Abstract
The determination of °Brix, pH, titratable acidity and antioxidant composition such as β-carotene, lycopene, and vitamin C was done in greenhouse “Savoura” tomatoes during maturation. The chromatic values L*, a*, b* were determined at the top surface of the tomato, and its strength-deformation curve was tested with a texturometer. Results pointed out that °Brix and titratable acidity did not change during postharvest, while β-carotene, lycopene, and vitamin C contents increased continuously toward the red stage of tomato. The colour values L*, a*, b*, and ratio a*/b* had a good correlation with the maturity stages. Also, a high correlation between lycopene content and (a*/b*) ratio was found, which was well represented with a second order polynomial function (r2 = 0.95). This equation permits to appropriately estimate lycopene content of tomato as a function of its color, without any chemical analysis. During ripening, tomato texture changed from firm turgid to soft and the puncture deformation degree increased. Tomato firmness and chromatic values had a good correlation, and the lycopene content was a logarithm function of firmness (r2 = 0.81). The strength in puncture test increased linearly with deformation until the bioyield point. The measured force was analyzed as the sum of the compression and the shear forces. The two force coefficients can be considered as tomato properties, independent of the puncture probe. These coefficients were found to decrease during tomato maturation.
Keywords:
INTRODUCTION
Tomato and its products (such as sauce, paste, and catsup) are the major source of lycopene in the human diet and are considered an important contributor of carotenoids. They also supply other important antioxidants for health such as vitamin C and β-carotene. There is emerging data supporting the connection between increased tomato consumption and reduced risk for both cardiovascular disease and prostate cancer.[Citation1–4] Depending on the frequency, and the extent of tomato consumption, the supporting data shows that tomato's role is of undeniable importance as part of a healthy diet.[Citation1,Citation2]
Tomato composition, structure and other quality characteristics change during maturation. Pigment composition, for example, changes markedly during ripening as progressively chlorophyll disappears with a concomitant increase in carotenoids until the fully ripe stage.[Citation5] The colour change is thus a visual sign of tomato maturation and is used as a maturation index. Based on the external color, the USDA established six visual ripening stages: green (100% green); breakers (a noticeable break in color from green with lesser than 10% of other than green color); turning (between 10 and 30% of surface of reddish color); pink (between 30 and 60% of the surface of reddish color); light red (between 60 and 90%) and red (more than 90% red).[Citation6]
Lycopene content of tomatoes depends on ripening conditions. Lycopene content is higher in tomatoes that have been ripened after harvesting than in vine-ripened ones.[Citation7] After passing the state of optimal maturation, the fruit quality deterioration increases. Research on postharvest maturation can certainly help to determine the optimal maturity stage. The distribution of vitamin C in tomato is usually similar to that of ascorbic oxidase, being two to three times higher in the skin than in the juice or flesh. Destruction of this vitamin depends on the concentration of oxidase.[Citation8] Previous reports on the relationship between mature stages and ascorbic acid content in tomato are conflicting. Some authors reported that vitamin C content was the highest at the yellow stage[Citation9] or before the red stage,[Citation8,Citation10–12] whereas other researchers showed that levels of vitamin C generally increased as the fruit ripens from the mature-green stage onward. When fruit ripens, the vitamin C level in the flesh increases up to the over-ripe stage.[Citation12–16] As reported by Giovanelli et al.,[Citation7] postharvest-ripened tomatoes had higher ascorbic acid content than vine-ripened tomatoes.
Carotenoids are synthesized during tomato maturation with a parallel appearance of pink skin. Lycopene accumulation during postharvest ripening followed an exponential rate, up to high concentration values at the very last period of ripening.[Citation7,Citation17] However, modification of β-carotene content during tomato maturity was reported differently. As reported by Dalal et al.,[Citation10] β-carotene content was maximum at the pink stage, while Abushita et al.[Citation9] showed that the concentration increased proportionally to the ripeness advance in accordance to the rapid accumulation of the red pigment.[Citation6] Lycopene and β-carotene accumulation was much higher in postharvest-ripened than in vine ripened tomatoes.[Citation7]
In postharvest studies, light and temperature may influence L*, a*, b* values.[Citation18,Citation19] It was reported that lycopene content and color CIE ratios a*/b* and (a*/b*)2 were significantly correlated,[Citation7,Citation17,Citation20,Citation21] which were exponential and linear functionalities, respectively. These good correlations allowed the estimation of lycopene content during tomato maturation without extraction procedures and chemical analysis. However, these correlations seemed to lose sensitivity at the red ripe stages.[Citation20] In the red stage, a very small modification of (a*/b*) corresponds to a very large change of lycopene content. Thus, these regression equations would not be enough exact at the red stage of tomato. Therefore, it is necessary to improve this correlation method to increase the exactitude at this stage.
When the tomato ripens at the red stage, the colour modification at the equatorial region is small. However, at the stem region the change in colour is much larger. Therefore, tomato could be chosen at red stage by measuring its colour at the stem zone, while many previous articles often reported the results with colour measurements done at the equator.[Citation17,Citation21]
Tomato maturation is also recognized by fruit softening as a result of the tissue structure modification. Exocarp and mesocarp of ripe tomato contribute principally to the mature fruit firmness. They modify profoundly during maturation and therefore the mechanical resistance of tomato changes also as function of the mature stage. Tomato mechanical resistance is also a tomato quality index, although such data are very rare in the literature. The modifications of skin and flesh resistances could certainly provide another method for evaluation of maturation. In addition, knowledge on mechanical resistance of tomato is very useful for proper handling of postharvest tomatoes.
Barret, Weakley, and Watnik[Citation22] in their research on qualitative and nutritional differences of tomatoes in organic and conventional production systems reported that the agricultural production system is a critical factor in determining the quality of fruit produced. There may be potential advantages to use of organically grown processing tomatoes because of higher levels of soluble solids, titratable acidity, and texture. In recent years, greenhouse tomatoes are successfully grown in Canada during winter. The ambient temperature and humidity as well as the lightning and the substrate are carefully controlled in order to obtain a high quality product with competitive prices. Unfortunately, the impact of the controlled environment on the quality of tomatoes has not been thoroughly studied, and the reported literature on quality of greenhouse tomatoes as a function of the maturity stage is not easily available in the literature.
The main goal of this work was to evaluate winter greenhouse tomato quality (texture, color, carotenoid, and vitamin C contents) during ripening in order to establish its best maturity stage. Attempt was also made to develop relationships between colour, texture, lycopene and vitamin C contents during maturation.
MATERIALS AND METHODS
“Savoura” tomatoes, Lycopersicum esculentum var beefsteak, at different maturities were bought in the local market (Québec, Canada). They were kept at room temperature for natural ripening until desired maturity for the experiment. Twenty-four (24) winter greenhouse tomatoes were used for the experiment, from turning to dark red stage. Each tomato fruit was analyzed to determine maturity parameters including colour, texture, carotenoids, vitamin C, soluble solids, water and acidity contents, as follows.
First, colour was determined by colorimetry at the stem region of a whole tomato. After the color measurement, each tomato was halved at the equator. The top half part was then used to measure the texture at the stem region. Afterwards, both parts (top and bottom half sections) were kept in ice for one hour prior to chemical analysis. The tomato sections were then grinded with a home mixer for two minutes. Tomato puree was transferred into a 60-ml beaker placed on ice. It was then homogenized with an Ultra Turrax (T25 Basic, Kika works Inc., Wilmington, NC, USA) for two minutes. The cold puree was used for analyzing carotenoids and ascorbic acid contents. Then, the puree was brought to room temperature to analyze soluble solids and water contents, pH and acidity.
Colour Measurements
Tomato color was determined from the L*, a*, b* coordinate system by using a colorimeter Minolta (CR-200 Series, Osaka, Japan). Before the measurement, the equipment was calibrated with a standard white tile (Y = 92.60, x = 0.3136, y = 0.3195). Reported colour data (L*, a*, b*) were the mean of five measurements.
Texture Measurements
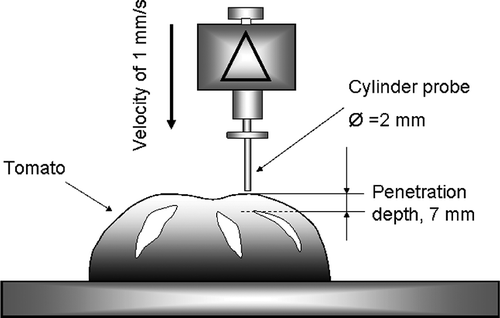
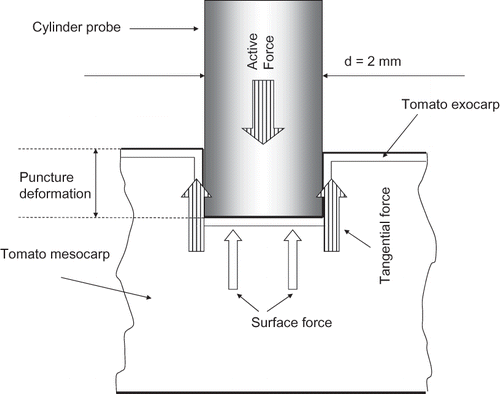
A compression texture profile (puncture force - deformation) was done with a texture analyzer (Model AX T2, Texture Tech. Corp., Scarsdale, NY, USA) on tomato halves (). The tomato and mesocarp firmness were measured at the stem region with a 2-mm diameter flat-face cylindrical probe, moving at a velocity of 1 mm/s up to a distance of 7 mm. The experiment was done at room temperature. Deformation was the puncture depth (mm) of the probe from first contact at tomato skin to the depth of skin rupture. The rupture strength is the highest force (N) at which the tomato skin was broken. Tomato firmness was computed as the initial slope (N/mm) of the force-deformation curve. The mesocarp strength is the resistance force (N) of the tomato mesocarp measured after the skin breaks. Reported data were a mean of five measurements. Because of the small probe diameter (2 mm), the pressure at the working point was approximately 2500 times the pressure on the whole sample, and thus the deformation of the half tomato did not affect the exactitude of the measurement.
Chemical Analysis
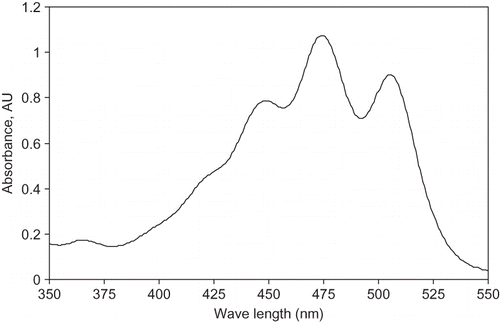
Carotenoids were determined by HPLC. Five (5) grams sample of the tomato puree was weighed with an analytical balance and then, twenty (20) ml 1% magnesium carbonate suspension was mixed in. After five minutes, the mixture was vacuum filtered through a Whatman No. 1 filter paper. The filtrate was clear without red or pink colour. All the insoluble carotenoids were left on the filter paper. The filter paper was transferred carefully into a 250-ml beaker for carotenoid extraction with 50 ml ethanol hexane (4:3 v/v) during one night. Then, the solution was collected and several extractions were repeated with 20 ml fresh solvent until all carotenoids were transferred into the liquid phase (the solvent had no colour after one-hour extraction and the pink colour on the filter paper disappeared). Extraction time depended on lycopene content. Total required solvent volume was 100–150 ml approximately. The solution was put in 250-ml separator funnel where 60 ml distilled water was added. The supernatant, a lipid phase containing hexane and carotenoids, was collected and then evaporated at 35°C to nearly dryness in a Rotavapor (Büchi, model RE 111, Brinkmann Instruments, Westbury, NY, USA) equipped with a water bath (Büchi, model B 461, Brinkmann Instruments, Westbury, NY, USA). The residue was first dissolved in 1 ml methylene chloride, followed by four times dissolution in 1 ml acetone. As carotenoids were totally recuperated, the solution obtained after the last dissolution was colourless. The carotenoid solution was adjusted exactly up 5 ml in a volumetric flask. The carotenoid solution was transferred into a closed vial and kept at 5–8°C before quick filtering through a 0.2-μm membrane filter with glass syringe. The filtrate was kept up to one week at 5–8°C in closed vials for HPLC analysis. Methylene chloride/acetone (20: 80 v/v, HPLC grade) was used to determine zero absorbance (blank sample). The diluted filtrate was scanned by Hewlett-Packard 8453 UV/visible spectrophotometer to determinate absorbance spectra (). The maximum absorbance of the solution was found to be at a wavelength of 476 nm.
Twenty (20) μl of carotenoid sample were injected at 5°C, using manual injector (Rheodyne 7725 Manual Injector Kit), into a HPLC System Gold Nouveau (Beckman Instrument Inc., Fullerton, CA, USA) with a inverse phase column, YMC30, 250 × 4.6 mm I.D, 5 μm particle (Waters Corporation, MA, USA) preceded by a YMC carotenoid S-5, 4.0 × 20 mm DC guard Cartridge, and YMC DIR-CON Guard Fitting (Waters Corporation, MA, USA). The column was placed in a column heater controlled at 40°C by a Precision Temperature Controller HTD 96-1. The HPLC system assembled also a solvent pump system (126P Solvent Module), a visible detector (166 detector with tungsten lamp, option part number 239370), and a software (Gold Nouveau Chromatography Data System v.1.0). A HPLC grade solvent mixture of 1-butanol and acetonitrile (210:490, v/v) (A) and 1-butanol, acetonitrile, and methylene chloride (84:196:70 v/v) (B), with a gradient elution of 70% A and 30% B, was used as the carrier. The flow-rate of the solvent was 1.0 ml/min. The output signal was detected at 476 nm. Lycopene (Chromadex, Santa Ana, CA, USA) and β-carotene (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada) standards were used for quantification. The identification of all-trans carotenoids and β-carotene was carried out by comparing the retention times and absorption spectra with reference standards.
The ascorbic acid content was analyzed by volumetric titration with sodium 2,6-dichloroindo phenol.[Citation23] Soluble solids content (°Brix) was measured by refractometry (Vista Vision, model 12777–984, Jenco International, Inc., Portland, OR, USA). The water content was analyzed in a vacuum oven at 60 °C until constant weight (± 0.005 g). The pH was measured at ambient temperature with a pH meter (SympHony, model SP20, Thermo Electron Corporation, USA). The acidity was determined by titration with 0.1N sodium hydroxide until pH 8.1 using the pH meter, expressing the results in grams anhydrous citric acid per 100 g of tomato.
Statistic Treatment
At least five (5) measurements of each tomato fruit were done at the stem region for color and texture measurments. Analysis of water content, °Brix, pH, titratable acidity, vitamin C, and carotenoids contents were done in duplicate. Reported data are the average values of each fruit. The regression equations obtained from those data were done by the Software of Microsoft Office Excel 2003 SP1, part of Microsoft Office Professional Edition 2003.
RESULTS AND DISCUSSION
°Brix, pH, and Acidity
The ANOVA analysis of the data of °Brix, water content, pH and acidity showed that there was no significant difference (p < 0.05) at different postharvest maturities. Therefore, the mean of these results at different maturity stages were 4.3, 94%, 4.25 and 0.5%, respectively for °Brix, water content, pH, and acidity. Although soluble solid content, acidity and pH were previously shown to increase with vine maturity,[Citation24,Citation25] in the present work they were practically constant during postharvest maturation. While wild tomatoes may attain very high soluble solid content (11–15%), common processing tomatoes exhibit moderate values, ranging between 4.5 and 6.25%.[Citation24] The greenhouse tomato soluble solids content obtained in this study is low, 4.3%.
Tomato water content and agricultural yield have a direct correlation.[Citation24,Citation26] On the other hand, soluble solids (°Brix) and total solids (%) are both negatively affected by irrigation.[Citation27] Greenhouse tomato growers use good irrigation to obtain a high agricultural yield, and therefore greenhouse tomatoes have high water and low soluble solids contents.[Citation28]
The taste of tomatoes is affected by the total soluble solids (°Brix) and titratable acidity. The work of Malundo, Shewfelt, and Scott[Citation29] on consumer acceptability of tomatoes showed that sugar level has a positive correlation with sweet taste while acid concentration positively correlates with sour taste. The sensory model generated from this research illustrates that at an acid level of 0.5%, as in the case of ‘Savoura’ tomatoes studied in this work, increasing °Brix increases flavor acceptance.
Tomato Colour
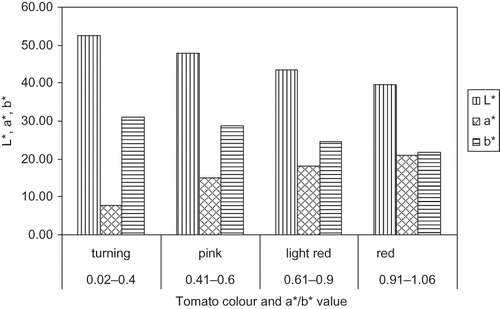
shows the L*, a*, b* value modification during tomato ripening stages. The lightness factor, L*, decreased during the ripening stages. This decrease reflects the darkening of the tomatoes with carotenoid synthesis and the loss of greenness. The a* value (green-red axe) increased during maturation as a consequence of the synthesis of lycopene (red) and depletion of chlorophyll (green), representing a color change from green to red. The b* value (blue yellow axe) decreased during ripening () showing that the effect of the yellow from the β-carotene was smaller when compared to the red of lycopene.
Lin and Chen[Citation2] found out that when color measurements were done at the stem region, L* and b* always decreased and a* always increased, while the measurement at the equatorial region showed that L* did not distinguish between the different red maturity stages, b* values increased through the first maturity stages and then decreased, and a* values were almost the same between the red maturity groups. Therefore, the colour measurements at tomato stem region seemed to be more suitable than at the equatorial region for describing maturation stages of tomato, especially at the red stages. The chromatic coordinates L*, a*, b* can all be used as maturation indexes. However, because a* increases and b* decreases during ripening, a*/b* or (a*/b*)2
ratio could be more appropriate as maturity indexes than a* and b* separately. The chroma value () changed slightly during maturation, and thus it did not show a good correlation with the ripening stages of tomatoes as well.
Antioxidant Content and Ripening
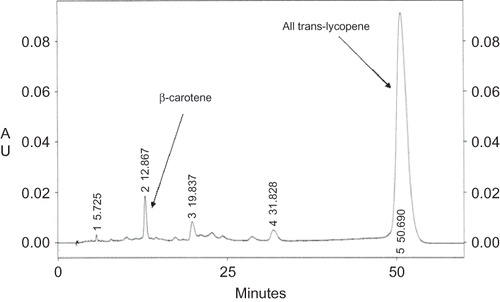
An example of the HPLC chromatogram for carotenoids obtained in this work is presented in . Several isomers of carotenoids were found but all-trans-lycopene and β-carotene were the most important carotenoids present in “Savoura” greenhouse tomatoes. Lycopene content changed during the ripening of tomato from 15 to 60 μg/g, showing that it was synthesized during maturation when the red colour of the tomato skin also increased.
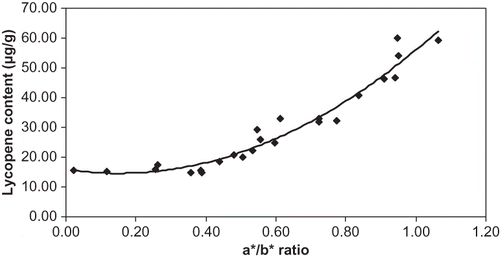
The value of a*/b* of the tomato skin had a good correlation with lycopene content. As shown in , the regression equation that better fitted the data was a second order polynomial (R2 = 95%) instead of an exponential as found when the color value was measured at the equatorial region[Citation10]:
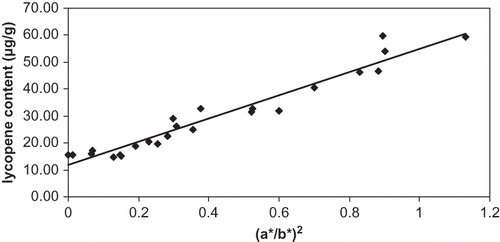
The change of regression equation type pointed out that measuring the color at the stem region could have a better accuracy at red stages. A linear regression of lycopene content and the quadratic function (a*/b*)2 had also a good determination coefficient (R2 = 94%), as shown in :
Therefore, the ratio a*/b* could prove not only useful as a maturation index, but also as a lycopene index of tomato. It permits the estimation of lycopene content of ripened tomato without chemical analysis. In addition, it pointed out that lycopene content increased importantly still at the end of maturation. These results were in agreement with those of Arias et al.[Citation17] However, Kaur et al.[Citation30] reported that lycopene content best correlated with Hunter a* values during ripening.
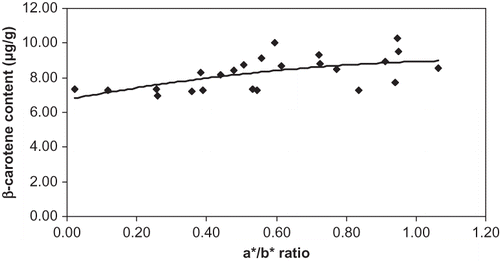
The HPLC results of β-carotene content were found to be from 7 to 10 μg/g, it tended to slightly increase during maturation (). However, the correlation between β-carotene content and a*/b* ratio was poor (R2 = 0.35), which means that the effect of β-carotene content on the visual colour was weak. The results found for β-carotene disagree with those of Dalal et al.,[Citation10] but agreed with the ones of Abushita et al.[Citation9]
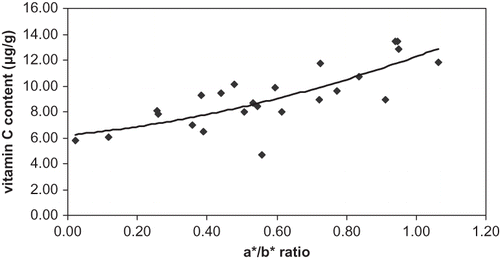
Vitamin C content of the tomato changed from 6 to 13 mg/100 g tomato. It tended to increase during maturation (). However, the correlation between β-carotene content and colour ratio, a*/b*, was not strong (R2 = 0.61). These results pointed out that the synthesis vitamin C in tomato continued during maturation and was more important than losses by oxidation after harvest. These results disagreed with the ones of Wokes and Organ,[Citation8] Abushita et al.,[Citation9] Dalal et al.,[Citation10] Lo Coco,[Citation11] Malewski and Markakis,[Citation12] but agreed with the ones of Clow and Marlatt,[Citation13] Hamner et al.,[Citation14] Liptay,[Citation15] and Liptay et al.[Citation16] The data showed that greenhouse “Savoura” tomatoes, harvested in winter, had the highest lycopene, β-carotene, and vitamin C contents at the red stage.
Texture and Ripening
In addition to compositional changes, tomato texture also transforms during ripening. In normal ripening, softening of the pericarp flesh is the major cause for the loss in texture. Texture is therefore considered as an appropriate index for the ripening process and for the associated tomato quality.[Citation31] On the other hand, decreased turgor due to water loss has also been suggested as a cause of tissue softening.[Citation32]
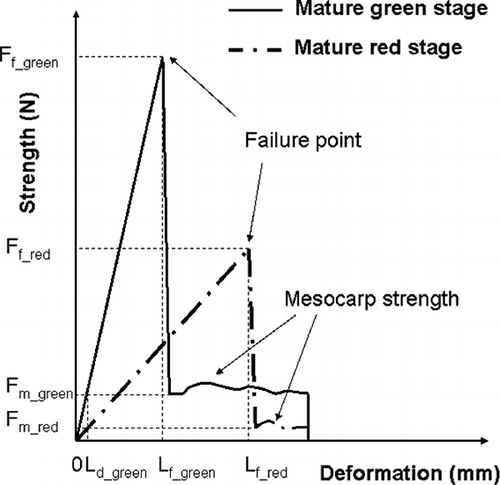
Strength-deformation curves of turning and red stages of tomato are shown in . Strength increased continuously until the bioyield point. Then, the strength decreased suddenly to reach the mesocarp resistance force. The resistance force varied slightly because of the heterogeneity of the mesocarp texture.
The relationship between tomato strength (N) and deformation (mm) was practically linear before the bioyield point (broken skin). Fruits with higher deformation value are softer. The initial slope of the curve (N/mm) represents the tactile tomato firmness. In the case of tomatoes, the initial slope was approximately the whole curve slope. The results showed that before the skin was broken, the overall resistance of tomato increased linearly. The deformation at the broken point was the maximum deformation level of the structure.
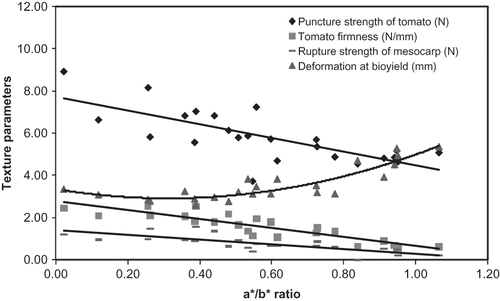
shows the rupture strength of skin and mesocarp, deformation level at bioyield and tomato firmness calculated from strength-deformation curves at different maturity stages of tomato. As shown in , the strength-deformation curve slope (firmness), skin, and mesocarp maximum strength importantly decreased during maturation. On the other hand, the maximum deformation level increased markedly. shows the firmness and maximum deformation as a function of the chromatic parameter (a*/b*). These results point out that there was an important textural change in the course of tomato ripening from firm to soft.
Table 1 Changes of tomato texture measurements as function of maturity
The two main parts of tomato resistance to deformation are the exocarp and the mesocarp, both changed profoundly during maturation decreasing their resistance and thus, the total tomato firmness decreased. The mesocarp resistance is always weaker than the exocarp resistance, pointing out the effective role of tomato skin in its mechanical protection. When an external mechanic shock is strong enough, the pulp will be damaged whereas the skin is still intact. From the point of view of postharvest handling, the external strength must be smaller than the strength of mesocarp. For the same reason, turning tomatoes having bigger resistance of the mesocarp, are more suitable for post harvest handling.
The firmness loss has been explained by several biochemical changes that lead to softening of fruits, accompanied by loss of neutral sugars, solubilisation and depolymerisation of the polysaccharides of the cell wall, and rearrangements of their associations, as the result of the combined action of several cell wall-modifying enzymes, acting in both pectic and hemicellulosic fractions.[Citation33]
Bourne[Citation34] showed that for most foods the bioyield point force, Ff , is proportional to both the area and perimeter of punch:
The tomato strength, F, includes tangential and perpendicular forces as shown in . The perpendicular force is created essentially by the compression of the mesocarp, whereas the tangential force, by the tension of the skin. Moreover, after the puncture point, the essential tangential force (skin tension) becomes zero, F becomes the strength of the mesocarp (Fm ). The mesocarp strength is small and practically constant with deformation ().
When L> Lf (puncture deformation level), EquationEq. (4) becomes:
The mesocarp strength, Fm, has the same value before the puncture point:
The value of the coefficient K2 can be thus calculated from EquationEq. (7). For L = Lf :
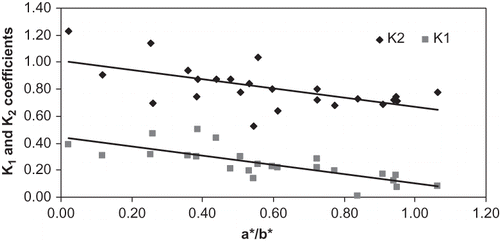
Coefficients K1 and K2 were then plotted as a function of maturation color parameter (a*/b*) in . Both coefficients decreased through maturation.
Figure 12 Modification of puncture coefficients, K1 compression coefficient (N/mm2) and K2 shear coefficient (N/mm), as function of maturity, a*/b*.
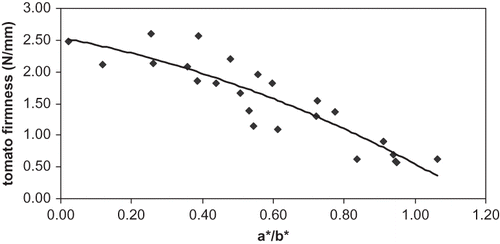
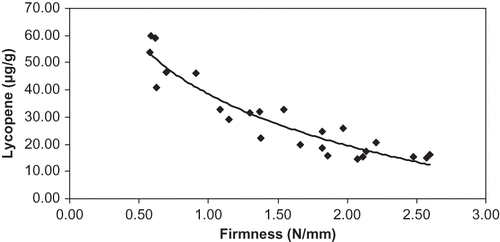
The tomato firmness and a*/b* ratio had a high correlation () described by (R2 = 0.81):
Both measurements, firmness and color ratio, can be done rapidly and are useful for evaluation of maturity degree of tomato. The lycopene content (μg/g) and tomato firmness also had a high correlation. When tomatoes ripened, the firmness decreased and the lycopene content increased, as shown in the . The regression equation was logarithmic-type (R2 = 0.89):
In addition to the relationship between lycopene content and a*/b* ratio (EquationEq. 1), the correlation between lycopene and firmness is also an alternative way capable of estimating lycopene content from fruit firmness of the greenhouse “Savoura” tomato.
CONCLUSION
This research showed the evolution of important properties during ripening of greenhouse tomatoes, such as °Brix, acidity, pH, water content, antioxidant composition, colour, firmness, and their interactions. Greenhouse tomato has low soluble solids content almost constant during postharvest maturity. Colour measurement at stem results in a polynomial regression of the correlation between lycopene content and (a*/b*). This regression equation is useful and exact at the red stage for estimating lycopene content. The ascorbic acid, lycopene and β-carotene contents of the greenhouse “Savoura” tomato increase during the postharvest maturation up to the red stage. The red stage is thus the best maturity stage of “Savoura” greenhouse tomato as a functional food. The strength-deformation curves and firmness of tomatoes were determined as a function of maturity. The puncture force decreases during maturity while the puncture deformation increases significantly. The firmness model and data of the winter greenhouse tomato obtained in this work can be used to choose a suitable maturity stage for the Canadian tomato industry.
ACKNOWLEDGMENTS
The authors wish to thank the PCBF, Canadian International Development Agency and NSERC (Natural Sciences and Engineering Research Council of Canada) for their financial support.
REFERENCES
- Goula , A.M. , Adamopoulos , K.G. , Chatzitakis , P.C. and Nikas , V.A. 2006 . Prediction of Lycopene Degradation During a Drying Process of Tomato Pulp . Journal of Food Engineering , 74 ( 1 ) : 37 – 46 .
- Lee , M.T. and Chen , B.H. 2002 . Stability of Lycopene During Heating and Illumination in a Model System . Food Chemistry , 78 ( 4 ) : 425 – 432 .
- Perera , C.O. and Yen , G.M. 2007 . Functional Properties of Carotenoids in Human Health . International Journal of Food Properties , 10 ( 2 ) : 201 – 230 .
- Rao , A.V. and Ali , A. 2007 . Biologically Active Phytochemicals in Human Health: Lycopene . International Journal of Food Properties , 10 ( 2 ) : 279 – 288 .
- Lai , A. , Santangelo , E. , Soressi , G.P. and Fantoni , R. 2007 . Analysis of the Main Secondary Metabolites Produced in Tomato (Lycopersicon esculentum, Mill.) Epicarp Tissue During Fruit Ripening Using Fluorescence Techniques . Postharvest Biology and Technology , 43 ( 3 ) : 335 – 342 .
- Watada , A.E. , Norris , K.H. , Worthington , J.T. and Massie , D.R. 1976 . Estimation of Chlorophyll and Carotenoid Contents whole Tomato by Light Absorbance Technique . Journal of Food Science , 41 ( 2 ) : 329 – 332 .
- Giovanelli , G. , Lavelli , V. , Peri , C. and Nobili , S. 1999 . Variation in antioxidant components of tomato during vine and postharvest ripening . Journal of the Science of Food and Agriculture , 79 ( 12 ) : 1583 – 1588 .
- Wokes , F. and Organ , J.G. 1943 . Oxidizing Enzymes and Vitamin C in Tomatoes . Biochemical journal , 37 ( 2 ) : 259 – 265 .
- Abushita , A.A. , Hebshi , E.A. , Daood , H.G. and Biacs , P.A. 1997 . Determination of Antioxidant Vitamins in Tomatoes . Food Chemistry , 60 ( 2 ) : 207 – 212 .
- Dalal , K.B. , Salunkhe , D.K. , Boe , A.A. and Olson , L.E. 1965 . Certain Physiological and Biochemical Changes in the Developing Tomato Fruit (Lycopersicon esculentum Mill.) . Journal of Food Science , 30 ( 3 ) : 504 – 508 .
- Lo Coco , G. 1945 . Composition of Northern California tomatoes . Journal of Food Science , 10 ( 2 ) : 114 – 121 .
- Malewski , W. and Markakis , P. 1971 . A Research Note Ascorbic Acid Content of the Developing Tomato Fruit . Journal of Food Science , 36 : 537
- Clow , B. and Marlatt , A.L. 1930 . Studies in Vitamin C in Fresh and Canned Tomatoes . Journal of Agricultural Research , 40 : 767 – 775 .
- Hamner , K.C. , Bernstein , L. and Maynard , L.A. 1945 . Effects of Light Intensity, Day Length, Temperature, and Other Environmental Factors on the Ascorbic Acid Content of Tomatoes . Journal of Nutrition , 29 ( 2 ) : 85 – 97 .
- Liptay , A. 1984 . Content and Distribution of vitamin C in Tomato Fruits During their Development . Acta Horticulturae (ISHS) , 163 : 271 – 276 .
- Liptay , A. , Papadopoulos , P. , Bryan , H.H. and Gull , D. 1986 . Ascorbic Acid Levels in Tomato (Lycopersicon esculentum Mill.) at low Temperature . Agricultural Biological Chemistry , 50 ( 12 ) : 3185 – 3187 .
- Arias , R. , Lee , T.-C. , Logendra , L. and Janes , H. 2000 . Correlation of Lycopene Measured by HPLC with the L*, a*, b*, Color Readings of a Hydroponic Tomato and the Relationship of Maturity with Color and Lycopene Content . Journal of Agricultural and Food Chemistry , 48 ( 5 ) : 1697 – 1702 .
- Camelo , A.F.L. and Gómez , P.A. 2004 . Comparison of Color Indexes for Tomato Ripening . Horticultura Brasileira , 22 ( 3 ) : 534 – 537 .
- Shewfelt , R.L. , Thai , C.N. and Davis , J.W. 1988 . Prediction of Changes in Color of Tomatoes during Ripening at Different Constant Temperatures . Journal of Food Science , 53 ( 5 ) : 1433 – 1437 .
- Davis , A.R. , Fish , W.W. and Perkins-Veazie , P. 2003 . A Rapid Spectrophotometric Method for Analyzing Lycopene Content in Tomato and Tomato Products . Postharvest Biology and Technology , 28 ( 3 ) : 425 – 430 .
- Thompson , K.A. , Marshall , M.R. , Sims , C.A. , Wei , C.I. , Sargent , S.A. and Scott , J.W. 2000 . Cultivar, Maturity, and Heat Treatment on Lycopene Content in Tomatoes . Journal of Food Science , 65 ( 5 ) : 791 – 795 .
- Barrett , D.M. , Weakley , C. , Diaz , J.V. and Watnik , M. 2007 . Qualitative and Nutritional Differences in Processing Tomatoes Grown under Commercial Organic and Conventional Production Systems . Journal of Food Science , 72 ( 9 ) : C441 – C451 .
- Askar , A. and Treptow , H. 1993 . Quality Assurance in Tropical fruit Processing , 231 Berlin, Heidelberg : Springer-Verlag .
- Garcia , E. and Barrett , D.M. 2006 . Evaluation of Processing Tomatoes from Two Consecutive Growing Seasons: Quality Attributes, Peelability and Yield . Journal of Food Processing and Preservation , 30 ( 1 ) : 20 – 36 .
- Renquist , A.R. and Reid , J.B. 1998 . Quality of Processing Tomato (lycopersicon esculentum) Fruit From Four Bloom Dates in Relation to Optimal Harvest Timing . New Zealand Journal of crop and Horticultural Science , 26 ( 2 ) : 161 – 168 .
- Rodica , S. , Apahidean , S.A. , Apahidean , M. , Maniutiu , D. and Paulette , L. Yield . Physical and Chemical Characteristics of Greenhouse Tomato Grown on Soil and Organic Substratum . 43rd Croatian and 3rd International Symposium on Agriculture . Edited by: Pospisil , M. pp. 439 – 443 . Opatija, Croatia : University of Zagreb, Faculty of Agriculture .
- Warner , J. , Tan , C.S. and Zhang , T.Q. Effect of Regulated Deficit Drip Irrigation on Processing Tomato Fruit Solids and Yield . 2004 ASAE Annual International Meeting. Fairmount Chateau Laurier, The Westin, government Center . Ottawa, Ontario, Canada. St. Joseph, MI : American Society of Agricultural and Biological Engineers .
- Cook , R. and Calvin , L. 2005 . Greenhouse Tomatoes Change the Dynamics of the North American Fresh Tomato Industry . Electronic Report from the Economic Research Service: USA , : 81
- Malundo , T.M.M. , Shewfelt , R.L. and Scott , J.W. 1995 . Flavor Quality of Fresh Tomato (Lycopersicon esculentum Mill.) as Affected by Sugar and Acid Levels . Postharvest Biology and Technology , 6 ( 1–2 ) : 103 – 110 .
- Kaur , D. , Sharma , R. , Wani , A.A. , Gill , B.S. and Sogi , D.S. 2006 . Physicochemical Changes in Seven Tomato (Lycopersicon esculentum) Cultivars During Ripening . International Journal of Food Properties , 9 ( 4 ) : 747 – 757 .
- Frenkel , C. and Jen , J.J. Tomatoes. 1989 . Quality and Preservation of Vegetable , Edited by: Eskin , N.A.M. 53 – 73 . Boca Raton, FL : CRC Press .
- Beaulieu , J.C. and Gorny , J.R. 2001 . “ Fresh-Cut Fruits ” . In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, Agriculture Handbook Number 66 , Edited by: Gross , K.C. , Saltveit , M.E. and Wang , C.Y. 1 – 49 . Washington, DC : USDA, ARS .
- Goulao , L.F. and Oliveira , C.M. 2008 . Cell Wall Modifications During Fruit Ripening: When a Fruit is not the Fruit . Trends in Food Science & Technology , 19 ( 1 ) : 4 – 25 .
- Bourne , M.C. 1975 . Method for Obtaining Compression and Shear Coefficients of Food Using Cylindrical Punches . Journal of Texture Studies , 5 ( 4 ) : 459 – 469 .