Abstract
Moisture sorption isotherms of ten un-modified and octenyl succinic anhydride substituted dextrin and gum acacia samples were determined gravimetrically via equilibration over saturated salt solutions (aw ranged from 0.11 to 0.85) at 23 and 35°C. The degree of octenyl succinic anhydride substitution did not affect moisture sorption characteristics for dextrin samples. For gum acacia, increased levels of octenyl succinic anhydride substitution resulted in a decreased affinity for moisture in the range of 0.3 to 0.7 aw. The sorption data were well described by the BET and GAB models.
INTRODUCTION
Moisture affects the properties of spray dried flavoring materials in a number of ways. Moisture affects the quality (i.e., caking, flowability, etc.) of finished flavor powders as well as the retention and release of flavor volatiles from these powders. For example, the theory of selective diffusion depends on decreasing moisture content to ensure the retention of flavors in hot air dried matrices.[Citation1–3] Also, the micro region theory is used to explain effects of moisture on volatile retention and release at a microscopic level. This theory is based on volatiles being entrapped in micro regions of an amorphous structure comprised of carbohydrate-carbohydrate hydrogen-bonds that can be disrupted by moisture contents above the monomolecular layer value of the matrix.[Citation4,Citation5] Moreover, water acts as a plasticizer to carbohydrates and can initiate phase transitions from a glassy to rubbery state, which can induce crystallization, and possibly collapse thereby causing various release phenomena of encapsulated flavors.[Citation6]
It is therefore reasonable to assume that an understanding of moisture sorption properties of common carrier materials could potentially benefit the flavor manufacturer and end user alike. The carbohydrates primarily used as carrier materials for spray drying of flavoring materials are gum acacia, maltodextrin, and modified starch [typically modified with octenyl succinic anhydride (OSAn)].[Citation7] The choice of carrier material to use for flavor encapsulation is based on a number of functional strengths and weaknesses (e.g., protection of flavor during storage, encapsulation efficiency, etc.); a summary of these characteristics can be found in the noted references.[Citation7,Citation8] A few researchers have published moisture sorption isotherms or equilibrium moisture content data to be interpreted as such for gum acacia,[Citation9,Citation10] maltodextrin,[Citation11–14] and OSAn-modified starch. [Citation15] However, to our knowledge, there are no published moisture sorption isotherm data for carrier materials of controlled OSAn-modification.
The objective of this study was to generate moisture sorption isotherms for un-modified and OSAn-substituted starch and gum acacia based carrier materials. This was accomplished by determining the static equilibrium moisture contents of each carrier material over a range of relative humidity (%RH) values (aw ranged from 0.11 to 0.85). Additionally, the data was modeled with the Brunauer-Emmett-Teller (BET) and Gugenheim-Anderson-de Boer (GAB) equations.
MATERIALS AND METHODS
Chemicals
Methyl alcohol (Optima grade, Fisher Scientific, Fair Lawn, NJ, USA), anhydrous sodium sulfate (Fisher Scientific, Fair Lawn, NJ, USA), and the chemicals comprising the pyridine free reagents kit (Photovolt Instruments Inc., Minneapolis, MN, USA) for the Karl Fischer titration system were used for Karl Fischer moisture analysis. Salts for the saturated salt solutions were also obtained from Fisher Scientific.
Carrier Materials
Ten carrier materials were utilized for this research; five were starch based and five were gum acacia based. lists the carrier materials and abbreviation codes used to describe them. The starch-based products are variations of dextrinized starch. CAPSUL™ (CAP) (National Starch Inc., Bridgewater, NJ, USA) is a commercially available dextrinized waxy maize starch octenyl succinate. The other four products were: an unmodified dextrin (D) (Amidex 182), and three custom dextrin products prepared by treating corn starch with one, two, and three percent OSAn (D1, D2, and D3, respectively).
Table 1 Carrier material abbreviation codes and identities
The gum acacia based products are derived from two species of acacia. Gum Arabic Spray Dry FCC Powder (GA) (TIC Gums, Inc., Belcamp, MD, USA) is a product harvested from the Acacia senegal species. Gum Arabic FT Powder (FT) (TIC Gums Inc., Belcamp, MD, USA) is harvested from the Acacia seyal species. TICAmulsion A-2010 (TA) (TIC Gums Inc., Belcamp, MD, USA) is a modified gum made by treating A. seyal with OSAn.[Citation16] Actual OSAn treatment levels are not published, but the treatment level is suspected to be no more than 3% as given by the United States of America Code of Federal Regulations for modified starch.[Citation17]
FT and TA were dry blended to create two additional A. seyal products with different levels of total (OSAn) substitution. Ratios (w/w) of 67:33 and 33:67 of FT and TA were blended to create intermediate levels of OSAn substitution. These blends were produced by placing the proper amount of each gum acacia product in a Hobart mixer (Model #: A-200, Hobart Mfg. Co., Troy, Ohio, USA) and then blending at the lowest speed with the paddle attachment for 15 min.
Initial Moisture Content of the Carrier Materials
Initial moisture contents of each carrier material were determined with Karl Fischer methodology using the Aquatest CMA instrument (Photovolt Instruments Inc., Minneapolis, MN, USA). Between 0.2 and 0.9 g of each carrier material were weighed into 20-mL headspace vials. To insure an instrument response that was in operational range, greater masses were used for powders that had lower moisture contents (as determined by previous trials). Approximately 10 g of methyl alcohol dried over anhydrous sodium sulfate was carefully weighed into the vial that contained the carrier material and tightly capped. The capped vials were placed on an orbital shaker (Lab-Line Orbit Shaker #3590, Lab-Line Instruments, Inc., Melrose Park, IL, USA) set for 200 rpm and left to shake overnight at room temperature. Aliquots of these extracts were weighed and injected into the Aquatest CMA instrument. Moisture content of each carrier material was calculated using the output from the Aquatest CMA and the data recorded during extract preparation. Methyl alcohol blanks were also run to correct for residual moisture in the solvent and for moisture absorbed during normal extraction procedures. Initial moisture content (g water/g solids) results for each carrier are calculated as an average of triplicate samples.
Moisture Sorption Isotherms of Carrier Materials
Moisture sorption isotherms were prepared for all carrier materials according to Bell and Labuza.[Citation18] Triplicate samples of approximately 0.3 g were weighed into 20 mL glass scintillation vials (Research Products International Corp., Mount Prospect, IL, USA) and placed in desiccators containing the following saturated salt solutions at 23°C and 35°C (aw at 23°C, aw at 35°C)[Citation19]: lithium chloride (0.113, 0.113); potassium acetate (0.225, 0.216); magnesium chloride (0.327, 0.321); potassium carbonate (0.432, 0.432); magnesium nitrate (0.533, 0.500); cobalt chloride (0.669, 0.586); sodium chloride (0.754, 0.752); potassium chloride (0.847, 0.830). After 21 days equilibrium time, samples were re-weighed. Data from the static desiccators and initial moisture content data were used to construct “working” isotherms.[Citation18] Isotherms were plotted using the equilibrium moisture contents of the products. BET and GAB models were fit using a Microsoft Excel spreadsheet based on the Water Analyzer Series – Isotherm/BET/GAB Program.[Citation20]
RESULTS AND DISCUSSION
Moisture Sorption Isotherms
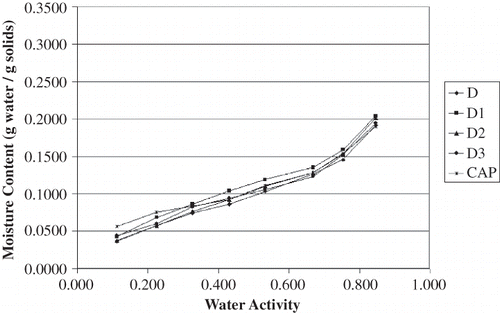
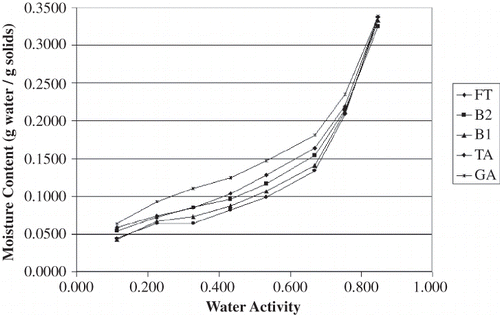
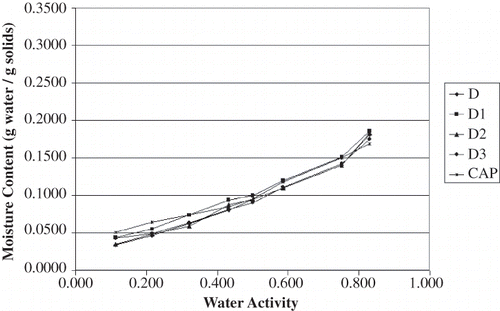
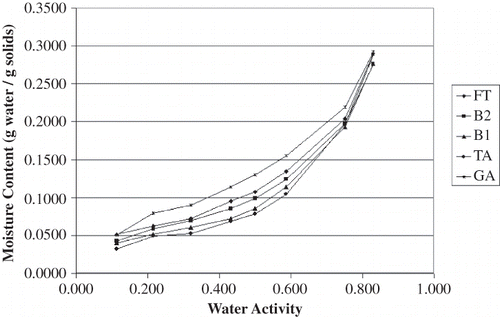
The equilibrium moisture content data for all carrier materials stored over the saturated salt solutions at 23°C and 35°C are shown in and , respectively. These data were plotted in to identify trends in the raw data; all four plots have the same scales to aid in visualizing differences among the results. In general, the gum acacia samples appeared to be more hygroscopic than the dextrin samples, particularly at water activity values greater than 0.7. The moisture sorption isotherms for all of the dextrin-based carrier materials are similar in shape to each other; the same is true for the gum acacia-based carrier materials. For the dextrin samples, the variations in water adsorption affinity do not appear to be strongly aligned with OSAn-treatment. Furthermore, there are several points where the isotherms from different products cross each other. These differences are suspected to be due to differences in the dextrinizing process, which was a batch treatment for each product. On the other hand, differences in the gum acacia carrier materials were very clear and trends were obvious. Between the water activity range of 0.3 to 0.7, acacia blends with increased OSAn result in a decreased affinity for moisture. This result is intuitive due to the hydrophobic nature of OSAn. Because variation of OSAn level for the acacia products was accomplished by blending FT and TA, these incredibly clear trends are likely explained by forming two weight-averaged (mass balance) isotherms for B2 and B1 in between FT and TA. Nonetheless, it still appears that a decrease in OSAn content will result in powder that is more hygroscopic.
Table 2 Equilibrium moisture content for the dextrin and gum acacia carrier materials equilibrated at 23°C
Table 3 Equilibrium moisture content for the dextrin and gum acacia carrier materials equilibrated at 35°C
The effect of temperature on sorption isotherms was quite evident. In comparing and and and , all products adsorbed less water at 35°C than at 23°C. This finding was expected as it is commonly accepted that as temperature increases at a given water activity (aw) the amount of sorbed water decreases based on the Clausius Clayperon equation.[Citation18,Citation21] Iglesias and Chirife[Citation22] commented that at higher temperatures, water sorption is less favorable and foods become less hygroscopic. Also, as temperature varies at a given aw, so does molecular excitation and attraction which leads to a variation in amount of water sorbed.[Citation23,Citation24] Other researchers have reported similar findings for comparable food products[Citation25,Citation26] with one exception; Perez-Alonso[Citation27] et al. reported that moisture decreased with increased temperature for dextrin (dextrose equivalence of 10) below an aw of 0.75 but the opposite for A. senegal. The authors attribute this result to the complexity of moisture adsorption in food polymers.
BET and GAB Modeling of Sorption Data
The BET and GAB equations are commonly used for description of moisture sorption isotherms. The data from the current experiment were inputted into an Excel spreadsheet based on the Water Analyzer Series program provided by Dr. Ted Labuza to determine the BET and GAB models. and summarizes the output of the spreadsheet; the output includes the calculated monolayer value (mo) and sorption constants C and K for the GAB model, as well as mo and constant C for the BET model for all products at 23°C and 35°C, respectively. According to the mean relative deviation modulus (%P-value),[Citation28,Citation29] the majority of the carrier material sorption data were explained by the models relatively well. The %P-value was less than 5 (a value considered to be a good fit) for all GAB models except D2 at 35°C with an average value of 3.41 and a range of 1.28 to 5.54. However, the BET models had more products with a %P-value greater than 5 (CAP, B1, and TA at 23°C and D2 and TA at 35°C) with an average %P-value of 3.63, and a range of 1.07 to 6.24.
Table 4 Estimated GAB and BET model parameters for all carrier materials at 23°C
Table 5 Estimated GAB and BET model parameters for all carrier materials at 35°C
The calculated GAB mo values for the dextrin based carrier materials ranged from 0.0649 to 0.0859 g water/g solids at 23°C and 0.0577 to 0.0714 g water/g solids at 35°C. The BET mo was less than the GAB mo for all products except for B1 and TA at 23°C and TA at 35°C. Collectively, the mo values for dextrin samples are comparable to the dextrin of Pérez-Alonso et al.[Citation27] who reported 0.0735 g water/g solids at 25°C, but higher than Abramovič and Klofutar[Citation13] who reported a range of 0.0463 to 0.0522 g water/g solids across a range of dextrins [dextrose equivalence (DE) range from 5 to 25]. The GAB mo values calculated for the gum acacia samples in the current research were from 0.0454 to 0.0807 g water/g solids at 23°C and 0.0400 to 0.0797 g water/g solids at 35°C. Pérez-Alonso et al.[Citation27] reported a mo value of 0.0811 g water/g solids for A. Senegal; this value was nearly identical to A. Senegal (GA) in the current research. However, as a group, the mo values for gum acacia samples in the current research were lower than Pérez-Alonso et al.[Citation27]
The aforementioned GAB mo comparisons must be considered with caution. Both Pérez-Alonso et al.[Citation27] and Abramovič and Klofutar[Citation13] determined true adsorption isotherms of the carbohydrate materials by gravimetric methods with samples stored over saturated salt solutions; however, the time taken for moisture equilibration was either not well defined[Citation27] or quite short.[Citation13] Wolf et al.[Citation30] commented that an equilibration period of at least 14 days is recommended to achieve equilibrium moisture content; this time is longer if the aw of the salt solution is greater than 0.65. Furthermore, Bell and Labuza recommended an equilibration time of 10 to 21 days or a sample mass change of no more than 2 mg/g of dry sample when sampled once every 7 days. Because these criteria were not met or defined in the previous studies,[Citation13, Citation27] the resultant calculated GAB mo values may not be indicative of the true sorption phenomena of the carbohydrate materials and therefore cannot be compared to the current study with complete confidence.
Other than the custom dextrin products (D, D1, D2, and D3) the calculated values for the C constant ranged considerably at 23°C. Conversely, at 35°C, the C constant range was far tighter than at 23°C. All C constants were within the normal range of 1 < C < 200.[Citation31] However, it should be noted that for the GAB model, when K constant values equal 1, the GAB equation reduces to the BET equation and at values above 1, the isotherm is physically unsound.[Citation32] Three of the products have K values that are just slightly (negligibly) greater than 1.
Linearity of the BET model depends on the maximum aw value used to model the data. In the literature, the maximum reported aw value declared acceptable for BET modeling ranges from 0.35 to 0.75.[Citation32,Citation33] However, the BET modeling criteria used for the data in this paper was recommended by Bell and Labuza[Citation18]; the authors recommend that if available, four aw values less than 0.45 should be used to calculate mo. The total range of aw values used for BET modeling for this research was from 0.11 to 0.43. Using this aw range, the calculated BET mo values for the dextrin based carrier materials ranged from 0.0559 to 0.0669 g water/g solids at 23°C and 0.0480 to 0.0578 g water/g solids at 35°C. The calculated mo values for dextrin samples were in consensus with other researchers who used the BET equation for dextrin products.[Citation13,Citation22] It should be noted that the aforementioned criteria used to generate BET models was not successful for CAP at 23°C; the C value was negative. In order to obtain a positive C value for CAP at 23°C, the BET modeling utilized aw range of 0.11 to 0.32. Using the un-modified criteria, the BET model parameters were 0.0534, −167.04, and 6.06 for mo, C, and %P-value, respectively. The BET mo values calculated for the gum acacia samples in the current research were from 0.0470 to 0.0759 g water/g solids at 23°C and 0.0407 to 0.0700 g water/g solids at 35°C. All of the BET mo values were less at 35°C than at 23°C, as expected since the excess enthalpy decreases with increasing moisture content. A decrease in mo may be due to temperature induced physical or chemical changes that lead to a reduction of active adsorption sites.[Citation24,Citation34,Citation35] Alternatively, this may be due to water molecules being in a higher energy state which would allow a detachment from adsorption sites.[Citation24]
CONCLUSIONS
The objective of this study was to generate moisture sorption isotherms for un-modified and OSAn-substituted starch and gum acacia used as carrier materials for spray dried flavoring materials. In general, gum acacia appeared to be more hygroscopic than the dextrin based samples. Furthermore, it appeared that varying the degree of OSAn-substitution for dextrin samples did not affect moisture sorption characteristics. However, for gum acacia samples, increased OSAn-substitution resulted in a decreased affinity for moisture in the range of 0.3 to 0.7 aw. GAB modeling indicated that mo values were typically higher for dextrin than for gum acacia samples; BET mo values could not be generalized as easily. Moisture sorption isotherm data for carrier materials is useful for processing, storing, and using flavor materials. When processing spray dried flavors, moisture sorption data can be used to formulate feed emulsions and optimize processing parameters to produce free-flowing powders less prone to stickiness and caking. During storage, moisture sorption data can aid in selecting proper storage conditions to prevent product failure (e.g., caking, chemical reaction, microbial growth, and flavor loss). Finally, moisture sorption data may be useful, in conjunction with glass transition data, when using dried flavors in controlled release applications.
ACKNOWLEDGMENTS
The authors thank Corn Products Brazil and TIC Gums, Inc., for their generous product donations.
REFERENCES
- Thijssen , H.A.C. 1965 . Inaugural Address , 1 – 19 . The Netherlands : Eindhoven University of Technology: Eindhoven .
- Thijssen , H.A.C. and Rulkens , W.H. 1968 . Retention of aromas in drying food liquids . De Ingenieur , 80 ( 47 ) : 45 – 56 .
- Kerkhof , P.J.A.M. and Thijssen , H.A.C. 1975 . “ The effect of process conditions on aroma retention in drying liquid foods ” . In Proceedings of the International Symposium on Aroma Research , 167 – 192 . Wageningen Zeist, , the Netherlands : Centre for Agricultural Publishing and Documentation .
- Flink , J. and Karel , M. 1970 . Retention of Organic Volatiles in Freeze-Dried Solutions of Carbohydrates. Journal of Agricultural and Food Chemistry , 18 ( 2 ) : 295 – 297 .
- Flink , J. and Karel , M. 1972 . Mechanisms of retention of organic volatiles in freeze-dried systems . Journal of Food Technology , 7 : 199 – 211 .
- Whorton , C. 1995 . “ Factors Influencing Volatile Release From Encapsulation Matrices ” . In Encapsulation and Controlled Release of Food Ingredients , Edited by: Risch , S.J. and Reineccius , G.A. 134 – 142 . Chicago : American Chemical Society .
- Reineccius , G.A. 1991 . Carbohydrates for Flavor Encapsulation . Food Technology , 45 ( 3 ) : 144 – 146 .
- Reineccius , G.A. 1999 . Source Book of Flavors , 605 – 613 . Gaithersburg : Aspen Publishers .
- Shotton , E. and Harb , N. 1965 . The effect of humidity and temperature on the equilibrium moisture content of powders . Journal of Pharmacy and Pharmacology , 17 : 504 – 508 .
- Minemoto , Y. , Adachi , S. and Matsuno , R. 1997 . Comparison of oxidation of methyl linoleate encapsulated with gum Arabic by hot-air-drying and freeze-drying . Journal of Agricultural and Food Chemistry , 45 : 4530 – 4534 .
- Raja , K.C.M. 1989 . Material Characterization Studies of Maltodextrin Samples for the Use of Wall Material . Starch/Starke , 41 ( 8 ) : 298 – 303 .
- Roos , Y. and Karel , M. 1991 . Phase Transitions of Mixtures of Amorphous Polysaccharides and Sugars . Biotechnology Progress , 7 ( 1 ) : 49 – 53 .
- Abramovic , H. and Klofutar , C. 2002 . Water Adsorption Isotherms of Some Maltodextrin Samples . Acta Chimica Slovenica , 49 ( 4 ) : 835 – 844 .
- Claude , J. and Ubbink , J. 2006 . Thermal degradation of carbohydrate polymers in amorphous states: a physical study including colorimetry . Food Chemistry , 96 ( 3 ) : 402 – 410 .
- Onwulata , C.I. and Holsinger , V.H. 1995 . Thermal properties and moisture sorption isotherms of spray-dried encapsulated milkfat . Journal of Food Processing and Preservation , 19 ( 1 ) : 33 – 51 .
- Ward , F.M. Water-Soluble Esterified Hydrocolloids . United States Patent No. 6,455,512 . 2002 . Filed: 5 March, 2001; Issued: 4 September
- Code of Federal Regulations . 2005 . Code of Federal Regulations Title 21 Part 172.892—Food starch-modified , 115 – 117 . Washington, DC : U.S. Government Printing Office .
- Bell , L.N. and Labuza , T.P. 2000 . Moisture Sorption: Practical Aspects of Isotherm Measurement and Use , 2nd , 122 St. Paul : American Association of Cereal Chemists .
- Greenspan , L. 1977 . Humidity Fixed Points of Binary Saturated Aqueous Solutions. Journal of Research of the National Bureau of Standards—A . Physics and Chemistry , 81A ( 1 ) : 89 – 96 .
- Nelson , K.A. and Labuza , T.P. 1991 . Water Analyzer Series Isotherm/BET/GAB Program version 2.09 , St. Paul : University of Minnesota Department of Food Science and Nutrition .
- Labuza , T.P. 1968 . Sorption phenomena in foods; Food Technology , 22 : 263 – 265 . 268 270 272
- Iglesias , H.A. and Chirife , J. 1982 . “ Handbook of food isotherms: water sorption parameters for food and food components ” . In Food Science and Technology—A Series of Monographs , Edited by: Schweigert , B.S. and Hawthorn , J. 3 New York : Academic Press .
- Mohsenin , N.N. 1986 . Physical properties of plant and animal materials: structure, physical characteristics and mechanical properties , 61 New York : Gordon and Breach Science Publishers .
- Palipane , K.B. and Driscoll , R.H. 1992 . Moisture sorption characteristics of in-shell macadamia nuts . Journal of Food Engineering , 18 : 63 – 76 .
- Iglesias , H.A. and Chirife , J. 1976 . Prediction of the effect of temperature on water sorption isotherms of food material . Journal of Food Technology , 11 : 109 – 116 .
- Bandyopadhyay , S. , Weisser , H. and Loncin , M. 1980 . Water adsorption isotherms of foods at high temperatures . Lebensmittel-Wissenschaft und Technologie , 13 : 182 – 185 .
- Perez-Alonso , C. 2006 . Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers . Journal of Food Engineering , 77 : 753 – 760 .
- Lomauro , C.J. , Bakshi , A.S. and Labuza , T.P. 1985 . Evaluation of food moisture sorption isotherm equations - part I: fruit, vegetable, and meat products . Lebensmittel-Wissenschaft und Technologie , 18 : 111 – 117 .
- Lomauro , C.J. , Bakshi , A.S. and Labuza , T.P. 1985 . Evaluation of food moisture sorption isotherm equations - part II: milk, coffee, tea, nuts, oilseeds, spices and starchy foods . Lebensmittel-Wissenschaft und Technologie , 18 : 118 – 124 .
- Wolf , W. , Spiess , W.E.L. and Jung , G. 1985 . “ Standardization of Isotherm Measurements (COST-Project 90 and 90 bis) ” . In Properties of Water in Foods , Edited by: Simatos , D. and Multon , J.L. 661 – 679 . Dordrecht, , The Netherlands : Martinus Nijhoff Publishers .
- Labuza, T.P. Creation of Moisture Sorption Isotherms for Hygroscopic Materials. University of Minnesota: St. Paul, 2008.- http://www.ardilla.umn.edu/Ted_Labuza/PDF_files/papers/Creation_Moisture_Isotherms.PDF (http://www.ardilla.umn.edu/Ted_Labuza/PDF_files/papers/Creation_Moisture_Isotherms.PDF) (Accessed: 3 August 2008 ).
- Al-Muhtaseb , A.H. , McMinn , W.A.M. and Magee , T.R.A. 2002 . Moisture sorption isotherm characteristics of food products: a review . Food and Bioproducts Processing , 80 ( C2 ) : 118 – 128 .
- Ikhu-Omoregbe , D.I.O. 2006 . Comparison of the sorption isotherm characteristics of two cassava products . International Journal of Food Properties , 9 : 167 – 177 .
- Iglesias , H.A. and Chirife , J. 1976 . Isoteric heats of water vapor sorption on dehydrated foods - Part I. Analysis of the differential heat curves . Lebensmittel-Wissenschaft und Technologie , 9 : 116 – 122 .
- Mazza , G. and LeMageur , M. 1978 . Water sorption properties of yellow globe onion (Allium cepa L.) . Canadian Institute of Food Science and Technology Journal , 11 ( 4 ) : 189 – 193 .