Abstract
Information on vitamin C chemical stability and deliquescence behavior in powder blends in the presence of amorphous ingredients is lacking. The effects of co-formulated ingredient phase transitions (deliquescence and/or glass transition) during exposure to selected relative humidities (RHs) on moisture sorption and ascorbate stability were investigated. Increasing storage RH promoted ascorbate degradation in blends, while co-formulation with maltodextrins enhanced ascorbate stability and reduced overall moisture sorption at high RHs compared to sodium ascorbate alone. Storage RH and deliquescence (RH0) affected ascorbate stability more than glass transition of the co-formulated maltodextrin. Consideration of the type and strength of water-solid interactions for amorphous ingredients is important to determine their impact on moisture sorption of blends and delivery of vitamin C.
INTRODUCTION
Increasing interest in fortified foods has resulted in the incorporation of vitamins into a variety of products. Additionally, the dietary supplement industry has demonstrated growth in recent years, particularly for vitamins.[Citation1, Citation2] Supplements and vitamin premixes are commonly supplied in powder form, often blended with multiple ingredients. Vitamin C is one such vitamin frequently added to foods or purchased in supplement form. It is well known that vitamin C is highly labile and susceptible to degradation in the presence of oxygen, heat, light, and moisture.[Citation3–6] Recent work in our lab has established the deliquescence behavior of several forms of vitamin C.[Citation7–9] Deliquescence is a first order phase transformation of the solid to a saturated solution which is triggered at a well defined relative humidity (RH0) which depends on the properties of the solid and the temperature.[Citation10–12] Chemical instability is enhanced once the deliquescence point (RH0) of a deliquescent ingredient is exceeded.[Citation7–9] Additionally, deliquescence behavior of multi-component crystalline systems and losses of chemical and physical stability have been reported.[Citation7–9] However, little information on chemical stability and deliquescence behavior of vitamin C in the presence of amorphous ingredients in powder blends is available. Due to the common practice of co-formulating deliquescent and amorphous ingredients in food premixes and supplements, it is important to characterize how these different solid states may influence the moisture sorption and chemical stability behavior of sensitive components.
Highly soluble crystalline substances will exhibit deliquescence, a first-order phase transformation from solid to solution at a relative humidity (RH) specific to that solid. Under certain RH conditions, amorphous ingredients can undergo a glass transition event.[Citation13] Amorphous materials lack the three-dimensional long range order characteristic of the crystalline state; instead consisting of a more random arrangement of molecules, somewhat like the liquid state, leading to physical properties that differ from those of a crystalline material.[Citation14] Generally, amorphous solids take up considerably more water than their crystalline counterparts below RH0[Citation15] since amorphous substances can also absorb water into their bulk while crystalline solids below RH0 only adsorb moisture at the surface.[Citation14] Moisture sorption upon exposure of amorphous ingredients to high RHs results in plasticization and a decrease in the glass transition temperature (Tg) for that solid. Following the transition from the glassy to the rubbery state, greater molecular mobility, increased free volume, and decreased viscosity have been reported, allowing reactions (especially diffusion-controlled reactions) to occur more readily.[Citation4, Citation16, Citation17]
Reports of conflicting mechanisms by which amorphous ingredients can influence moisture exposure in a system can be found.[Citation18–21] The character of the ingredient selected influences the overall moisture sorption behavior and potential phase transformations.[Citation19, Citation21, Citation22] Some have observed the capacity for amorphous ingredients to bring more moisture into a dry system, reducing chemical and physical stability.[Citation20, Citation23, Citation24] Others have reported the occurrence of amorphous ingredients tightly binding to available moisture, thus enhancing overall system stability.[Citation19] Of the five types of water-solid interactions (adsorption to a surface, absorption into the bulk phase, capillary condensation, crystal hydrate formation, and deliquescence) the formation of crystal hydrates, deliquescence, and absorption of water into amorphous solids are the most critical in affecting solid properties.[Citation17] Therefore, with the potential for occurrence of multiple modes of water-solid interaction, it would be useful to understand how the presence of an amorphous ingredient may alter the deliquescence behavior of vitamin C and, likewise, how the presence of a deliquescent ingredient may impact moisture sorption behavior of an amorphous ingredient. Knowledge of potential phase transitions in multi-component powder blends will help ensure proper formulation strategies to protect the quality and functionality of a food product, including delivery of the intended nutritional content.
In this study, a known deliquescent vitamin C form, sodium ascorbate, was selected for chemical stability studies in the presence of amorphous ingredients. Maltodextrins with different dextrose equivalents (DE) were chosen as common amorphous ingredients used in the food industry that would undergo glass transition near room temperature after exposure to a certain RH. The objective of this research was to characterize the impact of amorphous maltodextrins on the deliquescence behavior and chemical stability of sodium ascorbate following storage at particular RH conditions where the two ingredients would exist in varying solid states and phase transformations (solid/solution, glassy/rubbery). A second objective was to determine how exposure of an amorphous ingredient to high RH prior to blending with sodium ascorbate in a sealed container might affect dissolution and stability of vitamin C. Knowledge of how the presence of an amorphous ingredient such as maltodextrin will impact ascorbate dissolution and degradation will enhance understanding of storage stability and formulation concerns for vitamin C.
MATERIALS AND METHODS
Materials
Sodium ascorbate was obtained from Sigma-Aldrich, Inc. (St. Louis, MO) with a molecular weight of 198.11 and purity ≥98%. Maltodextrins with average dextrose equivalents of ∼10 (Maltrin® M100, carbohydrate profile >88.1% pentasaccharides and above, bulk density 0.54 g/cm3) and ∼18 (Maltrin® M180, carbohydrate profile >78.7% pentasaccharides and above, bulk density 0.61 g/cm3) were provided by Grain Processing Corp. (Muscatine, Iowa). The following salts were used to create saturated salt solutions and control RH in environmental chambers: K2CO3, KCl, and K2SO4 (Mallinckrodt-Baker, Phillipsburg, NJ); NaCl (Sigma-Aldrich, Inc., St. Louis, MO). Materials for preparing reagents for the microplate reader assay included: orthophosphoric acid (Alfa Aesar, Ward Hill, MA); trichloroacetic acid (TCA), and 2,2 bipyridine (Mallinckrodt-Baker, Phillipsburg, NJ); iron chloride (EMD Chemicals, Gibbstown, NJ); ethanol (Aaper Alcohol & Chemical Co., Inc., Shelbyville, KY); and potassium phosphate monobasic and dibasic (Mallinckrodt Baker, Inc., Paris, KY).
Sample Storage
Sodium ascorbate was stored individually or in binary mixtures with maltodextrins (M100 or M180). Equal parts by mass were used for binary mixtures, and all samples were prepared in triplicate. Samples were placed in controlled relative humidity (RH) environmental chambers prepared using saturated salt solutions: potassium carbonate (43% RH); sodium chloride (75% RH); potassium chloride (85% RH); and potassium sulfate (98% RH). The RH of each chamber was verified by digital hygrometer (Traceable humidity/Temperature/Dew point meter, Control Co., Friendswood, TX) or water activity (aw) (AquaLab 3TE, Decagon Devices, Inc., Pullman, WA). RH conditions and maltodextrin type were selected to be: below both RH0 of sodium ascorbate and maltodextrin Tg (43% RH); below RH0 but above Tg (75% and 85% RH); above both RH0 and Tg (98% RH). Samples were stored at the specified conditions at room temperature (22 ± 5°C) for 1, 2, 3, and 4 weeks. Additionally, 200 mg samples of maltodextrin were equilibrated at each RH in the controlled RH chamber for two weeks before mixing with an equal mass of sodium ascorbate, sealing in high density polyethylene (HDPE) 15 mL water activity meter cups with lids, and storing at room temperature for 2 weeks. Sample thickness is known to affect equilibration time,[Citation25] thus, efforts were made to have uniform thickness within triplicates. Controls were prepared by analyzing powder mixtures immediately after sample preparation. Physical observations and weight gain were recorded after each storage period.
Differential Scanning Calorimetry (DSC)
The glass transition temperature (Tg) of the maltodextrins after equilibration at various RHs was measured using a TA Q2000 DSC equipped with a refrigerated cooling system (TA Instruments, New Castle, DE). Operating in standard mode, the equipment was calibrated for temperature and the enthalpic response using indium and an empty hermetically sealed pan was used as the reference. Nitrogen served as the purge gas. A sample size of 3–5 mg was placed in hermetic pans and heated to 65°C at a rate of 10°C/min, then cooled to −20°C at 20°C/min to erase thermal history before heating to 85°C at a rate of 5°C/min. Duplicate samples were analyzed and the onset, midpoint, and end temperatures were used for reporting Tg.
Moisture Sorption Isotherms
Gravimetric sorption analysis was performed using a Symmetrical Gravimetric Analyzer (SGA-100) (VTI Corporation, Hialeah, FL) at 25°C in order to understand the moisture sorption behavior of ascorbate and maltodextrins, as well as maltodextrin-ascorbate mixtures. For the mixtures, larger quantities of individual components were blended at an equal mass ratio, and a 10–15 mg portion of the sample was used for analysis. Prior to sorption analysis, samples were dried at 60°C in the sorption analyzer. The settings for the sorption analyzer were: equilibrium criterion for the drying step of 0.01% w/w in 2 min, maximum drying time of 60 min, and step equilibrium criterion of 0.001% w/w in 5 min with a maximum step time of 100 min. During the experiment, samples were exposed to increasing RH (from 0 to 95% RH), increasing at 5% intervals. Predicted isotherms for the different mixtures were calculated from the experimental isotherms of each individual ingredient by adding their individual contributions to moisture sorption at each RH, as described by Ortiz et al.[Citation23] The predicted moisture sorption isotherms of the mixtures were then compared to the data obtained experimentally for the same mixture, and the difference was plotted against RH.
Ascorbate Stability Determination by Microplate Reader Analysis
Sodium ascorbate stability was measured using an AD/LD 340 Absorbance Detector microplate reader (Beckman Coulter, Inc., Fullterton, CA) according to the method of Stevens et al.[Citation26] for reduced ascorbate with minor modifications. Samples were dissolved in water before further dilutions with TCA. All samples were analyzed on the plate reader at 570 nm.
Statistical Analysis
A completely randomized three factor factorial design was used for studying the effects of RH, formulation, and time on the stability of individual ascorbate and in blends with maltodextrins. An ANOVA model was used for this analysis. Individual differences were tested using Tukey's multiple means comparison procedure. All statistical analysis procedures were conducted using PC SAS software and α = 0.05.
RESULTS AND DISCUSSION
Moisture Sorption of Ascorbate and Ascorbate: Maltodextrin Blends
Moisture sorption isotherms for maltdoextrins were sigmoidal in shape, indicative of a Type II isotherm. This isotherm shape is commonly observed with amorphous solids, and maltodextrin isotherms have been previously classified as type II [Citation18] (). Continuous moisture uptake was observed, along with minimal weight gain at low RHs, followed by increasing moisture sorption at high vapor pressures.[Citation18, Citation27] As moisture introduced into the system plasticizes the amorphous material to its Tg, increases in moisture sorption are observed.[Citation14, Citation28]. Sodium ascorbate demonstrated low water adsorption at low vapor pressures with a sharp break in the isotherm corresponding to rapid moisture sorption where deliquescence occurs (). RH0 for sodium ascorbate was 86% RH. Isotherms for mixtures of ascorbate with M100 and M180 maltodextrin were Type II in nature, though at higher RHs (above 75% RH) weight gain increased rapidly, suggesting that overall moisture sorption of the blend at higher RH was dominated by the ascorbate deliquescence event.
Figure 1 Adsorption isotherms for M100 and M180 maltodextrins, sodium ascorbate, and blends of M100 and M180 with ascorbate at 22°C. Data points are connected by trend lines. Formulations are shown by: ![]()
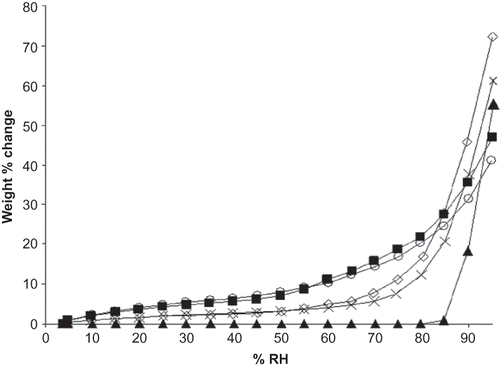
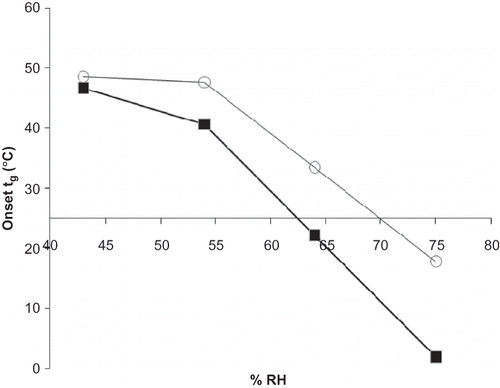
Overall moisture sorption at 95% RH from gravimetric sorption analysis for individual ascorbate and maltodextrins and their blends followed the order, from least to greatest moisture gain, of: M100 (41.1%), M180 (47.0%), sodium ascorbate (55.3%), M100+ascorbate (61.6%), M180+ascorbate (72.3%). Moisture uptake at low RHs and overall moisture sorption was increased in the maltodextrin:ascorbate mixtures versus individual ascorbate. The sharp break in the isotherm where ascorbate deliquescence occurs is less pronounced and more rounded in the isotherms for the blends. An increased amount of moisture is introduced into the system for the blends at RHs below RH0: compared to individual maltodextrin a mixture of maltodextrin and ascorbate exhibited greater moisture gain prior to the deliquescence event (). Below RH0, as expected, ascorbate did not contribute to moisture sorption of the blend and the reduced moisture uptake can be attributed to the decreased amount of maltodextrin in the mixture compared to individual maltodextrin samples. However, above RH0, the blends sorbed more moisture than the individual maltodextrins (). Interestingly, ascorbate mixed with M180 began to show increased moisture uptake compared to M180 alone at the RH step (at 85% RH) just prior to RH0 (). For a blend of M100:ascorbate, though, enhanced moisture sorption for the blend was not observed until after RH0, at 90% RH (). Just below RH0(at 85% RH) the difference in moisture uptake between the two binary formulations had minimized, decreasing from 7.9% at 80% RH, to 3.8% at 85% RH. This can be explained by the difference in Tg for the two maltodextrins at those RH conditions: M100 would undergo an onset of glass transition at room temperature between 64 and 75% RH, while the reduction in Tg to near room temperature for M180 would occur at lower RHs, between 54 and 64% RH[Citation25] (). The average glass transition onset, midpoint, and end temperatures for M100 at 64% RH were 47.7, 49.4, and 51.1°C, and at 75% RH were 22.2, 33.6, and 40.2°C, respectively. The onset, midpoint, and end Tg temperatures for M180 at 64% RH were 17.8, 29.2, and 36.5°C, and at 75% RH were 1.9, 3.9, and 8°C respectively. Therefore, M180 would be expected to bring more moisture into the system at lower RHs than M100, once its glass transition had taken place.
Figure 2 Glass transition temperature (Tg) for maltodextrins equilibrated at increasing RH analyzed by DSC. Tg values were determined from the onset temperature. Data points are connected by trend lines. Selected maltodextrins are shown by:![]()
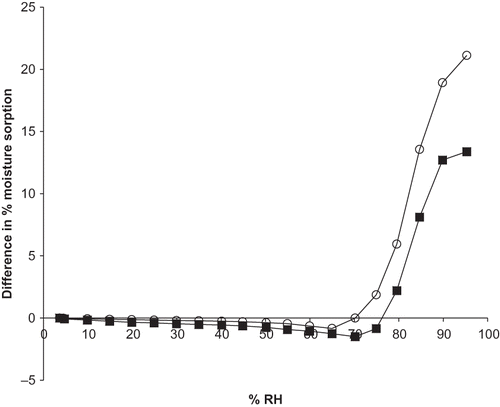
Additionally, a synergistic effect occurred in the blends at high RHs where Tg would be exceeded under the storage conditions used, whereby experimental moisture uptake was greater than that predicted from the isotherms of the individual components. Prior to glass transition of the maltodextrin, experimental values coincided closely with predicted moisture sorption. For example, at 25, 50, and 75% RH a mixture of M100 with ascorbate sorbed 0.4, 0.8, and 0.8% less moisture than predicted, respectively, and for the formulation of M180 with ascorbate, 0.2 and 0.4% less weight gain was observed for experimental data compared to that predicted from isotherms of the individual ingredients at 25 and 50% RH. However, above Tg but below RH0 at 80 and 85% RH, the M100:ascorbate blend exhibited differences between the experimental and predicted values of 2.2 and 8.1% greater sorption, respectively, while after the glass transition event for M180, at 75, 80, and 85% RH, differences of 1.9, 5.9, and 13.6% greater moisture uptake between the experimental and predicted values were observed (). Above ascorbate RH0 at 95% RH, the difference between experimental and predicted moisture sorption was 13.4% (). Thus, both glass transition and deliquescence phase transitions in a blend synergistically increased moisture sorption beyond what was expected from individual ingredient contributions.
Figure 3 Difference in percent moisture sorption between experimental and predicted moisture sorption isotherms of M100 maltodextrin+ascorbate and M180 maltodextrin+ascorbate blends exposed to 0–95% RH and 25C. Predicted isotherms were calculated from the experimental moisture sorption isotherms of the individual ingredients. The predicted moisture sorption isotherms of the mixtures were then compared to those obtained experimentally for the same mixture and the difference was plotted against RH. Data points are connected by trend lines. Different formulations are shown by: ![]()
Ascorbate Stability
Stability in premixed samples
Generally, sodium ascorbate was more stable in a mixture with maltodextrin (M100) at the highest storage RH conditions (85 and 98%) than individually, while at lower RHs (75 and 43%) sodium ascorbate was more stable when formulated alone (). Ascorbate demonstrated similar stability both individually and mixed with maltodextrin during the first 2 weeks of storage at 85 and 98% RH, with no significant differences in ascorbate stability observed between the two formulations (p = 1.000). However, at weeks 3 and 4 significantly more ascorbate had degraded when no maltodextrin was present (p < 0.0001) (, ). Endpoint degradation after 4 weeks of storage at 98% RH for ascorbate alone and in a mixture with M100 was 77.8 and 34.3%, respectively. At 85% RH, 21.9% ascorbate degradation was observed in a formulation with M100, while 51% degraded without M100 present (). No significant differences in stability were found in samples stored at 75 or 43% RH.
Figure 4 Sodium ascorbate stability at different storage RHs, formulated with and without maltodextrin (M100). A) 98% RH; B) 85% RH; C) 75% RH; D) 43% RH. Data points are connected by trend lines. Individual ascorbate and M100 maltodextrin mixtures are shown by: ![]()
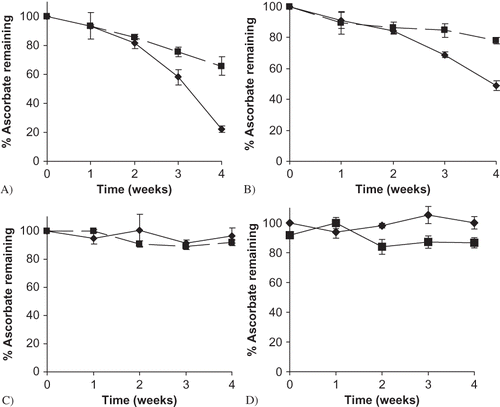
Table 1 Endpoint vitamin C degradation after 4 weeks of storage at select RHs for individual sodium ascorbate and binary mixtures of sodium ascorbate and M100 or M180 maltodextrin. Results are reported as % degradation with standard deviations, and different letters indicate significant differences.Footnote a
The chemical stability results appear to correlate with moisture sorption observations. A clear trend between increased moisture uptake and decreased ascorbate stability when stored at 98% RH for up to 4 weeks is demonstrated in . At higher RH conditions, near or above RH0, where greater moisture uptake was observed for ascorbate alone (123.6±4.2% to 223.9±1.3% endpoint weight gain) compared to mixtures with maltodextrin (71.2±1.5% to 130.8±0.8% endpoint weight gain), greater instability for ascorbate alone was seen. Generally at the higher storage RHs (85% and 98% RH) ascorbate exhibited greater moisture uptake alone than when mixed with maltodextrins (p < 0001) (). The enhanced moisture sorption for ascorbate alone above RH0 could also be due to the presence of hygroscopic degradation products. This differs from trends reported from gravimetric moisture sorption analysis, likely due to differences in moisture sorption kinetics between controlled RH storage and dynamic moisture sorption measurements and the longer time scale for the controlled RH storage studies. It appeared the maltodextrins decreased moisture sorption at these high RH conditions and thus exerted a slight protective effect on ascorbate. However, at lower RH conditions the maltodextrins introduced significantly greater amounts of moisture into the system than that observed for ascorbate alone (p = 0.0003 to p = 0.0050) () and therefore slightly greater degradation was observed for mixtures at 75% RH. At 43% RH, well below the RH0 of ascorbate and an RH that would result in the depression of maltodextrin Tg to room temperature, moisture sorption was minimal and did not differ significantly between ascorbate samples with and without maltodextrin (p = 1.000) (). The lack of a difference in moisture uptake resulted in no significant differences in ascorbate stability in the two formulations at 43% RH.
Figure 5 Weight change (%) versus % sodium ascorbate remaining for individual sodium ascorbate and its blend with M100 maltodextrin stored at 98% RH and 25°C up to 4 weeks. Data points are connected by trend lines. Trends for ascorbate and its blend are show by: ![]()
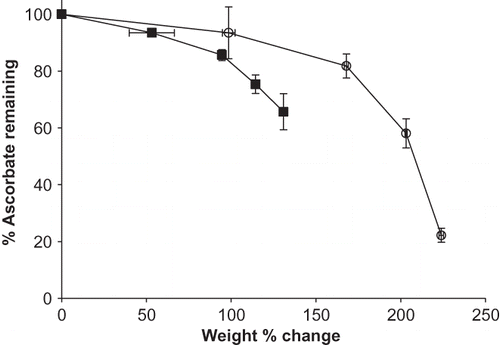
Figure 6 Weight change (%) over time for sodium ascorbate and its blends with M100 and M180 maltdoextrin stored up to 4 weeks at 22°C and select RH conditions. A) Weight change (%) over time for individual sodium ascorbate samples during controlled RH storage. Data points are connected by trend lines. Ascorbate samples at each RH are shown by: ![]()
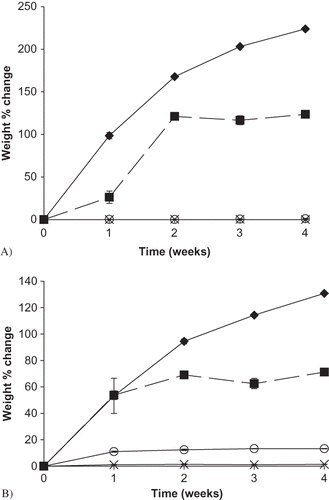
Sufficient moisture was sorbed by both ascorbate alone and ascorbate:maltodextrin blends at 98% and 85% RH to result in comparable amounts of dissolved vitamin. Therefore, the enhanced stability of ascorbate in the maltodextrin blend would not be attributable to a lack of ascorbate in the solution phase for subsequent degradation. An additional mechanism would then be responsible for the protective effect of maltodextrin observed. Others have reported on the ability of sugars to reduce vitamin C degradation in solution.[Citation29] They found that the rate of ascorbic acid oxidation was reduced in the presence of sugars and was further decreased with increasing concentration of sugar. Maltodextrins have been used in the food industry as encapsulating agents to protect against oxidation, primarily for their film-forming properties and were found to be useful for reducing vitamin C degradation.[Citation30] A possible explanation for the increased ascorbate stability at high RH in the presence of maltodextrins could be attributed to the ability of the maltodextrins to reduce oxidation once in solution. Additionally, while calculations of theorectical percent ascorbate dissolved in each formulation did not yield differences that would explain the enhanced ascorbate stability in the presence of maltodextrins, the maltodextrin:ascorbate blends had visibly increased viscosity. Enhanced viscosity has been related to reduced reactant mobility and chemical degradation,[Citation14, Citation31, Citation32] which may be a possible explanation for the protective effect of maltodextrins in these systems.
Ascorbate stability when mixed with pre-equilibrated maltodextrin
Exposure of maltodextrins to a specific environmental RH will result in water activity equilibration at that RH.[16] When sealed in an enclosed container, the headspace RH will be close to that of the equilibrated condition. The HDPE container used has low water permeability (1.37×1011 gm·cm/cm2·s at 37.8°C and 90% RH)[Citation30]; therefore, moisture content would not be expected to change during storage. Ascorbate content was reduced after 2 weeks of storage in a sealed container with maltodextrins previously equilibrated at 98, 75, and 43% RH. After 2 weeks of storage with M100 pre-equilibrated at 98% RH, 15.6 ± 3.2% ascorbate degradation occurred. Ascorbate degradation when mixed with M180 equilibrated at 98% RH equaled 9.6 ± 1.5% after 2 weeks (). Stability was significantly increased when ascorbate was stored with maltodextrin equilibrated at 43% RH compared to ascorbate stored with maltodextrin equilibrated at 98% RH after 2 weeks (p = 0.0251 to p = 0.0012). Degradation was not significantly different between ascorbate stored with maltodextrin equilibrated at 75% RH and maltodextrin equilibrated at 43% RH (p = 0.5028 to p = 1.000) ().
Table 2 Sodium ascorbate degradation after storage for 2 weeks in premixed ascorbate:maltodextrin samples and ascorbate blended with pre-equilibrated maltodextrin samples. Results are reported as % degradation with standard deviations, and different letters indicate significant differences.Footnote a
Compared to stability at 98% RH in premixed ascorbate:maltodextrin samples that were then stored in controlled RH chambers, ascorbate exhibited similar degradation after 2 weeks of storage in a sealed container with M100 pre-equilibrated at 98% RH: 14.3 ± 1.9% for premixed samples versus 15.6 ± 3.2% (p = 1.000) (). At 75% RH, whether premixed and stored at this condition or mixed with M180 pre-equilibrated at this RH, ascorbate was generally stable and degradation was not significantly different between the two mixing procedures (p = 0.5048 to p = 1.000). This was also true when stored with M100 at 43% RH: no significant differences were observed (p = 0.9987 to p = 1.000) ().
The similar stability observed between premixed ascorbate:maltodextrin samples and ascorbate stored in a sealed container with pre-equilibrated maltodextrin after 2 weeks of storage indicates that the introduction of increasing amounts of moisture present in the storage conditions for the premixed samples is responsible for decreased ascorbate stability. In this situation, the equilibrated maltodextrins were acting as a source of relative humidity within the enclosed space and were capable of dissolving any ascorbate they are in direct contact with. Moisture migration from the higher aw component (maltodextrin) to the lower aw environment occurred until equilibrium is reached.[Citation16] While it is important to distinguish between deliquescence and dissolution upon exposure to moisture present in the amorphous matrix of other ingredients in a blend, these results highlight how exposure of an amorphous ingredient to high RH conditions prior to mixing with a deliquescent ingredient may result in moisture-mediated degradation.
Relationship between Phase Transitions and Ascorbate Stability
The glass transition temperatures across RH conditions for each maltodextrin (M100 and M180) used in this study are reported in . Tg decreases after equilibration at increasing RH. The midpoint Tg would be reduced to near room temperature for M100 after exposure to RH between 74 and 85%, and for M180 at 64 and 75% RH, consistent with prior reports.[28] After exposure to RH between 64% and 75% RH, the onset Tg for M100 samples would be reduced to near room temperature, and a room temperature onset Tg for M180 occurs at a RH slightly below 64% RH. Roos and Karel[Citation28] studied the glass transition temperature of maltodextrins with varying dextrose equivalents after equilibration at a range of RHs. Based on their results, glass transition would occur at room temperature after exposure to a RH between 75% and 85% RH for M100, and 52% and 75%RH for M180.
Generally, RH and RH0 had a greater impact on ascorbate stability in mixtures with maltodextrins than relationship to Tg of the maltodextrin. This is likely due to the amount of ascorbate dissolved in the mixtures, with complete ascorbate dissolution only occurring in samples stored above the deliquescence RH. Ascorbate demonstrated greatest stability when stored well below both RH0 and a RH that would result in glass transition at room temperature: 43% RH. At a RH near Tg for M180 (75% RH), stability did not differ significantly (p = 1.000) in a mixture with maltodextrin compared to ascorbate alone, even though the maltodextrin mixture sorbed more moisture. However, once RH0 for sodium ascorbate was reached or exceeded, instability was observed. Significantly more degradation occurred at RHs of 85% and 98% after 4 weeks of storage for individual ascorbate than at 75% and 43% RH (p < 0.0001). Ascorbate stability was enhanced for mixtures of ascorbate with M100 maltodextrin at these high RHs, with mixtures demonstrating significantly less degradation after 4 weeks of storage than ascorbate alone at the same RH (p < 0.0001) (). At these high RH conditions, M100 maltodextrins appeared to have a protective affect on ascorbate stability, with less degradation occurring in binary mixtures than in samples of ascorbate alone. At 85% and 98%RH, M100 would be above its onset and midpoint Tg. Therefore, it appeared that once the maltodextrin underwent glass transition, it actually inhibited ascorbate degradation compared to ascorbate degradation in samples of ascorbate alone, whereas in the glassy state this protective effect was not observed.
Others have attempted to distinguish between the effects of aw and glass transition on ingredient stability. Sablani et al.[Citation34] investigated the impact of glass transition and water activity on vitamin C stability in infant formula. They found that these effects were correlated: vitamin C was stable at lower aws, which corresponded to the monolayer moisture content of the formula. This was also the moisture content at which the glass transition temperature of the powder was above room temperature. Overall they concluded that both aw and glass transition concepts predicted similar conditions for improved retention of vitamin C. Bell and Hageman[35] suggested that aspartame rearrangement and subsequent degradation appeared to be more dependent on the aw of a system than Tg. In contrast, thiamin stability in a solid system was affected by both Tg and aw; however, glass transition appeared to have a larger effect on molecular mobility and overall thiamin degradation.[Citation36]
The influence of aw versus Tg on a particular chemical reaction is dependent on the reaction mechanism[Citation35] and food system. Due to limitations in broadly applying glass transition and aw concepts for determining food stability, Rahman[Citation37] proposed the additional concept of using macro-micro region state diagrams to evaluate food stability; however, much more information is needed to identify stability for each food or ingredient in each macro and micro region. Little work has been performed investigating the particular phenomenon of deliquescence as related to aw versus Tg in a powder system. One previous report found that the presence of amorphous green tea powder introduced sufficient moisture into a powder system to promote dissolution of crystalline ingredients at RHs below RH0.[Citation23] The protective effect observed during this study demonstrates the variability in functionality of amorphous components on ingredient chemical stability during exposure to moisture.
CONCLUSION
In summary, the presence of amorphous maltodextrin in a blend with sodium ascorbate increased ascorbate stability and reduced overall moisture sorption of the blends at high RHs compared to sodium ascorbate alone. Both premixing ascorbate with maltodextrin and exposing it in a sealed container to maltodextrin equilibrated at various RHs resulted in decreased ascorbate stability in the presence of high environmental moisture content. In general, RH and RH0 appeared to influence ascorbate stability more than Tg of the maltodextrin. The strength of water-solid interactions for specific amorphous ingredients may impact moisture sorption of ascorbate:maltodextrin mixtures and final delivery of vitamin C, and should therefore be considered during formulation of powder blends. Storage of sodium ascorbate and maltodextrin blends below Tg and RH0 for enhanced vitamin stability is recommended.
ACKNOWLEDGMENT
The authors acknowledge support by USDA-NRICGP Grant #07-35503-18405. We also thank Mohamad Abiad for his support in DSC analyses and data interpretation.
| NOMENCLATURE | ||
| RH | = |
Relative humidity |
| RH0 | = |
Deliquescence point of an individual crystalline ingredient |
| Tg | = |
Glass transition temperature |
REFERENCES
- Marigny Research Group. Nutritional Supplements in the U.SInc.: 06. Rockville, MD, USA, 2010; 1–200 MarketResearch.com (http://MarketResearch.com)
- Muth , M.K. , Anderson , D.W. , Domanico , J.L. , Smith , J.B. and Wendling , B. 1999 . Economic Characterization of the Dietary Supplement Industry , NC, USA : Research Triangle Institute, Research Triangle Park . 6673-03
- Dennison , D.B. and Kirk , J.R. 1978 . Oxygen Effect on Degradation of Ascorbic-Acid in A Dehydrated Food System . Journal of Food Science , 43 ( 2 ) : 609 – 618 .
- Fennema , O. R. 1996 . Food Chemistry , 3rd , 531 – 616 . New York : Marcel Dekker .
- Kirk , J. , Dennison , D. , Kokoczka , P. and Heldman , D. 1977 . Degradation of Ascorbic-Acid in A Dehydrated Food System . Journal of Food Science , 42 ( 5 ) : 1274 – 1279 .
- Laing , B. M. , Schlueter , D. L. and Labuza , T. P. 1978 . Degradation Kinetics of Ascorbic-Acid at High-Temperature and Water Activity . Journal of Food Science , 43 ( 5 ) : 1440 – 1443 .
- Hiatt , A. N. , Ferruzzi , M. G. , Taylor , L. S. and Mauer , L. J. 2008 . Impact of deliquescence on the chemical stability of vitamins B-1, B-6, and C in powder blends . Journal of Agricultural and Food Chemistry , 56 ( 15 ) : 6471 – 6479 .
- Ortiz , J. , Ferruzzi , M.G. , Taylor , L.S. and Mauer , L.J. 2008 . Interaction of environmental moisture with powdered green tea formulations: Effect on catechin chemical stability (vol 56, pg 4068, 2008) . Journal of Agricultural and Food Chemistry , 56 ( 16 ) : 7586
- Salameh , A.K. and Taylor , L.S. 2006 . Role of deliquescence lowering in enhancing chemical reactivity in physical mixtures . Journal of Physical Chemistry B , 110 ( 20 ) : 10190 – 10196 .
- Zografi , G. and Hancock , B. Topics in Pharmaceutical Sciences . Proceedings of International Congress on Pharmaceutical Sciences F.I.P . 1993 . Water-Solid Interactions in Pharmaceutical Systems , Edited by: Crommelin , D.J.A. , Midha , K.K. and Nagai , T. pp. 405 – 419 . Stuttgart, , Germany : Medpharm Scientific .
- McNaught , A.D. and Wilkinson . 1997; 2183 . A. IUPAC compendium of chemical terminology , Cambridge : Blackwell Science .
- Salameh , A.K. , Mauer , L.J. and Taylor , L.S. 2006 . Deliquescence lowering in food ingredient mixtures . Journal of Food Science , 71 : E10 – E16 .
- Wexler , A.S. and Seinfeld , J.H. 1991 . 2Nd-Generation Inorganic Aerosol Model . Atmospheric Environment Part A-General Topics , 25 ( 12 ) : 2731 – 2748 .
- Hancock , B.C. and Zografi , G. 1997 . Characteristics and significance of the amorphous state in pharmaceutical systems . Journal of Pharmaceutical Sciences , 86 ( 1 ) : 1 – 12 .
- Ahlneck , C. and Zografi , G. 1990 . The Molecular-Basis of Moisture Effects on the Physical and Chemical-Stability of Drugs in the Solid-State . International Journal of Pharmaceutics , 62 ( 2–3 ) : 87 – 95 .
- Labuza , T. P. and Hyman , C. R. 1998 . Moisture migration and control in multi-domain foods . Trends in Food Science & Technology , 9 ( 2 ) : 47 – 55 .
- Zografi , G. 1988 . States of Water Associated with Solids . Drug Development and Industrial Pharmacy , 14 ( 14 ) : 1905 – 1926 .
- Airaksinen , S. , Karjalainen , M. , Shevchenko , A. , Westermarck , S. , Leppanen , E. , Rantanen , J. and Yliruusi , J. 2005 . Role of water in the physical stability of solid dosage formulations . Journal of Pharmaceutical Sciences , 94 ( 10 ) : 2147 – 2165 .
- Jorgensen , A.C. , Airaksinen , S. , Karjalainen , M. , Luukkonen , P. , Rantanen , J. and Yliruusi , J. 2004 . Role of excipients in hydrate formation kinetics of theophylline in wet masses studied by near-infrared spectroscopy . European Journal of Pharmaceutical Sciences , 23 ( 1 ) : 99 – 104 .
- Otsuka , M. and Matsuda , Y. 1994 . The Effect of Humidity on Hydration Kinetics of Mixtures of Nitrofurantoin Anhydride and Diluents . Chemical & Pharmaceutical Bulletin , 42 ( 1 ) : 156 – 159 .
- Otsuka , M. , Ohfusa , T. and Matsuda , Y. 2000 . Effect of binders on polymorphic transformation kinetics of carbamazepine in aqueous solution . Colloids and Surfaces B-Biointerfaces , 17 ( 3 ) : 145 – 152 .
- Dalton , C.R. and Hancock , B.C. 1997 . Processing and storage effects on water vapor sorption by some model pharmaceutical solid dosage formulations . International Journal of Pharmaceutics , 156 ( 2 ) : 143 – 151 .
- Ortiz , J. , Kestur , U. S. , Taylor , L. S. and Mauer , L. J. 2009 . Interaction of environmental moisture with powdered green tea formulations: relationship betwen catechin stability and moisture-induced phase transformations . Journal of Agricultural and Food Chemistry , 57 ( 11 ) : 4691 – 4697 .
- Zografi , G. and Kontny , M. J. 1986 . The Interactions of Water with Cellulose-Derived and Starch-Derived Pharmaceutical Excipients . Pharmaceutical Research , 3 ( 4 ) : 187 – 194 .
- Bell , L. N. and Labuza , T. P. 2000 . Practical aspects of moisture sorption isotherm measurement and use , 2nd , 1 – 222 . Eagan : AACC Eagan Press .
- Stevens , R. , Buret , M. , Garchery , C. , Carretero , Y. and Causse , M. 2006 . Technique for rapid, small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection . Journal of Agricultural and Food Chemistry , 54 ( 17 ) : 6159 – 6165 .
- Mathlouthi , M. and Roge , B. 2003 . Water vapour sorption isotherms and the caking of food powders . Food Chemistry , 82 ( 1 ) : 61 – 71 .
- Roos , Y.H. and Karel , M. 1991 . Phase-Transitions of Mixtures of Amorphous Polysaccharides and Sugars . Biotechnology Progress , 7 ( 1 ) : 49 – 53 .
- Miller , J. and Joslyn , M.A. 1949 . Effect of Sugars on Oxidation of Ascorbic Acid .3. Influence of Ph and Type of Buffer . Food Research , 14 ( 4 ) : 354 – 363 .
- Righetto , A.M. and Netto , F.M. 2006 . Vitamin C stability in encapsulated green West Indian cherry juice and in encapsulated synthetic ascorbic acid . Journal of the Science of Food and Agriculture , 86 ( 8 ) : 1202 – 1208 .
- Karel , M. , Anglea , S. , Buera , P. , Karmas , R. , Levi , G. and Roos , Y.H. 1994 . Stability-Related Transitions of Amorphous Foods . Thermochimica Acta , 246 ( 2 ) : 249 – 269 .
- Soesanto , T. and Williams , M.C. 1981 . Volumetric Interpretation of Viscosity for Concentrated and Dilute Sugar Solutions . Journal of Physical Chemistry , 85 ( 22 ) : 3338 – 3341 .
- Kamal , M.R. , Jinnah , I.A. and Utracki , L.A. 1984 . Permeability of Oxygen and Water-Vapor Through Polyethylene Polyamide Films . Polymer Engineering and Science , 24 ( 17 ) : 1337 – 1347 .
- Sablani , S.S. , Al-Belushi , K. , Al-Marhubi , I. and Al-Belushi , R. 2007 . Evaluating stability of vitamin C in fortified formula using water activity and glass transition . International Journal of Food Properties , 10 ( 1 ) : 61 – 71 .
- Bell , L.N. and Hageman , M.J. 1994 . Differentiating Between the Effects of Water Activity and Glass-Transition Dependent Mobility on A Solid-State Chemical-Reaction - Aspartame Degradation . Journal of Agricultural and Food Chemistry , 42 ( 11 ) : 2398 – 2401 .
- Bell , L.N. and White , K.L. 2000 . Thiamin stability in solids as affected by the glass transition . Journal of Food Science , 65 ( 3 ) : 498 – 501 .
- Rahman , M.S. 2009 . Food stability beyond water activity and glass transition: Macro-micro region concept in the state diagram . International Journal of Food Properties , 12 : 726 – 740 .