Abstract
Juices made from fruits of 30 Tunisian accessions of pomegranate were studied for their organic acids, sugars, and anthocyanin contents, using high performance liquid chromatography. Among the detected organic acids, malic acid was the major one (>50%) followed by citric acid (>22%), while among sugars, fructose and glucose were most present in pomegranate juice contributing 53.9 and 43.4% of the total sugar content, respectively. The total anthocyanin content ranged from 9–115 mg per litre of juice with the following ranges of the six compounds found: cyanidin-3,5-diglucoside (3.1–74.4 mg/L), delphinidin-3-glucoside (0.7–22.0 mg/L), cyanidin-3-glucoside (0.8–21.0 mg/L), pelargonidin-3-glucoside (0.5–16.1 mg/L), pelargonidin-3,5-diglucoside (0.0–11.8 mg/L), and delphinidin-3,5-diglucoside (0.0–5.4 mg/L). Based on the analyzed parameters, cluster analysis allowed grouping cultivars into two main clusters. One was made of sour cultivars and the second of the sweet ones. Principle component and cluster analyses suggested that the composition of the pomegranate fruits is determined by cultivar rather than cultivation location.
INTRODUCTION
The pomegranate (Punica granatum L., Punicaceae) is one of the oldest known edible fruits (latin: pomum “apple” and granatus “seeded”). This species, originated from Persia to the Himalayas in northern India and surrounding areas, is now extensively cultivated in other countries (e.g., Spain, Tunisia, Turkey, China, Russia, and USA). Pomegranate trees are adapted to saline and poor soils in arid and semi-arid climates, as in the case of the large area of Tunisia, without special requirements.[Citation1] Thus, the pomegranate tree can be an adequate cultivation choice for agricultural lands to contribute to the reduction of desertification.
Currently, there is an increased interest in pomegranate fruit because it is considered as a functional product of great health benefits because of its richness of several groups of substances useful in disease prevention and health promotion.[Citation2–4] Pomegranate fruits are known for high antioxidant and radical scavenging activities related to high levels of anthocyanins,[Citation2,Citation5] punicalagin and ellagitannins,[Citation2] and other unexplored phenolic compounds. Several health-promoting effects have been reported for pomegranate juices (e.g., protection against cancer, diabetes, cardiovascular disease, inflammations, dental conditions, erectile dysfunction, bacterial infections, antibiotic resistance, UV-induced skin damage, infant brain ischemia, male infertility, Alzheimer's disease, arthritis).[Citation3,Citation6–9] Pomegranate extracts were not found to be toxic since the no observed-adverse-effect level (NOAEL) for a standardized pomegranate fruit extract was determined as 600 mg/kg body weight/day.[Citation10]
Pomegranate fruits are mainly consumed fresh but they are also used in the preparation of fresh juices, canned beverages, jellies, jams, etc.[Citation11–13] The edible parts of the fruit are called arils (∼50% of the fruit) and are composed of 22% seeds and 78% juicy fraction.[Citation14] Malic and citric acids are reported as the main organic acids in pomegranate fruits. These two acids are known to be correlated with the sensory perception of sourness.[Citation15] Two types of sugars, fructose and glucose, are known as the main sources of energy and sweetness.[Citation16] Six anthocyanins, namely the 3-glucosides and 3,5-diglucosides of cyanidin, delphinidin, and pelargonidin, were identified as the main compounds responsible for the colour of the pomegranate juice.[Citation17,Citation18] In combination, organic acids, sugars, and anthocyanins are important for the sensory attributes and authenticity of fruit products. The types and amount of organic acids, sugars, and anthocyanins also contribute to the nutritional quality of both fresh fruits and juices.[Citation12]
In Tunisia, pomegranate has been cultivated in the coastal regions of the north, center, and south since ancient times. The pomegranates are propagated by cuttings that are selected by the farmers mainly aiming at desirable fruit qualities.[Citation19] Actually, acreage of nearly 15,000 ha with 5 million trees produces about 50,000 tons of fruit that are mainly consumed locally.[Citation20] Characterization of main Tunisian pomegranate cultivars by amplified fragment length polymorphism analysis revealed that cultivars are clustered independent on their geographical origin or their denomination and suggested a common genetic basis.[Citation21] The aim of the present work was to study the variability in the amounts of organic acids, sugars, and anthocyanins in samples belonging to 30 main Tunisian pomegranate cultivars using HPLC technique.
MATERIAL AND METHODS
Fruit Samples
The present study was carried out on fruits of a set of 30 pomegranate accessions collected at maturity from the main regions of pomegranate cultivation in Tunisia (). Maturity is determined based on visible fruit characteristics, mainly peel colour and size. Aril colour and taste were also included for each fruit included in the present study.
Table 1 Cultivar names, sample codes, and collection sites and regions of 30 Tunisian pomegranate samples
Reagents
Organic acids (oxalic, tartaric, malic, ascorbic, acetic, citric, fumaric, and succinic acids) and sugars (arabinose, fructose, galactose, glucose, maltose, and sucrose) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Standards of anthocyanins (delphinidin 3-glucoside and 3,5-diglucoside, cyaniding 3-glucoside and 3,5-diglucoside, and pelargonidin 3-glucoside and 3,5-diglucoside) as well as HPLC grade solvents were also obtained from Sigma. Ultrapure water was purified with the MilliQ system (Millipore, Billerica, MA, USA).
Juice Extraction
The weights of the whole fruit and of 100 arils were determined. The peel colour was described using a rating scale ranging from yellow-green (01) to red-purple (16). To obtain the juice, pomegranate fruits were peeled by hand and the seeds were liquefied using a Moulinex food processor. The obtained juice was pre-filtered and then centrifuged at 14,000 rpm for 15 min. Furthermore, it was filtered through 0.45 μm filters before being stored at −18°C upon analyses. The pH was measured using a pH meter (Jenway, Cambridge, UK). Titratable acidity, expressed as percentage of malic acid, was performed by titrating 10 mL of pomegranate juice with a 0.1 M NaOH to a pH point of 8.1. Morphological measures are performed on ten fruits and pH and titratable acidity in triplicates. HPLC analyses were performed in duplicates, on juices of two different fruits.
HPLC Analysis of Organic Acids, Sugars, and Anthocyanins
Juice samples (1 mL) were centrifuged at 10,000 rpm for 2 min and filtered through a 0.45-μm Millipore filter. Organic acids and sugars were analyzed by the HPLC apparatus (model HP 1100, Hewlett Packard, Wilmington, DE, USA) equipped with a refractive index detector for sugars detection, and UV/Vis detector for organic acids analysis. The injection volumes were 10 μL for both. Organic acids were separated on a SupelcogelTM C-610H column (30 cm × 7.8 mm i.d., Supelco, Bellefonte, PA, USA) run isocratically at 30°C using 0.1% phosphoric acid as a mobile phase at a flow rate of 0.5 mL/min and were detected at 210 nm. Sugars analysis was performed on a μBondapak-NH2 column (30 cm × 3.9 mm i.d., Waters, Milford, MA, USA) using acetonitrile/water (85:15, v/v) as the mobile phase.
Anthocyanins were analysed on the same model of HPLC apparatus, equipped with a diode array detector (DAD). Twenty μL of each juice sample was injected. Anthocyanins separation was performed on LiChroCART 100 RP-18 column (12.5 cm × 0.4 cm i.d.; 5 μm particle size; Merck, Darmstadt, Germany) using 5% formic acid (solvent A) and methanol (solvent B) in a gradient starting with 15% B in A, reaching 35% B in 15 min, then isocratically until 20 min at a flow rate of 1 mL/min. Anthocyanin peaks were detected at 520 nm as described by Hernandez et al.[Citation22] The different organic acids, sugars, and anthocyanins were identified and quantified by reference to authentic standards using external calibration. All determinations were performed in duplicates and compound identification was made by comparison of their retention time to those of pure standards. Standard curves of the respective organic acids, sugars, and anthocyanins have been used to calculate the concentrations of these compounds.
Statistical Analysis
Results were reported as average of collected data as well as mean, coefficient of variation, and relative percentage of chemical compounds. The interdependence of the variables was investigated by the analysis of Pearson correlations. Besides, using XLSTAT software, Principal Components Analysis and cluster analysis were performed including all the investigated parameters.
RESULTS AND DISCUSSION
Variability in Tunisian Pomegranate Fruit and Juice Characteristics
Tunisian pomegranate fruits vary considerably in size and characteristics (). The weight of the fruits varied from 196–674 g and the colour of the peels varied from 6–11 measured against an arbitrary scale ranging from yellow-green (01) to red-purple (16). The juice colours varied considerably ranging from white-yellow to dark red-purple (). The pH of the juice varied between 2.9 and 4.6, and its titratable acidity varied between 0.2 and 3.35% of malic acid equivalents with a coefficient of variation of 105%. Five of the 30 cultivars (GB1, GR2, MZ1, MZ2, and MZ3) were characterized by sour taste. The samples of the 30 cultivars studied here showed a larger variability than have been reported before.[Citation23–26] Variability in fruit characteristics is well documented, e.g., for mulberry (Morus alba L.)[Citation27] and bayberry.[Citation28]
Table 2 Values of measured characters on fruits and juices of samples of 30 Tunisian pomegranate cultivars
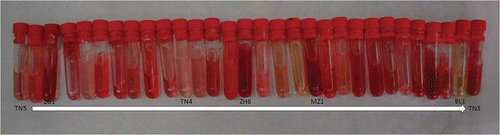
Figure 1 Photo showing the colours of the juices of the 30 Tunisian pomegranate samples. Starting from the left, juice order is: TN5, GB1, ZG1, GB8, CH3, GR2, ZH1, GB4, MZ2, ZH3, TN4, GB2, GB9, GB5, ZH6, MZ3, GR1, EP1, MZ1, TN1, CH1, ZH2, JB1, KL1, CH2, GB3, JB3, TN6, BL1, TN3 (color figure available online).
Variability in the Content of Organic Acids and Sugars
Organic acids and sugars are the main components determining the taste of fruit juice. The total organic acids in Tunisian pomegranate juices varied 12–63 g/L with a mean of 26 g/L and a coefficient of variation of ∼50% (). Six organic acids were identified, including malic acid (∼50% of total), citric acid (∼23% of total), succinic acid (∼17% of total), oxalic acid (∼7% of total), tartaric acid (∼2% of total), and ascorbic acid (∼0.3% of total). All organic acids showed large variability, expressed by the coefficient of variation that was lowest for malic acid (22%) and highest for citric acid (155%). While malic acid was the prominent organic acid in these Tunisian pomegranates, citric acid was found to be the major acid in Turkish pomegranates.[Citation29] The highest malic acid content, 62.6 g/L, was found in the cultivar ‘Mezzi 2’ (MZ2), which also showed the highest titrable acidity. Tunisian pomegranates seem to be rich in oxalic acid (0.3–3.9 g/L) compared with 40 Spanish cultivars with the highest value of 0.34 g/L.[Citation29] Oxalic acid has been reported as the main organic acid in ‘Assaria’ cultivar from Portugal.[Citation30] In this study, acetic acid could not be quantified and fumaric acid was detected only as traces, which is quite different from previous results on Spanish,[Citation12] and Iranian pomegranate cultivars,[Citation31] where both acids have been quantified.
Table 3 Contents of organic acids and sugars in the 30 pomegranate fruit juices (g/L)
Interestingly, tartaric acid was detected in all samples of the sweet fruits but not in any of the five sour cultivars. Thus, sour taste may be negatively correlated with tartaric acid content. On the other hand, citric, malic, and succinic acid concentrations were higher in the sour cultivars suggesting an association, as is the case for citric acid and sourness in orange juice.[Citation32] Indeed, it is known that acidity modifies the sensory properties by affecting the perception of volatile compounds in juice and improving the appeal of the juice.
The total sugars in Tunisian pomegranate juices varied from 131.3–199.8 g/L (). Fructose and glucose, previously identified as the two major sugars, represented 53.9 and 43.3% of total sugars in the studied samples, respectively. The concentrations of fructose varied from 72–106 g/L (mean 90.5 g/L) and those of glucose varied from 57–86 g/L (mean 72.8 g/L) in the 30 samples with average concentrations of 90.5 and 72.8 g/L, respectively. These results are in agreement with previous reports on Spanish and Turkish pomegranates showing fructose concentrations higher than those of glucose but contrast results on samples from Russia, Saudi Arabia, and Turkey indicating that glucose was the dominant sugar.[Citation12,Citation33,Citation34] On the other hand, these Tunisian pomegranates contained more sugars than the Spanish fruits, where the highest fructose and glucose concentrations were 82.2 and 78.0 g/L and the overall means were 65.8 and 61.4 g/L, respectively.
Low concentrations of arabinose (0–9.19) and sucrose (0–3.62) and sometimes traces of galactose were found. The variation of very low sucrose content may be explained by the fact that it may be converted to invert sugars during the ripening process. Sorbitol and maltose were not detected in any of the studied pomegranate juices. The limited number of five sour fruit samples tended to have higher average sugar content than sweet ones; 189.17 and 163.97 g/L, respectively, which disagreed with values found for Spanish pomegranate reported by Melgarejo et al.[Citation12]
Variability in the Content of Anthocyanins
Anthocyanins (glycosylated anthocyanidins) are the main phenolic compounds responsible for the purple-red colours of pomegranate. Previous studies reported 3-glucosides and 3,5-diglucosides of delphinidin, cyanidin, and pelargonidin () as the anthocyanins involved in the colouring of pomegranate aril colouring during maturation.[Citation18,Citation22] In the present study, these anthocyanins were quantified in the 30 Tunisian cultivars of pomegranate showing a large diversity in aril and juice colour at maturity stage (). Supporting this visual appearance, the total anthocyanin content of the 30 samples ranged from 9–115 mg per liter of juice. The values of total anthocyanins in the Tunisian samples were lower than those of 493, 306, and 252 mg/L reported for pomegranate from Turkey,[Citation35] the American ‘Wonderful’ variety and TML variety from Iran,[Citation36] respectively. In a recent study, Iranian pomegranates were found to be much richer in anthocyanins than Tunisian and Spanish fruits, with a total anthocyanin content reaching 7760 mg/L.[Citation37] For most of the Tunisian samples, the total anthocyanin content agreed with colour (; ). However, this was not always true as it was the case of ‘Mezzi 1’ (MZ1) that had a dark red juice while its anthocyanins content was only 43.7 mg/L. This can be explained by the importance of co-pigmentation phenomenon resulting in redder hues.[Citation38] For example, delphinidin was reported to exist in egg plant also as sasunin (delphinidin-3-(p-coumaroyl-rutinoside)-5-glucose).[Citation39]
Figure 2 Structures of pomegranate anthocyanins.
R1 = R2 = H; Pelargonidin (3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-1-benzopyrylium cation)
R1 = H, R2 = OH; Cyanidin (3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-1-benzopyrylium cation)
R1 = R2 = OH; Delphinidin (3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-1-benzopyrylium cation).
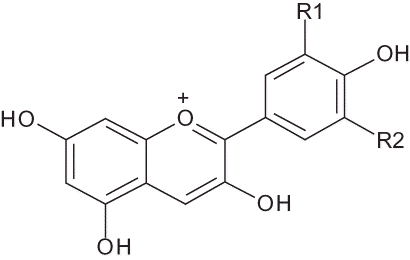
Table 4 Contents of anthocyanins in the studied pomegranate fruit juices (mg/L)
The contents of the individual anthocyanins were as follows: cyanidin-3,5-diglucoside (3.1–74.4 mg/L), delphinidin-3-glucoside (0.7–22.0 mg/L), cyanidin-3-glucoside (0.8–21.0 mg/L), pelargonidin-3-glucoside (0.5–16.1 mg/L), pelargonidin-3,5-diglucoside (0.0–11.8 mg/L), and delphinidin-3,5-diglucoside (0.0–5.4 mg/L). With the exception of one sample “Gabsi 5,” cyanidin-3,5-diglucoside was the most abundant pigment representing >50% of the total anthocyanins, whereas delphinidin-3,5-diglucoside was the minor one representing only about 3% of the total anthocyanins. The anthocyanins profiles of Tunisian cultivars were markedly different from those reported for Iranian and Spanish cultivars. In eight Iranian cultivars, the most abundant pigment was delphinidin-3,5-diglucoside followed by cyanidin-3,5-diglucoside while cyanidin-3-glucoside was reported as the major pigment in four Spanish clones as well as ‘Wonderful’ variety.[Citation2,Citation18,Citation22,Citation37] It might be possible that anthocyanin, alone or in combination with other phytochemicals, can be used for fingerprinting and differentiating of pomegranate juices.
Multivariate Analysis
The inter-relations between the variables studied were investigated by the analysis of Pearson correlation (). Correlation values ranged from 0 to 0.936. The highest correlations were obtained between titratable acidity and citric acid (0.936), followed by the ones between malic and succinic acids (0.901), fructose and glucose (0.881), and pH and citric acid content (−0.858). There is also a very strong correlation between pH and titratable acidity.
Table 5 Pearson correlations between studied parameters
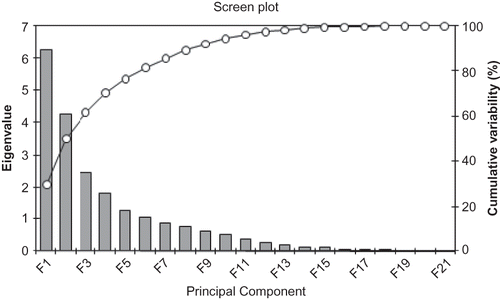
Principal components analysis (PCA) and cluster analyses were performed over the whole data set. The total variability is explained by 21 principal components with the first three explaining 61.8% of the cumulative variance (). The distribution of the studied pomegranates along the first two principle components, explaining 51.14% of the total variability, is shown in . As can be seen, sour and sweet pomegranates are located separately. Interestingly, all pomegranates varieties studied are differentiated as a consequence of the large range of studied traits independent of the geographic origin, suggesting that differences are mainly dependant on the cultivar. In fact, organic acids, sugars, anthocyanins, or morphometric traits in all samples studied are similarly differentiated depending on cultivar (data not shown).
Figure 4 Biplot of the first two principal components F1 and F2s showing dispersion of Tunisian pomegranates based on their organic acids, sugars, anthocyanins contents, and some morphological traits.
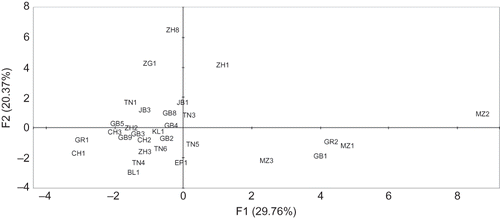
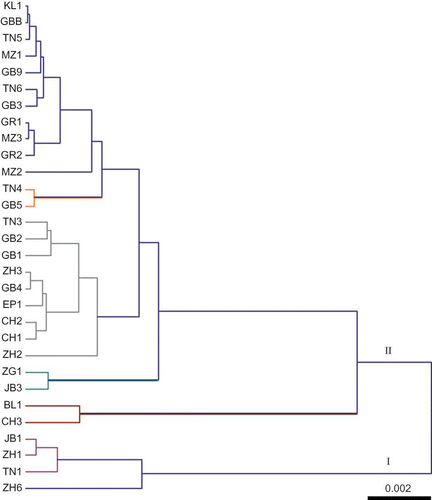
Similarly, hierarchical ascendant classification leads to two main groups (). Although the first main group (I) is made of four cultivars originated from the north of Tunisia (region R4), there is no clear weight of geographic origin on clustering. Indeed, the grouping inside the second main group (II) shows no clear correlation with origin. The straddling of the samples belonged to different regions given evidence of the exchange of pomegranate plant material between and within a region in a small country like Tunisia, and cultivar characteristics are conserved due to the vegetative propagation of the pomegranate.
CONCLUSION
Fructose and glucose were the two abundant sugars and malic acid and citric acids were the most frequent organic acids in Tunisian pomegranate juice. This study suggests that pomegranate juice sourness or sweetness is due to high or low concentrations of citric acid, respectively, and not to low or high sugar content. Furthermore, PCA including all parameters studied, the majority of studied pomegranate accessions were clearly distinguished into groups independent of the cultivation location suggesting the importance of cultivar. Besides, the quite large ranges of contents' values of different studied chemical compounds reflect the large diversity of local pomegranate germplasm. It is important for future research to describe the complete phenolic profile of different cultivars and how the juice colour is determined by different phenolic components and by co-pigmentation. These results may be helpful for further works on pomegranate valorization, for example by mixing its juice with other fruit juices to produce a drink with high nutritive and health benefits.[Citation40,Citation41]
ACKNOWLEDGMENT
This work was funded through a cooperation project (Ref. 7/04/P) between AECID (Agencia Española de Cooperación Internacional para el Desarrollo, Spain) and the Ministry of Higher Education, Scientific Research, and Technology (Tunisia). NH is thankful to F. Gaaliche for his assistance in the collection and preparation of juice samples and to Pedro for his technical support in HPLC analyses.
Notes
To the memory of Professor Mohamed Marrakchi, who passed away in April 2008
REFERENCES
- Tous , J. and Ferguson , L. 1996 . “ Mediterranean fruits ” . In Progress in New Crops , Edited by: Janick , J. 416 – 430 . Arlington : ASHS Press .
- Gil , M.I. , Tomas-Barberan , F.A. , Hess-Pierce , B. , Holcroft , D.M. and Kader , A.A. 2000 . Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing . Journal of Agricultural and Food Chemistry , 10 : 4581 – 4589 .
- Lansky , E.P. and Newman , R.A. 2007 . Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer . Journal of Ethnopharmacology , 109 : 177 – 206 .
- Wang , R. , Wang , W. , Wang , L. , Liu , R. , Ding , Y. and Du , L. 2006 . Constituents of flowers of Punica granatum . Fitoterapia , 77 : 534 – 537 .
- Noda , Y. , Kaneyuki , T. , Mor , A. and Packer , L. 2002 . Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin . Journal of Agricultural and Food Chemistry , 50 : 166 – 171 .
- Aviram , M. , Dornfeld , L. , Rosenblat , M. , Volkova , N. , Kaplan , M. , Coleman , R. , Hayek , T. , Presser , D. and Fuhrman , B. 2000 . Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice . American Journal of Clinical Nutrition , 71 : 1062 – 1076 .
- Aviram , M. , Rosenblat , M. , Gaitini , D. , Nitecki , S. , Hoffman , A. , Dornfeld , L. , Volkova , N. , Presser , D. , Attias , J. , Liker , H. and Hayek , T. 2004 . Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation . Clinical Nutrition , 23 : 423 – 433 .
- Jurenka , J. 2008 . Therapeutic applications of pomegranate (Punica granatum L.) . A review. Alternative Medicine. Review , 13 : 128 – 144 .
- Basu , A. and Penugonda , K. 2009 . Pomegranate juice: A heart-healthy fruit juice . Nutrition Review , 67 : 49 – 56 .
- Patel , C. , Dadhaniya , P. , Hingorani , L. and Soni , M.G. 2008 . Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies . Food and Chemical Toxicology , 46 : 2728 – 2735 .
- Maestre , J. , Melgarejo , P. , Tomás-Barberán , F.A. and Garcia-Viguera , C. 2000 . New food products derived from pomegranate . Options Méditeranéennes , 42 : 243 – 245 .
- Melgarejo , P. , Salazar , D.M. and Artés , F. 2000 . Organic acids and sugars composition of harvested pomegranate fruits . European Food Research and Technology , 211 : 185 – 190 .
- Vardin , H. and Fenercioğlu , H. 2003 . Study on the development of pomegranate juice processing technology: Clarification of pomegranate juice . Nahrung , 47 : 300 – 303 .
- El-Nemr , S.E. , Ismail , I.A. and Ragab , M. 1990 . Chemical composition of juice and seeds of pomegranate fruit . Nahrung , 34 : 601 – 606 .
- Esti , M. , Messia , M.C. , Sinesio , F. , Nicotra , A. , Conte , L. , La Notte , E. and Palleschi , G. 1997 . Quality evaluation of peach and nectarines by electrochemical and multivariate analysis: Relationship between analytical measurements and sensory attributes . Food Chemistry , 60 : 659 – 666 .
- Tezcan , F. , Gültekin-Özgüven , M. , Diken , T. , Özçelik , B. and Erim , F.B. 2009 . Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices . Food Chemistry , 115 : 873 – 877 .
- Du , C.T. , Wang , P. and L; Francis , F.J. 1975 . Anthocyanins of pomegranate, Punica granatum . Journal of Food Science , 40 : 417 – 418 .
- Gil , M.I. , Garciá-Viguera , C. , Artés , F. and Tomás-Barberán , F.A. 1995 . Changes in pomegranate juice pigmentation during ripening . Journal of the Sciences of Food and Agriculture , 68 : 77 – 81 .
- Mars , M. and Marrakchi , M. 1998 . Conservation et valorisation des ressources génétiques du grenadier (Punica granatum L.) en Tunisie . Plant Genetic Resources Newsletters , 114 : 35 – 39 .
- Mars , M. and Marrakchi , M. 1999 . Diversity of pomegranate (Punica granatum L.) germplasm in Tunisia . Genetic Resources and Crop Evolution , 46 : 461 – 476 .
- Jbir , R. , Hasnaoui , N. , Mars , M. , Marrakchi , M. and Trifi , M. 2008 . Characterization of Tunisian pomegranate (Punica granatum L.) cultivars using amplified fragment length polymorphism analysis . Scientia Horticulurae , 115 : 231 – 237 .
- Hernandez , F. , Melgarejo , P. and Tomas-Barberan , F.A. 1999 . Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum L.) . European Food Research and Technology , 210 : 39 – 42 .
- Fadavi , A. , Barzegar , M. , Azizi , M.H. and Bayat , M. 2005 . Physico-chemical of ten pomegranate cultivars (Punica granatum L.) grown in Iran . Food Science and Technology International , 11 : 113 – 119 .
- Opara , L.U. , Al-Ani , M.R. and Al-Shuaibi , Y.S. 2008 . Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.) . Food and Bioprocess Technology , 2 : 315 – 321 .
- Al-Said , F.A. , Opara , L.U. and Al-Yahyai , R.A. 2009 . Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman . Journal of Food Engineering , 90 : 129 – 134 .
- Ozgen , M. , Durgaç , C. , Serçe , S. and Kaya , C. 2008 . Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey . Food Chemistry , 111 : 703 – 706 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits . International Journal of Food Properties , 11 ( 1 ) : 44 – 52 .
- Shao , Y , He , Y. and Bao , Y. 2007 . A new approach to predict acidity of bayberry juice by using vis/near infrared spectroscopy . International Journal of Food Properties , 10 ( 3 ) : 631 – 638 .
- Poyrazoglu , E. , Gokmen , V. and Artik , N. 2002 . Organic acids and phenolic compounds in pomegranate (Punica granatum) grown in Turkey . Journal of Food Composition and Analysis , 15 : 267 – 275 .
- Miguel , G. , Fontes , C. , Antunes , D. , Neves , A. and Martins , D. 2004 . Anthocyanin concentration of “Assaria” pomegranate fruits during different cold storage conditions . Journal of Biomedicine and Biotechnology , 5 : 338 – 342 .
- Aarabi , A. , Barzegar , M. and Azizi , M.H. 2008 . Effect of cultivar and cold storage of pomegranate (Punica granatum L.) juices on organic acids . Asean Food Journal , 15 : 45 – 55 .
- Dominguez , A.L. 2002 . “ Caractérisation et optimisation de la flaveur du jus d'orange non fait de concentré ” . In Thèse Ph.D. Faculté des Sciences de l'Agriculture et de l'Alimentation , Université Laval (Laval, Canada) .
- Al-Maiman , S.A. and Dilshad , A. 2002 . Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation . Food Chemistry , 76 : 437 – 441 .
- Cam , M. , Hisil , Y. and Durmaz , G. 2009 . Characterization of pomegranate juices from ten cultivars grown in Turkey . International Journal of Food Properties , 12 ( 2 ) : 388 – 395 .
- Orak , H.H. 2008 . Evaluation of antioxidant acitivity, colour and some nutritional characteristics of pomegranate (Punica granatum L.) juice and its sour concentrate processed by conventional evaporation . International Journal of Food Sciences and Nutrition , 60 : 1 – 11 .
- Aligourchi , H. , Barzegar , M. and Abbasi , S. 2008 . Anthocyanins characterisation of 15 Iranian pomegranate (Punica granatum L.) varieties and their variation after storage and pasteurization . European Food Research and Technology , 227 : 881 – 887 .
- Mousavinejad , G. , Emam-Djomeh , Z. , Rezaei , K. and Khodaparast , M.H.H. 2009 . Identification and quantification of phenolic compounds and their effects on antioxidant acitivity in pomegranate juices of eight Iranian cultivars . Food Chemistry , 115 : 1274 – 1278 .
- Delgado-Vargas , F. and Paredes-López , O. CRC Press 2003 . Natural colorants for food and nutraceutical uses
- Noda , Y. , Kaneyuki , T. , Igarashi , K. , Mori , A. and Packer , L. 1998 . Antioxidant activity of nasunin, an anthocyanin in eggplant . Research Communication in Molecular Pathology and Pharmacology , 102 : 175 – 187 .
- González-Molina , E. , Moreno , D.A. and García-Viguera , C. 2009 . A new drink rich in healthy bioactive combining lemon and pomegranate juices . Food Chemistry , 115 : 1364 – 1372 .
- Jaiswal , V. , DerMarderosian , A. and Porter , J.R. 2009 . Anthocyanins and polyphenol axidase from dried arils of pomegranate (Punica granatum L.) . Food Chemistry , 118 : 11 – 16 .