). Cassava starch was considered to be A-type crystalline structure by Hizukuri,[Citation24] Defloor et al.,[Citation25] and Jyothi et al.[Citation26] However, Zobel,[Citation27] and Gebre-Mariam et al.[Citation28] considered cassava starch as C-type crystalline structure. The different crystalline structures may result from different varieties and planting conditions. The X-ray diffraction of semi-crystalline cassava starch was characterized by the presence of both relatively sharp reflections and broad diffuse maxima. The former were the result of coherent scattering from crystalline structure and the latter originated from incoherent scattering caused by amorphous material.[Citation29,Citation30] Additionally, there was no obvious change in the X-ray diffraction patterns after homogenization. This revealed the crystalline structure of starch granules was much resistant to high-pressure homogenization and the absorption of water and radial swelling occur primarily within the amorphous region.
CONCLUSION
The present work investigated the effect of high-pressure homogenization (up to 100 MPa) on the structure of cassava starch. The noticeable temperature rise of starch suspension (18.7°C per 100 MPa) ascribed to high-pressure homogenization partly gelatinized cassava starch, which was evident in granule deformation (observed by microscopy) and swelling (size increase determined by laser scattering technique). And the degree of gelatinization increased with increasing homogenizing pressure. The result of microscopy also showed that small particles were more resistant to high-pressure homogenization. X-ray powder diffraction patterns implied that homogenization induced gelatinization occurred in the amorphous region of starch granule. The crystalline structure of starch granule was much resistant to high-pressure homogenization at a pressure not more than 100 MPa. Future research may focus on high-pressure homogenization at pressures higher than 100 MPa.
ACKNOWLEDGMENTS
Research support was provided by the Key Project of Chinese Ministry of Education, (No. 105014), the Funding System for Scientific Research Projects of Doctor Subject of Chinese Advanced University (No. 20050019029) and Funding System for Scientific Research Projects of China Agricultural University (No. 2004010).
REFERENCES
- Paquin , P. 1999 . Technological Properties of High Pressure Homogenizers: The Effect of Fat Globules, Milk proteins, and Polysaccharides . International Dairy Journal , 9 : 329 – 335 .
- Pandolf , W.D. and Kinney , R.R. 1998 . High-Pressure Homogenization . Chemical Processing , 61 ( 3 ) : 39 – 43 .
- Brookman , J.S.G. 1974 . Mechanism of Cell Disintegration in a High Pressure Homogenizer . Biotechnology and Bioengineering , 16 : 371 – 383 .
- Jyothi , A.N. , Sasikiran , K. , Sajeev , M.S. , Revamma , R. and Moorthy , S.N. 2005 . Gelatinisation Properties of Cassava Starch in the Presence of Salts, Acids and Oxidising Agents . Starch/Stärke , 57 : 547 – 555 .
- Mandala , I.G. and Palogou , E.D. 2003 . Effect of Preparation Conditions and Starch/xanthan Concentration on Gelation Process of Potato Starch Systems . International Journal of Food Properties , 6 ( 2 ) : 311 – 328 .
- Maaruf , A.G. , Che Man , Y.B. , Asbi , B.A. , Junainah , A.H. and Kennedy , J.F. 2001 . Effect of Water Content on the Gelatinisation Temperature of Sago Starch . Carbohydrate Polymers , 46 : 331 – 337 .
- Stolt , M. , Stoforos , N.G. , Taoukis , P.S. and Autio , K. 1999 . Evaluation and Modelling of Rheological Properties of High Pressure Treated Waxy Maize Starch Dispersions . Journal of Food Engineering , 40 : 293 – 298 .
- Katopo , H. , Song , Y. and Jane , J. 2002 . Effect and Mechanism of Ultrahigh Hydrostatic Pressure on the Structure and Properties of Starches . Carbohydrate Polymers , 47 : 233 – 244 .
- Bauer , B.A. and Knorr , D. 2005 . The Impact of Pressure, Temperature and Treatment Time on Starches: Pressure-induced Starch Gelatinisation as Pressure Time Temperature Indicator for High Hydrostatic Pressure Processing . Journal of Food Engineering , 68 : 329 – 334 .
- Błaszczak , W. , Fornal , J. , Valverde , S. and Garrido , L. 2005 . Pressure-Induced Changes in the Structure of Corn Starches with Different Amylose Content . Carbohydrate Polymers , 61 : 132 – 140 .
- Błaszczak , W. , Valverde , S. and Fornal , J. 2005 . Effect of High Pressure on the Structure of Potato Starch . Carbohydrate Polymers , 59 : 377 – 383 .
- Singh , J. , McCarthy , O.J. and Singh , H. 2006 . Physico-chemical and Morphological Characteristics of New Zealand Taewa(Maori Potato) Starches . Carbohydrate Polymers , 64 : 569 – 581 .
- Floury , J. , Desrumaux , A. and Lardières , J. 2000 . Effect of High-Pressure Homogenization on Droplet Size Distributions and Rheological Properties of Model Oil-in-Water Emulsions . Innovative Food Science & Emerging Technologies , 1 : 127 – 134 .
- Sandra , S. and Dalgleish , D.G. 2005 . Effects of Ultra-High-Pressure Homogenization and Heating on Structural Properties of Casein Micelles in Reconstituted Skim Milk Powder . International Dairy Journal , 15 : 1095 – 1104 .
- Hayes , M.G. and Kelly , A.L. 2003 . High Pressure Homogenisation of Raw Whole Milk (a) Effects on Fat Globule Size and Other Properties . Journal of Dairy Research , 70 : 297 – 305 .
- Thiebaud , M. , Dumay , E. , Picart , L. , Guiraud , J.P. and Cheftel , J.C. 2003 . High-Pressure Homogenisation of Raw Bovine Milk. Effects on Fat Globule Size Distribution and Microbial Inactivation . International Dairy Journal , 13 : 427 – 439 .
- Kolakowski , P. , Dumay , E. and Cheftel , J.-C. 2001 . Effects of High Pressure and Low Temperature on β-lactoglobulin Unfolding and Aggregation . Food Hydrocolloids , 15 : 215 – 232 .
- Freudig , B. , Tesch , S. and Schubert , H. 2003 . Production of Emulsions in High-Pressure Homogenizers—Part II: Influence of Cavitation on Droplet Breakup . Engineering in Life Sciences , 3 ( 6 ) : 266 – 270 .
- Sriroth , K. , Santisopasri , V. , Petchalanuwat , C. , Kurotjanawong , K. , Piyachomkwan , K. and Oates , C.G. 1999 . Cassava Starch Granule Structure-Function Properties: Influence of Time and Conditions at Harvest on Four Cultivars of Cassava Starch . Carbohydrate Polymers , 38 : 161 – 170 .
- Santisopasri , V. , Kurotjanawong , K. , Chotineeranat , S. , Piyachomkwan , K. , Sriroth , K. and Oates , C.G. 2001 . Impact of Water Stress on Yield and Quality of Cassava Starch . Industrial Crops and Products , 13 : 115 – 129 .
- Spigno , G. and De Faveri , D.M. 2004 . Gelatinization Kinetics of Rice Starch Studied by Non-isothermal Calorimetric Technique: Influence of Extraction Method, Water Concentration and Heating Rate . Journal of Food Engineering , 62 : 337 – 344 .
- Stolt , M. , Oinonen , S. and Autio , K. 2001 . Effect of High Pressure on the Physical Properties of Barley Starch . Innovative Food Science & Emerging Technologies , 1 : 167 – 175 .
- Stute , R. , Klingler , R.W. , Boguslawski , S. , Eshtiaghi , M.N. and Knorr , D. 1996 . Effects of High Pressures Treatment on Starches . Starch/Stärke , 48 : 399 – 408 .
- Hizukuri , S. 1985 . Relationship between the Distribution of the Chain Length of Amylopectin and the Crystalline Structure of Starch Granules . Carbohydrate Research , 141 : 295 – 306 .
- Defloor , I. , Dehing , I. and Delcour , J.A. 1998 . Physico-Chemical Properties of Cassava Starch . Starch/Stärke , 50 : 58 – 64 .
- Jyothi , A.N. , Moorthy , S.N. and Vimala , B. 2003 . Physicochemical and Functional Properties of Starch from Two Species of Curcuma . International Journal of Food Properties , 6 ( 1 ) : 135 – 145 .
- Zobel , H.F. 1988 . Molecules to Granules: A Comprehensive Starch Review . Starch/Stärke , 40 : 44 – 50 .
- Gebre-Mariam , T. and Schmidt , P.C. 1998 . Some Physico-Chemical Properties of Dioscorea Starch from Ethiopia . Starch/Stärke , 50 : 241 – 246 .
- Bayer , R.K. and Baltá Calleja , F.J. 2005 . Comment on the Structure of Amorphous Starch as Derived from Precursors of Crystallization: The Role of the Entanglement Network . Journal of Macromolecular Science: Physics , 44 ( 4 ) : 471 – 479 .
- Che , L.M. , Li , D. , Wang , L.J. , Chen , X.D. and Mao , Z.H. 2007 . Micronization and Hydrophobic Modification of Cassava Starch . International Journal of Food Properties , 10 ( 3 ) : 527 – 536 .