Abstract
Angiotensin I-converting enzyme (ACE) inhibitory activities of soybeans fermented by Bacillus subtilis B1 with pretreatment of soaking in electrolyzed oxidizing (EO) water, electrolyzed reduced (ER) water, chemically modified acidic and alkaline solutions as well as conventional tap water were investigated. Using electrolyzed water improved significantly (p < 0.05) the ACE inhibitory activities of fermented soybeans as compared with using tap water. The highest ACE inhibitory activity was recorded as an IC50 value of 0.065 mg/mL in the sample, which had been pretreated by soaking in ER water prior to fermentation. The ACE inhibitory activity increased to and tended to keep at a higher level during fermentation of EO water-soaked soybeans. However, there was no evident advantage of chemically modified acidic and alkaline solutions over tap water. Results suggested that electrolyzed water possessed potent ability to improve the ACE inhibitory activity of fermented soybeans, which might be through providing an appropriate microenvironment for the hydrolytic actions of microorganism to generate ACE inhibitors. In addition, this work also suggested a potential ability of electrolyzed water to be applied into the production of functional foods.
INTRODUCTION
Hypertension is related to the incidence of cardiovascular diseases. Angiotensin I-converting enzyme (ACE, EC 3.4.15.1) plays an important physiological role in the regulation of blood pressure. It can raise blood pressure by converting the inactive angiotensin I to the potent vasoconstrictor, angiotensin II, and by catalyzing the degradation of bradykinin.[Citation1] The inhibition of ACE activity is therefore considered as a useful approach to lower blood pressure. Because of the adverse effects caused by synthetic ACE inhibitors currently used as clinical antihypertensive drugs, ACE inhibitors from food resources have been paid dramatic attention in connection with the prevention and remedy of hypertension.
Soybean is a subject of extensive scientific research due to its contribution to good nutrition and health. Several literatures had reported the ACE inhibitory activities of soybean protein hydrolysates.[Citation2–4] Many fermented soybean foods were also confirmed to exhibit ACE inhibitory activities, in which the ACE inhibitors were mainly recognized as bioactive peptides.[Citation5–8] Processing techniques, such as starter cultures used,[Citation9] fermentation temperature,[Citation10] salt supplementation,[Citation11] and so on, could affect the production of bioactive substances in fermented soybean foods. Soaking is an indispensable pretreatment step in the preparation of fermented soybean foods, as it can make soybeans expand sufficiently and render them digestive, and thus facilitate the actions of microorganisms on soybeans in combination with the subsequent treatment of steam cooking. During soaking process, changes in the structure and composition of chemical ingredients in soybeans may take place due to the action of hydration.[Citation12,Citation13] Vijayakumari and co-workers[Citation14] reported the reduction of antinutritional compounds in Bauhinia purpurea L. seeds by soaking in distilled water and NaHCO3 solution. The glucoside conjugates of soybean isoflavones could be converted into the aglycone under the action of β-glucosidase present in the soybeans during soybean soaking.[Citation15]
Conventionally, tap water is used for soybean soaking. Today, electrolyzed water, a relatively new type of water, has been developed and applied to various fields ranging from agriculture[Citation16] to food industry[Citation17] due to its special physicochemical properties. Electrolyzed water is produced through the electrolysis of NaCl solution in a chamber where positive and negative electrodes are separated by a membrane. When a current is passed across the electrodes, two types of water possessing different characteristics are generated: the cathode produces electrolyzed reduced (ER) water with a reducing potential that leads to a reduction of free radicals in biological systems and may be useful in the treatment of organ malfunctions, while the anode produces electrolyzed oxidizing (EO) water with an oxidizing potential and strong bactericidal effect that has been extensively used for sanitation and food quality control.[Citation17,Citation18] Generally, electrolyzed water generated by the electrolysis of NaCl solution is strongly acidic and alkaline electrolyzed water. Besides, another type of electrolyzed water, weakly electrolyzed water which is produced by the electrolysis of tap water without electrolytes added, could be also utilized into improving the eating qualities of bread,[Citation19] cooked rice,[Citation20] boiled noodles[Citation21] and tofu.[Citation22] Therefore, electrolyzed water could be considered as an alternative in order to improve the properties of final food products due to its different physicochemical characteristics from tap water.
In the previous work, Bacillus subtilis B1, a new strain isolated from the koji of douchi (a Chinese traditional salt-fermented soybean food), was found to produce strong ACE inhibitory activity in douchi. However, the ACE inhibitory activity of douchi started with B. subtilis B1 decreased significantly during salting and ripening.[Citation23] Recently, the authors attempted to improve the ACE inhibitory activity of fermented soybeans started with B. subtilis B1 by modifying the soaking technique. To their knowledge, no information on the potential application of electrolyzed water to improve the functional properties of fermented soybean foods is available. The present study aimed at evaluating the effect of various soaking water, including EO water, ER water, chemically modified acidic and alkaline solutions as well as tap water, on the ACE inhibitory activities of soybeans fermented by B. subtilis B1. The changes in viscosity and the hydrolysis of soy protein during soybean fermentation were also analysed.
MATERIALS AND METHODS
Materials
Black soybeans were purchased from the Center of Soybean Research, Agricultural Academy of Jilin Province (Jilin, China). Bacillus subtilis B1 was isolated from douchi koji previously in our laboratory[Citation24] and identified by the Institute of Microbiology of Chinese Academy of Sciences (Beijing, China) using 16S rDNA PCR-RFLP. Hippuryl-His-Leu (HHL), angiotensin I-converting enzyme (ACE, from rabbit lung), o-phthaldialdehyde (OPA) and 2,4,6-trinitrobenzenesulfoinc acid (TNBS) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). All other reagents were of analytical grade.
Preparation of Starter Culture and Pretreatment Solutions
B. subtilis B1 was inoculated into Luria-Bertani liquid medium (Oxoid, England) and allowed to grow at 37°C for 12 h with shaking at 150 rpm in an incubation shaker. The enriched culture was diluted with sterile 0.1% peptone solution to prepare the starter suspension with a concentration of around 107 cfu/mL. Electrolyzed water was prepared by the electrolysis of 0.1% NaCl solution using a commercial electrolyzed water generator (CE-7001, Saiai Environment-Protecting Technology Co., Ltd., Guangzhou, China). The pH values of EO water and ER water were 2.98 ± 0.01 and 11.53 ± 0.01, respectively, measured by using a digital pH meter (F-23, Horiba, Ltd., Kyoto, Japan). Chemically modified solutions, namely HCl, NaOH and KOH solutions with pH values similar as that of EO or ER water, were also prepared. The pH value of tap water used in the present work was 7.70 ± 0.01. All pretreatment solutions were used within 10 min after preparation.
Laboratorial Fermentation of Soybeans
Soybeans were washed and soaked in tap water, EO water, ER water, HCl solution, NaOH solution and KOH solution at 28°C for 8 h, respectively, according to a ratio of 1:3 (w/w). After draining, soybeans were steamed at 121°C for 40 min in an autoclave (YXQ-SG46-280A, Boxun Medical Instrument Co., Ltd., Beijing, China), and then cooled to 30°C. Subsequently, soybeans were inoculated with B. subtilis culture suspension (1 mL/100 g cooked beans) and incubated at 37°C and 90% relative humidity for 96 h in an incubator (EYELA KCL-1000, Tokyo Rikakikai Co., Ltd., Tokyo, Japan). Each experiment was conducted in duplicate.
Extraction of ACE Inhibitor
Samples were lyophilized with a freeze dryer (EYELA FDU-540, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and ground into powder. One gram of fermented soybean powder was suspended in 10 mL of distilled water, followed by homogenization (T25 basic, IKA Labortechnik, Staufen, Germany) at 15,000 rpm for 2 min and sonication (KQ-100E, Ultrasonic Instrument Co., Ltd., Kunshan, China) for 5 min. The mixture was extracted in an orbital shaker for 1 h at room temperature. After boiling for 15 min, the suspension was centrifuged at 12,000 × g for 15 min. The resulted supernatant was collected after filtration by a 0.45 μm membrane and then used for assaying. The concentration was labeled as 100 mg/mL.
ACE Inhibitory Activity Assay
ACE inhibitory activity assay was measured using the modified method described by Horie.[Citation25] A 20 μL of sample extract was mixed with 40 μL of ACE solution (12.5 munits/mL) and 40 μL of substrate solution (4.66 mM HHL in 400 mM phosphate buffer containing 600 mM NaCl, pH 8.5) and then incubated at 37°C for 1 h. The reaction was terminated by adding 150 μL of 1.2 M NaOH solution. The mixture was incubated for 20 min at room temperature after the addition of 40 μL of 2% OPA dissolved in methanol. The derivation reaction was terminated by adding 40 μL of 6 M HCl solution. The fluorescence intensity was measured using a spectrofluorophotometer (RF-5300PC, Shimadzu Co., Kyoto, Japan) under the following conditions: Ex, 340 nm; Em, 455 nm; slit width, 5 nm. ACE inhibitory activity was calculated as follows:
Determination of pH and Viscosity of Fermented Soybeans
Five grams of sample powder were homogenized with 45 mL of demineralised water. The pH value of the homogenate was determined with the pH meter (F-23, Horiba, Ltd., Kyoto, Japan). The viscosity of this suspension was measured by a digital viscosimeter (SNB-1, Precise Scientific Instrument Co., Ltd., Shanghai, China).
Determination of Protease Activity and Amounts of Free Amino Groups
One gram of fermented soybean sample was suspended in 10 mL of distilled water. After shaking for 1 h, the suspension was centrifuged at 4°C and 5000 × g for 10 min. The supernatant was used as crude enzyme extract. Protease activity was measured using the modified method described by Yang et al.[Citation26] A 2 mL of crude enzyme extract was mixed with the same volume of 2% casein dissolved in 0.2 M phosphate buffer (pH 7.5). The mixture was incubated at 37°C for 10 min. The reaction was terminated by the addition of 4 mL of 0.4 M trichloroacetic acid solution, and the mixture was centrifuged. The soluble peptide in the supernatant was assessed with tyrosine as the reference compound. Amounts of free amino groups in fermented soybeans were measured according to the method of Haynes et al.[Citation27] using TNBS, the specific reagent for amino groups, on a spectrophotometer (UV 1240, shimadzu, Co., Kyoto, Japan). Leucine was used as the standard.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using the general linear model (Version 8.0, SAS Institute Inc., NC, USA). Duncan's multiple range test was used to determine the differences among samples. Significant levels were defined as probabilities of 0.05 or less.
RESULTS
ACE Inhibitory Activities of Fermented Soybeans
shows the ACE inhibitory activities of B. subtilis B1 fermented soybeans with pretreatment of soaking in various types of water. Through the entire fermentation process, soybeans pretreated with soaking in EO water and ER water showed much higher ACE inhibitory activities than those pretreated with soaking in HCl solution, NaOH solution, KOH solution and tap water. However, there was no significant difference of the ACE inhibitory activities in the samples pretreated with HCl, NaOH and KOH solutions from that pretreated with tap water. The weakest ACE inhibitory activity was observed in soybeans, which had been soaked in NaOH solution prior to steam-cooking and fermentation.
Figure 1 Changes in ACE inhibitory activities during fermentation of soybeans pretreated with soaking in various types of water. Values were measured with a sample concentration of 0.1 mg/mL. No ACE inhibitory activities were detected (values below zero) at the first four hours of fermentation under the experimental sample concentration. The error bars indicated the standard deviation of three replicates.
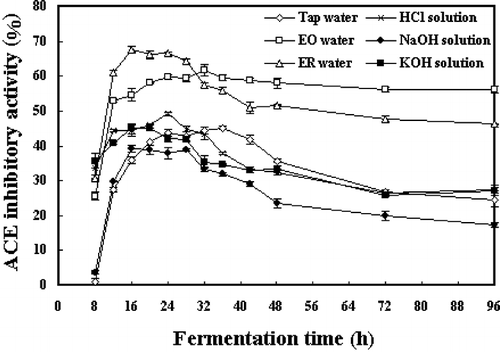
In general, the ACE inhibitory activities increased rapidly in the first 16 hours of soybean fermentation, and then kept relatively constant in the following several hours before entering an attenuation period. However, there was an exception that no obvious decline of the ACE inhibitory activity in the EO water-soaked soybean sample was observed, after its ACE inhibitory activity elevated to a high level. It was also showed that the ACE inhibitory activities of soybeans pretreated with soaking in electrolyzed water, HCl and KOH solutions tended to increase much more rapidly than that pretreated with tap water in the early period of fermentation. For example, the ACE inhibitory activity of soybeans soaked in ER water could be up to 60.9% after 12 h of fermentation, which was more than twice higher than that soaked in tap water with a value of 27.5%.
Among all samples, the highest ACE inhibitory activity was recorded in an ER water-soaked sample with an IC50 value of 0.065 mg/mL, which had been fermented for 16 h by B. subtilis B1. presents the IC50 values for ACE inhibitory activities of soybeans after 24 h of fermentation. It was showed that samples pretreated by electrolyzed water possessed significantly lower (p < 0.05) IC50 values of 0.081 and 0.067 mg/mL than other samples, while there was no significant difference in IC50 values of samples pretreated with soaking in tap water (0.118 mg/mL) and KOH solution (0.122 mg/mL). The highest IC50 value (0.140 mg/mL) was detected in the sample with pretreatment of soaking in NaOH solution, as the lowest ACE inhibitory activity among the fermented soybeans prepared.
Table 1 IC50 values for ACE inhibitory activities of soybeans fermented for 24 h with pretreatment of soaking in various types of water
pH and Viscosity of Fermented Soybeans
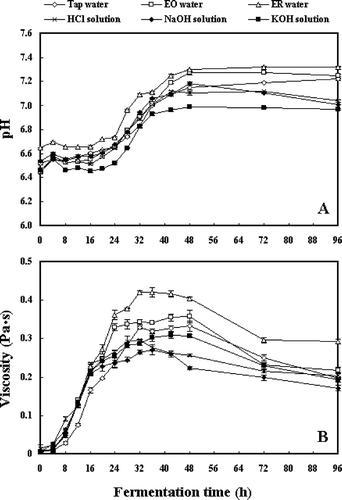
presents the changes in pH (A) and viscosity (B) during fermentation of soybeans pretreated by various types of water. The pH values of soaked soybeans ranged from 6.44 to 6.65 as indicated at 0 h of fermentation (). During fermentation, relatively higher pH values were observed in soybeans soaked by ER water, while comparatively lower pH values were found in KOH-soaked soybeans. The pH value of EO water-soaked soybeans increased to a level close to fermented soybeans pretreated by ER water after 42 h of fermentation. According to , the viscosity of tap water-soaked soybeans was relatively lower in the early period of fermentation. During the whole fermentation, higher viscosities were observed in soybeans pretreated by soaking in electrolyzed water, with the highest viscosity detected in ER water-soaked soybeans.
Protease Activities and Amounts of Free Amino Groups in Fermented Soybeans
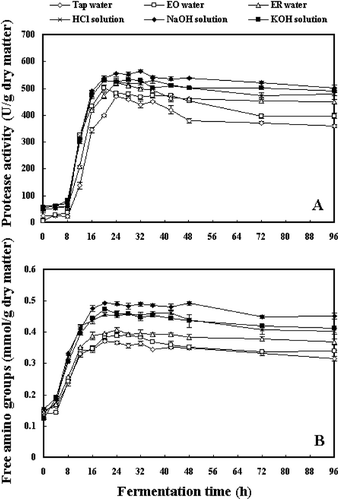
exhibits the changes in protease activities (A) and amounts of free amino groups (B) during fermentation of soybeans. From , the levels of amounts of free amino groups were in line with the production of protease activities. Higher protease activities and more amounts of free amino groups were found in soybeans soaked by HCl, NaOH and KOH solutions during fermentation. The lowest protease activity was observed in tap water-soaked soybeans, while fermented soybeans pretreated with electrolyzed water showed moderate protease activities.
DISCUSSION
Soybean soaking is of practical importance since it may not only be an initial hydration stage, but also induce changes in chemical ingredients and thus affect the quality of the final product.[Citation12,Citation13] The results in this work exhibited that soaking soybeans with various types of water may vary the ACE inhibitory activities in soybeans fermented by B. subtilis B1, and the application of electrolyzed water for soybean soaking could enhance enormously the ACE inhibitory activities of fermented soybeans (). It was suggested that the soaking water with different physicochemical properties might play different roles in the production of ACE inhibitors from soybeans during fermentation.
It is well known that the pH value is an important factor for the growth of microorganisms. The optimum pH value for the growth of B. subtilis B1 on soybeans was 6.4–7.6 (data not shown). In the present study, the pH values of various soybean suspensions at 0 h of fermentation ranged from 6.44 to 6.65 when various types of water were used as soaking water (), which was the common pH value for the growth of B. subtilis B1 on soybeans, suggesting that the soaking procedure itself did not change significantly the acidic and alkaline properties of soybeans. Therefore, it might be reasonable to presume that the different levels of the ACE inhibitory activities in fermented soybeans might not be resulted from the difference in pH values of soaking water used in this work. It had been reported that EO water possessed high oxidation-reduction potential (ORP) and high dissolved oxygen, while ER water had low ORP and high dissolved hydrogen.[Citation17] Nowadays, electrolyzed water has been used to improve the organoleptical properties of various foods. It was found that the use of alkaline electrolyzed water could increase the protein content of tofu and thus the eating quality of tofu was modified.[Citation22] For the preparation of noodles, acidic electrolyzed water promoted the dissolution of gliadin and glutenin subunits, which participated in the formation of gluten-matrix and so imparted a favorable texture to the product. Conversely, the use of alkaline electrolyzed water resulted in less springiness because it changed the gelatinization conditions of the starch, which was a negative point.[Citation21] Onishi and co-workers[Citation19] reported that using acidic electrolyzed water would produce bread with higher elasticity, while soft bread could be produced by the use of alkaline electrolyzed water. Hence, whereas the mechanism of the enhanced ACE inhibitory activities by using electrolyzed water as soaking water needed to be further investigated, the special physicochemical properties of electrolyzed water might be contributing to its evident advantage for producing fermented soybeans with strong ACE inhibitory activities over chemically modified acidic and alkaline solutions and tap water. Tentatively, the higher ACE inhibitory activities in electrolyzed water-soaked soybeans might be due to the favorite microenvironments provided by electrolyzed water for the actions of microorganism.
Although the highest ACE inhibitory activity was found in fermented soybeans pretreated with soaking in ER water, it was interesting to note that the ACE inhibitory activity of EO water-soaked soybeans elevated to a high level and then nearly kept constant till the end of fermentation without obvious decline observed, suggesting that the two types of electrolyzed water might produce diverse ACE inhibitory activities in fermented soybeans. It seemed that ER water exhibited more potential to be utilized into obtaining the most abundant ACE inhibitors from fermented soybeans, while EO water might be favorable to keep the ACE inhibitory activity of fermented soybeans at a high level for a long time, which would be beneficial for commercialized soybean food. In addition, the results that the ACE inhibitory activities of electrolyzed water-soaked soybeans reached to a much higher level than that soaked by tap water at approximate 12 h of fermentation, indicated that the fermentation time could be shortened to obtain stronger ACE inhibitory activity in fermented soybeans by using electrolyzed water as soybean soaking water.
In previous studies, bioactive peptides were paid considerable attention for the ACE inhibitory activities of many fermented soybean foods. Peptides were generated from the hydrolysis of soy protein by proteases derived from microorganisms.[5–8] The amounts of free amino groups could reflect the degrees of protein hydrolysis, to a certain extent. In the present work, higher amounts of free amino groups were found in samples pretreated with soaking in chemically modified acidic and alkaline solutions, which was corresponding with the higher protease activities (). In contrast, samples pretreated by ER water and EO water with potent ACE inhibitory activities contained relatively less amounts of free amino groups and moderate protease activities. These results implied that soy proteins pretreated by chemically modified solutions might be degraded more easily than those pretreated by electrolyzed water. It had been reported that the structures of peptides were quite responsible for their ACE inhibitory activities.[Citation28] The inordinate protein hydrolysis, largely due to high protease activity, might result in unexpected loss of ACE inhibitory activity, which was especially confirmed by the result that the weakest ACE inhibitory activity was observed in NaOH-soaked soybeans exhibiting the highest protease activity in this work. The present results indicated that protease activity at a proper level might be helpful to generate ACE inhibitors from soybeans without excessive hydrolysis of proteins.
On the other hand, a visible phenomenon was the production of viscous materials covered the surface of fermented soybeans, which were mainly secondary metabolites produced by B. subtilis B1 during soybean fermentation. The viscous materials in natto, a Japanese B. subtilis fermented soybean food, had been reported to be a complex mainly composed of poly-γ-glutamate (PGA) and other biopolymers including polysaccharides. B. subtilis subsp. chungkookjang, utilized as starter for the production of a Korean soy-fermented food (chungkookjang), was also used to synthesize PGA.[Citation29] The viscosity of samples could be an indication of the production of viscous materials. Okamoto et al.[Citation30] reported that the viscous fraction of natto showed higher ACE inhibitory activity than the residual bean extract. Their further research also elucidated that the fraction with strong ACE inhibitory activity from the viscous materials of natto was of high molecular weight and was supposed to be a proteinaceous thing, while the fractions with low molecular weight showed much lower activities. In the present work, higher viscosities were found in fermented soybeans with pretreatment of soaking in ER water and EO water, which correspondingly exhibited much stronger ACE inhibitory activities than other samples. Considering the results that fermented soybeans pretreated by electrolyzed water showed moderate protein hydrolysis, it was implied that, except for peptides derived from soy proteins, the viscous materials might be also contributing to the potent ACE inhibitory activities observed in electrolyzed water-soaked soybeans during fermentation, which was also indicated by the weakest viscosity detected in NaOH-soaked soybeans with the lowest ACE inhibitory activity. In addition, soybean itself contained ACE inhibitors such as nicotianamine, which was reported to be responsible for the ACE inhibitory activity of soy sauce.[Citation31] Although the effect of soaking water on all the potential ACE inhibitors still remained unclear in this work, it was suggested that the total ACE inhibitory activities of fermented soybeans may occur from the combined function of various bioactive substances.
It was noticeable that the IC50 values for ACE inhibitory activities of samples fermented for 24 h ranged from 0.067 to 0.140 mg/mL (). It is well known that natto is also produced by using B. subtilis as starter culture. Ibe et al.[Citation32] prepared natto by using eleven species of B. subtilis as starters, and the products showed ACE inhibitory activities with IC50 values at a range of 0.270 to 0.440 mg/mL. Okamoto and co-workers[Citation30,Citation33] reported the IC50 values of 0.19 and 0.40 mg/mL for natto samples. Compared with these data, fermented soybeans possessed much higher ACE inhibitory activities by using B. subtilis B1 as starter culture in this work. The previous study found that the IC50 value of douchi started with the same strain and then subjected to salting and ripening for 30 d was 0.315 mg/mL for ACE inhibitory activity.[Citation23] Moreover, the high level of salt supplementation in douchi manufacturation was a serious health concern to consumers, especially to hypertensive patients. So, it might be an alternative strategy to prepare foods favorable for hypertensive patients by producing salt-free soybeans fermented with B. subtilis B1 through modified processing technique, such as using electrolyzed water as soybean soaking water, although the mechanism of electrolyzed water for increasing ACE inhibitory activities in fermented soybeans needed to be further investigated. In addition, since weakly electrolyzed water has been developed recently, it is now in progress to evaluate the effect of various electrolyzed water with different characteristics on the ACE inhibitory activities of fermented soybeans.
CONCLUSIONS
The ACE inhibitory activities of fermented soybeans started with B. subtilis B1 were influenced by pretreatment of soaking soybeans in various types of water. Using electrolyzed water to soak soybeans could improve significantly (p < 0.05) the ACE inhibitory activities of fermented soybeans, however, there was no obvious advantage of chemically modified acidic and alkaline solutions over conventional tap water. The highest ACE inhibitory activity with an IC50 value of 0.065 mg/mL was observed in soybeans fermented for 16 h with pretreatment of soaking in electrolyzed reduced water, while electrolyzed oxidizing water was favorable to keep the ACE inhibitory activity at a higher level during fermentation. These results indicated that soaking soybeans in electrolyzed water could be an alternative pretreatment procedure to improve the ACE inhibitory activity in fermented soybeans when B. subtilis B1 was used as starter culture. In addition, this work also expanded the application of electrolyzed water to the production of functional foods.
ACKNOWLEDGMENTS
This study was conducted within the framework of the collaborative research project between Japan and China entitled “Development of sustainable production and utilization of major food resources in China” supported by Japan International Research Center for Agricultural Sciences (JIRCAS). This work was also supported by the National Key-technologies R&D Program (2006BAD27B09 & 2007BAD83B03) of the 11th 5-year Plan of the People's Republic of China.
REFERENCES
- Erdos , E.G. 1975 . Angiotensin-I converting enzyme . Circulation Research , 36 : 247 – 255 .
- Fan , J.F. , Saito , M. , Tatsumi , E. and Li , L.T. 2003 . Preparation of Angiotensin I-converting enzyme inhibiting peptides from soybean protein by enzymatic hydrolysis . Food Science and Technology Research , 9 ( 3 ) : 254 – 256 .
- Kuba , M. , Tana , C. , Tawata , S. and Yasuda , M. 2005 . Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase . Process Biochemistry , 40 : 2191 – 2196 .
- Chiang , W.D. , Tsou , M.J. , Tsai , Z.Y. and Tsai , T.C. 2006 . Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor . Food Chemistry , 98 : 725 – 732 .
- Shin , Z.I. , Yu , R. , Park , S.A. , Chung , D.K. , Ahn , C.W. and Nam , H.S. 2001 . His-His-Leu, an angiotensin I converting enzyme inhibitory peptide derived from Korean soybean paste, exerts antihypertensive activity in vivo . Journal of Agricultural Food Chemistry , 49 : 3004 – 3009 .
- Kuba , M. , Tanaka , K. , Tawata , S. , Takeda , Y. and Yasuda , M. 2003 . Angiotensin I-converting enzyme inhibitory peptides isolated from tofuyo fermented soybean food . Bioscience, Biotechnology, and Biochemistry , 67 ( 6 ) : 1278 – 1283 .
- Gibbs , B.F. , Zougman , A. , Masse , R. and Mulligan , C. 2004 . Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food . Food Research International , 37 : 123 – 131 .
- Zhang , J.H. , Eizo , T. , Ding , C.H. and Li , L.T. 2006 . Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product . Food Chemistry , 98 : 551 – 557 .
- Chen , J. , Cheng , Y.Q. , Yamaki , K. and Li , L.T. 2007 . Anti-α-glucosidase activity of Chinese traditionally fermented soybean (douchi) . Food Chemistry , 103 : 1091 – 1096 .
- Yin , L.J. , Li , L.T. , Liu , H. , Saito , M. and Tatsumi , E. 2005 . Effects of fermentation temperature on the content and composition of isoflavones and β-glucosidase activity in sufu . Bioscience, Biotechnology, and Biochemistry , 69 ( 2 ) : 267 – 272 .
- Yin , L.J. , Li , L.T. , Li , Z.G. , Tatsumi , E. and Saito , M. 2004 . Changes in isoflavone contents and composition of sufu (fermented tofu) during manufacturing . Food Chemistry , 87 : 587 – 592 .
- Bayram , M. , Kaya , A. and Öner , M.D. 2004 . Changes in properties of soaking water during production of soy-bulgur . Journal of Food Engineering , 61 : 221 – 230 .
- Bayram , M. , Öner , M.D. and Kaya , A. 2004 . Influence of soaking on the dimensions and colour of soybean for bulgur production . Journal of Food Engineering , 61 : 331 – 339 .
- Vijayakumari , K. , Pugalenthi , M. and Vadivel , V. 2007 . Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seeds . Food Chemistry , 103 : 968 – 975 .
- Toda , T. , Sakamoto , A. , Takayanagi , T. and Yokotsuka , K. 2000 . Changes in isoflavone compositions of soybean foods during cooking process . Food Science and Technology Research , 6 ( 4 ) : 314 – 319 .
- Buck , J.W. , van Iersel , M.W. , Oetting , R.D. and Hung , Y.C. 2003 . Evaluation of acidic electrolyzed water for phytotoxic symptoms on foliage and flowers of bedding plants . Crop Protection , 22 : 73 – 77 .
- Huang , Y.R. , Hung , Y.C. , Hsu , S.Y. , Huang , Y.W. and Hwang , D.F. 2008 . Application of electrolyzed water in the food industry . Food Control , 19 : 329 – 345 .
- Al-Haq , M.I. , Seo , Y. , Oshita , S. and Kawagoe , Y. 2002 . Disinfection effects of electrolyzed oxidizing water on suppressing fruit rot of pear caused by Botryosphaeria berengeriana . Food Research International , 35 : 657 – 664 .
- Onishi , R. , Hara , Y. and Arai , E. 1999 . Effect of weak electrolyzed water on the properties of bread . Food Science and Technology Research , 5 ( 4 ) : 388 – 392 .
- Kobayashi , K. , Tosa , N. , Hara , Y. and Horie , S. 1996 . An examination of cooked rice with electrolyzed water . Nippon Shokuhin Kagaku Kogaku Kaishi , 43 : 930 – 938 . (in Japanese)
- Hara , Y. , Watanuki , A. and Arai , E. 2003 . Effects of weakly electrolyzed water on properties of Japanese wheat noodles (Udon) . Food Science and Technology Research , 9 ( 4 ) : 320 – 326 .
- Hara , Y. , Matsuda , H. and Arai , E. 2003 . Effects of weakly electrolyzed water on properties of tofu (soybean curd) . Food Science and Technology Research , 9 ( 4 ) : 332 – 337 .
- Li , F.J. , Yin , L.J. , Lu , X. and Li , L.T. 2010 . Changes in angiotensin I-converting enzyme inhibitory activities during the ripening of douchi (a Chinese traditional soybean product) fermented by various starter cultures . International Journal of Food Properties. , 13 ( 3 ) : 512 – 524 .
- Fan , J.F. 2006 . “ Thesis, China Agricultural University ” . In Antioxidant activity and gel-forming ability of douchi peptides and soy peptides , Beijing : Ph.D .
- Horie , H. 1999 . Handbook of functional evaluation of food , 117 – 121 . Japanese Ministry of Agriculture, Forestry and Fisheries. Agriculture, Forestry and Fishery Research Council. National Food Research Institute, Tokyo . (in Japanese)
- Yang , J.K. , Shih , I.L. , Tzeng , Y.M. and Wang , S.L. 2000 . Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes . Enzyme and Microbial Technology , 26 : 406 – 413 .
- Haynes , R. , Osuga , D.T. and Feeney , R.E. 1967 . Modification of proteolytic enzyme . Biochemistry , 6 : 541 – 547 .
- Li , G.H. , Le , G.W. , Shi , Y.H. and Shrestha , S. 2004 . Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects . Nutrition Research , 24 : 469 – 486 .
- Park , C. , Choi , J. C. , Choi , Y.H. , Nakamura , H. , Shimanouchi , K. , Horiuchi , T. , Misono , H. , Sewaki , T. , Soda , K. , Ashiuchi , M. and Sung , M.H. 2005 . Synthesis of super-high-molecular-weight poly-γ-glutamic acid by Bacillus subtilis subsp. chungkookjang . Journal of Molecular Catalysis B: Enzymatic , 35 : 128 – 133 .
- Okamoto , A. , Hanagata , H. , Kawamura , Y. and Yanagida , F. 1995 . Anti-hypertensive substances in fermented soybean, natto . Plant Foods for Human Nutrition , 47 : 39 – 47 .
- Kinoshita , E. , Yamakoshi , J. and Kikuchi , M. 1993 . Purification and identification of an angiotensin I-converting enzyme inhibitor from soy sauce . Bioscience, Biotechnology, and Biochemistry , 57 : 1107 – 1110 .
- Ibe , S. , Yoshida , K. and Kumada , K. 2006 . Angiotensin I-converting enzyme inhibitory avtivity of natto, a traditional Japanese fermented food . Nippon Shokuhin Kagaku Kogaku Kaishi , 53 ( 3 ) : 189 – 192 . (in Japanese)
- Okamoto , A. , Hanagata , H. , Matsumoto , E. , Kawamura , Y. , Koizumi , Y. and Yanagida , F. 1995 . Angiotensin I converting enzyme inhibitory activities of various fermented foods . Bioscience, Biotechnology, and Biochemistry , 59 ( 6 ) : 1147 – 1149 .