Abstract
The following study was conducted to investigate the efficacy of several leaf and berry extracts against a range of food-borne pathogens and food spoilage bacteria. The methanol, ethanol, and ethyl acetate extracts of Myrtus communis leaves and berries were examined for in vitro antibacterial activity. The methanolic leaf extract of M. communis, which was seen to have antibacterial activity against Listeria monocytogenes CECT 4032 and Pseudomonas aeruginosa IH, was further investigated to determine the effect of the extract on viable counts of bacteria using the bacterial cell-death time. Most of the extracts showed relatively high antibacterial activity against most of the tested microorganisms. None of the extracts was active against Escherichia coli K12. The results obtained confirm the antibacterial potential of the extracts of M. communis.
INTRODUCTION
Food contamination and spoilage by microorganisms is a yet unresolved problem, despite the range of preservation techniques available. The microbiological safety of food continues to be a major concern to consumers, regulatory agencies and food industries throughout the world.[Citation1] Microbial contamination still poses important public health and economic concerns for the human society. Among many strategies to inhibit the growth of undesirable microorganisms, is the use of chemical agents that exhibit antimicrobial activity. These chemicals may be either synthetic compounds intentionally added to foods or naturally occurring and biologically derived substances. Traditional antimicrobials such as acetic, benzoic, lactic, propionic and sorbic acids and nitrite and sulfites have been used for many years to control the growth of microorganisms in foods.[Citation2,Citation3] However, current consumer demand for more natural and fresh-like foods containing fewer synthetic additives but increased safety and shelf life, have encouraged food manufacturers to use natural or mild preservation techniques, with the results that alternative sources of safe, effective and acceptable natural preservatives need to be explored.
Plant extracts and essential oils have long constituted a natural source of antimicrobial compounds.[Citation4,Citation5] Essential oils and purified components are used as natural antimicrobials in food systems, as well as to prevent the growth of food-borne bacteria and moulds, resulting in a longer shelf life for processed foods.[Citation6,Citation7] These medicinal herbs constitute indispensable components of traditional medicine practised worldwide due to their low cost and easy access.[Citation8] In recent decades, antimicrobial plant products have gained special interest because of the resistance to antibiotics that some microorganisms have acquired[Citation9], and due to increasing popular concern about the safety of food and the potential impact of synthetic additives on health.[Citation10]
Morocco has a rich flora that is widely distributed throughout the country. Many plants or their components are used as folk medicines in many parts of the world to cure various infectious diseases such as urinary tract infections, bronchitis, diarrhea, cutaneous abscesses and parasitic diseases. Myrtus communis a member of myrtaceae family is an evergreen sclerophyll shrub or small tree.[Citation11] It is traditionally used as an antiseptic, disinfectant drug and hypoglycaemic agent.[Citation12] Different parts of the plant find have been used in the food industry, for example for flavouring meat and sauces, and in the cosmetic industry.[Citation13] Foods flavored with the smoke of myrtle are common in rural areas of Italy or Sardinia.[Citation14]
To the best of the authors' knowledge and following a survey of the pertinent literature, only a few reports about the antibacterial activity of the essential oils and crude methanol extract of M. communis leaves have been published[Citation15–17], although several authors have found that the solvent used and the extraction system may modify the antibacterial activity of the extracts.[Citation18] For these reasons, the aim of the work was to test the efficacy of various leaf and berry extracts against a diverse range of food-borne pathogens and food spoilage bacteria.
MATERIAL AND METHODS
Plant Material
Myrtus communis L. (Myrtaceae) leaves and berries were collected from Chefchaouen region (NW Morocco) during their vegetation period. The taxonomic identification of plant material was confirmed by A. Ennabili (from The National Institute of Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University). The collected plant materials were dried in an oven (Selecta, Barcelona, Spain) at 35°C and then the leaves and berries of plants were separated and ground in a grinder (Moulinex, France).
Preparation of Extracts
Dried powders of leaves and berries from M. communis were extracted with different solvents (methanol, ethanol, and ethyl acetate). A twenty-five gram aliquot of each dried sample was extracted using 100 ml of methanol, ethanol, or ethyl acetate, respectively, at room temperature for 7 days. Two extraction replicates of each solvent were prepared for each plant sample. The extracts were filtered through a Whatman #1 filter paper and the solvents were eliminated using a rotary evaporator (Buchi Rotvapor R-200) to obtain a dry extract. The extracts were stored at −20°C until use.
Microorganisms
Thirteen bacterial pathogens, including the food spoilage bacteria Pseudomonas aeruginosa IH, Pseudomonas aeruginosa CECT 118, Pseudomonas aeruginosa CECT 110T, Pseudomonas fluorescence CECT 378 and Bacillus subtilis DCM 3366, and the food-borne pathogenic bacteria, namely Escherchia coli K12, Listeria innocua CECT 4030, Listeria monocytogenes CECT 4032, Enteroccus faecium CECT 410, Staphylococcus aureus MBLA, Staphylococcus aureus CECT 976 Staphylococcus aureus CECT 794 and Proteus vulgaris CECT 484, were tested.
Bacillus subtilis DCM 3366 was obtained from the German Collection of Micro-organisms. Escherichia coli K12 and Staphylococcus aureus MBLA were obtained from the Laboratory of Food Microbiology, UCL, Belgium. Pseudomonas aeruginosa IH was obtained from the Institute of Hygiene, Rabat, Morocco. P. aeruginosa CECT 110T, P. aeruginosa CECT 118, Pseudomonas fluorescens CECT 378, Proteus vulgaris CECT 484, S. aureus CECT 976, S. aureus CECT 794, Listeria innocua CECT 4030, Listeria monocytogenes serovar CECT 4032 and Enterococcus faecium CECT 410 were obtained from the Spanish Type Culture Collection.
Antibacterial Activity Assay
The antibacterial tests were performed using the agar-well diffusion assay,[Citation19] preparing a basal layer with Muller-Hinton agar. After the agar plates had solidified, sterile 8 mm diameter cylinders were deposited. Six ml of LB medium in superfusion containing 0.8% agar were inoculated with a fresh culture of indicator bacterial strain (final concentration 106 CFU/ml). After solidification, the wells were filled with 50 μl of extracts. Negative controls were prepared using the same solvents as used to prepare the extracts. After standing at 4°C for 2 h, the plates were incubated at 37°C for 24 h. The plates were then inspected for bacterial growth inhibition, as indicated by a clear zone around the wells. The size of any inhibition zone was measured and the antibacterial activity was expressed in terms of their average diameter of inhibition zones. The absence of such a zone was interpreted as the absence of inhibitory activity. Each extract was tested in triplicate.
Determination of Minimum Inhibitory Concentration (MIC)
The minimal inhibition concentration (MIC) values were determined for the bacterial strains which were sensitive to the extract in well diffusion assay, as reported by Koneman[Citation20] and Camporese,[Citation21] following the recommendations of the National Committee for Clinical Laboratory Standard.[Citation22] Dry extracts were initially diluted in Mueller Hinton Broth, to reach the final concentration of the first test well of each microtiter line, and then 50 μl/ml scalar dilutions were successively transferred from the second to the 7th well, while the 8th tube was considered as growth control, since no extracts solution was added. Then, 50 μl of a 106 CFU/ml bacterial suspension were added to each well. The final concentration of the extracts ranged from 5 mg/ml to 0.019 mg/ml. Plates were incubated for 18 h at 37°C. The lowest concentration of the sample which permitted growth of test organisms was taken as the MIC (expressed in μl/ml).
Effect of Extracts on Viable Counts of Bacteria
This effect was determined by the bacterial cell-death time assay for the methanolic Myrtus leaf extract against Listeria monocytogenes CECT 4032 and Pseudomonas aeruginosa IH. Approximately 106 CFU/ml of test bacterial strain was introduced into 20 ml of LB containing MIC, 2 MIC or 4 MIC of methanol leaf extract for Pseudomonas aeruginosa IH and Listeria monocytogenes CECT 4032. The controls consisted of two sets of duplicate cultures, one without extract (negative control). All experiments were incubated in a shaker water bath at 37°C. At 30 min intervals, 1 ml of sample was withdrawn, diluted in ten-fold series and 1 ml of each dilution was plated on MHA. After 24 h of incubation at 37°C, emergent bacterial colonies were counted and compared with the count of the control culture without extract. The results were expressed as negative or positive log10.
Statistical Analysis
The data obtained for antibacterial activity of the extracts were statistically analysed by and ANOVA and mean values were calculated. A Student's t-test was computed for the statistical significance of the results. Statistical data analysis was undertaken using the statistical package Statgraphics plus 2.0.
RESULTS AND DISCUSSION
The in vitro antibacterial activities of M. communis extracts against the bacteria used were qualitatively and quantitatively assessed from the presence or absence of inhibition zones. shows the results obtained of the antibacterial activity for the various extracts obtained from M. communis leaves and berries. All the assayed extracts significantly inhibited the growth of at least some of the bacterial strains tested. Only one of the bacteria, E. coli K12, was not inhibited by any of the extracts assayed. However, methanol and ethanol extracts of both leaves and berries exhibited a potential antibacterial effect against the rest of the bacteria tested. No antibacterial effect of the ethyl acetate extracts (leaves or berries) was observed against any of the Pseudomonas strains (Ps. aeruginosa IH, Ps. aeruginosa CECT 118, Ps. aeruginosa CECT 110T, Ps. aeruginosa CECT 378), Proteus vulgaris CECT 484, and S. aureus MBLA.
Table 1 Antibacterial activity of different leaf and berry extracts of Myrtus communis against food-borne and spoiling bacteria.Footnote a
Of the extracts obtained from leaves of M. communis, the methanol extracts showed the highest (P<0.05) antibacterial activity (the diameter of the inhibition zones ranging from 12 to 50 mm) while, of the extracts obtained from berries, the ethanol extracts showed the most potent antibacterial activity (the diameter zones of inhibition ranging from 14–37 mm). In general, the extracts obtained from leaves of M. communis showed stronger antibacterial activity (P < 0.05) than extracts obtained from berries, the diameter of the respective zones of inhibition being in the range from 12–50 mm and 11–37 mm, respectively ().
In this study, all the extracts of M. communis exhibited significantly higher antibacterial activity (P<0.05) against the Gram-positive bacteria (Bacillus subtilis DCM 3366, Listeria innocua CECT 4030, Listeria monocytogenes CECT 4032, Enteroccus faecium CECT 410, Staphylococcus aureus MBLA, Staphylococcus aureus CECT 976 and Staphylococcus aureus CECT 794) than the Gram-negative bacteria (Ps. aeruginosa IH, Ps. aeruginosa CECT 118, Ps. aeruginosa CECT 110T, Ps. aeruginosa CECT 378, E. coli K12, and Proteus vulgaris CECT 484). It is often reported that Gram-negative bacteria are more resistant to plant-based extracts and essential oils,[Citation23,Citation24] since the hydrophilic cell wall structure of Gram-negative bacteria is constituted essentially of a lipo-polysaccharide (LPS) that blocks the penetration of hydrophobic oil and avoids the accumulation of essential oils in target cell membrane.[Citation25] This is the reason that Gram-positive bacteria were found to be more sensitive to the extracts of M. communis than Gram-negative bacteria.
Minimal inhibition concentration assays were performed to determine the concentration at which the extracts are effective. As shown in , the minimum inhibitory concentration values of leaf extracts against the bacterial strains used were lower than those of the berry extracts. Methanol and ethanol extracts exerted higher (P < 0.05) antibacterial activity than the ethyl acetate extracts. The solvents did not inhibit the growth of any of the bacteria tested at the concentrations used. MIC values ranged from <0.075 mg/ml to 2.5 mg/ml for the methanolic leaf extracts and from <0.075 mg/ml to 5 mg/ml for the ethanol and ethyl acetate leaf extracts. The MIC values obtained using the methanol extract from berries ranged from 0.075 to 5 mg/ml, for the ethanol extract from 0.15 to 5 mg/ml and for the ethyl acetate extract from 0.3 to 5 mg/ml. The methanol, ethanol and ethyl acetate extracts of M. communis leaves exhibited similar antibacterial effect against L. innocua CECT 4030 and S. aureus CECT 794 (MIC value of 0.15 mg/ml and <0.075 mg/ml, respectively). For the same extracts obtained from berries of M. communis a similar antibacterial effect was found against the two strains of Listeria assayed (L. innocua CECT 4030 and L. monocytogenes CECT 4032) with respective MIC values of 2.5 mg/ml and 1.25 mg/ml.
Table 2 Minimum inhibition concentrations (MICs) of different leaf and berry extracts of Myrtus communis against food-borne and spoiling bacteria
Overall, methanol extracts from leaves of M. communis showed the highest antibacterial activity of all the extracts. Moreover, this extract exhibited its highest bacterial effect against one food-borne Gram-positive bacterium (L. monocytogenes CECT 4032) and one spoiling Gram-negative bacterium (Ps. aeruginosa IH). The effect on viable counts of these two bacteria is shown in .
Figure 1 Viable count of (a) Listeria monocytogenes CECT 4032 and (b) Pseudomonas aeruginosa IH cells (log CFU/ml) at different times after the addition of three concentrations (▪: control; ♦: MIC; ○: 2 MIC; and Δ: 4 MIC) of methanolic leaf extracts.
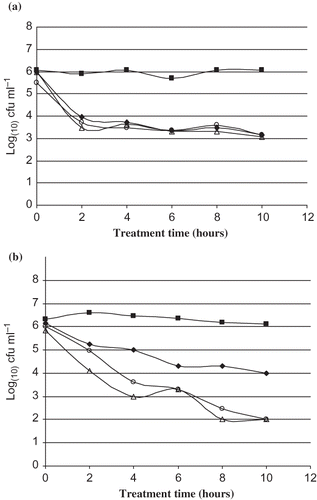
Listeria monocytogenes is widely distributed in nature and is frequently found in a large number of food products, as well as in processing plants.[Citation26–29] Listeriosis is recognized as an important public health problem, affecting primarily pregnant women, newborns and adults with weakened immune systems.[Citation30–32] The majority of human listeriosis infections are caused by the consumption of contaminated food.[Citation33]
Pseudomonas aeruginosa is a ubiquitous environmental bacterium. It can be recovered, often in high numbers, from common food, especially vegetables and in low numbers in drinking water.[Citation34] As a result of their metabolic diversity, ability to grow at low temperatures and ubiquitous nature, many Pseudomonas can cause food spoilage.[Citation35]
Based on susceptibility, the results of the viable counts assay revealed that L. monocytogenes CECT 4032 and Ps.aeruginosa IH displayed different sensitivities to the methanol extracts of M. communis leaves. The effect of the extract on the growth of L. monocytogenes CECT 4030 demonstrated the reduced viability of the bacterium at the MIC concentration of the extract, the extent of reduction not increasing when the extract was added at higher concentrations (2 or 4 MIC). However, in the case of Ps. aeruginosa IH the reduction in bacterial viability was dose dependent since when the extract was added at 2 or 4 MIC, the reduction was higher (P < 0.05) than when it was added at 1 MIC. Methanol extracts from leaves exerted maximum bactericidal activity against Ps. aeruginosa IH at 2 MIC, as seen from the significant reduction in microbial counts after 10 h exposure. However, at 1 MIC, the maximum bactericidal activity of the extract was against L. monocytogenes CECT 4030.
Various publications have documented the antimicrobial activity of essential oils and plant extract constituents.[Citation36–38] In recent years, several researchers have reported their major constituents to be phenolic compounds. M. communis L. leaves are characterized by the presence of flavonols (myricetin and quercetin glycosides) and galloyl derivatives, which include galloyl-glucosides, ellagitannins, and galloyl-quinic acids.[Citation39] Thus, myrtle leaves contain different polyphenolic classes as previously described by Romani et al.[Citation40] It seems reasonable to assume that their antimicrobial mode of action was related to the phenolic compounds present.[Citation41] Most of the studies on the mechanism of phenolic compounds have focused on their effects on cellular membranes. They have been seen to attack not only cell walls and cell membranes, thereby affecting their permeability and the release of intracellular constituents, but also to interfere with membrane functions such as electron transport, enzyme activity or nutrient uptake.[Citation24,Citation42] Thus, active phenolic compounds might have several targets which could lead to the inhibition of bacteria.
CONCLUSION
The Myrtus communis extracts evaluated in this study showed a varying degree of inhibitory activity against all the tested bacteria, except against E. coli K12. Methanol and ethanol extracts from the leaves of Myrtus communis exhibited the highest antibacterial activity against the food-borne pathogenic and spoilage bacteria tested. Therefore, extracts from Myrtus communis may act as an alternative to synthetic bactericides for use in the food industry, where bacterial pathogens cause great loss. The use of plant extracts as additives in food processing is expected to increase in the future due to the worldwide demand to reduce or eliminate chemically synthesized additives in foods, and to the recent phenomenon of “green consumption” which stimulates the use and development of products derived from plants. However, it is still unknown about the compounds are responsible for the antibacterial activity in different solvent extracts. The isolation and identification of the active principles of these plants extracts is now in progress. However, it must always be borne in mind that if plant extracts are to be used for food preservation or medicinal purposes, issues of safety and toxicity will always need to be addressed.
ACKNOWLEDGMENTS
The authors are grateful to Dr. A. Ennabili (from The National Institute of the Medicinal and Aromatic Plants, Sidi Mohamed Ben Abdellah University) for the identification of the plant. The authors would like to thank the Spanish Agency for International Cooperation (AECI) for financing this research project (A/9934/07) and the Centre Nacional pour la Recherche Scientifique et Technique (CNRST) from Morocco for support the doctoral grant of one of the authors.
REFERENCES
- Soliman , K.M. and Badeaa , R.I. 2002 . Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi . Food and Chemical Toxicology , 40 : 1669 – 1675 .
- Davidson , P.M. 1997 . Chemical Food Microbiology: Fundamentals and Frontiers , 520 – 556 . Washington : ASM .
- Sofos , J.N. , Beuchat , L.R. , Davidson , P.M. and Johnson , E.A. 1998 . Naturally occurring antimicrobials in food . Regulatory Toxicology and Pharmacology , 28 : 71 – 72 .
- Tepe , B. , Daferera , D. , Sökmen , M. , Polissiou , M. and Sökmen , A. 2004 . In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii . Journal of Agricultural and Food Chemistry , 52 ( 5 ) : 1132 – 1137 .
- Mandic , A.I. , Dilas , S.M. , Cetkovic , G.S. , Canadanovic-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 : 713 – 726 .
- Kalemba , D. and Kunicka , A. 2003 . Antibacterial and antifungal properties of essential oils . Current Medicinal Chemistry , 10 : 813 – 829 .
- Burt , S. 2004 . Essential oils: Their antibacterial properties and potential applications in foods . International Journal of Food Microbiology , 94 : 223 – 253 .
- Martin-Bettolo , G.B. 1980 . Present aspects of the use of medicinal plants in traditional medicine . Journal of Ethnopharmacology , 2 : 5 – 7 .
- Essawi , T. and Srour , M. 2000 . Screening of some Palestinian medicinal plants for antibacterial activity . Journal of Ethnopharmacology , 70 : 343 – 349 .
- Reische , D.W. , Lillard , D.A. and Eintenmiller , R.R. 1998 . “ Antioxidants in food lipids ” . In Chemistry, Nutrition and Biotechnology , Edited by: Ahoh , C.C. and Min , D.B. 423 – 448 . New York : Marcel Decker .
- Mendes , M.M. , Gazarini , L.C. and Rodrigues , M.L. 2001 . Acclimation of Myrtus communis to contrasting Mediterranean light environments—effects on structure and chemical composition of foliage and plant water relations . Environmental and Experimental Botany , 45 : 165 – 178 .
- Elfalah , M.S. , Akhtar , M.H. and Khan , M.T. 1984 . Antihyperglycaemic effect of an extract of Myrtus communis in streptozotocin-induced diabetes in mice . Journal of Ethnopharmacology , 11 : 275 – 281 .
- Chalchat , J. , Garry , R.P. and Michet , A. 1998 . Essential Oils of myrtle (Myrtus communis L.) of the mediterranean littoral . Journal of Essential Oil Research , 10 : 613 – 617 .
- Olga , G. , Stavros , L. , Ioanna , C. and John , T. 2008 . Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes . European Food Research and Technology , 226 ( 3 ) : 583 – 590 .
- Mansouri , S. , Foroumadi , A. , Ghaneie , T. and Gholamhosseinian , A.N. 2001 . Antibacterial Activity of the Crude Extracts and Fractionated Constituents of Myrtus communis . Pharmaceutical Biology , 395 : 399 – 401 .
- Deriu , A. , Branca , G. , Molicotti , P. , Pintore , G. , Tirillini , B. , Paglietti , B. , Mura , A. , Sechi , L.A. , Fadda , G. and Zanetti , S. 2007 . In vitro activity of essential oil of Myrtus communis L. against Helicobacter pylori . International Journal of Antimicrobial Agents , 30 : 562 – 565 .
- Salvagnini , L.E. , Oliveira , J.R.S. , Santos , L.E. , Moreira , R.R.D. and Pietr , R.C.L.R. 2008 . Avaliação da Atividade Antibacteriana de folhas de Myrtus communis L. (Myrtaceae) . Brazilian Journal of Pharmacognosy , 18 ( 2 ) : 241 – 244 .
- Muthuvelan , B. and Balaji Raja , R. 2008 . Studies on the efficiency of different extraction procedures on the antimicrobial activity of selected medicinal plants . World Journal of Microbiology and Biotechnology , 24 : 2837 – 2842 .
- Tagg , J.R. and Macgiven , A.R. 1971 . Assay system for bacteriocins . Applied Microbiology , 21 : 934
- Koneman , E.W. 1995 . Testo Atlante di Microbiologia Diagnostica , 2nd , 550 – 605 . Roma : A. Delfino .
- Camporese , A. 1997 . L'aromatogramma: metodi, corretto utilizzo, prospettive di ricerca . Rivista Italiana EPPO , 21 : 4 1997
- National Committee for Clinical Laboratory Standards . 2001 . Performance standards for antimicrobial disk susceptibility tests , 102 – 103 . Wayne, PA : NCCLS .
- Reynolds , J.E.F. 1996 . Martindale the extra harmacopoeia , 31st , 1681 – 1682 . London : Royal Pharmaceutical Society of Great Britain .
- Bajpai , V.K. , Rahman , A. and Kang , S.C. 2008 . Chemical composition and inhibitory parameters of essential oil and extracts of Nandina domestica Thunb. to control food-borne pathogenic and spoilage bacteria . International Journal of Food Microbiology , 125 ( 2 ) : 117 – 122 .
- Bezic , N. , Skocibusic , M. , Dinkic , V. and Radonic , A. 2003 . Composition and antimicrobial activity of Achillea clavennae L. essential oil . Phytotherapy Research , 17 : 1037 – 1040 .
- Johnson , J.L. , Doyle , M.P. and Cassens , R.G. 1990 . Listeria monocytogenes and other Listeria spp. in meat and meat products . Journal of Food Protection , 53 : 81 – 91 .
- Beuchat , L.R. 1996 . Pathogenic microorganisums associated with fresh produce . J. Food Protection , 59 : 204 – 216 . 1996
- Kells , J. and Gilmour , A. 2004 . Incidence of Listeria monocytogenes in two milk processing environments, and assessment of Listeria monocytogenes blood agar for isolation . International Journal of Food Microbiology , 91 : 167 – 174 .
- Pagotto , F. , Ng , L. , Clark , C. and Farber , J. 2006 . Canadian Listeriosis Reference Service . Foodborne Pathogens and Disease , 3 ( 1 ) : 132 – 137 .
- Cox , R.M. , Spavold-Tims , J. and Hughes , R.N. 1989 . Acid fog and ozone: their possible role in birch deterioration around the bay of Fundy, Canada . Water, Air, and Soil Pollution , 48 : 263 – 276 .
- ICMFS Microorganisms in Foods 7 . 2002 . Microbiological Testing in Food Safety Management , 862 New York : Kluwer Academic/Plenum Publishers .
- Peccio , A. , Autio , T. , Korkeala , H. , Rosmini , R. and Trevisani , M. 2003 . Listeria monocytogenes occurrence and characterization in meat-producing plants . Letters in Applied Microbiology , 37 ( 3 ) : 234 – 238 .
- McLauchlin , J. , Mitchell , R.T. , Smerdon , W.J. and Jewell , K. 2004 . Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods . International Journal of Food Microbiology , 92 : 15 – 33 .
- Hamouda , A. , Vali , L. , Walker , D.J. , Mateus , A. , Dave , J. , Gibb , A.P. , Dancer , S. and Amyes , S.G.B. Antibiotic resistance in Pseudomonas aeruginosa from food-producing animals: is it a threat for hospital-acquired infections? . Proceedings of the 18th European Congress of Clinical Microbiology and Infectious Diseases . Sept 13–20 2008 , Barcelona, Spain. Barcelona, , Spain : European Society of Clinical Microbiology and Infectious Diseases .
- Gennari , M. and Dragotto , F. 2008 . A study of the incidence of different fluorescent Pseudomonas species and biovars in the microflora of fresh and spoiled meat and fish, raw milk, cheese, soil and water . Journal of Applied Microbiology , 72 ( 4 ) : 281 – 288 .
- Morris , J.A. , Khettry , A. and Seitz , E.W. 1979 . Antimicrobial activity of aroma chemicals and essential oils . Journal of the American Oil Chemists' Society , 56 ( 5 ) : 595 – 603 .
- Viuda-Martos , M. , Ruiz-Navajas , Y. , Fernández-López , J. and Pérez-Álvarez , J. A. 2008 . Antibacterial activity of different essential oils obtained from spices widely used in Mediterranean diet . International Journal of Food Science and Technology , 43 : 526 – 531 .
- Viuda-Martos , M. , Ruiz-Navajas , Y. , Fernández-López , J. and Pérez-Álvarez , J. A. 2008 . Antibacterial activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils . Journal of Food Safety , 28 : 567 – 576 .
- Hayder , N. , Bouhlel , I. , Skandrani , I. , Kadri , M. , Steiman , R. , Guiraud , P. , Mariotte , A. , Ghedira , K. , Dijoux-Franca , M. and Chekir-Ghedira , L. 2008 . In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray . Toxicology in vitro , 22 ( 3 ) : 567 – 581 .
- Romani , M. , Marchi , J.V. , Banelli , B. and Casciano , I. 1999 . Identification and quantitation of polyphenols in leaves of Myrtus communis L . Chromatographia , 49 : 17 – 20 .
- Cakir , A. , Kordali , S. , Zengin , H. , Izumi , S. and Hirata , T. 2004 . Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum . Flavour & Fragrance Journal , 19 : 62 – 68 .
- Fung , D.Y.C. , Taylor , S. and Kahan , J. 1977 . Effects of butylated hydroxyanisole (BHA) and Butylated hydroxytoluene (BHT) on growth and aflatoxina production of Aspergillus flavus . Journal of Food Safety , 1 : 39 – 51 .