Abstract
Antioxidant and bioadhesive properties of onion, garlic, leek, and ramsons, processed under low pH conditions were investigated. For investigation of antioxidant activity DPPH free radical scavenging activity and reducing power were used. The most active radical scavenger (EC50 = 2.07 mg/ml) and reductant was the ethanolic extract of leek leaf. Positive correlation between antioxidant activity and phenol content in the extracts (P < 0.01) was found. To determine bioadhesive properties of the extracts, tensile studies were performed and work of adhesion (Wa) was measured. The most pronounced bioadhesive properties were determined for garlic and ramsons bulbs extracts (Wa > 8 μJ).
INTRODUCTION
The role of reactive oxygen species (ROS) in many diseases such as cardiovascular diseases,[Citation1,] diabetes,[Citation2,] and many others has been in focus of research interest in the last decade. However, it seems that natural antioxidants are effective in protecting the body or even reversing the damage induced by ROS. Thus, they inhibit most of the processes that underlie different pathological conditions.[Citation3,] Among them, phenolic substances are of special interest because of their wide distribution in the plant kingdom. They have the structural requirements for free radical scavengers and have potential as food antioxidants.[Citation4,] However, besides phenolic compounds, other substances can contribute to antioxidant activity of some plants. For example, Allium L. genus is rich in thiosulfinates, important antioxidant constituents.[Citation5,Citation6,] In addition to that, thiols, that are also present in Allium species, may contribute to antioxidant activity.[Citation7,Citation8,]
Plants of genus Allium are a common ingredient of human nutrition. They are used as food or spices and are especially appreciated because of their reputed antibacterial and other biological activities.[Citation6,Citation9,Citation10,] They have also been reported to protect against stomach and colorectal cancers.[Citation6,Citation11,] Allium species are often applied in traditional medicine in treatment of cardiovascular diseases, as well as for prevention and elevation of symptoms of infective diseases. Some of those effects may be explained by their antioxidant activity. Antioxidant activity of oil,[Citation12,Citation13,] ethanolic,[Citation14,] and methanolic extract[Citation15,Citation16,] of garlic and several other plants of genus Allium[Citation17,] was confirmed. Besides that, some other mechanisms may contribute to the observed biological properties of Allium plants. One among them may be bioadhesion.
The term bioadhesion has been defined as attachment of a synthetic or natural macromolecule to mucus and/or epithelial surface. Over the last two decades, bioadhesion has become of interest for its potential to optimize drug delivery, by retaining a formulation in a close proximity to the mucosal surface of the body for a prolonged period.[Citation18,] Among bioadhesives, there are many polymers of natural origin, including chitosan and different gums such as guar, xanthan, gellan, carragenan, pectin, and alginate.[Citation19,] Some natural polymers, such as chitosan,[Citation20,] or the polymer extracted from tamarind seed[Citation21,] have been proven to facilitate the transport of biologically active molecules across the mucosal surfaces of the body. Plant lectins extracted from potatoes, tomatoes, stinging nettle or different leguminosae species have showed the cell-specific adhesion in the gastronintestinal tract.[Citation19,] All this may contribute to the absorption of the herbal extracts and thereby increase their activity
It has been found that the antioxidant activity of some Allium plants may be reduced at lower pH.[Citation17,] Under the same pH conditions allinase, the enzyme that converts sulfur oxides to thiosulfinates is inactivated.[Citation22,] In addition to that, transformation of sulfhydryl to disulfide groups, which would occur in neutral or basic media, is inhibited.[Citation23,] Therefore, the aim of this study was to evaluate composition, antioxidant potential and bioadhesive properties of some onions used in human nutrition, namely: onion (A. cepa L.), garlic (A. sativum L.), leek (A. porrum L.) and ramsons (A. ursinum L.), simulating low pH conditions that may occur during the preparation of some foods with acidic ingredients. An additional goal was to determine which of the reputed antioxidant substances present in onions may contribute mainly to their antioxidant activity.
MATERIALS AND METHODS
Materials
Butylated hydroxyanisol (BHA), l-cysteine, 5,3-dithio-bis-(2-nitrobenzoic acid) (DTNB), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent and gallic acid were purchased from Sigma Chemical Co. (USA). Other chemicals and solvents were of analytical grade. Extracts were freeze dried using Freeze Dryer Alpha 1–4 M. (Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). For absorbance measurements, Lambda 25 (Perkin Elmer, Waltham, USA) spectrophotometer was used. Samples were compressed using a hydraulic press (Beckman Press, Glenrothes, UK). A precise balance (Sartorius BP 221S, Göttingen, Germany) was used for measuring the work of adhesion.
Preparation of Herbal Extracts
Bulbs and leaves of fresh plants (obtained at local market) were separated, finely chopped and mixed with 5 mM solution of HCl in water or 20% ethanol. After stirring for 1 h, the extracts were filtered and freeze dried.
Total Phenol Content
For determination of total phenol content, a solution of extract (0.5 ml) was mixed with the Folin–Ciocalteau reagent (0.5 ml) and 10% (w/v) Na2CO3 (0.5 ml). The absorbance was measured at 760 nm after 1 h of incubation at room temperature. Total phenolic content was calculated from calibration curve of gallic acid and expressed as gallic acid equivalents.[Citation24,]
Total Sugar Content
Total sugar content was investigated according to Dubois et al.[Citation25,] To 2 ml of 1 mg/ml (w/v) extract solution, 1 ml of 5% phenol solution (w/v) was added, followed by 5 ml of concentrated sulfuric acid. Tubes were allowed to stand first for 10 min at room temperature and then for 10 min at water bath at 30°C. Absorbance was measured at 490 nm. Blanks were prepared in the same manner but instead of extracts, 2 ml of water was used. Total sugar content was calculated from calibration curve of glucose and expressed as glucose equivalents.
Total Thiols and Thiosulfinates
The concentration of total thiosulfinates and thiols was estimated using the method of Han et al. (26) with slight modifications. To 1800 μl of TRIS/HCl buffer (50 mM, pH = 7.5) (determination of thiols) or 1800 μl of 100 μM cysteine solution in TRIS/HCl buffer (determination of thiosulfinates), 200 μl of freshly prepared extract solution (1 mg/ml) in TRIS/HCl buffer was added. The solution was incubated for 10 min at room temperature in dark. Following that, 200 μl of 1.5 mM DTNB solution in TRIS/HCl buffer was added and solution incubated again for 10 min. Absorbance was measured at 412 nm. The concentration of thiousulfinates was equivalent to half the decrease in cysteine concentration. It was expressed as μmol allicin equivalents in g of extract. The content of thiols was calculated from calibration curve of cysteine and expressed as μmol cysteine equivalents in g of extract.
DPPH Radical-Scavenging Activity
For determination of scavenging of DPPH radicals, 2.0 ml of 0.16 mM DPPH ethanolic solution (at concentrations of 0.1 to 2.5 mg/ml) was added to 2.0 ml of either ethanolic solution of extract (sample) or ethanol (control). The mixtures were incubated at room temperature in the dark. After 30 min, absorbance was read at 517 nm. Radical scavenging activity (RSA) was calculated using the equation:
The Reducing Power of the Extracts
Extracts (2–16 mg) were dissolved in 1.0 ml of distilled water and mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide. The mixtures were incubated at 50°C for 20 min. After incubation, 2.5 ml of a 10% (w/v) trichloroacetic acid was added to the mixtures. Following that, samples were centrifuged for 10 min. Aliquots of 2.5 ml of the upper layer were combined with 2.5 ml of water and 0.5 ml of the 0.1% (w/v) solution of ferric chloride. Absorbance was measured at 700 nm (27).
In Vitro Bioadhesion Test
To evaluate the bioadhesive properties of prepared hydrophilic extracts, a tensile study was performed using bovine buccal mucosa. 100 mg of the prepared extracts were compressed into flat-faced discs of 5.0 mm diameter by means of a hydraulic press, applying a compression pressure of 160 kPa cm−2. The discs were attached to the stainless steel support connected to a precise balance using cyanoacrylate glue. The bovine buccal mucosa was fixed to the glass dish mounted on the mobile support. The mucosa surface was wetted with 0.1 ml of simulated saliva (an aqueous solution containing 2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl per litre; pH-value was adjusted to 6.75 with phosphoric acid) and brought in contact with the sample. The sample and the tissue were left in contact for 5 min, allowing the formation of a mucoadhesive bond. The force of detachment was measured as a function of displacement, by lowering the mobile support at a constant rate of 5 mm min−1 until total separation of the components was achieved. The work of bioadhesion (W a) was calculated as the area under the force/distance curve. The obtained values were compared to a negative control (work of adhesion value of blank stainless steel support).
Statistical Analysis
The experiments were performed in triplicate. Results were expressed as mean ± standard deviation. The data were compared by ANOVA followed by Tukey posttest. Unless otherwise stated, P values <0.05 were considered statistically significant. For purposes of linear regression, the antioxidant activity was expressed as slope of the trendline in reducing power assay (RPS) and RSA of the extracts as the activity at the highest investigated concentration. All calculations were performed using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, USA).
RESULTS AND DISCUSSION
Allium L. extracts were obtained by extracting fresh bulb or leaves with 5 mM solution of HCl in water (A) or 20% ethanol (E), simulating the low pH conditions that may occur during preparation of some foods. The extracts were prepared from bulbs (B) of A. sativum (AS-BA, AS-BA) and A. cepa (AC-BA, AC-BE), as well as bulbs or leaves (L) of A. porrum (AP-BA, AP-BE, AP-LA, AP-LE) and A. ursinum (AU-BA, AU-BE, AU-LA, AU-LE).
The total phenols content in the extracts was determined since the phenolic substances are reputed antioxidants (). It ranged from 0.6% in AU-BA to 4.5% in AC-BE, respectively. With the exception of ramsons bulbs, addition of ethanol to extraction media resulted in significantly higher (P < 0,001) quantity of phenols in extracts. Moreover, statistically significant difference in phenolic content between extracts obtained from bulbs and extracts obtained from leaves was found. That is not surprising since phenols are moderately polar substances, usually located in the leaves or other aerial organs of the plant.[Citation9,]
Table 1 Total quantity of phenols (TP), sugars (TS), thiosulfinates (TSH), and thiols (SH) in the extracts and radical scavenging activity (EC50) of the extracts
According to some previous reports, non-structural carbohydrates in bulbs of onions consisted mostly of monosaccharide and oligomeric fructans.[Citation28,] The content of sugars in extracts was high () and ranged from 57.4 in to 93.7 in AU-BA and AU-LE, respectively. With the exception of leek aqueous extract, if both leaves and bulbs were included in investigation, the content of sugar was higher in leaves than in bulbs.
For determination of thiosulfinates, they were first reacted with an excess amount of cysteine. In that reaction one molecule of allicin reacts with two molecules of cysteine to form two molecules of S-allylmercaptocysteine. The decrease in cysteine concentration was then measured in reaction with DTNB, and corresponded to twofold amount of thiosulfinates. For purposes of this study, the extracts were prepared in acidic solution (pH = 3). According to Ellmore and Feldberg,[Citation22,] that pH leads to inactivation of allinase, the enzyme that converts sulfur oxides to thiosulfinates. Although there are reports that garlic extracts do not contain free sulfhydril groups, even if alliinase is inhibited or inactivated,[Citation26,] detectable amounts of sulfhydril groups were found in all the extracts (). Besides inhibition of alliinase, that finding could also be attributed to the acidic inhibition of transformation of sulfhydryl to disulfide groups, the reaction that would normally occur in neutral or basic media.[Citation23,] However, even though the amount of thiosulfinates in some extracts was lower than in some previous reports,[Citation26,] it was still somewhat higher than the amount of thiols which could indicate that inhibition of alliinase was partial.
Being a stable free radical, DPPH is frequently used to determine radical scavenging activity of natural compounds. In its radical form, DPPH absorbs at 517 nm, but upon reaction with an antioxidant the absorption decreases due to the formation of its non-radical form, DPPH–H. Thus, the radical scavenging activity can be monitored as a decrease in absorbance of DPPH solution. shows the EC50, the concentration that scavenges 50% of free radicals in solution. Aqueous and ethanolic extracts of onions demonstrated moderate antiradical activity, which is in accordance with the results of some previous studies that the antioxidant activity of some Allium plants may be reduced at lower pH.[Citation17,] For example, in the final concentration of 1.25 mg/ml, the RSA of the most active extract, AP-LA, was 35.0% while the RSA of BHA, which was used as a standard, was approximately 100% even at concentration of 50 μg/ml. Among the extracts included in investigation, the most active were the extracts obtained from A. porrum leaves, followed by extracts obtained from A. ursinum leaves. If radical scavenging activity was compared between leaves and bulbs, the activity of leaves was significantly higher. On the other hand, extraction solvent did not significantly affect antiradical activity of the extracts.
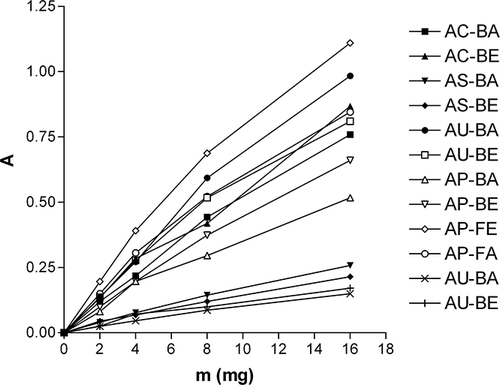
The reducing power of a compound is related to its electron transfer ability and may serve as a significant indicator of its potential antioxidant activity. In this assay, the yellow color of the test solution changes to green depending on the reducing power of test specimen. Greater absorbance at 700 nm indicated greater reducing power. presents the reductive capabilities of the investigated extracts. In the concentration range investigated, all the extracts demonstrated reducing power that increased linearly with concentration (r 2 was between 0.97 and 1.00 for all the extracts). Again, AP-LE and AU-LA were the most active extracts, while the activity of A. ursinum bulb extracts was the lowest. If leaf and bulb extract were included in investigation, then the activity of leaf extracts was significantly higher than the activity of bulbs.
Figure 1 Reducing power of aqueous (A) and 20% ethanolic (E) extracts of bulbs (B) and leaves (L) of A. cepa (AC), A. sativum (AS), A. porrum (AP), and A. ursinum (AU).
The bioadhesive properties of prepared extracts were assessed according to the work of adhesion necessary for detachment of a sample and mucosal surface. Since some of the extracts could not be compressed into discs, those samples were omitted from the experiment. The obtained work of adhesion values for other extracts, as well as for the negative control is presented at .
Figure 2 In vitro bioadhesive properties of solid aqueous (A) and 20% ethanolic (E) extracts of bulbs (B) and leaves (L) of A. cepa (AC), A. sativum (AS), A. porrum (AP), and A. ursinum (AU), expressed as a work of adhesion (W a). Statistically significant differences: *P < 0.001 compared to the negative control (n.c.); **P < 0.001 compared to the negative control; and P < 0.05 compared to AP-BA and AP-BE, respectively.
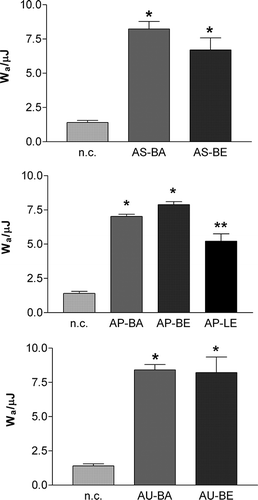
From the in vitro test, it is clearly evident that all extracts tested showed bioadhesive characteristics. In all the cases, work of adhesion values were significantly different form that of negative control (P < 0,001). The total sugar content determination has showed that sugars are the main component of the extracts and they are probably responsible for their bioadhesive properties. In onions, non-structural sugars are mostly present in form of mono and oligosaccharides[Citation28,] and the mechanism of mucoadhesive bond formation may be explained by mechanical interpenetration between oligosaccharides and mucine strands.[Citation18,] The type of media used during the extraction procedure did not show significant influence on the bioadhesive properties of the samples. In case of A. porum extracts, the difference between bioadhesive properties of extracts from bulbs and leaves was observed. This may be related to different chemical composition of the extracts and higher molecular weight of carbohydrates in the subterranean organs. The extracts with the most pronounced bioadhesive properties were aqueous extracts of bulbs of A. sativum and A. ursinum (W a > 8 μJ). That could be associated with higher degree of oligosaccharide polymerization A. sativum in than in A. cepa and A. porrum.[Citation28,]
Several studies have revealed a positive correlation between the content of phenolic compounds and the antioxidant activity in plants.[Citation16,Citation29,Citation30,] In this experiment, it was tried to establish if there was a correlation between antioxidant activity of Allium L. plants processed under acidic conditions and content of phenols, sugars, thiosulfinates or thiols. The relations between quantity of investigated compounds and antioxidant capacity varied between extracts. The results showed strong correlations between total phenolic content and RPS (r 2 = 0.98; P < 0,001) for the extracts of A. cepa, ethanolic extracts of A. porrum and A. ursinum as well as aqueous extracts of bulbs of A. porrum and A. ursinum. In addition to that, significant correlations between RSA and total phenols (r 2 = 0.60), as well as total sugars (r 2 = 0.75) were also found in those extracts. If all the extracts were included in analysis, correlation between total phenolic content and RPS were significant but much lower (r 2 = 0.47). Antioxidant activity in the tests did not correlate neither with the amount of thiols nor thiosulfinates, even though they are known to have antioxidant properties.[Citation5–8 Citation Citation Citation8, Positive correlation was observed between RPS and RSA (r 2 = 0.75) in all tests, which might lead to conclusion that similar mechanisms or compounds were responsible for the activity in both tests.
CONCLUSION
The results of this present study showed that inhibition of alliinase in acidic (pH = 3) aqueous and 20% ethanolic media resulted in Allium extracts that contained free sulfhydril groups. However, even though the extracts prepared under acidic conditions are moderately effective reductants and radical scavengers, content of thiols or thiosulfinates did not correlate with that activity. It seems that more than one class of chemical constituents is involved in the antioxidant activity of investigated extracts, phenols and sugars being the most important among the investigated compounds. Since the leaves of A. porrum and A. ursinum demonstrated greatest antioxidant activity in present research, it would be justifiable if they would be more seriously considered as flavoring agents and multifunctional ingredients in foods prepared or preserved at low pH. All the extracts were capable of binding to mucosal surface. Therefore, it is possible that the in vivo biological actions of onions prepared under such conditions may be enhanced by their pronounced bioadhesive characteristics.
ACKNOWLEDGMENTS
This work was supported by Grant “Nanodelivery systems” and Grant “Research on Bioactive Compounds and Biological Activities of Medicinal Plants” of the Ministry of Science, Education, and Sport of the Republic of Croatia.
REFERENCES
- Kaliora , A.C. , Dedoussis , G.V.Z. and Schmidt , H. 2006 . Dietary antioxidants in preventing atherogenesis. Atherosclerosis , 187 ( 1 ) : 1 – 17 .
- Robertson , R.P. and Harmon , J.S. 2006 . Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet β cell . Free Radical Biology and Medicine , 41 ( 2 ) : 177 – 184 .
- Issa , A.Y. , Volate , S.R. and Wargovich , M.J. 2006 . The role of phytochemicals in inhibition of cancer and inflammation: New directions and perspectives . Journal of Food Composition and Analysis , 19 ( 5 ) : 405 – 419 .
- Sun , J. , Chu , Y.-F. , Wu , X. and Liu , R.H. 2002 . Antioxidant and antiproliferative activities of fruits . Journal of Agricultural and Food Chemistry , 50 ( 25 ) : 7449 – 7454 .
- Jacob , C. 2006 . A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms . Natural Product Reports , 23 ( 6 ) : 851 – 863 .
- Rahman , M.S. 2007 . Allicin and other functional active components in garlic: health benefits and bioavailability . International Journal of Food Properties , 10 ( 2 ) : 245 – 268 .
- Granvogl , M. , Christlbauer , M. and Schieberle , P. 2004 . Quantitation of the intense aroma compound 3-mercapto-2-methylpentan-1-ol in raw and processed onions (Allium cepa) of different origins and in other Allium varieties using a stable isotope dilution assay . Journal of Agricultural and Food Chemistry , 52 ( 10 ) : 2797 – 2802 .
- Sendl , A. 1995 . Allium sativum and Allium ursinum: Part 1. Chemistry, analysis, history, botany . Phytomedicine , 4 : 323 – 339 .
- Bruneton , J. 1999 . Pharmacognosy, Phytochemistry, Medicinal Plants , 207 Paris , , France : Lavoisier Publishing .
- Corzo-Martınez , M. , Corzo , N. and Villamiel , M. 2007 . Biological properties of onions and garlic . Trends in Food Science & Technology , 18 ( 12 ) : 609 – 625 .
- Bianchini , F. and Vainio , H. 2001 . Allium vegetables and organosulfur compounds: do they help prevent cancer? . Environmental Health Perspectives , 109 ( 9 ) : 893 – 902 .
- Helen , A. , Krishnakumar , K. , Vijayammal , P.L. and Augusti , K.T. 2000 . Antioxidant effect of onion oil (Allium cepa Linn) on the damage induced by nicotine in rats as compared to alpha-tocopherol . Toxicology Letters , 116 ( 1–2 ) : 61 – 68 .
- Godevac , D. , Vujisić , L. , Mojović , M. , Ignjatović , A. , Spasojević , I. and Vajs , V. 2008 . Evaluation of antioxidant capacity of Allium ursinum L. volatile oil and its effect on membrane fluidity . Food Chemistry , 107 ( 4 ) : 1692 – 1700 .
- Imai , J. , Ide , N. , Nagae , S. , Moriguchi , T. , Matsuura , H. and Itakura , Y. 1994 . Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Medica , 60 ( 5 ) : 417 – 420 .
- Nuutila , A.M. , Puupponen-Pimia , R. , Aarni , M. and Oksman-Caldentey , K.M. 2003 . Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity . Food Chemistry , 81 ( 4 ) : 485 – 493 .
- Bozin , B. , Mimica-Dukic , N. , Samojlik , I. , Anackov , G. and Igic , R. 2008 . Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) . Food Chemistry , 111 ( 4 ) : 925 – 929 .
- Yin , M.C. and Cheng , W.S. 1998 . Antioxidant activity of several Allium members . Journal of Agricultural and Food Chemistry , 46 ( 10 ) : 4097 – 4101 .
- Smart , J.D. 2005 . The basic and underlying mechanism of mucoadhesion . Advanced Drug Delivery Reviews , 57 ( 11 ) : 1556 – 1568 .
- Lee , J.W. , Park , J.H. and Robinson , J.R. 1999 . Bioadhesive-based dosage forms: The next generation . Journal of Pharmaceutical Sciences , 89 ( 7 ) : 850 – 866 .
- Thanou , M. , Verhoef , J.C. and Junginger , H.E. 2001 . Oral drug absorption enhancement by chitosan and its derivatives . Advanced Drug Delivery Reviews , 52 ( 2 ) : 117 – 126 .
- Gheraldi , E. , Tavanti , A. , Davini , P. , Celandroni , F. , Salvetti , S. , Parisio , E. , Boldrini , E. , Senesi , S. and Campa , M. 2004 . A mucoadhesive polymer extracted from tamarind seed improves the intraocular penetration and efficacy of rufloxacin in topical treatment of experimental bacterial keratitis . Antimicrobial Agents and Chemotherapy , 48 ( 9 ) : 3396 – 3401 .
- Ellmore , G.S. and Feldberg , R.S. 1994 . Alliin lyase localization in bundle sheaths of the garlic clove (Allium sativum) . American Journal of Botany , 81 ( 1 ) : 89 – 94 .
- Bernkop-Schnürch , A. , Scholler , S. and Biebel , R.G. 2000 . Development of controlled drug release systems based on thiolated polymers . Journal of Controlled Release , 66 ( 1 ) : 39 – 48 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent . Methods in Enzymology , 299 : 152 – 178 .
- Dubois , M. , Gilles , K.A. , Hamilton , J.K. , Rebers , P.A. and Smith , F. 1956 . Colorimetric method for determination of sugars and related substances . Analytical Chemistry , 28 ( 3 ) : 350 – 355 .
- Han , J. , Lawson , L. , Han , G. and Han , P. 1995 . A spectrophotometric method for quantitative determination of allicin and total garlic thiosulfinates . Analytical Biochemistry , 225 ( 1 ) : 157 – 160 .
- Yen , G.-C. and Chen , H.-Y. 1995 . J. Antioxidant activity of various tea extracts in relation to their antimutagenicity . Journal of Agricultural and Food Chemistry , 43 ( 1 ) : 27 – 32 .
- Darbyshire , B. and Henry , R.J. 1981 . Differences in fructan content and. synthesis in some Allium species . New Phytologist , 87 ( 2 ) : 249 – 256 .
- Mandic , A.I. , Ðilas , S.M. , Cetkovic , G.S. , Canadanovic-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 ( 4 ) : 713 – 726 .
- Gulcin , I. , Tel , A.Z. and Kirecci , E. 2008 . Antioxidant, antimicrobial, antifungal, and antiradical activities of Cyclotrichium Niveum (BOISS.) Manden and Scheng . International Journal of Food Properties , 11 ( 2 ) : 450 – 471 .