Abstract
Dairy foams are complex aerated materials where the liquid matrix is an emulsion made by oil droplets dispersed in a water system. An innovative application of these systems leads to an interesting product derived from instant whipped creams that are stored and consumed at low temperatures (typically between −4 and −18°C) like an ice cream. This novel product requires a specific texture due to the particular conditions related to its consumption. In the present work, the effects of some relevant ingredients (emulsifiers, sugars, and fats) on rheological properties and freezing temperature of dairy emulsions were investigated. Samples were prepared on lab scale and it was found that structure extension is affected strongly by stabilizers (carrageenan and guar gum) and in a lower measure by fat content. As far as freezing point is concerned a significant effect only of sugars (type and amount) and fats was measured. A formulation having interesting properties for low temperature applications was obtained and it was prepared on a pilot plant scale to investigate the potential effects of the industrial production. These samples exhibited a relevant reduction in both viscosity and elasticity; it was speculated that this effect could be attributed to the whey protein thermal damage (induced by the UHT treatment) and to the homogenisation conditions, different from those adopted on lab scale.
INTRODUCTION
Food foams are systems showing particular organoleptic and structural characteristics. Their relevant element is the presence of air, incorporated as small bubbles to provide specific texture.[Citation1–4] Among them a relevant role is played by dairy foams, based on oil-in-water emulsions that can be consumed either in the frozen state, ice cream, or in the unfrozen state, such as whipped cream. Even though they are characterised by similar void fractions[Citation5] of approximately 50% (v/v), they have different properties related to the consumption temperature and to the different mechanisms of formation and stabilization. Ice cream production is usually obtained by pressure beating,[Citation2] i.e., dissolution of air or gas under pressure, followed by a freezing process and a hardening period.[Citation3] The foamed structure, obtained using this procedure, is very fine and it is usually stabilized through the combined action of ice crystals, fat globule network, and highly viscous aqueous phase. On the contrary, whipped cream can be produced both by partial aeration of the liquid phase during whipping or, most commonly, by gas injection,[Citation2] i.e., expansion of gas bubbles initially introduced, under pressure, into the liquid matrix (instant whipped cream); in both cases the product is stabilized only by a network made up of fat particles without the presence of ice crystals.[Citation1]
A novel interesting product is the so-called “soft ice cream” having properties that are intermediate between traditional ice creams and whipped creams. They are characterised by a particular smooth texture and a very low amount of ice crystals and are produced by aeration of the liquid phase under moderate over-pressure (i.e., 4–8 bar) and then extruded either by mechanical systems or by using the same gas introduced for the aeration. In both cases, bubble expansion is due to the turn-off in pressure during extrusion and gas retention is mainly due to emulsion film properties because of the presence of an unfrozen structure even at temperatures usually ranging between −4 and −18°C. Therefore, an “optimal” rheological behaviour is necessary to obtain the desired product consisting in an aerated elastic system entrapping the proper amount of gas, but still liquid at a given temperature, as a consequence of a low freezing point. In fact, usually high elasticity, particularly at the interface, is required to improve gas retention[Citation6] and control the texture, but low viscosity at low temperature is necessary to allow flowing through a nozzle under a relatively small pressure drop.
It is known in the literature that foam properties are strictly related to both the aeration mechanism and the formulation of the liquid matrix, whilst the dynamic of gas bubbles is often neglected.[Citation2] However, the knowledge of the mechanism related to the growth and the shrinkage of the gas cells (during process or food consumption) is necessary to properly design both the product (in terms of organoleptic properties related to void fractions) and the process (guaranteeing the desired bubble distribution).[Citation2] It is known that dynamic behaviour of a single gas bubble in a viscoelastic medium can be modelled by solving a mechanical problem involving the momentum balance equation, where the rheological properties of the matrix have to be described by a proper constitutive equations.[Citation6, Citation7] Moreover, flow properties of aerated systems, necessary to design process units, can be often related to those of the liquid phase by using proper rules (e.g., Gardiner et al.[Citation8]). As a consequence, the main characteristics of aerated systems are strictly related to the rheological properties of the matrix; therefore, when designing new foams, the rheological characterization of the intermediate product can give important information about the final behaviour of these materials.
Starting from these considerations, in this work the effects of selected ingredients on the rheological properties of different emulsions were studied on lab scale, aiming to propose a formulation having the proper rheological characteristics for “soft ice cream” applications. However, the rheological properties of a product, and the texture as a consequence, depend also on process conditions[Citation9]; therefore, some samples were prepared by using a pressure homogenizer and a pilot plant, available in the Cremal Research Center (Marcianise, Italy), to carry out a preliminary investigation on the potential effects of pressure homogenisation and sterilisation (typically adopted in industrial production) on rheological properties of these emulsions.
MATERIALS AND METHODS
The samples were prepared using a base recipe, adopted for commercial dairy emulsions, based on milk and powder skim milk (77.5 g/100 g), hydrogenated vegetable fats (4 g/100 g), a mixture (18 g/100 g) of glucose syrup and dextrose (having approximately 1:4 ratio), a mixture (0.5 g/100 g) of emulsifiers (0.374 g/100 g mono and diglycerides of fatty acids, Grindsted HP40, Danisco, Denmark), and stabilizers (0.063 g/100 g carrageen CL 220, Danisco, Denmark and 0.063 g/100 g guar gum, Danisco, Denmark). Concentrations are based on final formulation. This base recipe was modified, as shown in , changing the emulsifier/stabilizer ratio or replacing some ingredients, according to empirical and literature information and practical knowledge concerning different cream formulations. In samples E1–E3, the relative amount of emulsifier and stabilizers was modified investigating their effects on emulsion “consistency”; in sample E4, dextrose was replaced by sucrose (less expensive, better tasting for consumers, and with a higher sweetness rating[Citation3]); cream milk was added in sample E5 to increase fat content, stabilising the structure[Citation1, Citation3, Citation10] and decreasing the freezing point.
Table 1 Sample description and freezing point (FP) values
Each sample was prepared, on lab scale, by mixing all the ingredients in a fixed order, at 70°C for 55 min by Ultra Turrax (T50 Basic, IKA-Werke, Germany) to pre-emulsify the sample; the mixture was then sharply cooled down to 0°C in an ice bath and hold at this temperature for 15 min. Afterward it was put in an ultrasound bath (Transsonic T310, ELMA, Germany) for 15 min to further decrease particle size,[Citation11–13] then cooled down to 0°C in an ice bath and hold at this temperature for 3 h. All samples were stored for 24 h at 4°C before testing.
To evaluate the effect of different homogenisation procedures, sample E6 was prepared replacing the ultrasound bath by a pressure homogenizer (APV Gaulin, APV, Crawley, UK) having three pistons and working at 150 atm (first stage) and 30 atm (second stage). In this case, the emulsification is achieved by forcing the liquid through a small valve opening[Citation3]; no rest time was allowed between mixing and homogenisation. Sample E6 was prepared, following the described procedure, starting from sample E3 and replacing 4.5 g/100 g milk with glycerol (to decrease the freezing point). Finally, sample E7, having the same composition of sample E6, was prepared on a pilot plant replicating the industrial process and consisting of the following unit operations sequence: mixing of ingredients at 70°C, sterilisation (UHT treatment working at 150°C), homogenisation, and cooling. A double-stage pressure driven homogeniser, having three pistons and working at 150 atm (first stage) and 30 atm (second stage), was adopted in the pilot plant.
All ingredients (kindly supplied by Codap S.p.A., Marcianise, Italy) are commercial products, commonly adopted in industrial processes, and used without further purification with the aim of describing the behaviour of commercial dairy emulsions and not “model systems.” Total fat content in final emulsions was determined according to the Gerber test[Citation14] and it is approximately 6 g/100 g formulation for samples E1–E4 and approximately 12 g/100 g formulation for samples E5–E7.
A rheological characterization of all samples was performed by using a controlled strain rheometer (ARES-RFS, TA Instruments, New Castle, DE, USA), equipped with parallel plate geometry (φ = 50 mm) and Peltier-effect temperature control. Frequency sweep tests were performed, increasing frequency from 0.1 to 10 Hz, at 4, 0, −5°C in the linear viscoelastic region (previously determined by strain sweep tests at 1 Hz). The viscosity was evaluated, at the same temperatures, increasing shear rate from 0.1 to 100 s−1, allowing, for each point, the proper measurement time (determined by step shear rate tests) to reach steady state conditions.
Time cure tests at 1 Hz with a ramp rate of 1°C/min (decreasing temperature from 4°C to emulsion freezing) and time sweep tests were used to detect a “dynamic” freezing temperature by rheological measurements.[Citation15] All tests were not affected by water loss problems due to the low temperatures adopted. The preparation of all samples was carried out in triplicate and all results presented are average values; differences of measurement are shown by standard deviations, typically lower than 10%.
RESULTS AND DISCUSSION
Dynamic Freezing Point
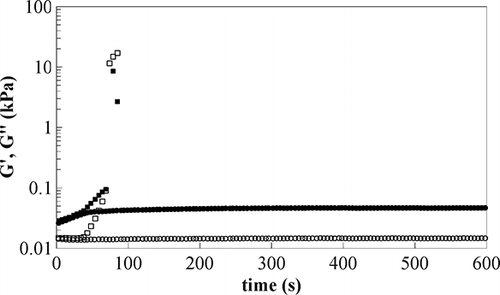
Freezing point determination was carried out by dynamic rheological tests at 1 Hz, decreasing the sample temperature at controlled rate (time cure test); results obtained for sample E1 are reported in and the decreasing trend of the loss tangent when decreasing T, evidences that the structure is slightly changing and the sample becomes more “solid-like”; this is also due to initial freezing effects like crystallisation centres forming. When a critical low temperature is reached approaching −10°C, both moduli diverge, due to the potential formation of ice crystals, and the rheological behaviour of the emulsion changes greatly, giving torque values usually higher than the rheometer upper limit (maximum torque 0.1 N m). This critical temperature, however, could be affected by the adopted temperature ramp rate, oscillation frequency, and gap between plates. According to Hetzel et al.,[Citation15] the dynamic freezing point shows no dependence on frequency and gap, whilst an effect of the ramp rate was found only for values greater than 5°C/min. The ramp adopted in this work (1°C/min), lower than that limit, should be adequate; however, to ensure that the measured property did not depend on the ramp rate, time sweep tests were performed at different increasing temperatures, starting from the critical value previously detected by the time cure. This experiment allowed the freezing point to be set as the temperature at which dynamic moduli diverge during time sweep in a time window of 10 min. Results obtained for sample E1 () show that at −8°C, loss and storage moduli are almost constant in the considered time range, whilst at −9°C a sharp increase is found, for both of them after 40 s; therefore, the value of −9°C was assumed as freezing temperature for this sample.
Figure 1 Time cure test, sample E1 at 1 Hz (−1°C/min), G′ full circles, G″ open circles, loss tangent open triangle.
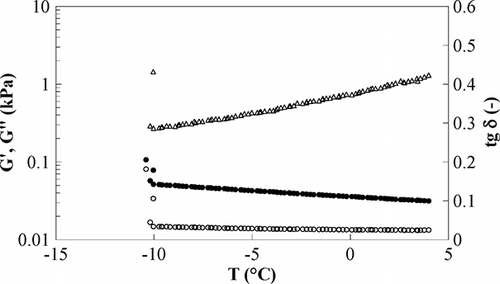
Figure 2 Time sweep test, sample E1 at 1 Hz, T = −8°C (circle) and T = −9°C (square), G′ full symbols, G″ open symbols.
The values obtained by this rheological method are usually different from the classic freezing points determined in static conditions by thermal analysis,[Citation15] probably because of the different test conditions (surface to volume ratio, sample amount) and to the transition criterion: a sharp change in enthalpy owing to the melting latent heat is used for thermal analysis, a sharp change in rheological properties is used in the present case. The latter, being related to mechanical properties, probably could be more useful when the fluid dynamic behaviour of these materials is studied. In addition, the dynamic freezing point should be less affected by crystal growth inhibition problems (small volume and extremely smooth clean surface in differential scanning calorimeter, DSC), than the DSC transition temperature; therefore, it should be much closer to the real situation and much more practice-oriented when rheology or mechanical characteristics are involved.[Citation15]
All samples were subjected to the illustrated sequence of rheological tests and the freezing temperatures (FP), reported in , evidenced how different formulations affect them. It is well-known that dextrose is able to decrease water activity, giving a higher cryoscopic depression than sucrose,[Citation3, Citation16] therefore, sample E4 (−6°C) has a FP higher than sample E1. On the contrary, the addition of fats (sample E5) or glycerol (E6–E7) has the opposite effect, reducing the FP. It should be remembered that samples E6 and E7 were prepared by using a pressure homogeniser, therefore, the reduced FP is probably due also to a different structure and droplet size and distribution. Finally, no relevant effect of different amounts of stabilizers and emulsifiers (sample E1–E3) was found on sample freezing temperature.
Dynamic Rheological Tests
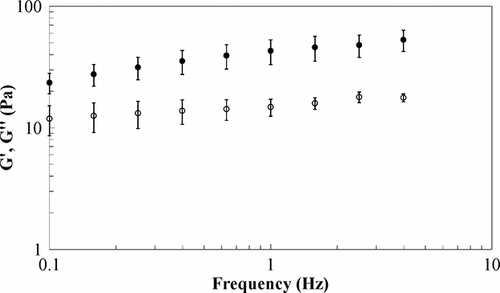
A typical frequency sweep is shown in for sample E1 at −5°C; it can be seen that, in the considered frequency range, both moduli have an almost linear trend on a log-log scale with G′ larger than G″. A similar trend was found also at different temperatures and for all samples. According to the weak gel model,[Citation17] many foods can be described as weakly structured systems having a three-dimensional network with rheological “units” connected by weak bonds. The network extent and the strength of these bonds can be evaluated by fitting dynamic data, in terms of complex modulus G*, by using a power law equation:
Table 2 Weak gel model parameters (A and z) for tested samples at different temperatures
The effects of stabilizers are evident in sample E2 (), where a reduction in guar gum content is balanced by the carrageenan increase; in this sample, greater values of both z and A, when compared to E1, were obtained because of the gelling effects of carrageenan.[Citation18] This trend is more evident in sample E3 () where the structure enhancement (evidenced by larger z value) is coupled to the thickening effects of guar gum.[Citation18]
As far the temperature dependence is concerned, it can be seen that, in the tested range, sample E1 and E2 are characterised by an increase in both network strength and interactions, as confirmed also by time cure test ( and ) showing a reduction in loss tangent; as far as sample E3 is concerned, both network extent, measured by z, and loss tangent are almost constant with temperature, probably due to a more structured system, less sensitive to temperature change until the freezing point is reached. No relevant difference is found in rheological properties of samples E1 and E4 (), because they differ only in the sugar type (a disaccharide instead of a monosaccharide) having a poor influence on microstructure; for both samples an increase in z, at decreasing temperatures, was obtained as confirmed also by the loss tangent behaviour during the time cure ().
Figure 4 Time cure test, samples E1, E2, E3: (a) complex modulus (full symbols) and (b) loss tangent (open symbols).
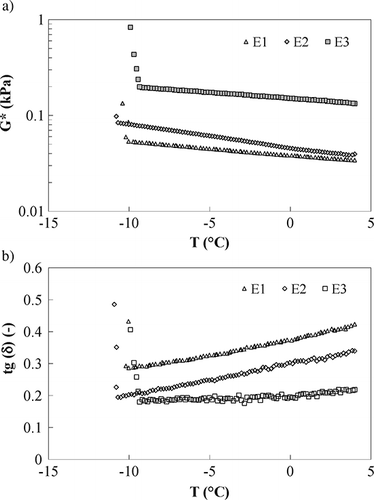
Figure 5 Time cure test, samples E1, E4, E5, E6, E7: (a) complex modulus (full symbols) and (b) loss tangent (open symbols).
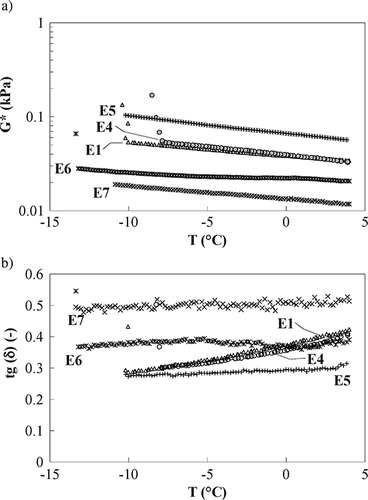
Milk cream (E5 sample) slightly increases emulsion structure and elasticity at a higher temperature, as shown by z value (), whilst no difference was found at −5°C; in addition, the strength of the interactions (as shown by A parameter) is greater than the previous sample value, probably owing to the increased amount of fat globules.[Citation19] Both frequency sweep tests, at different temperatures, and time cure ( and ) show a larger temperature stability, in the considered range, evidenced by the almost constant loss tangent trend; this is probably due to the increase in fat content that reinforces the gel strength, increasing bulk protein concentration due to excluded volume effects.[Citation20] When sample E6 is compared to the previous one, lower values of both A and z () were found at all tested temperatures. Aiming to discuss potential reasons for these differences, it is worth reminding that rheological behavior of emulsions mainly depends on both composition (i.e., nature and quantity of oil phase, emulsifiers, stabilizers, etc.) and drop size and distribution. A sample having the same composition of E6 was prepared on lab scale and it was found that the change in formulation, limited to a reduced amount of glycerol, did not affect the rheological properties in a relevant way (data not shown); therefore, even though no experimental data is available, a potential explanation for this difference could be related to a different drop size distribution caused by the different homogenisation device.
It is well-known that the homogenisation process had a relevant effect on droplet size and size distribution mainly depending on the effective amount of energy received by the sample.[Citation3, Citation21] It was found that at equal power, applied per unit volume, the high pressure homogenisers are efficient devices giving the smallest droplets, whilst the sonifiers and the rotor-stator systems seem to be less effective[Citation21]; however, for high pressure homogenisers, a dependence on the adopted valve was found by Behrend and Schubert,[Citation12] which also showed that the sonifier gives smaller droplets than pressure devices except when a Microfluidizer® is adopted. Investigating nano-emulsion production by sonication and microfluidization, Jafari et al.[Citation13] found a critical pressure, depending on the specific emulsion, above which drop size can increase as a result of the competition between the drop formation and the drop-drop coalescence yielding particles larger than those obtained by sonication.
Furthermore, it is known that severe processing conditions in the pressure homogeniser (such as high pressure, shear stress, and temperature) can damage the whey protein structure, affecting their interaction at the oil-water interface and, as a consequence, a reduction in emulsion stability and viscosity was found increasing homogenisation pressure.[Citation22] Starting from these considerations, the lower values of both moduli could be attributed to the different drop size distribution and, also in agreement with Pal,[Citation23] which found that upon increase of droplet size the emulsions become much less elastic and viscous, larger particles could be expected in sample E6.
However, the same temperature stability as sample E5 was found, as confirmed by an almost constant loss tangent (), probably because it is more dependent on formulation than on drop size distribution, in the considered temperature range. It is worth reminding that the rheological properties of the sample are not affected in a relevant way by the reduced amount of added glycerol, as discussed in the previous paragraph. Finally, sample E7 shows values of both A and z () lower than sample E6; this effect is probably due to the thermal damage of the structure. In fact, milk proteins are excellent emulsifiers and they give an important contribution in emulsion formation and stabilisation;[Citation24, Citation25] however, even though casein is insensitive to heating, whey proteins can be denaturated when heated at high temperatures[Citation24] and this could affect their emulsifying properties reducing the protein stabilising role. As a consequence, owing to the high shear and impact forces during the process in the pilot plant, the interfacial membranes could break yielding a broader size distribution.[Citation22] However, also for this sample a good temperature stability was found in the considered range ().
Steady Tests
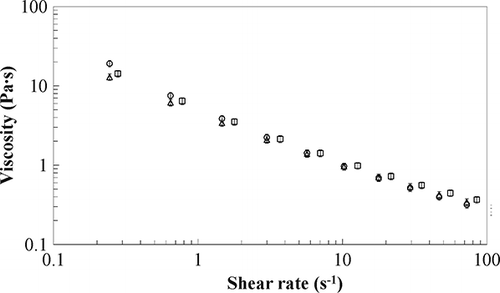
Flow curves were run at temperatures ranging between −5°C and 4°C for all the samples. All the investigated materials showed a marked shear thinning behaviour because, from a structural point of view, they may be roughly considered as dispersions of fat particles in a water medium; the increasing shear action destroys the weak bonds among the particles and a decreasing viscosity is found. Experimental viscosity data, for all samples in the tested shear rate window, showed a linear trend in a log-log plot, therefore, they were fitted by a power law model[Citation26]:
Table 3 Viscosity power law parameters (k and n) for tested samples at different temperatures
Sample E4 is characterised by viscosity values slightly larger than E1 (as shown by consistency index value, ) probably owing to the effect of sucrose that usually gives viscosity higher than dextrose at the same weight concentration in aqueous solutions,[Citation27] whilst no relevant difference in flow index is found confirming that sugars mainly act as thickening agents without affecting emulsion structure. Moreover, a greater temperature dependence when compared to E1 is found, probably owing to the different sucrose behaviour as confirmed by data obtained in water solution.[Citation28]
Sample E5 is characterised by an increase in consistency index () owing to the higher fat content,[Citation3] whilst flow index decreases confirming the presence of a structured network more shear sensitive than in sample E1. Sample E6 and E7 (), prepared by using the pressure homogenizer and the pilot plant, show consistency index values lower than previous samples; this is due to the different preparation as discussed for dynamic tests in the previous paragraph. Indeed, it is well-known that emulsion viscosity increases with decreasing droplet size[Citation29] and that samples with a broader size distribution have lower viscosities than samples with narrow distributions.[Citation26] Therefore, viscosity data are in agreement with dynamic moduli suggesting that both pressure homogeniser and high temperature treatment bring to a weaker structure. Both samples exhibit, also, almost constant flow index values, confirming a good thermal stability in the considered temperature range.
Experimental results have shown that emulsion consistency is strongly affected by carrageenan and guar gum that can have a structuring or thickening effect depending on their specific characteristics; fat content, acting on dispersed phase fraction, change the three-dimensional network affecting both viscosity and elasticity but at a lower extent than stabilizers; different sugars such as dextrose or sucrose slightly change only viscosity. It is worth reminding that the increase in fat and stabilizers content seems to enhance sample stability at low temperatures as evidenced by the time cure test. Freezing point is significantly affected by sugars (or glycerol), able to decrease water activity in a different way, and by fat content, both of them are able to decrease the freezing temperature; on the contrary, a change in emulsifier and stabilizers (in the tested conditions) does not have relevant effects on low temperature behaviour.
As far as the preparation procedure is concerned, the pressure homogenizer seems to reduce viscosity and elasticity probably owing to a lower control on drop size and distribution attributed to the different homogenisation device; a similar effect is caused also by the high temperature treatment in the pilot plant, probably owing to the whey protein damage that reduces their emulsifying capability.
CONCLUSION
Different dairy emulsions were prepared and characterized by rheological tests aiming to individuate a formulation suitable for commercial preparation of “low temperature” whipping products. The effects of either main ingredients (i.e., stabilizers, fats, sugars) or production process changes were investigated by using rheological characterisation. The “ideal” emulsion for “low temperature” applications should have a low dynamic freezing point (to avoid ice crystal formation), enough elasticity to entrap gas, a rather high viscosity (to enhance emulsion stability and gas retention), and a low temperature dependence. However, it is worth noticing that high viscosity and elasticity could make the emulsion flow through a nozzle difficult, therefore, for these properties, a good compromise between two opposite needs is necessary. Among tested emulsions, sample E4 has high FP (usually cold applications range between −4 and −18°C) and sample E3 has too high viscosity and dynamic moduli, both appearing unsuitable for the considered aim. As a consequence, among the tested samples, the best formulation seems to be E5 having a low freezing point and rheological properties apparently proper for the considered application. It is worth noticing that the industrial production, including additional operations (such as the UHT treatment) or modifying the preparation steps (no rest time between different operations, different homogenisation devices), can alter final emulsion properties as shown by sample E6 and E7. A further investigation on scale-up effects and drop size distribution seems to be necessary in order to better clarify some issues. However, the results obtained in this work are very promising for industrial applications, as confirmed by preliminary tests carried out by the company.
REFERENCES
- Stanley , D.W. , Goff , H.D. and Smith , A.K. 1996 . Texture-structure relationships in foamed dairy emulsions . Food Research International , 29 : 1 – 13 .
- Campbell , G.M. and Mougeot , E. 1999 . Creation and characterisation of aerated food products . Trends in Food Science & Technology , 10 : 283 – 296 .
- Berger , K.G. 2000 . “ Ice cream ” . In Food Emulsions , 3rd , Edited by: Friberg , S.E. and Larsson , K. 413 – 490 . New York : Marcel Dekker Inc .
- Kloek , W. , van Vliet , T. and Meinders , M. 2001 . Effect of bulk and interfacial rheological properties on bubble dissolution . Journal of Colloid and Interface Science , 237 : 158 – 166 .
- van Aken , G.A. 2001 . Aeration of emulsions by whipping . Colloids and Surfaces A. Physicochemical and Engineering Aspects , 190 : 333 – 354 .
- Fyrillas , M.M. , Kloek , W. , van Vliet , T. and Mellema , J. 2000 . Factors determining the stability of a gas cell in an elastic medium . Langmuir , 16 : 1014 – 1019 .
- de Cindio , B. and Correra , S. 1995 . Mathematical modelling of leavened cereal goods . Journal of Food Engineering , 24 : 379 – 403 .
- Gardiner , B.S. , Dlugogorski , B.Z. and Jameson , G.J. 1998 . Rheology of fire-fighting foams . Fire Safety Journal , 31 : 61 – 75 .
- Dalgleish , D.G. 2006 . Food emulsions—their structure and structure forming properties . Food Hydrocolloids , 20 : 415 – 422 .
- McClements , D.J. 1999 . Food Emulsions: Principle, Practice and Techniques , Boca Raton : CRC Press .
- Abismail , B. , Canselier , J.P. , Wilhelm , A.M. , Delmas , H. and Gourdon , C. 1999 . Emulsification by ultrasound: Drop size distribution and stability . Ultrasonics Sonochemistry , 6 : 75 – 83 .
- Behrend , O. and Schubert , K.A.H. 2000 . Influence of continuous phase viscosity on emulsification by ultrasound . Ultrasonic Sonochemistry , 7 : 77 – 85 .
- Jafari , S.M. , He , Y. and Bhandari , B. 2006 . Nano-emulsion production by sonication and microfluidization—a comparison . International Journal of Food Properties , 9 : 475 – 485 .
- British Standards Institution, B.S. 696 . 1955 . Gerber Method for the Determination of Fat in Milk and Milk Products, Part II , London : British Standards Institution .
- Hetzel , F. , Nielsen , J. , Wiesner , S. and Brummer , R. 2000 . Dynamic mechanical freezing points of cosmetic O/W emulsions and their stability at low temperatures . Applied Rheology , 10 : 114 – 118 .
- Spiliotis , N. and Tassios , D. 2000 . A UNIFAC model for phase equilibrium calculations in aqueous and non aqueous sugar solutions . Fluid Phase Equilibria , 173 : 39 – 55 .
- Gabriele , D. , de Cindio , B. and D'Antona , P. 2001 . A weak gel model for foods . Rheologica Acta , 40 : 120 – 127 .
- Ross-Murphy , S.B. 1995 . Structure-property relationships in food biopolymer gels and solutions . Journal of Rheology , 39 : 1451 – 1463 .
- Adapa , S. , Dingeldein , H. , Schmidt , K.A. and Herald , T.J. 2000 . Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers . Journal of Dairy Science , 83 : 2224 – 2229 .
- Reiffers-Magnani , C.K. , Cuq , J.L. and Watzke , H.J. 1999 . Composite structure formation in whey protein stabilized O/W emulsions. I. Influence of the dispersed phase on viscoelastic properties . Food Hydrocolloids , 13 : 303 – 316 .
- Asua , J.M. 2002 . Miniemulsion polymerization . Progress in Polymer Science , 27 : 1283 – 1346 .
- Floury , J. , Desrumaux , A. and Lardieres , J. 2000 . Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions . Innovative Food Science & Emerging Technologies , 1 : 127 – 134 .
- Pal , R. 1997 . Viscosity and storage/loss moduli for mixtures of fine and coarse emulsions . Chemical Engineering Journal , 67 : 37 – 44 .
- Euston , S.R. , Finnigan , S.R. and Hirst , R.L. 2001 . Aggregation kinetics of heated whey protein-stabilised emulsions: Effect of low molecular weight emulsifiers . Food Hydrocolloids , 15 : 253 – 262 .
- Firebaugh , J.D. and Daubert , C.R. 2005 . Emulsifying and foaming properties of a derivatized whey protein ingredient . International Journal of Food Properties , 8 : 243 – 253 .
- Barnes , H.A. , Hutton , J.F. and Walters , K. 1989 . An Introduction to Rheology , Amsterdam, , the Netherlands : Elsevier Science .
- Chirife , J. and Buera , M.P. 1997 . A simple model for predicting the viscosity of sugar and oligosaccharide solutions . Journal of Food Engineering , 33 : 221 – 226 .
- Migliori , M. , Gabriele , D. , Di Sanzo , R. , de Cindio , B. and Correra , S. 2007 . Viscosity of multi-component solutions of simple and complex sugars in water . Journal of Chemical Engineering and Data , 52 : 1347 – 1353 .
- Ford , L.D. , Borwankar , R. , Martin , R.W. and Holcomb , D.N. 2000 . “ Dressing and sauces ” . In Food Emulsions , 3rd , Edited by: Friberg , S.E. and Larsson , K. 361 – 412 . New York : Marcel Dekker Inc .