Abstract
This research evaluated the chemical, technological, and thermal properties of rice flour modified chemically using phosphorus oxychloride (0.018; 0.030; 0.059; 0.088 and 0.100 g/100 g flour). The phosphorylated rice flours (PRF) obtained showed a decrease in viscosity, but the stability increased significantly as compared to the unmodified flour. The gel force and adhesiveness values decreased with the increase in phosphate groups incorporated. However, cohesiveness and thermal properties did not show significant differences among the native and modified rice flours, with exception for gelatinization enthalpy values. The phosphorylated flours obtained can be used as ingredient, especially in cream soups, salad dressing, and filling.
INTRODUCTION
In Brazil, the by-products of the rice milling industry (broken grains and flour) have low commercial value, and large volumes are often used for feed. One of the alternatives to add value to this raw material and to expand its applications would be the chemical modification of the starch, transforming these co-products into food ingredients with enhanced functional properties.[Citation1] Starch is the predominant component of rice, and it contributes to the rice product properties.[Citation2]
Generally, phosphorylation enhances the physicochemical properties of the modified starches as compared to their native counterparts. The solubility and swelling power greatly increase when phosphorylation is carried out to a low degree of substitution, and then gradually decrease with increasing degree of substitution. However, the values for the monoesters were still higher than those of the corresponding native polysaccharides.[Citation3]
Crosslinked starch is more resistant to acid, heat and shearing than native starch. The reaction conditions used for the production of crosslinked starch vary widely, depending on the specific bi or polyfunctional reagents used.[Citation4] Starch phosphates are derived from starches, widely used in diverse food products such as gravies, cream soups, sauces, oriental foods, and baby foods. These modified starches can be divided into two broad categories: starch phosphate monoesters, and starch phosphate multiesters, in which more than one of the acid groups of the phosphoric acid is esterified. The latter is usually a mixture of mono-, di-, and triesters of phosphoric acid.[Citation5]
Starch modification is permitted using a bi or polyfunctional (phosphorus oxycloride, sodium trimetaphosphate, or mixtures of adipic and acetic anhydride) reagent, capable of reacting with two or more different hydroxyl groups in the same or different starch polymer. The introduction of negatively charged phosphate groups reduces inter-chain associations and facilitates starch hydration. Starch phosphates can be grouped into two classes: mono-starch phosphates and di-starch phosphates. The starch phosphate monoester is formed when one starch hydroxyl group is esterified to phosphate and the starch phosphate di-ester is formed when two starch hydroxyl groups are esterified to the same phosphate group. During phosphorylation, pH plays a dominant role in determining the ratio of starch monoester to di-ester. Phosphate monoesters are produced in a pH range of 5.0–6.5 with mixtures of orthophosphates, and in a pH range of 5.0–9.0 with sodium tripolyphosphate (STPP).[Citation6]
The preparation of starch phosphates has included diverse starch sources such as waxy, common, and high-amylose cornstarches, wheat, sago, cassava, and rice starches. However, the effects of the phosphorous oxychloride concentration on the chemical, functional, and thermal properties of rice flours have not been evaluated. Thus, the aim of this research was to prepare phosphorylated rice flours and to analyse their chemical, rheological, and thermal properties.
EXPERIMENTAL
Raw Materials
The rice (Oriza sativa L. var. Agulha) was donated byCerealista Coradine Ltda. (Porto Alegre-RS-Brazil). The rice grains were milled using a Brabender mill (Quadrumat Senior), with a particle size of <250 microns.
Chemical Analysis of the Rice Flour
Moisture, total ash, protein, crude fiber and lipids were measured using[Citation7] methods 44-15A, 08-01, 46-13, 32-10, and 30-10, respectively. The carbohydrate content was determined by difference. Amylose was determined following the methodology described by Bhattachrya et al.[Citation8] All measurements were carried out in triplicate.
Preparation of Phosphorylated Rice Flour (PRF)
Rice flour (350 g, dry base) was suspended in 700 mL of distilled water with 7 g of Na2SO4, the pH adjusted to 11.5 using 2N NaOH, and phosphorus oxychloride added at different concentrations, as shown in. The mixture was stirred for 90 min at 45°C and the suspension then adjusted to pH 5.5 with 1N HCl. The rice flour was washed fourtimes with distilled water, centrifuged (Fanen, model 20NR), filtered and oven dried at 40°C.[Citation4] The modified rice flour was milled using a Brabender mill (Quadrumat Senior), with a particle size <250 microns for further analyses.
Table 1 Percent phosphorous, degree of substitution and viscosity properties of unmodified rice and phosphorylated and flours
Determination of Phosphorus
The starch sample was washed with 65% ethanol and with methanol to remove the excess salt solution, then defatted and incinerated at 550°C.[Citation9] All the measurements were performed using ICP trace analyser emission spectrometer (Model ICAP 61E, Thermo-Jarrel Ash, Waltham, MA) and the results expressed as mg/100 g flour.[Citation1]
Degree of Substitution (DS)
The degree of substitution was calculated according to Rutenberg and Solarek[Citation10] using the following equation:
Viscosity Profiles
The viscosity of the samples was determined using a rapid visco-analyser model 3C (RVA; Newport Scientific Pty Ltd., Sidney, Australia) using the Thermocline program-Windows version 1.10. The suspensions were prepared using 2.5 g of sample and adjusted to 27.5 mL with distilled water (corrected to 14 g/100 g de humidity, w.b.). The standard 1 test was used involving 13 min of analysis. The following parameters were measured: peak viscosity (PV), hot paste viscosity (HPV), cold paste viscosity (CPV), breakdown, retrogradation, and stability. All tests were performed in triplicate.
Thermal Properties
Differential Scanning Calorimetry (DSC 821, Birefrigerated, Mettler Toledo Lab Plant, Huddersfield, England) was used to measure the thermal properties of the unmodified and modified rice flours. The samples were heated from 30 to 100°C at a rate of 10°C/min and onset (To), peak (Tp), conclusion (Tc) temperatures of gelatinization, and enthalpy (ΔH, J/g) were determined using software provided with the equipment.[Citation11] The range Tr was calculated as Tc-To. All measurements were replicated twice.
Gel Texture
After RVA testing, the starch pastes were covered and kept at 4°C for 12 hr, and hardness and cohesiveness of the gel were registered using a universal TA–XT2 Texture Analyser (Stable Micro System, Godalming, UK), equipped with the texture Expert software program (Version 1.19). For texture analysis was used cylindrical probe (20 mm, ser.121) and operated in the compression mode. The probe speed was 3.0 mm s−1, and compression distance was 10 mm. Three repetitions were made for each analysis.
Statistical Analysis
All data were statistically analysed using the Statistica, a computer software package (StatSoft, Inc., Tulsa, OK, USA), using the Tukey test to differentiate the means different treatments (P < 0.05)
RESULTS AND DISCUSSION
The chemical composition (d.b., %, three repetitions) of rice flour was 0.49 total ash, 7.2 protein (N × 6.25), 0.63 lipids, 0.01 crude fiber, and 91.67 total carbohydrates. These values are between the ranges established by the Brazilian legislation.[Citation12] The amylose value was 22.70%, classified as rice with intermediate amylose content,[Citation13] the most common rice produced in Brazil. Cameron and Wang[Citation14] analysed eight long-grain rice cultivars and reported no differences in the chemical composition with respect to crude protein (6.6–9.3%), crude fat (0.18–0.51%), and apparent amylose content determined by iodine colorimetry (19.6–27.0%)
Properties of Phosphorylated Rice Flours
The degree of substitution varied from 9 × 10−3 to 24.8 × 10−4 (), the maximum limit for the addition of the reagent being according to the Brazilian and USA legislation. These legislations specify that the addition of phosphorous oxychloride must be no higher than 0.1%. The percent phosphorous introduced was increased with increase in amount of phosphorous oxychloride added and significant differences were found between the modified samples, except for the last two samples, modified with the highest concentrations of phosphorous oxychloride, which showed no significant differences between them (0.047 and 0.046 mg/100 g flour), probably indicating that the reaction capacity was saturated under the conditions used. The degree of substitution, evaluated from the average number of derived D-glucopyranosil units, followed the same trend. The degreeof substitution found in the starch phosphates was associated with the percentage of phosphorous oxychloride.
These values were lower than the values reported for wheat and corn phosphates reported by Lim and Seib[Citation15] using 5% (based on dry starch) sodium tripolyphosphate at pH 6. Also, Chang and Lii[Citation16] reported that the degree of substitution was 0.047 for cassava starch phosphates prepared by conventional methods and 0.040 by extrusion methods. Corresponding values for corn were 0.048 and 0.040 using 4 and 12.6 g of sodium tripolyphosphate/100 g starch in the extrusion and conventional processes, respectively. These differences were attributed to the differences in raw materials and the phosphorylation process used.
Yeh and Yeh[Citation17] prepared crosslinked rice starch using a 35% starch solution, 0.1% of phosphorous oxychloride, alkaline pH of 10.5, 5.0% sodium sulphate, reaction temperature of 35°C and different reaction times (15, 30, 60, and 120 minutes). The degree of substitution increased with the increase in reaction time, varying from 2.86 to 3.66. These researchers reported that crosslinking increased the heat of gelatinization, shear stability, and reduced the solubility in dimethyl sulfoxide and the freeze-thaw stability.
Viscosity Profiles
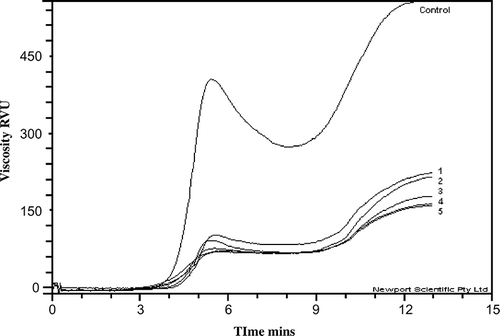
As compared to the unmodified flour the values for peak viscosity (PV), hot paste viscosity (HPV), cold paste viscosity (CPV), paste temperature (PT), breakdown and retrogradation decreased but the PRF stability increased significantly. The phosphorylated samples were characterized by a lack of peak viscosity. All the viscosity values were significantly different to that of the control and followed a similar trend between them. The lower viscosity values for the PRF with higher degrees of substitution, suggests that the components of the starch were negatively charged for the phosphate ester groups and formed a weak gel due to repulsion between the negative charges. Similar results were reported by Sitohy et al.,[Citation3] Marusza and Tomasik,[Citation18] Lim and Seib,[Citation15] Lin and Czuzhajowska,[Citation19] and Landerito and Wang,[Citation20–21] showing that the introduction of phosphate groups diminished the starch gelatinization temperature.
In addition, Wurzburg[Citation4] reported that the introduction of crosslinking strengthened the starch granule structure, preventing it from swelling, and consequently the viscosity decreased with increase in these chemical linkages. According to this research, the presence of a medium level of crosslinking (1 link per 440 units of anhydroglucose), is sufficient to reinforce the starch granule structure and critically inhibit the swelling of the starch granule, thus decreasing the viscosity.
Starches from normal maize, maize with high amylose, maize with high amylopectin, rice and potato, were phosphorylated with different degrees of substitution using monosodium orthophosphate and disodium hydrogen phosphate at 160°C under vacuum. The modified starches showed the highest viscosity values with the lowest degree of substitution, except for the high amylose maize starch, for which increasing degrees of substitution increased the values of viscosity.[Citation3]
The retrogradation values of PRF were lower than those of the raw rice flours, suggesting that during the modification with phosphorous oxychloride, more monoester groups than diester groups (crosslinking) were produced, as previously reported by other researchers.[Citation5,Citation22] The presence of only crosslinking would increase these values, since this type of linkage increases the degree of interaction between the molecules, whilst the monoester linkages decrease these interactions.[Citation4]
Phosphorylation
One of the most important properties of the crosslinked flour/starch is its resistance to shear during prolonged heating, this property being used as a way of characterizing this type of linkage.[Citation4] The values of breakdown and stability showed this behaviour ( and). The paste viscosity values of the PRF during heating at a constant temperature of 95°C followed by cooling, suffered little change due to the modification, whilst with the unmodified rice flour, the viscosity decreased during heating at a constant temperature of 95°C and then significantly increased.
Figure 1 Viscosity profiles of unmodified and acetylated rice flours. 1- 0.46, 2- 1.50, 3- 4.00, 4- 6.50, 5- 7.53 g kg−1 Acetic anhydride and control = unmodified rice flour.
Chatakanonda et al.[Citation23] crosslinked rice starch with 300 mg of sodium trimetaphosphate and sodium tripolyphosphate (0, 18, 27, and 39 g) dissolved in 300 mL of water at a pH value of 11 and temperature of 50°C for 20 min. The degrees of substitution were 0.0, 9.2, 26.2, and 29.2%, respectively. The crosslinking of the starch significantly raised the gelatinization temperature, but did not significantly affect the enthalpy. The delay in gelatinization and retrogradation was due to the restricted swelling and reduced hydration of the rice starch granules.
Muhammad et al.[Citation24] reported that during the phosphorylation of sago starch, the reaction conditions and reagents used modified the viscosity values. Sago starch phosphorylated with 2% sodium trimetaphosphate and 5% sodium trypolyphosphate at pH 9.5, showed lower hot paste viscosity and higher cold paste viscosity than for sago starches phosphorylated at pH 9 using only sodium trypolyphosphate. Contrary to our findings, some researchers (6, 25, 14, 26, 3, 27) have reported that phosphorylated starches exhibited much higher peak, final and total setback viscosities and swelled earlier, compared with their parent starches. Also, Landerito and Wang[Citation20] reported that, in general, waxy, common, and high-amylose cornstarches phosphorylated with blends of sodium tripolyphosphate (STPP) and sodium trimetaphosphate (STMP) by the oven-heating method, showed higher viscosity properties as compared to the unmodified starch.In general, the phosphorylated starch prepared by the slurry treatment exhibited a lower gelatinization temperature, a higher peak viscosity, a reduced degree of retrogradation and improved freeze-thaw stability as compared to those prepared by the dry-mixing treatment. The slurry treatment more efficiently incorporated phosphorous into the starch as compared to the dry mixing treatment under the reaction conditions studied. Waxy starch was more prone to phosphorylation, followed by common and high-amylose starches, respectively.
In the present research, the amount and location of the incorporated phosphate groups varied with the starch type according to the amylose-amylopectin ratio, starch source, microstructural characteristics, starch purity, different phosphate salts and the conditions during the phosphorylation treatments. Reacting with STPP and STMP at pH 10 produced starch phosphates with excessive crosslinking, indicating that starch diester formation prevailed in alkali-catalysed reactions with STPP and STMP.[Citation6]
The crosslinking of starch with STMP using extrusion process increased the levels of STMP from 5 to 15% reduced the water solubility index and sectional expansion index of extrudates.[Citation28]
Gel Texture
The values for firmness and adhesiveness () decreased with increase in the numbers of phosphate groups incorporated during phosphorylation. These values were significantly different between samples and from the control. However, cohesiveness showed values from 0.492 to 0.558 and did not show significant differences between them or with the control. Similar findings were reported by Liu et al.[Citation27]
Table 2 Gel texture properties of unmodified and phosphorylated rice flours
These researchers reported that after phosphorylation, the hardness of normal starch gels decreased and that ofwx starch gels increased, both these effects being relatively independent of the pH level. Also, the adhesiveness, springiness, and cohesiveness of all the starch gels increased, except for the adhesiveness of the normal starch gels and the springiness of thewx starch gels, which decreased, and the cohesiveness of thewx starch gels, which remained unchanged.
According to these authors the inclusion of negatively charged phosphate groups can cause inter-chain repulsion that prevents the close association necessary for inter-chain hydrogen bonding, inhibiting double helix formation through hydrogen bonding. Muhrbeck et al.[Citation29] studied potato starches that differed in their degree of phosphorylation, and concluded that crystallinity decreased linearly with increasing degree of phosphorylation.
Thermal Properties
The PRF showed no significant differences at the To, Tp, Tc, or in Tr, with relation to the unmodified flour, and only significantly modified the values of enthalpy in relation to the unmodified flour (). After phosphorylation, the gelatinization enthalpy of all the starches significantly decreased, especially forae and normal starches. By inhibiting double helix formation through hydrogen bonding, phosphorylation greatly reduced the stability of the starch structure and, consequently, reduced the energy required for the structural transitions occurring in gelatinization.[Citation27] The present results agreed with previous studies that reported that the introduction of phosphorus decreased the starch gelatinization temperature.[Citation16,Citation19,Citation20] Also, Cooke and Gidley[Citation30] using X-ray and NMR spectroscopy, demonstrated that gelatinization enthalpy was proportional to granule stability, thus supporting the function of the phosphate groups in destabilizing starch granules.
Table 3 Thermal properties of native and phosphorylated rice flours
Blennow et al.[Citation31] reported that the gelatinization temperature of native starches determined by differential scanning calorimeter positively correlated (R2 = 0.75) with the phosphate content of starch, while that the crystallinity (gelatinization enthalpy) and the heterogeneity of the crystal (width of the endotherm) did not show any correlation with the phosphate starch content. The addition of phosphate side chains to starch granules caused the properties of the modified starches to change as compared to the native starches. The starch phosphates of cassava and corn, whether produced by the conventional method or by extrusion, showed slightly lower gelatinization temperatures and lower enthalpies of gelatinization than those of native starch.[Citation17]
CONCLUSIONS
Increases in phosphorous oxychloride decreased the gel properties of the phosphorylated rice flours. However, the gel stability values during prolonged shear and heating increased significantly in comparison to the unmodified rice flour. The introduction of phosphate groups weakened the force and adhesiveness of the gels. The enthalpy values were significantly decreased in relation to the raw rice flour.
ACKNOWLEDGMENTS
The authors are grateful to CNPq for their financial support.
REFERENCES
- Juliano , B.O. and Sakurai , J. 1995 . “ Miscellaneous rice products ” . In Rice: Chemistry and Technology , 2nd , Edited by: Juliano , B.O. 569 – 618 . Saint Paul, , United States : American Association of Cereal Chemistry .
- Juliano , B.O. 1985 . Rice: Chemistry and Technology , 774 Saint Paul, MN : American Association of Cereal Chemistry .
- Sitohy , M.Z. , El-Saadany , S.S. , Labib , S.M. , Ramadan , M.F. and Mohamed , Z. 2000 . Physicochemical properties of different types of starch phosphate monoester . Starch , 52 ( 4 ) : 101 – 105 .
- Wurzburg , O.B. 1986 . “ Cross-linked starches ” . In Modified Starches: Properties and Uses , Edited by: Wurzburg , O.B. 41 – 54 . Boca Raton, FL : CRC Press .
- Hamilton , R.M. and Paschall , E.F. 1967 . “ Production and uses of starch phosphates ” . In Starch, Chemistry and Technology , Edited by: Whistler , R.L. and Paschall , E.F. Vol. 2 , 351 – 368 . New York : Academic Press .
- Solarek , D.B. 1986 . “ Phosphorylated starches and miscellaneous inorganic esters ” . In Modified starches: properties and uses , Edited by: Wurzburg , O.B. 98 – 108 . Boca Raton, , United States : CRC Press .
- American Association of Cereal Chemists . 2000 . Approved Methods of the AACC , 10th , 1 – 2 . Saint Paul, MN : AACC .
- Bhattachrya , K.R. , Sowbhagya , C.M. and Indudhara-Swany , Y.M. 1972 . Interrelationship between certain physicochemical properties of rice . Journal of Food Science , 37 ( 5 ) : 733 – 735 .
- Nabeshima , E.H. and Grossmann , M.E. 2001 . Functional properties of pregelatinized and cross-linked cassava starch obtained by extrusion with sodium trimetaphosphate . Carbohydrate Polymers , 45 ( 4 ) : 347 – 353 .
- Rutenberg , M.W. and Solarek , D. 1984 . “ Starch derivatives: Production and uses ” . In Starch: Chemistry and Technology , Edited by: Whistler , R.L. , Bemiller , J.N. and Paschall , E. F. 312 – 388 . London : Academic Press .
- Kasemsuwan , T. , Jane , J. , Schnable , I. , Stinard , P. and Robertson , D. 1995 . Characterization of the dominant mutant extender (Ael-5180) maize starch . Cereal Chemistry , 72 ( 5 ) : 457 – 464 .
- Brazil . Ministério da Agricultura. Secretaria Nacional de Abastecimento. Normas de classificação, embalagem e marcação de arroz. Portaria n° 269, de 17 de novembro de 1988
- Castro , E.M. , Vieira , N.R.A. , Rabelo , R.R. and Silva , S.A. 1999 . “ Qualidade de grãos em arroz ” . In Embrapa Arroz e Feijão , 30 Brazil : Santo Antônio de Goiás .
- Cameron , D.K. and Wang , Y.J. 2005 . A better understanding of factors that affect the hardness and stickiness of long-grain rice . Cereal Chemistry , 82 ( 2 ) : 113 – 119 .
- Lim , S. and Seib , P.A. 1993 . Preparation and pasting properties of wheat and corn starch phosphates . Cereal Chemistry , 70 ( 2 ) : 137 – 144 .
- Chang , Y.H. and Lii , C.Y. 1992 . Preparation of starch phosphates by extrusion . Journal of Food Science , 57 ( 1 ) : 203 – 205 .
- Yeh , A. and Yeh , S. 1993 . Some characteristics of hydroxypropylated and cross-linked rice starch . Cereal Chemistry , 70 ( 5 ) : 596 – 601 .
- Marusza , K. and Tomasik , P. 1991 . Highly phosphorylated starch . Starch , 43 ( 2 ) : 66 – 69 .
- Lin , P. and Czuzhajowska , Z. 1998 . Role of phosphorus in viscosity, gelatinization and retrogradation of starch . Cereal Chemistry , 75 ( 5 ) : 705 – 709 .
- Landerito , N.A. and Wang , Y.J. 2005 . Preparation and properties of starch phosphates using waxy, common and high-amylose corn starches. I. Oven-heating method . Cereal Chemistry , 82 ( 3 ) : 264 – 270 .
- Landerito , N.A. and Wang , Y.J. 2005 . Preparation and properties of starch phosphates using waxy, common and high-amylose corn starches. II . Reactive extrusion method. Cereal Chemistry , 82 ( 3 ) : 271 – 276 .
- Hahn , D.E. and Hood , L.F. 1980 . Physical characteristics of α-amylase hydrolysates from unmodified and chemically-modified waxy maize starches . Journal of Food Science , 45 ( 3 ) : 518 – 522 .
- Chatakanonda , P. , Varavinit , S. and Chinachoti , P. 2000 . Relationship of gelatinization and recrystallization of cross-linked rice to glass transition temperature . Cereal Chemistry , 77 ( 3 ) : 315 – 319 .
- Muhammad , K. , Hussin , F. , Man , Y.C. , Ghazali , H.M. and Kennedy , J.F. 2000 . Effect of pH on phosphorylation of sago starch . Carbohydrate Polymers , 42 ( 1 ) : 85 – 90 .
- Wu , Y. and Seib , P.A. 1990 . Acetylated and hydroxypropylated distarch phosphates from waxy barley: paste properties and freeze-thaw stability . Cereal Chemistry , 67 ( 2 ) : 202 – 208 .
- Liu , H. and Ramsden; Corke , H. 1999 . Physical properties and enzymatic digestibility of phosphorylated ae, wx, and normal maize starch prepared at different pH levels . Cereal Chemistry , 76 ( 6 ) : 938 – 943 .
- Becker , A. , Hill , S.E. and Mitchell , J.R. 2001 . Milling: A further parameter affecting the Rapid Visco Analyser (RVA) Profile . Cereal Chemistry , 78 ( 2 ) : 166 – 172 .
- Seker , M. , Sadikoglu , H. , Ozdemir , M. and Hanna , M.A. 2003 . Cross-Linking of Starch with Reactive Extrusion and Expansion of Extrudates . International Journal of Food Properties , 6 ( 3 ) : 473 – 480 .
- Muhrbeck , P. , Svensson , E. and Eliasson , A.C. 1991 . Effect of the degree of phosphorylation on the crystallinity of native potato starch . Starch , 43 ( 12 ) : 466 – 468 .
- Cooke , D. and Gidley , M.J. 1992 . Loss of crystalline and molecular order during starch gelatinization: Origin of enthalpic transition . Carbohydrate Research , 227 : 103 – 112 .
- Blennow , A. , Bay-Smidt , A.M. , Olsen , C.E. and Moller , B.L. 2000 . The distribution of covalently bound phosphate in the starch granule in relation to starch crystallinity . International Journal of Biological Macromolecules , 27 ( 3 ) : 211 – 218 .