Abstract
Antioxidative activities of E- and Z-ajoene prepared from Japanese garlic were studied using various radical scavenging effects. Among the various antioxidative activities tested, ajoene was found to show the highest hydroxyl radicals scavenging activity (E-ajoene = 50% and Z-ajoene = 63%). These ajoene were 5.2-13 times less efficient at reducing DPPH radical and 1.1-1.4 times less efficient at hydroxyl radical as compared with authentic α–tocopherol. Essentially E- and Z-ajoene were capable of scavenging superoxide anion (E- ajoene = 0.11%, Z-ajoene = 3.74%), but 80 minutes later antioxidant activity could not be detected. The effectiveness of Z-ajoene is significantly higher than E-ajoene.
INTRODUCTION
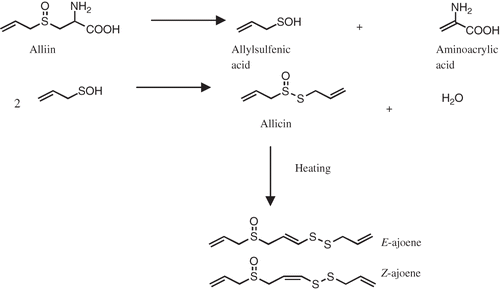
In recent years, there has been increasing interest in antioxidants derived from fruits, vegetables, herbs, and beverages. Epidemiological studies have indicated that dietary intake of antioxidants from plants is inversely associated with mortality from coronary heart disease.[Citation1] Garlic (Allium sativum) has been advocated as a remedy for the treatment and prevention of a number of diseases, including atherosclerosis and cancer. Several clinical studies conducted during the last three decades, however, have shown contradictory lipid-lowering activity and beneficial effects on cancer incidence.[Citation2–4] Ajoene is a well-established antiplatelet agent, and its inhibitory effect on platelet aggregation has been extensively studied and documented both by in vivo and in vitro experiments.[Citation5–9] Ajoene is considered as a major natural compound derived from garlic through the conversion of alliin into allicin by an alliinase-induced cleavage,[Citation10] as shown in .
The antioxidant activities in Allium tissue extracts have been of particular interest because of the relationship between oxidative stress and pathologies such as atherosclerosis, cancer, and aging, in which free radicals and reactive oxygen species are implicated.[Citation11–14] Many recent studies on antioxidant activities of Allium tissue components have used crude extracts or tissue derivatives. Despite the complexity of such preparations, many investigators have reached unequivocal conclusions[Citation15–17] or have insinuated[Citation18–20] that the thiosulfinates or related organosulfur components are primarily responsible for the observed antioxidant effects, even though many other endogenous components may have antioxidant properties.[Citation21] Pure alliin (S-allyl-L-cysteine sulfoxide) has no antioxidant activity in a linoleic acid emulsion and has only weak reducing power, despite earlier claims that alliin is an effective antioxidant.[Citation22] Pure thiosulfinates[Citation23] and related organosulfur compounds[Citation24] exhibit antioxidant properties under specific conditions. However, allicin is found the most effective antioxidant and PrS(O)SPr is the least effective thiosulfinate.[Citation25] The nonenzymatic antioxidant activity of diallyl sulfide, diallyl disulfide, S-ethyl cysteine and N-acetyl cysteine in the liposome system is examined.[Citation26] Until now, no study has been undertaken to compare the antioxidant activities of two ajoene isomers. The purpose of this study was to examine the antioxidant property of E- and Z-ajoene derived from Japanese garlic.
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma or Aldrich (Japan), Wako (Japan) and Nacalai Tesque, Inc. (Kyoto, Japan) Chemical Companies. Precoated kieselgel 60 on aluminum sheet for thin-layer chromatography (TLC) were purchased from Merck (Germany). Japanese garlic (Allium sativum L) was obtained from a local market in Japan. Rice oil was obtained from Fine Foods Co., Ltd. (Osaka, Japan).
Preparation and Purification of E-and Z-ajoene from Japanese Garlic
Preparation and purification of E-and Z-ajoene from Japanese garlic was followed by Naznin et al.[Citation27] In brief, garlic cloves were cut into 3-4-mm thick slices, and then ground to mix oil using SMT High-Flex disperser apparatus (SMT Co., Tokyo, Japan) mechanically driven at 700 rpm by a drill press. To minimize frictional heating of the sample during the grinding process, the tissue grinder was chilled prior to and during the grinding process with an ice bath. Garlic samples were mixed with oil (0.25 kg/L) directly in the disperser apparatus. Then the mixture was stored at 80 °C for 4 h to allow complete ajoene formation. Ten ml samples were extracted with ethyl acetate and analyzed by HPLC and TLC according to Naznin et al.[Citation27] In brief, extracted compounds were analyzed by Si-HPLC using a LiChrospher Si 60 column (250 mm X 4.0 mm, Kanto Chemical Co., Inc. Tokyo). The eluent was n-hexane/2-propanol (85:15, v/v) at a flow rate of 1.0 ml/min, and was monitored at 240 nm. TLC was developed with ethyl acetate as a solvent, and each spot on the plate was detected by spraying coloring reagent (molibdo phosphoric acid/85% phosphoric acid/conc. sulfuric acid/water, 2.4: 1.5: 5, v/v/v) and heated at 70°C for 20 minutes. E- and Z-ajoene were separated on a silica gel column chromatography. After washing the column with 200 ml of 40% ethyl acetate in n-hexane, the Z-ajoene was eluted with 100 ml of 50% ethyl acetate/ n-hexane (1:1, v/v), whereas E-ajoene was eluted with 150 ml of 65% ethyl acetate and found to be over 95% pure. Purified E-and Z-ajoene were analyzed by 1H NMR, HPLC and TLC.[Citation27]
Scavenging of DPPH Radical
Antioxidant activity of the ajoene was measured in terms of hydrogen donating or radical scavenging ability, using the stable radical, DPPH. According to Blois,[Citation28] as the odd electron of DPPH becomes paired off in the presence of a hydrogen donor, namely the antioxidant, the absorption disappears and the resulting discoloration with regard to the number of electrons captured. The method was similar to the previous report[Citation29] with some modifications. In briefly, Test compounds were incubated in 3 ml of solution containing DPPH radical (500 μM) in ethanol for 30 minutes, and the absorbance of the solution was measured at 517 nm with a Shimadzu spectrophotometer UV-160A (Kyoto, Japan). The scavenging activity was calculated using EquationEq. (1),[Citation30]
Scavenging of Hydroxyl Radical (OH•)
The scavenging activity of the OH• was measured by using deoxyribose method[Citation31] with a slight modification. Sample solution in 10 mM sodium phosphate buffer (pH 7.4) was added to the same buffer containing 2.5 mM 2-deoxyribose, 1.0 mM iron ammonium sulfate premixed with 1.04 mM EDTA was added. Samples were incubated in the water bath at 37°C, the reaction started by adding 1.0 mM ascorbic acid and 0.1 M H2O2. The solution was maintained at 37°C for 10 min, and then cold 2.8% TCA (Trichloroacetic acid) was added, followed by addition of 1% thiobarbituric acid (TBA). The solution was heated for 8 minutes and the absorbance was measured at 532 nm. The scavenging activity was calculated using EquationEq. (1).
Scavenging of Superoxide Anion (O2 −)
The ability of E- and Z-ajoene to scavenge superoxide anion (O2 −) was determined on the basis of inhibiting the reduction of nitro blue tetrazolium (NBT).[Citation32] Superoxide was generated in a nonenzymic system. According to Xiao and Parkin,[Citation25] the reaction mixture contained, at final concentrations, NADH (117 μM), NBT (37.5 μM), and phenazine methosulfate (PMS, 15 μM) in sodium phosphate (0.1 M, pH 7.4). The test compounds or standard antioxidant (1 mM) were added and the reaction was started by the addition of PMS. After incubation at ambient temperature for 5 min, the absorbance at 560 nm was measured against appropriate blank samples. The scavenging activity was calculated using EquationEq. (1).
Scavenging of H2O2
The level of H2O2 remained was measured after 10 min incubation by the Fe3+-xylenol orange (FOX) complex assay.[Citation33] E-and Z-ajoene were incubated in the presence of H2O2 (0.5 mM) in sodium phosphate-buffered saline (PBS, pH 7.4) for 10 min. According to Xiao and Parkin,[Citation25] 0.1 ml of reaction mixture was mixed with 0.9 ml of FOX reagent that contained 90% methanol, 100 μM xylenol orange, 4 mM butylated hydroxytoluene (BHT), and 25 mM H2SO4, and incubated for 30 min at 20–22 °C. The absorbance at 560 nm was determined against a blank solution. The scavenging activity was calculated using EquationEq. (1).
Statistical Analysis
The statistical analysis of the measured parameters was made using analysis of variance (ANOVA). Significant differences among samples were evaluated by Duncan's multiple-range test (P < 0.05) using SPSS software (v. 11.0, SPSS, Chicago, IL).
RESULTS AND DISCUSSION
Scavenging of DPPH Radical
The tested E- and Z-ajoene reduced DPPH radical in a concentration-dependent response (). The DPPH radical-scavenging effect increased as the concentration of the ajoene increased to a certain extent, and then leveled off even with further increase in the concentration (0.2–1 mM). For example, at a concentration of 0.2-1 mM, the Z-ajoene showed 2–17% scavenging activity of DPPH radical. Further increasing the dosage did not significantly increase the DPPH radical-scavenging effect (data not shown).
Figure 2 Scavenging activity of ajoene toward DPPH radical. The standard reaction mixture contained 500μM DPPH in ethanol. Ajoene was added to the final concentrations indicated on the axis. The results represent the mean values ± SD from three independent experiments.
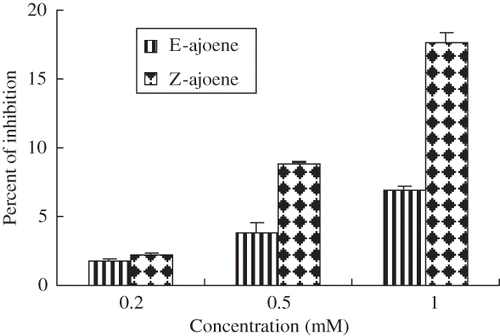
Z- Ajoene was more effective than E-ajoene. The sulfinyl functional unit may have a role in DPPH radical reduction. In ajoene, hydrogen atoms bonded to central double bonded carbon atoms have the largest positive excess charge (about +0.15e) among all the hydrogen atoms in the molecule; these hydrogen atoms are expected to leave the molecule first in a proton transfer reaction. This may be related to potential antioxidant activity.[Citation34]
The effectiveness of ajoene was 5–11 times lesser than that of more commonly used antioxidants α-tocopherol (1 mM) (). The ineffectiveness of the ajoene in the DPPH radical quenching assay was likely associated with the lack of reactivity of the sulfinyl functional unit with DPPH radical and/or the relative oxidation-reduction potentials for the alk(en)yl groups compared to respective α-tocopherol.[Citation35]
Table 1 Antioxidant activities of E- and Z-ajoene and known antioxidant α-tocopherol
Scavenging of Hydroxyl Radical (OH•)
The OH•-scavengers, namely antioxidants compete with deoxyribose for the OH• produced and diminish chromogen formation. OH•-scavenging activities of E- and Z-ajoene are shown in , in which Z-ajoene showed greater inhibition activity than E-ajoene. Ajoene has cis-(Z-) and trans-(E-) isomers, both with varying degrees and specificities of bioactivity.[Citation34]
Figure 3 Scavenging activities of ajoene toward hydroxyl radical. The standard reaction mixture contained, at final concentrations, 10 mM sodium phosphate (pH 7.4), 2.5 mM 2-deoxyribose, 1.0 mM iron ammonium sulfate, 1.04 mM EDTA, 1.0 mM ascorbic acid, 0.1 M H2O2, 2.8% TCA% and 1% TBA. Ajoene were added to the final concentrations indicated on the axis. The results represent the mean values ± SD from three independent experiments. Error bars indicate standard deviations; where no error bars are visible, the standard deviation is less than the size of the symbol.
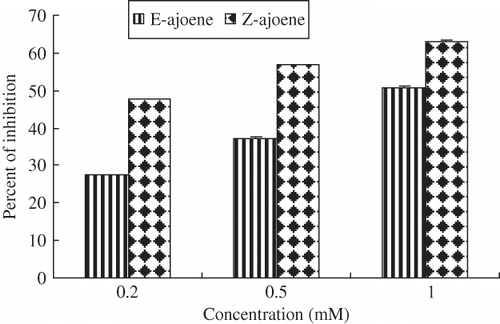
Comparing with two isomers Z-isomer is slightly more potent than E-isomer.[Citation36] Perhaps, for these reasons Z-ajoene showed greater antioxidant activity than E-ajoene.
The inhibitory properties of E- and Z-ajoene against OH• rivaled of those of the common antioxidant α-tocopherol tested (). The higher effectiveness of the E-and Z-ajoene (51% and 63%) implies that the scavenging of OH• is based on the functional unit. In fact, OH• reacts with virtually all organic compounds at or near the rate of diffusion,[Citation37] so it is not surprising that the components had evaluated higher inhibitory effects.
Scavenging of Superoxide Anion and H2O2
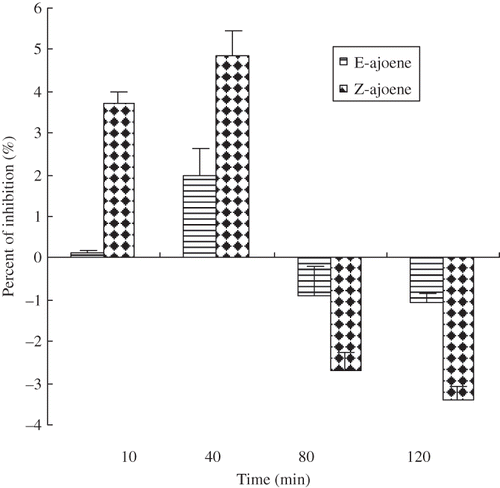
Very less O2 − scavenging activity based on inhibition of NBT reduction was observed for E- and Z-ajoene (). In fact, 1mM E- and Z-ajoene after 40 minutes incubation period, the amount of NBT reduction was 1.9% and 4.8%. However, after 80 minutes incubation period no antioxidant activity was observed. It is possible that ajoene scavenges O2 − and yield O2 −. Perhaps, for these reasons there was no antioxidant effect observed after incubation period for 80 and 120 min. In ajoene, there is a large positive charge development (about +1.4e) on the sulfur atom bonded to oxygen; other two sulfur atoms have relatively less excess charge. Oxygen atom has negative excess charge of about –0.8e, which is the only atom in ajoene molecule having attractive potential. Oxygen atom in ajoene may play in important role in the interaction of ajoene and its environment.[Citation34]
Figure 4 Scavenging activities of ajoene toward superoxide anion. The standard reaction mixture contained, at final concentrations, NADH (117 μM), NBT (37.5 μM), and phenazine methosulfate (PMS, 15 μM) in sodium phosphate (0.1 M, pH 7.4). The test compounds or standard antioxidant (1mM) were added and the reaction was started by the addition of PMS. The results represent the mean values ± SD from three independent experiments. Error bars indicate standard deviations.
Hydrogen peroxide can be generated in biological and food systems. Being a non-radical oxygen-containing reactive agent, it can form hydroxyl radical, the most highly reactive oxygen radical known, in the presence of transition metal ions and participate in free-radical reaction.[Citation38] High levels of hydrogen peroxide can attack several cellular energy-producing systems.[Citation39] H2O2 scavenging activity was not observed for E- and Z-ajoene examined at levels over 1mM (data not shown). However, ajoene was not capable of scavenging H2O2, no corresponding analyses for any common antioxidants were conducted. Xiao and Parkin[Citation25] support this result.
CONCLUSION
This study demonstrated that E- and Z-ajoene have various extents of antioxidant properties, including scavenging activities for DPPH radicals and hydroxyl radicals. These antioxidative effects may vary with the concentration. In general, within a certain concentration range, the antioxidative activity increased with the concentration. Furthermore, ajoene with low concentration exhibited a higher DPPH radicals and OH• radicals scavenging activity than those with higher. These E- and Z-ajoene may be used as a source of antioxidants, as a possible food supplement or in the pharmaceutical industry, especially in areas where the garlic and its derivatives are used.
ACKNOWLEDGEMENT
The authors are greatly indebted to the Ministry of Education, Science, Sports and Culture, Japan for the award of scholarship to Most Tahera Naznin. We reverentially thank assistant professor Mitsugu Akagawa (Department of Biological Chemistry, Osaka Prefecture University) for his valuable suggestions. We specially thank Fine Foods Co., Ltd. (Osaka, Japan) for supplying the oil.
REFERENCES
- Giugliano , D. 2000 . Dietary antioxidants for cardiovascular prevention . Nutr. Metab. Cardiovasc. Dis. , 10 : 38 – 44 .
- Agarwal , K. C. 1996 . Therapeutic actions of garlic constituents . Med. Res. Rev. , 16 : 111 – 124 .
- Buiatti , E. , Palli , D. , Decarli , A. , Amadori , D. , Avellini , C. , Blanchi , S. , Biserni , R. , Cipriani , F. , Cocco , P. , Glacosa , A. , Marubini , E. , Puntoni , R. , Vindigni , C. , Fraumenti , J. and Blot , W. 1989 . A case-control study of gastric cancer and diet in Italy . Int. J. Cancer , 44 : 611 – 616 .
- You , W.C. , Blot , W.J. , Chang , Y.S. , Ershow , A. , Yang , Z.T. , An , Q. , Henderson , B.E. , Fraumeni , J.F. and Wang , T.G. 1989 . Allium vegetables and reduced risk of stomach cancer . J. Natl. Cancer Inst. , 81 : 162 – 164 .
- Apitz-Castro , R. , Escalante , J. , Vargas , R. and Jain , M.K. 1986a . Ajoene, the antiplatelet principle of garlic, synergistically potentiates the antiaggregatory action of prostacyclin, forskolin, indomethacin and dypiridamole on human platelets . Thromb Res. , 42 : 303 – 311 .
- Apitz-Castro , R. , Ledezma , E. , Escalante , J. and Jain , M.K. 1986b . The molecular basis of the antiplatelet action of ajoene: direct interaction with the fibrinogen receptor. Biochem . Biophys. Res. Commun. , 14 : 145 – 150 .
- Apitz-Castro , R. , Ledezma , E. , Escalante , J. , Jorquera , A. , Pinate , F. M. , Morenorea , J. , Carrillo , G. , Leal , O. and Jain , M.K. 1988 . Reversible prevention of platelet activation by (E, Z)-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (ajoene) in dogs under extra- corporeal circulation. Arzneimittel-Forschung . 38 : 901 – 904 .
- Apitz-Castro , R. , Jain , M.K. , Bartoli , F. , Ledezma , E. , Ruiz , M. C. and Salas , R. 1991 . Evidence for direct coupling of primary agonist-receptor interaction to the exposure of functional IIb-IIIa complexes in human blood platelets. Results from studies with the antiplatelet compound ajoene. Biochim . Biophys. Acta , 1094 : 269 – 280 .
- Apitz-Castro , R. , Badimon , J. J. and Badimon , L. A. 1994 . Garlic derivative, ajoene, inhibits platelet deposition on severely damaged vessel wall in an in vivo porcine experimental model . Thrombosis Res. , 75 : 243 – 249 .
- Lawson , L. D. , Wang , Z.-Y. J. and Hughes , B. G. 1991 . Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products . Planta Med. , 57 : 363 – 370 .
- Maeda , H. , Katsuki , T. , Akaike , T. and Yasutake , R. 1992 . High correlation between lipid peroxide radical and tumor-promoter effect: suppression of tumor promotion in the Epstein-Barr virus/B-lymphocyte system and scavenging of alkyl peroxide radicals by various vegetables extracts . Jap. J. Cancer Res. Gann , 52 : 923 – 928 .
- Halliwell , B. , Gutteridge , J. M. C. and Cross , E. E. J. 1992 . Free radicals, antioxidants, and human disease: where are we now? . J. Lab Clinical. Med. , 119 : 598 – 620 .
- Cavalieri , E. L. and Rogan , E. G. 1992 . The approach to understanding aromatic hydrocarbon carcinogenesis . The central role of radical cations in metabolic action. Pharmacol. Ther. , 55 : 183 – 199 .
- Salvemini , D. and Botting , R. 1993 . Modulation of platelet function by free radicals and free-radical scavengers . Trends Pharmacol. Sci. , 14 ( 2 ) : 36 – 42 .
- Prasad , K. , Laxdal , V. A. , Yu , M. and Raney , B. L. 1995 . Antioxidant activity of allicin, an active principle in garlic . Mol. Cellular Biochem. , 148 : 183 – 189 .
- Kourounakis , P. N. and Rekka , E. A. 1991 . Effect on active oxygen species of alliin and Allium sativum (garlic) powder . Res. Commun. Chem. Pathol. Pharmacol. , 74 : 249 – 252 .
- Rekka , E. A. and Kourounakis , P. N. 1994 . Investigation of the molecular mechanism of the antioxidant activity of some Allium sativum ingredients . Die Pharmazie , 49 : 539 – 540 .
- Siegers , C.-P. , Röbke , A. and Pentz , R. 1999 . Effects of garlic preparations on superoxide production by phorbol ester activated granulocytes. Phytomedicine . Int. J. Phytother. Phytopharmacol. , 6 : 13 – 16 .
- Popov , I. , Blumstein , A. and Lewin , G. 1994 . Antioxidant effects of aqueous garlic extract. 1st communication: direct detection using the photochemiluminescence . Arzneimittel-Forschung , 44 : 602 – 604 .
- Kim , S. M. , Kubota , K. and Kobayashi , A. 1997 . Antioxidative activity of sulfur-containing flavor compounds in garlic . Biosci. Biotech. Biochem. , 61 : 1482 – 1485 .
- Yin , M.-C. and Cheng , W.-S. 1998 . Antioxidant activity of several Allium members . J Agric Food Chem. , 46 : 4097 – 4101 .
- Hirata , R. and Matsushita , S. 1996 . Reducing activity level of alliin. Biosci . Biotech. Biochem . , 60 : 484 – 485 .
- Rabinkov , A. , Miron , T. , Konstantinovski , L. , Wilchek , M. , Mirelman , D. and Weiner , L. 1998 . The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins . Biochim. Biophys. Acta , 1379 : 233 – 244 .
- Imai , J. , Ide , N. , Nagae , S. , Moriguchi , T. , Matsuura , H. and Itakura , Y. 1994 . Antioxidant and radical scavenging effects of aged garlic extract and its constituents . Planta Med. , 60 : 417 – 420 .
- Xiao , H. and Parkin , K. L. 2002 . Antioxidant functions of selected Allium thiosulfinates and S-alk(en)-L-cysteine sulfoxides . J. Agric. Food Chem. , 50 : 2488 – 2493 .
- Yin , M. , Hwang , S. and Chan , K. 2002 . Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic . J. Agric. Food Chem. , 50 : 6143 – 6147 .
- Naznin , M. T. , Akagawa , M. , Okukawa , K. , Maeda , T. and Morita , N. 2008 . Characterization of E- and Z-Ajoene obtained from different varieties of garlics . Food Chem. , 106 : 1113 – 1119 .
- Blois , M. S. 1958 . Antioxidant determinations by the use of a stable free radical . Nature , 181 : 1199 – 1200 .
- Yamaguchi , T. , Takamura , H. , Matoba , T. and Terao , J. 1998 . HPLC method for evaluation of the free radical- scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl . Biosci. Biotechno. Biochem. , 62 : 1201 – 1204 .
- Lin , H. -Y. and Chou , C. -C. 2004 . Antioxidative activities of water-soluble disaccharide chotosan derivatives . Food Res. Int. , 37 : 883 – 889 .
- Halliwell , B. , Gutteridge , J. M. C. and Aruoma , O. I. 1987 . The deoxyribose method: a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals . Anal. Biochem. , 165 : 215 – 219 .
- Yen , G.-C. and Chen , H.-Y. 1995 . Antioxidant activity of various tea extracts in relation to their antimutagenicity . J. Agric. Food Chem. , 43 : 27 – 32 .
- Jiang , Z.-Y. , Hunt , J. V. and Wolff , S. P. 1992 . Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low-density lipoprotein . Anal. Biochem. , 202 : 384 – 389 .
- Erkoç , Ş. , Sümer , S. and Erkoç , F. 2003 . Structural and electronic properties of ajoene molecule . J. Mol. Struc. (Theochem) , 631 : 271 – 276 .
- Buettner , G. R. 1993 . The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate . Arch. Biochem. Biophys. , 300 : 535 – 543 .
- Block , E. and Ahmad , S. 1984 . (E, Z)-Ajoene a potent antithrombotic agent from garlic . J. Amer. Chem. Soc. , 106 : 8295 – 8296 .
- Youngman , R. J. 1984 . Oxygen activation: is the hydroxyl radical always biologically relevant? . Trends Biochem. Sci. , 9 : 280 – 283 .
- Halliwell , B. , Murcia , M. A. , Chirico , S. and Aruoma , O. I. 1995 . Aruoma, free radicals and antioxidants in food and in vivo: What they do and how they work . Crit. Rev. Food Sci. Nutr. , 35 : 7 – 20 .
- Hyslop , P. A. , Hinshaw , D. B. , Halsey , W. A. J. , Schraufstater , I. U. , Sauerheber , R. D. , Spragg , R. G. , Jackson , J. H. and Cochrane , C. G. 1988 . Mechanisms of oxidant-mediated injury . J. Biologic. Chem. , 263 : 1665 – 1675 .