Abstract
Adding whey protein concentrate (WPC80) and cashew pulp (CP) to extruded snacks can reduce overall carbohydrate content. In this study, barley, cassava, corn meal and quinoa were blended with WPC80 (12.5 wt%) or with CP (12.5 wt%), then extruded and baked. The products' rapidly available glucose values or potential glycemic index were: quinoa (70%), barley (61%), corn (54%), and cassava (48%). Adding WPC80 with or without CP improved the glycemic potential values for barley and quinoa, but not for cassava, which increased from 61 to 77%. Adding WPC80 or CP had no effect on corn products.
INTRODUCTION
Snack products may be developed to help mitigate the growing worldwide nutritional imbalance associated with negative health effects such as high incidence of obesity. The increasing consumption of low-nutrient-dense snacks such as puffed flavored corn curls or potatoes comprised of mostly starches, are implicated in the growing obesity trend. Obesity is associated with the epidemiology of other metabolic syndromes.[Citation1] Consumption of starch-based high-energy low-nutrient-dense foods raises blood sugar rapidly and are termed high glycemic foods, e.g., corn puffs. High glycemic foods are reported to play a role in increasing obesity and diabetes in the U.S.[Citation2–5] Collinearly, underconsumption of micronutrients such as iron, zinc, and vitamin A has been reported in other parts of the world where snack consumption is replacing traditional meals.[Citation6]
The glycemic index measures the rapid increase in blood glucose following consumption of carbohydrates.[Citation7] It has been recommended that metabolically-at-risk consumers such diabetics and the elderly need to consume low glycemic response foods or whole grain and dietary fiber foods associated with reduced disease risks.[Citation8] The glycemic response, the effects that carbohydrate-containing foods have on blood glucose concentration during the digestion process, can be estimated through in vivo response to a reference food, typically white bread, over a 2 hour period.[Citation9] Glycemic index and response, by definition, require the use of human subjects to measure the glucose response in vivo. But, good estimates of the glycemic response of a food product can be made in vitro, without using human subjects, to predict the potential glycemic responses of a food product, such as rapidly available glucose (RAG), or slowly available glucose (SAG).[Citation10,Citation11]
Nutrient-rich cheese whey proteins, available in different concentrations, such as whey protein concentrates, isolates, and lactalbumin, can be combined with starchy snacks to improve their nutrient profile when used to make products for human diets. Such products were demonstrated by combining whey protein and others starches such as corn or wheat or rice using the twin extrusion process to increase the potential nutrient value of snack foods.[Citation6,Citation12] Recent research has shown that diets with a reduced ratio of carbohydrates to protein or whole meals are beneficial for weight loss; also, whey proteins rich in leucine, a branched chain amino acid (BCAA) are reported to help in weight loss diets and glucose management.[Citation3,Citation14,Citation15] Whey proteins contain higher levels of BCAA and leucine (26 g and 14 g) per 100 grams of protein than in any other proteins.[Citation16]
Though unrefined grains such as corn, rice, and wheat high in fiber content are needed for healthful snack products, mostly refined fast digesting carbohydrate sources deficient in fiber are used.[Citation17] Population studies have shown that consumption of whole grain products diminishes the risk of serious diet-related diseases.[Citation18] To boost the fiber content on healthy snack products fibers such as cereal wheat bran are substituted.[Citation6,Citation18,Citation19] Associated healthful benefits of increasing fiber consumption may include: reducing risk of some types of diseases including breast cancer, coronary heart disease, regulating blood glucose and insulin, lowering the concentration of blood lipids, reduced risk of cardiovascular disease and controlling diabetes, and alleviating constipation and weight management.[Citation20]
Extrusion processing offers versatility in forming materials for various functional uses; it is well suited for creating a high complex fiber product.[Citation21] A complex product combining cereal grains, flours, whey protein concentrates, and cashew pulp may perform as a low glycemic potential snack particularly by reducing the rate of glucose digestion in the gut. Specifically, this research investigated the use of cassava, quinoa, and barley, as an alternate source of carbohydrate, along with cashew pulp (Anacardium occidentale L., 41% fiber) as an alternate source of fiber, and whey protein concentrate (80% protein) to increase protein content in extruded, partially cooked (half-product) snacks, which were subsequently fully cooked by baking or frying. The process conditions, quality attributes, and potential glycemic response effects were identified.
MATERIALS AND METHODS
Malted barley flour (10% moisture, 2% fat, 14% protein, 1.5% ash, 1.5% non-soluble fiber, carbohydrate by difference) was purchased from ConAgra Foods (Omaha, NE). Commercial tapioca pearl made from cassava starch (13% moisture, 1% fat, 1% protein, 1% ash, 3% non soluble fiber, carbohydrate by difference) with small particles ranging from 1 – 2 mm was received gratis from AKFP (American Key Food Products, Closter, NJ). Degermed medium yellow cornmeal (12% moisture, 1% fat, 8% protein, 1% ash, 1% non-soluble fiber, carbohydrate by difference) was purchased from AGRICOR, Inc. (Marion, IN). Quinoa, a whole grain grown in the high Andes of South America (11% moisture, 7% fat, 16% protein, 3% ash, 4% non-soluble fiber, carbohydrate by difference) was purchased from Quinoa Corporation (Gardena, CA). Whey Protein Concentrate (WPC80), (5% moisture, 8% fat, 80% protein, 4% ash, 0% non-soluble fiber, carbohydrate by difference) was purchased from Davisco Foods International, Inc. (Eden Prairie, MN). Cashew pulp (Dwarf cashew variety developed by Embrapa, Brazil Ministry of Agriculture, Anacardium occidentale L.), received from Embrapa Food Technology (Rio de Janeiro, Brazil), was prepared from cashew fruits purchased from Nolem Comercial Importadora e Exportadora S.A. (Fortaleza, Brazil; www.nolem.com.br). The cashew fruits were stored in plastic trays at ∼10°C. After unpacking and nut removal, cashew “apples” fruit portion were soaked in a 30-ppm chlorine solution for 20 min before pulverization using a Geiger UM 12 cutter (Pinhais, Brazil). The pulp was separated from the juice with a Bonina 025 DF Itametal de-pulping machine (Itabuna, Brazil) fitted with a 0.6 mm aperture sieve. The pulp was dried at 60°C for 24 h in an Embrapa patented electric fruit fan dryer (Brazil #: MU 8302917-6) (Rio de Janeiro, Brazil). Dried pulp with 7% moisture was milled in a TREU hammer/knife mill (Rio de Janeiro, Brazil) fitted with a 0.8 mm sieve. The resulting cashew pulp (CP) (5% moisture, 1.5% fat, 9% protein, 1.7% ash, 41% non-soluble fiber, carbohydrate by difference) was blended with the barley, cassava, corn, or quinoa for extrusion of half-products. Detailed proximate compositions of the ingredient formulations are presented in .
Table 1 Proximate composition of starch materials blended with whey protein concentrate (W) and cashew pulp (CP)
Extrusion Processing
A ZSK-30 twin-screw extruder (Krupp Werner Pfleiderer Co., Ramsey, NJ) with a smooth barrel was used. The extruder has nine zones, and the effective forming zone temperatures were set to 100, 90, 70, and 60°C, respectively, for zones 6, 7, 8, and 9. Zones 1 to 2 were set to 35°C, 3 to 50°C, 4 to 80°C, and 5 to 90°C. Melt temperature was monitored behind the die. The die plate was fitted with a single flat die of 3 × 30 mm. The screw elements were selected to provide low shear at 300 rpm; the screw profile was published by Onwulata et al.[Citation19] Feed was conveyed into the extruder with a series 6300 digital feeder, type T-35 twin screw volumetric feeder (K-Tron Corp., Pitman, NJ). The feed screw speed was set at 600 rpm, corresponding to a rate of 6.3 kg/h for all products. Water was added into the extruder at the rate of 1.0 L/h with an electromagnetic dosing pump (Milton Roy, Acton, MA). Half-products were collected after 25 min of processing, dried in a laboratory oven at 60°C for 10 min, and stored at 20°C until thawed at ambient temperature, and analyzed.
Moisture content was measured as per Method #925.09, AOAC, 1998, using a vacuum oven. Water absorption (WAI) index was determined using a modification of the AACC method #56-20 (27). Products were ground in a laboratory blender and sifted through a 210 micron sieve. Samples (1.0 g ± 0.005 g) were placed in tared 50 mL polystyrene centrifuge tubes and wet with 10 mL distilled water. After standing for 15 min (with shaking every 5 min), the samples were centrifuged for 15 min at 1000 g (Econospin Model, Sorvall Instruments, Wilmington, DE). Weight gain in the pellet was determined after the supernatant was decanted into a tared aluminum pan. The supernatant was dried overnight under vacuum at 90°C. WAI was calculated as follows: WAI = [(Weight gain of the material)/(dry weight)].
A Rapid Visco Analyser (RVA, Newport Scientific, Warriewood, Australia) was used to measure the apparent viscosity of samples as a function of temperature. The dried samples from stored extrudates were milled using a laboratory disc mill (Model 3600, Perten Instruments, Huddinge, Sweden). Measurements were made on products passing through a 250-μm sieve and retained on 106 μm. A 2.5 g specimen, adjusted to 14% moisture basis, was added to 25 g distilled water. The time-temperature profile followed the RVA general pastry method #1 (ICC Standard Method #162) and included initially holding the sample with the paddles rotating at 160 rpm, at 50°C for 4 min, to investigate the cold-swelling starch peak. The specimen was heated to 95°C at a constant rate of 12°C/min, held at that temperature for 3 min, and then cooled to 50°C in 4 min at the same rate.
Thermal transitions generated by dynamic scanning calorimeter (DSC) using 25–30 mg samples of the raw blends mixtures and extruded specimen were analyzed in a Q100 DSC (TA Instruments, New Castle, DE) calibrated with indium. The powders and extruded products were placed in hermetically sealed aluminum pans and scanned using the modulated DSC program (MDSC) with a modulation period of ±0.16°C and a scanning rate of 1°C/min from −20°C to 200°C. Differences in the gelatinization enthalpy (J/g), values for the raw carbohydrate sources and their respective half-products were calculated as percent gelatinization.
The in vitro rate of the digestion of ground specimens of barley, cassava, corn or quinoa and their blends with WPC80 and CP were measured using methods described by Englyst et al.[Citation22] determining rapidly available glucose (RAG), glucose available in 20 min, and slowly available glucose (SAG), glucose available in 120 min, as in vitro predictors of the glycemic response of food formulations. The chemometric hydrolysis methods are described in detail by Englyst.[Citation23] For the hydrolysis, 0.8 to 1.5 g milled specimen was incubated with enzyme cocktail consisting of 2.8 ml amyloglucosidase (Sigma Aldrich, St. Louis, MO) and 8.0 mL deionized water (140 AGU/ml amyloglucosidase), 3.0 g pancreatin (Sigma Aldrich, St. Louis, MO), and 20 ml sodium acetate buffer (prepared by dissolving 16.6 g sodium acetate trihydrate (Sigma Aldrich, St. Louis, MO) in 250 ml saturated benzoic acid and made up with deionized water to 1 L. RAG was measured with 0.5 ml hydrolysate removed after 20 min. incubation at 37°C. Another 0.5 ml was removed after 120 min. incubation at 37°C for determining SAG. RAG values are highly correlated to the glycemic index when tested against human subjects.[Citation10,Citation22,Citation23,Citation24]. After the hydrolysis and digestion procedure, glucose values for RAG and SAG were determined using YSI analyzer model 2700 Select (YSI Life Sciences, Yellow Springs, OH). Glycemic potential was determined as the ratio of glucose in approximately 1 g carbohydrate in dry basis.[Citation11,Citation24]
For confocal laser scanning microscopy (CSLM), extruded half products (4 × 10 mm) were immersed in 20 mL aliquots of 2.5% glutaraldehdye-0.1 M imidazole solution (pH 7.0), to crosslink the protein components and amplify autofluorescence, then stored in sealed vials for 2 h. For imaging, pieces of the products were transferred to MatTek microwell dishes (MatTek Corp., Ashland, MA) and mounted on the stage of a model IRBE optical microscope system equipped with a 20× lens and coupled to a model TCS NT/SP confocal scanner head (Leica Microsystems, Exton, PA). Samples were illuminated with the 488 nm line of an Argon laser and reflection was collected in one channel, green autofluorescence (500–540 nm) and in some cases yellow-orange fluorescence (580–620 nm) from Nile Red staining was collected in a second and third channel. Overlays of the two or three channels were visualized in extended focus images to resolve the arrangement of components in the powder particles.
Baking/Frying
The extruded half-products were removed from refrigerated storage and left at ambient temperature (21°C) to equilibrate for 24 h before cutting into pieces of 30 × 30 mm. Then, 25 pieces of each product were fried and another 25 pieces were baked. The 25 half-product specimens were baked at 205°C for 24 min in a Despatch™ Rotary oven model #150 (Despatch, Minneapolis, MN). For frying, small batches of 4 to 5 pieces, were deep fried in vegetable oil at 190°C for 60 s in a De'Longhi Fryer, model D14427DZ (Saddle Brook, NJ). Frying was repeated until 25 specimens were obtained. The baked and fried specimens were subsequently analyzed for density, porosity, and breaking strength.
Bulk and particle or substance densities of the specimens were determined by an air pycnometer on ground fully cooked extrudates (Horiba Instruments Inc, model VM 100, Irvine, CA). Density tests were repeated in quadruplicates. Porosity was determined from density measurements (g/cm3) made with an air pycnometer (Horiba Instruments Inc. Model VM 100, Irvine, CA). A 4 g specimen of the baked or fried extrudate (as is) was used to measure the whole piece density (ρw) and the ground particle or solid density (ρs) of the same piece was determined. Porosity was then calculated as: Porosity (Ρ) = [1 – (ρw/ρs)] * 100.
Breaking strength (hardness) was determined using a TA-XT2 Texture Analyzer (Stable Micro Systems, Surrey, England), outfitted with a 500 N load cell, running at a cross-head speed of 0.2 mm/sec, and fitted with a Warner-Bratzler shear cell (1 mm thick blade). Breaking strength (N) was measured as the maximum force required for breaking the extruded samples (30 mm pieces) in the shear cell. Data reported are averages of 10 specimens. A full factorial design was used for the experiments; the design was replicated, and samples were analyzed in triplicate. Analysis of variance was used to identify differences in the physical properties for the different materials at various processing conditions. The Bonferroni LSD method was used to test for mean separation, and the CORR procedure was used to test correlations. The statistical analysis system package was used (SAS Institute Inc, Cary, NC).
RESULTS
Formulating with WPC80 and dried cashew pulp (CP) boosted the protein and fiber content of all products significantly (p < 0.05), especially corn meal (), a major grain used for puffed snack products. As formulated, barley (13.4% protein, 0.8% fiber) and quinoa (13.2% protein, 0.6% fiber) were higher in protein content than either corn (6.6% protein, <1.0% fiber) or cassava pearl (1.4% protein, 1.0% fiber). Cassava flour, not used for this work, has higher protein (2.3%) and fiber (1.7%) content.[Citation25] Substituting 12.5 wt% of the material with WPC80 increased protein content 45 to 125% for all products. Similarly, fiber content was increased significantly (p < 0.01) for all products; the weight ratios ranged from 1.6 to 2.2 wt% with the added CP.
Representative photographic images of the baked products, barley, cassava, corn and quinoa, top view (), show different coloration and expansion for the products. Barley (1A) was most puffed, pillow-like; cassava (1B) was similarly puffed, but with ridges. Corn (1C) was not as puffed as the other products. Quinoa (1D) was puffed in the middle, but not as much as barley or cassava. The typical effects of adding WPC80 and CP to products are shown for barley products in . The expanded puffed barley (2A) is followed by a flat shrunken barley and WPC80 (2B), showing the effect of adding WPC80, and a slightly expanded but discolored product, barley with WPC80 and CP (2C). The same patterns were observed for the others not shown. Similar effects were reported previously, for expanded corn, milk proteins and wheat bran fiber;[Citation12] barley and oat bran added to wheat flour dough decreased thickness and spread, and caused discoloration.[Citation6] Puffing and crust wall thickness created internal voids that entrapped moisture, resulting in rather large values for the products. Others have reported similar values for barley substituted for wheat in extruded “bread” product, noting that beta-glucans in barley binds an appreciable amount of water tightly.[Citation56]
Figure 1 Photographs of Baked barley (A), cassava (B), corn (C), and quinoa (D); illustrating natural colorations and peculiar expansion modes of crusted tops with pillow-like expansion of the products.
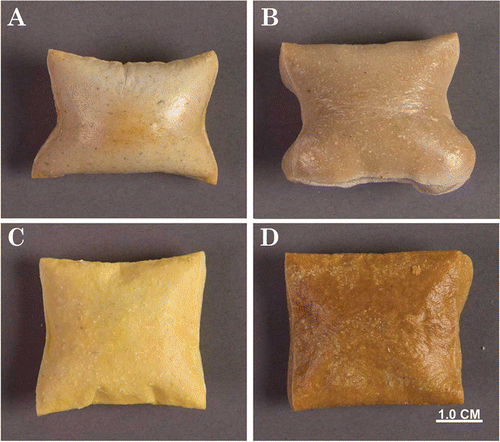
Figure 2 Photographs of baked barley products (A) control, (B) barley with whey protein concentrate, 80% protein (WPC80), and (C) Barley with WPC80 and Cashew Pulp (CP). Typical shrinkage and uneven puffing with associated with WPC80, and intense discoloration with WPC80 and CP.

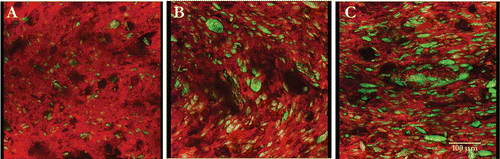
In , the confocal laser scanning images of dyed raw products, barley, cassava, corn and quinoa, show both starch (red) and protein (autofluorescence green). The intensity of the protein follows in increasing order: 3A, cassava (1.4%); 3B, corn (6.6%); 3C, quinoa (13.2%), and 3D, barley (13.4%). Correspondingly, as the protein content of the formulation increased with the addition of WPC80 the green autofluorescence increases. A typical image and material distribution in extruded half-products is shown for barley (). A is the non extruded barley grain; 4B is the extruded barley half-product with WPC80, showing directional flow patterns from extrusion shear plane and increased green autofluorescence from proteins. Barley containing both WPC80 and CP (4C), show large green autofluorescence bodies and more fiber-like structures from CP. Images containing CP have increased bright yellow areas that correspond to areas where protein, starch and sometimes CP, comingle.
Figure 3 Confocal scanning laser micrographs of partially-cooked (half-products) of barley (A), cassava (B), corn (C), and quinoa (D); showing distribution of protein bodies, starch, and protein-starch complex.
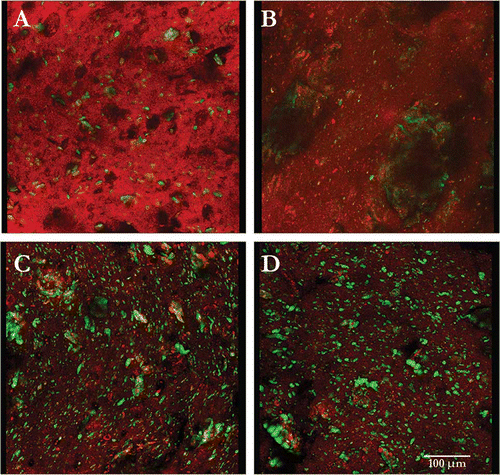
Figure 4 Confocal scanning laser micrographs of partially-cooked (half-products) barley (A), barley with whey protein concentrate, 80% protein (WPC80), and (C) Barley with WPC80 and Cashew Pulp (CP); showing distribution of protein bodies, starch, and protein-starch complex, and CP fiber as long slender lines.
Barley
The moisture, solubility, viscosity, gelatinization, and glycemic potentials (RAG or SAG) of barley specimens are presented in . The moisture content of the formulations were not significantly different, but the addition of WPC80 at 12.5 wt% increased moisture content approximately 8%, and adding CP at 12.5 wt% added another 5% for a combined 14% increase in moisture during extrusion. Solubility and WAI patterns were significantly different (p < 0.05) when comparing the extruded half-products to their control specimens. Barley was significantly less soluble before extrusion possibly due to the presence of ungelatinized starch and intact fiber cells. It was shown previously that gelatinized starch increases water absorption.[Citation26] When barley was combined with WPC80 or CP, the solubility of the raw products increased, but decreased significantly (p < 0.05) after extrusion. This decrease in solubility can be attributed to denaturation of the proteins which has been shown to cause loss of solubility.[Citation27] Water absorption index (WAI) increased significantly with extrusion for all barley formulations; possibly from the presence of gelatinized starch and fiber from CP. The effect of protein (whey proteins) on gelatinization is in line with the findings that higher amounts of protein in wheat starch increased onset and peak temperatures and decreased enthalpy.[Citation57]
Table 2 Physical properties and glycemic responses of barley, whey protein concentrate (WPC80), and cashew pulp (CP) products.Footnote*
Peak viscosity, as expected, was mostly higher in non extruded barley formulations containing non gelatinized starch, except for barley with WPC80; extrusion reduced peak viscosity significantly (p < 0.05) and adding both WPC80 and CP reduced viscosity even more (p < 0.05). Breakdown viscosity of non extruded barley was significantly (p < 0.05) higher than barley with WPC80, and significantly (p < 0.05) higher than barley, or barley with WPC80 and CP. Breakdown viscosity increased after extrusion for the three formulations, barley, barley and WPC80, and barley with WPC80 and CP; however, the increase was significant (p < 0.05) only with the addition of WPC80. Combining CP with WPC80 decreased breakdown viscosity. Peak viscosity, attributable to the thermal effect on starch, is an indication of degree of starch conversion and corresponds to the gelatinization peak.[Citation28] Breakdown viscosity is related to the starch response to shear at constant rotation, and measures heat induced swelling and rupture of starch molecules, which results in the fall of viscosity.[Citation29] Added protein from WPC80 protected starch breakdown in barley, maintaining peak viscosity. The presence of WPC80 and CP increased starch breakdown from increased moisture absorption. Dietary fibers are known to increase a formulation's water absorption capacity when incorporated into foods, making the surrounding food components dry and brittle, especially the insoluble fibers. For example, Sangnark and Noomhorn[Citation30] reported that bread fortified with wheat bran at levels between 5 to 15 g/100 g resulted in significantly decreased expansion, and stickiness.
Extrusion cooking provided the thermal conditions necessary for starch gelatinization for all formulations, as peak gelatinization temperatures were similar, ranging from 50.1 to 82.3°C (). Barley was 90.3% gelatinized, adding WPC80 interfered with gelatinization (98.0%) of barley starch (26.6%), but adding CP with WPC80 to barley increased gelatinization significantly (p < 0.05) more than barley alone. Perhaps barley starch cooked in the presence of protein lacked water due to competition for water by protein; hence, there was limited gelatinization of starch cooked in the presence of protein.[Citation31] The glycemic potentials, both RAG and SAG were similar, but adding WPC80 reduced RAG by 12%, and adding both WPC80 and CP reduced RAG by 31%. There was no advantage with adding WPC80 and CP after 120 min digestion, SAG. Protein and dietary fiber are reported to markedly affect the glycemic index of foods, and reduces blood glucose response.[Citation32]
The baking and frying properties of barley half-products are presented in . Baking extruded barley products reduced their moisture content significantly (p < 0.05) from 37% to less than 2%. Frying reduced moisture significantly (p < 0.05) from 37% to 16%. Bulk and substance densities were unchanged by baking and frying of barley extruded half-products. Overall, baking increased apparent porosity slightly for all products. Frying decreased apparent porosity, significantly (p < 0.05), particularly barley with WPC80. Extruded barley products, with whey and CP were very soft, less than 5 N. Baking and frying increased hardness. Frying increased hardness significantly (p < 0.05). Increased hardness may be the result of complete protein denaturation coupled with the texturing (increased denseness) imparted during extrusion.
Table 3 Physical properties of baked or fried extruded barley, whey protein concentrate (WPC80) or cashew pulp (CP) half-products.Footnote*
Cassava
The moisture, solubility, viscosity, gelatinization, SAG, and RAG of cassava specimens are presented in . Moisture content of cassava formulations varied, but were similar after extrusion. The addition of WPC80 decreased moisture by 14%. Adding WPC80 and CP did not change moisture content; WPC80 and CP counteracted, leaving moisture unchanged. Extrusion cooking of cassava increased its solubility and WAI, but decreased peak and breakdown viscosity very significantly (p < 0.05). Reduction in peak viscosity was 36% with the addition of WPC80, and 57% with WPC80 and CP combined. A similar pattern was observed with breakdown viscosity, which was reduced 66% with addition of WPC80 and 80% with WPC80 and CP. Cassava was significantly less soluble before extrusion. When combined with WPC80 and or with CP, the solubility of cassava products increased. Extrusion cooking of cassava provided the melt conditions for partial gelatinization, less than 46% for all formulation. Peak gelatinization temperatures ranged from 52.7 to 68.6°C. Cassava was only 42.4% gelatinized, adding WPC80 reduced gelatinization to 40.7%, but adding CP along with WPC80 increased gelatinization slightly to 45.6%. RAG and SAG for cassava differed. Adding WPC80 increased RAG by 58% and adding both WPC80 and CP increased RAG by 26%; but with SAG adding WPC80 reduced SAG by 2%, and WPC80 with CP reduced it by 22%. Though Cui et al.[Citation32] and others have reported decreased SAG with protein addition, in this study, we find that the effect of adding WPC80 and CP depends on the subject carbohydrate source, and does not result in lowering RAG in all cases. In cassava for instance, RAG increased.
Table 4 Physical properties and glycemic responses of cassava, whey protein concentrate (WPC80), and cashew pulp (CP) products.Footnote*
The baking and frying properties of cassava products are presented in . Baking extruded cassava half-products reduced their moisture moderately from an average of 19% to between 5.8 and 9.8%, with cassava, WPC80 and CP being the lowest. Frying reduced moisture slightly from 16.9 to 14.7%. Bulk and substance densities were similar for cassava products, with the exception of cassava with WPC80 and CP which was significantly (p < 0.05) denser than the others. Apparent volume and expansion were all similar, but hardness varied. Baking reduced overall hardness. Frying increased hardness. Baking reduced hardness significantly (p < 0.05) for cassava (47%), and very significantly (p < 0.01) for cassava with WPC80 (66%) and cassava with WPC80 and CP (86%).
Table 5 Physical properties of baked or fried extruded cassava, whey protein concentrate or cashew pulp half-products.Footnote*
Corn
The moisture content of corn and its half-products were about 22%. Water solubility and WAI increased for the extruded products. Solubility was suppressed with addition of WPC80 and CP to corn (). Peak viscosity was decreased 30% by extrusion; it was decreased further significantly (p < 0.05) by the addition of WPC80 and CP. Breakdown viscosity or starch rupturing was highest for corn (98.7 cP), but was significantly (p < 0.05) reduced with addition of WPC80 (47.3 cP), and WPC80 and CP (29.0 cP). Peak gelatinization temperatures ranged from 50.1 to 84.8°C; cornstarch gelatinization was moderate, ranging from 50.4 to 52.2%. Adding WPC80 or WPC80 and CP did not affect corn gelatinization. RAG decreased with addition of WPC80 (12%) and with WPC80 and CP (31%). SAG was not affected.
Table 6 Physical properties and potential glycemic responses of corn, whey protein concentrate (WPC80), and cashew pulp (CP) products.Footnote*
The baking and frying properties of corn products are presented in . Baking reduced moisture more significantly (p < 0.05) than frying from a range of 14.4 to 24.0%, to between 1.3 to 3.7%. Frying reduced moisture only to a range of 8.7–11.6%. Bulk density was reduced by baking and frying; the addition of WPC80 to corn reduced density by 24%, and WPC80 with CP by 48%. Particle density was unchanged. Baking and frying increased apparent porosity, but more so frying. Baking expanded corn products, but the addition of WPC80 (20%), and WPC80 and CP (37%) suppressed their apparent expansion. Frying, the addition of WPC80 (13%) and WPC80 and CP (65%) increased apparent expansion. Frying increased expansion significantly (p < 0.05) over baking, except for fried corn with WPC80 and CP, which was not expanded.
Table 7 Physical properties of baked or fried extruded corn, whey protein concentrate or cashew pulp half-products.Footnote*
Quinoa
The moisture content of quinoa products did not change with extrusion, but solubility decreased and WAI increased significantly (p < 0.05) (). Peak and breakdown viscosity increased significantly with extrusion, but both were decreased by the addition of WPC80 (35 and 93%), and WPC80 and CP (59 and 54%), respectively. Peak gelatinization temperatures ranged from 52.1 to 87.2°C; gelatinization increased from 57.9% to 60.1% with addition of WPC80, and to 90% with WPC80 and CP. RAG decreased progressively from 61.2 % to 54.1% with WPC80, and to 42% with WPC80 and CP. SAG was increased with addition of WPC80, but decreased with the addition of WPC80 and CP.
Table 8 Physical properties and potential glycemic responses of Quinoa, whey protein concentrate (WPC80), and cashew pulp (CP) products.Footnote*
Baking and frying properties of quinoa half-products are presented in . Baking extruded quinoa products reduced their moisture content very significantly (p < 0.01) from 19% to less than 4%, while frying reduced moisture significantly (p < 0.05) to 15% or less. Bulk density was increased, and volume expansion decreased by frying. Baking increased apparent porosity and volume expansion. Substance densities were the same for quinoa products. Frying increased hardness significantly (p < 0.05) more than baking, but both baking and frying reduced hardness at least four-fold when compared to the extruded quinoa half-products which were somewhat pliable but very hard.
Table 9 Physical properties of baked or fried extruded quinoa, whey protein concentrate (WPC80) or cashew pulp (CP) half-products.Footnote*
DISCUSSION
There are health benefits from eating products made with whole grains such as improving atherosclerotic cardiovascular diseases and decreased body weight.[Citation33] These health benefits are consistent with epidemiologic studies showing similar effects with incorporating cereal fibers in foods.[Citation17] Blending and extruding carbohydrate products with whey proteins and fiber results in widely varying physical property responses such as moisture, solubility and water absorption, mainly from interaction or interference of the blended materials with the starch matrix.[Citation34] Our results also show that the effect of blending WPC80 with CP depended on each product type, carbohydrate source, and that these effects changed. For example, with corn meal, adding WPC80 with or without CP increased moisture, and decreased solubility; but with cassava, there was a small decrease in moisture and a big increase in solubility. Solubility decreased for barley products. These different responses may be due to varying chemical and physical interactions occurring for extrusion, particularly as the moisture available to protein and starch during gelatinization and denaturation decreased. Diminishing available moisture for denaturation and gelatinization may account for the reduction or increase in percent gelatinization. Allen et al.[Citation35] showed that protein concentration and starch type (corn starch or pre-gelatinized waxy starch) affected the water absorption index and water-soluble carbohydrate significantly, and that a covalent complex formed between amylose and whey protein. Extrusion lowers the water absorption water vapor due to decreased specific surface area associated with increasing expansion; this resulted in new structures formed. The new structures formed exhibited increased water vapor sorption capacity and specific surface area.[Citation36]
The differences in physical properties such as solubility, moisture content, and WAI, which varied with the products, are attributable to quality changes. For example, moisture content increased for barley, cassava, and corn, but not quinoa. Quinoa with WPC80 and CP was significantly less soluble. Incorporation of WPC80 and CP increased WAI. Chang et al.[Citation37] described twin-screw processing of cassava starch and soy protein isolates, and reported decreased water absorption. Our results with cassava flour and WPC80 showed increased solubility and WAI, and decreased solubility for corn or barley blended with WPC80. Water content of barley half-products inversely affected hardness (R2 = 0.82), but WAI was moderately related to breakdown viscosity (R2 = 0.66); in the finished products, baked or fried, the higher the density, the less the porosity (R2 = 0.95). With quinoa half-products, moisture content correlated with hardness (R2 = 0.86). In the finished products, WAI correlated with breakdown viscosity (R2 = 0.95), and inversely between WSI and peak viscosity (R2 = 0.67). These correlations show that the effects of processing on functional properties are carbohydrate source dependent. These responses are not limited to temperature effects only, as suggested by Becker et al.[Citation28] that starch cold swelling peak viscosity was the result of thermomechanical processes. The presence of extra protein and fiber also affects viscosity. This seems to be a generalized effect as Indrani et al.[Citation38] showed that adding greater than 5% whey protein concentrate (37%) protein adversely affected the characteristics of flat bread (parotta) by decreasing peak viscosity, breakdown and setback values. Pogaku et al.[Citation39] in a critical review of whey protein isolate-starch systems concluded that decreased viscosity and elastic modulus (G') was the generalized effect of increasing protein concentration.
Baking and deep fat frying are widely used in food processing both in the industry and in homes in many places in the world. Frying in an edible oil or fat above the boiling point of water facilitates the dehydration cooking process.[Citation40,Citation41] These conditions lead to high heat-transfer rates, rapid cooking, browning, texture and flavor development. High heat-transfer rates are largely responsible for the development of the desired sensorial and textural properties in fried potatoes.[Citation40] Frying increased hardness for all products; possibly, due to case hardening. Fried foods undergo physicochemical changes, which affects structural, textural and porosity by the heat-transfer induced phenomenon of shrinkage or puffing.[Citation42] Kawas and Moireira[Citation43] reported that during frying, tortilla chips experienced both shrinkage in the radial direction and expansion in thickness. Baik and Mittal[Citation44] showed that frying at higher temperatures resulted in greater shrinkage at the same frying period in tofu samples. Product porosity is reported to affect the system energy and mass transport decreasing bulk density and increasing volume (puffiness). The crispy structure of potato chips is the result of changes at the cellular and sub-cellular levels in the outermost layers of the product. But, due to consumer health concerns in mitigating obesity from high energy dense processes and increased formation of acrylamide, a possible carcinogen, alternate processes such as baking, are now favored.[Citation45] Baking increased the porosity of barley, corn and cassava products, except for quinoa and barley with WPC80 and CP where volume decreased. Formation of air cells and internal fissures play an important role in improving texture of baked products, particularly at elevated temperatures over 220°C.[Citation46] Incorporation of protein-rich cowpea (24–26 wt%) to baked wheat flour bread resulted in increased water absorption in the dough, and reduced volume in the baked bread, leading to compact structures.[Citation47] Sufficiently high baking temperatures (>200°C) are needed to create good texture in baked snacks, but effort is needed to understand and mitigate the quality losses that may come from incorporating WPC80 or CP.
Dietary carbohydrates are digested at different rates in the human small intestine and depending on the botanical source the rate at which blood glucose elevates differ.[Citation24] In one review, it was shown that slowly absorbed carbohydrate produces beneficial effects on managing chronic diseases, and that fast-release carbohydrate, highly processed, results in higher blood glucose levels and greater insulin demand.[Citation48] Nutrition researchers have developed and correlated in vitro digestion methods that estimate or predict the glycemic response of starch based food products using in vivo glycemic responses.[Citation24] For example, Bornet[Citation49] showed excellent correlation of the percentage of starch hydrolysis in vitro to the in vivo glycemic response after 30 minutes. Epidemiological studies show strong links between type 2 diabetes and other metabolic syndromes to consumption of products exhibiting rapidly available glucose, RAG.[Citation50] Looking strictly at the potential glycemic index, RAG, cassava (48) products would seem to offer an advantage, but including WPC80 negates that benefit, increasing RAG to 77. Adding WPC80 and CP to cassava increased RAG to 61. However, with barley (61) or corn (54), adding WPC80 and CP reduced RAG to 42 for barley and 48 for corn. Englyst et al.[Citation24] determined that rapidly available glucose (RAG) is an excellent predictor of the glycemic response and explains approximately 61% of the glycemic response measured in vivo. RAG is highly correlated to the glycemic index when tested against human subjects.[Citation10] For instance, the addition of lentil flour to an extruded snack product lowered the glycemic index, RAG.[Citation11] Others have found high correlations of in vitro starch hydrolysis to the glycemic index and response.[Citation51,Citation52,Citation53,Citation54,Citation55]
CONCLUSION
The physical properties and glycemic potential responses observed for the selected extrusion process conditions, and the inclusion of WPC80 or CP to the products, was carbohydrate-source dependent. Clearly, the solubility and gelatinization of each carbohydrate source differed, and how rapidly or slowly the glucose was digested depended on those responses. The effect of adding WPC80 or CP depended on the interaction of starch, protein, and fiber. Adding WPC80 and CP was positive (decreasing RAG) in barley and corn, neutral to quinoa, and negative (increasing RAG) for cassava products. Each carbohydrate source needs independent study for its characteristic interactions and effects. For low-glycemic product application, mixtures of corn meal, barley flour, quinoa, and cashew pulp or other starches in proportions needed to create expanded snack products can help alleviate sucrose-surge stress and may help fight obesity. Product quality characteristics are carbohydrate-source dependent, meaning that the response depends on the choice of grain or tuber selected. Inclusion of WPC80 or CP may or may not decrease the potential glycemic indices RAG or SAG. Ultimately, in some products like cassava with WPC80 or CP, the initial glucose surge (20 min), RAG, may increase, but after 120 min, SAG, decreases.
ACKNOWLEDGMENT
The assistance of Eric Tilman with extrusion processing is appreciated, as well as Guoping Bao with microscopy.
Notes
This article not subject to United States copyright law.
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information, and this does not imply a recommendation or an endorsement by the U.S. Department of Agriculture.
REFERENCES
- Flegal , K.M. 2005 . Epidemiologic Aspects of Overweight and Obesity in the United States . Physiology & Behavior , 86 : 599 – 602 .
- McLellan , F. 2002 . Obesity Rising to Alarming Levels around the World . Lancet , 359 : 1412 2002
- Rolls , B.J. , Roe , L.S. , Kral , T.V.E. , Meengs , J.S. and Wall , D.E. 2004 . Increasing the Portion Size of a Packaged Snack Increases Energy Intake in Men and Women . Appetite , 42 : 63 – 69 .
- Sothern , M.S. 2004 . Obesity Prevention in Children: Physical Activity and Nutrition . Nutr. , 20 : 704 – 708 .
- Heilbronn , L.K. , Noakes , M. and Clifton , P.M. 2002 . The Effect of High- and Low-Glycemic Index Energy Restricted Diets on Plasma Lipid and Glucose Profiles in Type 2 Diabetic Subjects with Varying Glycemic Control . J. Am. Coll. Nutr. , 21 ( 2 ) : 120 – 127 .
- Sudha , M.L. , Ventrimani , R. and Leelavathi , K. 2007 . Influence of Fibre from Different Cereals on the Rheological Characteristics of Wheat Flour Dough and on Biscuit quality . Food Chem. , 100 : 1365 – 1370 .
- Frost , G. , Wolever , T.M.S. and Leeds , A.R. 1993 . Review: the Glycaemic Index. Is it Time to Take a New Look? Dietary Fiber Biblio . Rev. , 1 : 66 – 71 .
- Qi , L. , Rimm , E. , Liu , S. , Rifal , N. and Hu , F.B. 2006 . Dietary Glycemic Index, Glycemic Load, Cereal Fiber, and Plasma Adiponectin Concentration in Diabetic Men . Diabetic Care , 28 ( 5 ) : 1022 – 1028 .
- Wolever , T.M. , Katman-Relle , L. , Jenkins , A.L. , Vuksan , V. , Josse , R.G. and Jenkins , D.J.A. 1994 . Glycaemic Index of 102 Complex Carbohydrate Foods in Patients with Diabetes . Nutr. Res. , 14 ( 5 ) : 651 – 669 . 1994
- Englyst , K.N. , Vinoy , S. , Englyst , H.N. and Lang , V. 2003 . Glycaemic Index of Cereal Products Explained by their Content of Rapidly and Slowly Available Glucose . British Journal of Nutrition , 89 : 329 – 339 .
- Hardacre , A.K. , Clark , S.M. , Riviere , S. , Monro , J.A. and Hawkins , A.J. 2006 . Some Textural, Sensory and Nutritional Properties of Expanded Snack Food Wafers made from Corn, Lentil and Other Ingredients . Journal of Texture Studies , 37 : 94 – 111 .
- Onwulata , C.I. , Konstance , R.P. , Smith , P.W. and Holsinger , V.H. 2001 . Co-extrusion of Dietary Fiber and Milk Proteins in Expanded Corn Products . Lebensm.-Wiss. U-Technol. , 34 : 424 – 429 .
- Wolever , T.M. and Jenkins , D.J. 1986 . The Use of Glycemic Index in Predicting the Blood Glucose Response to Mixed Meals . Am J. Clin. Nutr. , 43 : 167 – 172 .
- Lehmann , U. and Robin , F. 2007 . Slowly Digestible Starch – its Structure and Health Implications . Trends in Food Science & Technol. , 18 : 346 – 355 .
- Wester , T.J. , Lobley , G.E. , Birnie , L.M. and Lomax , A.X. 2000 . Insulin Stimulates Phenylalanine Uptake Across the Hind Limb in Feed Lambs . J. Nutr. , 130 ( 3 ) : 608 – 611 .
- Layman , D.K. 2003 . The Role of Leucine in Weight Loss Diets and Glucose Homeostatsis . J. Nutr. , 133 : 261S – 267S .
- Jacobs , D.R. Jr. and Gallaher , D.D. 2004 . Whole Grain Intake and Cardiovascular Disease: A Review . Current Atherosclerosis Reports , 6 : 415 – 423 .
- Topping , D. 2007 . Cereal Complex Carbohydrates and Their Contribution to Human Health . J. Cereal Sci. , 46 : 220 – 229 .
- Onwulata , C.I. , Konstance , R.P. , Smith , P.W. and Holsinger , V.H. 1998 . Physical Properties of Extruded Products as Affected by Cheese Whey . J. Food Sci. , 63 ( 5 ) : 814 – 818 .
- Wolk , A. , Manson , J.E. , Stampfer , M.J. , Colditz , G.A. , Hu , F.B. , Speizer , F.E. , Hennekens , C.H. and Willett , W.C. 1999 . Long Term Intake of Dethary Fiber and Decreased Risk of Coronary Heart Disease Among Women . JAMA , 281 ( 21 ) : 1998 – 2004 .
- Breitenbach , J. 2002 . Melt Extrusion: From Process to Drug Delivery Technology . European J. Pharmaceutics and Biopharmaceutics , 54 : 107 – 117 .
- Englyst , H.N. , Veenstra , J. and Hudson , G.J. 1996 . Measurement of Rapidly Available Glucose (RAG) in Plant Foods: A Potential In vitro Predictor of the Glycaemic Response . British J. Nutr. , 75 : 327 – 337 .
- Englyst , H.N. , Kingman , S.M. and Cummings , J.H. 1992 . Classification and Measurement of Nutritionally Important Starch Fractions . European Journal of Clinical Nutrition , 46 ( Supplement 2 ) : S33 – S50 .
- Englyst , K.N. , Englyst , H.N. , Hudson , G.J. , Cole , T.J. and Cummings , J.H. 1999 . Rapidly Available Glucose in Foods: an In vitro Measurement that Reflects the Glycemic Response . Am. J. Clin Nutr. , 69 : 448 – 454 .
- Aryee , F.N.A. , Oduro , I. , Ellis , W.O. and Afuakwa , J.J. 2006 . The Physicochemical Properties of Flour Samples from the Roots of 31 Varieties of Cassava . Food Control , 17 : 916 – 922 .
- Rosa , D.A. , Guedes , C.G.F. , Pedroso , A.G. and Calil , M.R. 2004 . The Influence of Starch Gelatinization on the Rheological, Thermal, and Morphological Properties of Poly(ϵ-caprolactone) with Corn Starch Blends . Material Sci. Eng. C , 24 : 663 – 670 .
- Pelegrine , D.H.G. and Gasparetto , C.A. 2004 . Whey Proteins Solubility as Function of Temperature and pH . Lebensm.-Wiss. U-Technol. , 38 : 77 – 80 .
- Becker , A. , Hill , S.E. and Mitchell , J.R. 2001 . Milling - A Further Parameter Affecting the Rapid Visco Analyser (RVA) Profile . Cereal Chemistry , 78 ( 2 ) : 166 – 172 .
- Mishra , S. and Rai , T. 2006 . Morphology and Functional Properties of Corn, Potato and Tapioca Starches . Food Hydrocolloids , 20 : 557 – 556 .
- Sangnark , A. and Noomhorn , A. 2003 . Effect of Dietary Fiber from Sugarcane Bagasse and Sucrose Ester on Dough Bread Properties . Libensmittel – Wissenschaft und-Technologie , 37 ( 7 ) : 697 – 704 .
- Watson , C.A. and Johnson , J.A. 1965 . Studies on the Gelatinization of Starch I. Competition for water by protein and cellulose derivatives . J. Food Sci. , 30 ( 3 ) : 450 – 456 .
- Cui , H. , Yang , Y. , Bian , L. and He , M. 1999 . Effect of Food Composition of Mixed Food on Glycemic Index . J. Hygiene Research , 28 ( 6 ) : 356 – 358 .
- Katcher , H.I. , Legro , R.S. , Kunselman , A.R. , Gillies , P.J. , Demers , L.M. , Bagshaw , D.M. and Kris-Etherton , P.M. 2008 . The Effects of a Whole Grain-Enriched Hypocaloric Diet on Cardiovascular Disease Risk Factors in Men and Women with Metabolic Syndrome . Am. J. Clin. Nutr. , 87 : 79 – 90 .
- Matthey , F.P. and Hanna , M.A. 1997 . Physical and Functional Properties of Twin-Screw Extruded Whey Protein Concentrate-Corn Starch Blends . Lebens. -Wiss. U. -Technol. , 30 ( 4 ) : 359 – 366 .
- Allen , K.E. , Carpenter , C.E. and Walsh , M.K. 2007 . Influence of Protein Level and Starch Type on an Extrusion-Expanded Whey Product . Int'l J. Food Sci. & Technol. , 42 ( 8 ) : 953 – 960 .
- Wlodarczyk-Stasiak , M. and Jamroz , J. 2008 . Analysis of Sorption Properties of Starch-Protein Extrudates with the Use of Water Vapour . J. Food Eng. , 85 : 580 – 589 .
- Chang , Y.K. , Hashimoto , J.M. , Moura-Alcioli , R. and Martinez-Bustos , F. 2001 . Twin-Screw Extrusion of Cassava Starch and Isolated Soybean Blends . Nahrung/Food , 45 : 234 – 240 .
- Indrani , D. , Prabhasankar , P. , Jyotsna , J. and Rao , G.V. 2007 . Influence of Whey Protein Concentrate on the Rheological Characteristics of Dough, Microstructure and Quality of Unleavened Flat Bread (Parotta) . Food Res. Int'l. , 40 : 1254 – 1260 .
- Pogaku, P.; Seng, C.E.; Boonbeng, L.; Kallu, U.R. Whey Protein Isolate-Starch System-A Critical Review. Int'l J. Food Eng. 2007, 3 (6), Available at http://www.bepress.com/cgi/siteview.cgi/ijfe/vol3/iss6/art1
- Pedrechi , F. , Kaack , K. and Granby , K. 2006 . Acrylamide Content and Color Development in Fried Potato Strips . Food Res. Int'l. , 39 : 40 – 46 .
- Quayson , E.T. and Ayernor , G.S. 2007 . 2007. Non-Enzymatic Browning and Estimated Acrylamide in Roots, Tubers and Plantain Products . Food Chem. , 105 : 1525 – 1529 .
- Farinu , A. and Baik , O.D. 2007 . Heat Transfer Coefficients during Deep Fat Frying of Sweet Potato: Effects of Product Size and Oil Temperature . Food Res. Int'l. , 40 : 989 – 994 .
- Kawas , M.L. and Moreira , R.G. 2001 . Characterization of Product Quality Attributes of Tortilla Chips during the Frying Process . J. Food Eng. , 47 : 97 – 107 .
- Baik , O.D. and Mittal , G.S. 2005 . Heat and Moisture Transfer and Shrinkage Simulation of Deep-Fat Tofu Frying . Food Res. Int'l. , 38 : 183 – 191 . 2005
- Taiwo , K.A. and Baik , O.D. 2007 . Effects of Pre-treatment on the Shrinkage and Textural Properties of Fried Sweet Potatoes . LWT , 40 : 661 – 668 .
- Kayacier , A. and Singh , R.K. 2003 . Textural Properties of Baked Tortilla Chips. Lebensm.-Wiss . U-Technol. , 36 : 403 – 466 .
- Hallen , E. , Ibanoglu , S. and Ainsworth , P. 2004 . Effect of Fermented/Germinated Cowpea Flour Addition on the Rheological and Baking Properties of Wheat Flour . J. Food Eng. , 63 : 177 – 184 .
- Augustin , I.S. , Franceschi , S. , Jenkins , D.J.A. , Kendall , C.W.C. and Vecchia , C.L. 2002 . Glycemic Index in Chronic Disease: A Review . Eur. J Clin. Nutr. , 56 : 1049 – 1071 .
- Bornet , F.R.J. , Fontvieille , A.M. , Rizkalla , S. , Colonna , P. , Blayo , A. , Mercier , C. and Slama , G. 1989 . Insulin and Glycemic Responses in Healthy Humans to Native Starches Processed in Different Ways: Correlation with In vitro α-amylase Hydrolysis . The American Journal of Clinical Nutrition , 50 : 315 – 323 .
- Salmeron , J. , Ascherio , A. , Rimm , E.B. , Coldtz , G.A. , Spiegleman , D. , Jenkins , D.J. , Wing , A.L. and Willett , W.C. 1997 . Dietary Fiber, Glycemic Load, and Risk of NIDDM in Men . Diabetes Care , 20 ( 4 ) : 545 – 550 .
- Goni , I. , Garcia-Alonso , A. and Saura-Calixto , F. 1997 . A Starch Hydrolysis Procedure to Estimate Glycemic Index . Nutrition Research , 17 ( 3 ) : 427 – 437 .
- Akerberg , A.K.E. , Liljeberg , H.G.M. , Granfeldt , Y.E. , Drews , A.W. and Bjorck , I.M.E. 1998 . An In Vitro Method, Based on Chewing, To Predict Resistant Starch Content in Foods Allows Parallel Determination of Potentially Available Starch and Dietary Fiber . The Journal of Nutrition. , 128 : 651 – 660 .
- Sharavathy , M.K. , Urooj , A. and Puttaraj , S. 2001 . Nutritionally Important Starch Fractions in Cereal Based Indian Food Preparations . Food Chemistry , 75 : 241 – 247 .
- Seal , C.J. , Daly , M.E. , Thomas , L.C. , Bal , W. , Birkett , A.M. , Jeffcoat , R. and Mathers , J.C. 2003 . Postprandial Carbohydrate Metabolism in Healthy Subjects and Those with Type 2 Diabetes Fed Starches with Slow and Rapid Hydrolysis Rates Determined In vitro . British Journal of Nutrition , 90 : 853 – 864 .
- Brand-Miller , J. and Holt , S. 2004 . Testing the Glycaemic Index of Foods: In vivo, not In vitro . European Journal of Clinical Nutrition , 50 : 700 – 701 .
- Gill , S , Vasanthan , T. , Ooraikul , B. and Rossnagel , B. 2002 . Wheat bread quality as influenced by substitution of waxy and regular barley flours in their native and extruded forms . Journal of Cereal Science , 36 : 219 – 237 .
- Mohammed , A.A. and Rayas-Duarte , P. 2003 . The effect of mixing and wheat protein/gluten on the gelatinization of wheat starch . Food Chemistry , 81 : 533 – 545 .